Abstract
AIM: To investigate the mechanisms involved in a possible modulator role of interleukin (IL)-6 signalling on CYR61-CTGF-NOV (CCN) 2/connective tissue growth factor (CTGF) expression in hepatocytes (PC) and to look for a relation between serum concentrations of these two parameters in patients with acute inflammation.
METHODS: Expression of CCN2/CTGF, p-STAT3, p-Smad3/1 and p-Smad2 was examined in primary freshly isolated rat or cryo-preserved human PC exposed to various stimuli by Western blotting, electrophoretic mobility shift assay (EMSA), reporter-gene-assays and reverse-transcriptase polymerase chain reaction.
RESULTS: IL-6 strongly down-regulated CCN2/CTGF protein and mRNA expression in PC, enhanceable by extracellular presence of the soluble IL-6 receptor gp80, and supported by an inverse relation between IL-6 and CCN2/CTGF concentrations in patients’ sera. The inhibition of TGFβ1 driven CCN2/CTGF expression by IL-6 did not involve a modulation of Smad2 (and Smad1/3) signalling. However, the STAT3 SH2 domain binding peptide, a selective inhibitor of STAT3 DNA binding activity, counteracted the inhibitory effect of IL-6 on CCN2/CTGF expression much more pronounced than pyrrolidine-dithiocarbamate, an inhibitor primarily of STAT3 phosphorylation. An EMSA confirmed STAT3 binding to the proposed proximal STAT binding site in the CCN2/CTGF promoter.
CONCLUSION: CCN2/CTGF is identified as a hepatocellular negative acute phase protein which is down-regulated by IL-6 via the STAT3 pathway through interaction on the DNA binding level.
Keywords: Hepatocytes, Interleukin-6, Connective tissue growth factor, STAT3, Liver fibrosis, Acute phase reaction
INTRODUCTION
Fibrogenic restructuring of the liver is commonly caused by chronic inflammatory processes. Upon perpetuation of the initial inflammatory attack, a rapid synthesis of several proteins, which is stimulated by cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, and particularly IL-6, takes place in order to restore homeostasis. This process is widely known as the hepatocellular acute phase reaction upon the initial tissue injury, infection or inflammation[1].
CYR61-CTGF-NOV (CCN) 2/connective tissue growth factor (CTGF), a member of the CCN superfamily of secreted, cysteine-rich glycoproteins, has been implicated in the pathogenesis of hepatic fibrosis and is currently suggested to be an important downstream amplifier of the effects of the profibrogenic master cytokine transforming growth factor (TGF)-β[2,3]. Its molecular mechanism of action is still not known in detail, but it very likely strengthens the binding of TGFβ1 to its cognate receptors[4]. Its crucial role in fibrogenesis is documented by strong upregulation in fibrotic liver tissue[5-7], and even more importantly by recent studies, in which knock-down of CCN2/CTGF by siRNA lead to substantial attenuation of experimental liver fibrosis[8,9]. Recently, we were among the first to identify that hepatocytes (PC) substantially synthesize CCN2/CTGF in cell culture and in injured liver, and that CCN2/CTGF is sensitively up-regulated by TGFβ1[10-12].
IL-6, originally identified as a B cell differentiation factor in 1981[13] is a pleiotropic cytokine, which is in the liver mostly synthesized by hepatic macrophages (Kupffer cells) or CD4+ T-helper (Th) cells[14-16]. IL-6 signals through a cell-surface type I cytokine receptor complex consisting of the ligand-binding IL-6Rα chain (gp80, CD126), and the signal-transducing component gp130, which is the common signal transducer for several cytokines including leukemia inhibitory factor (LIF), oncostatin M, or IL-11, and which is almost ubiquitously expressed in most tissues[17]. In contrast, the expression of gp80 is restricted to certain cells such as PC, neutrophils, monocytes/macrophages and some lymphocytes. However, naturally occurring soluble IL-6R together with IL-6 can stimulate cells lacking gp80 receptor part, a process termed trans-signalling[18]. As IL-6 interacts with its receptor gp80, it triggers the gp130 and IL-6R proteins to form a complex, thus activating the receptor. These complexes bring together the intracellular regions of gp130 to initiate a signal transduction cascade through certain transcription factors, Janus kinases (JAKs) and Signal Transducers and Activators of Transcription (STATs), but may also lead to an activation of MAP-kinase (MAPK) and phosphoinositide 3-kinase (PI3K) signalling cascades[19].
In the present study, we investigated the anti-fibrogenic effect of IL-6 comparing with the effect of other selected cytokines (IL-12, IL-2) on CCN2/CTGF. The down-regulating effect of IFN-γ on CCN2/CTGF in hepatocytes and hepatic stellate cells was already shown by others[11,20]. On the other hand, the up-regulation of IL-6 induced by CCN2/CTGF was shown in pancreatic stellate cells[21].
Earlier reports by van Gool et al[22] gave evidence that the stereotypical rat acute phase reactant α2-macrofetoprotein acts as an inhibitor of experimental hepatitis; however, the impact of this or other acute phase proteins such as IL-6 on CCN2/CTGF production in PC and the molecular basis of CCN2/CTGF involvement in the acute phase reaction are yet unknown.
We therefore investigated the mechanisms involved in a possible modulator role of IL-6 signalling on CCN2/CTGF expression in rat and human PC and looked for a possible association between serum concentrations of these two parameters in patients with acute inflammation. Our findings propose that CCN2/CTGF serves as a hepatocellular negative acute phase protein which is down-regulated by IL-6 via the STAT3 pathway.
MATERIALS AND METHODS
Reagents
Cytokines or soluble cytokine receptors: rrIL-6 (506-RL), IL-2, IL-12, rhTGFβ1 (240-B) and rhsgp130 (228-GP) were all from R&D Systems (Minneapolis, MN); rhsgp80 was prepared as described by Weiergräber et al[23] and kindly provided by the Department of Biochemistry, University Hospital of RWTH Aachen, Germany. rhIL-6 (1131567) was from Roche (Mannheim, Germany). STAT3 inhibitors: PDTC was from Sigma-Aldrich (St. Louis, MO); cell permeable STAT3 inhibitor peptide (PY*LKTK, Cat. No. 573096) was from Calbiochem (Darmstadt, Germany). Specific MAP Kinase inhibitors used in this study were: PD98059 (Cat. No. 513000), SB203580 (Cat. No. 559398), and UO126 (Cat. No. 662005) were all from Calbiochem (Darmstadt, Germany), Edelfosine (PKI-ET18) was from Biaffin (Kassel, Germany).
Antibodies for Western blotting: rabbit anti-Smad3 (ab28379) and chicken anti-α1-AT (ab14226) (Abcam, Cambridge, UK); goat anti-CTGF/CCN2 (L-20, sc-14939) (Santa Cruz, CA); rabbit anti-p-Smad3 (Ser423/425)/p-Smad1 (Ser463/465) (#9514), rabbit anti-p-Smad2 (Ser465/467) (#3101), rabbit anti-Smad2 (#3102), rabbit anti-phospho-STAT3 (Tyr705) (#9145) and mouse anti-STAT3 (#9139) (Cell Signalling/New England Biolabs, Ipswich, MA); mouse anti-β-actin (AC-15, Cat. No. 5441, Sigma-Aldrich).
Animals
Adult male Sprague-Dawley rats (body weight 180 to 220 g, between 0.5 and 0.8 years of age) had free access to a standard laboratory chow diet and normal tap water throughout the experimental period. All animals received care and treatment in compliance with the German Animal Protection Act, which is in accordance with the German Research Council’s criteria.
Isolation and culture of rat hepatocytes
Primary rat PC were isolated from male Sprague-Dawley rats by the two-step collagenase method of Seglen[24] modified as described before[25]. Cell culture was performed under serum-free conditions as previously described[10]. Supplementation of the culture medium with rrIL-6, rhTGFβ1 or STAT3 inhibitors was performed as described in the respective figure legends.
Western blotting analysis
Preparations of cytoplasmic cell extracts, determination of protein concentrations, and Western blotting analysis were performed exactly as previously described[12,26]. Densitometric quantification of the blot results was done with the Lumi-Imager (Roche, Mannheim, Germany) and the LumiAnalyst 3.0 Software (Roche).
Reverse-transcriptase polymerase chain reaction for rat CCN2/CTGF
Total cellular RNA was extracted with the Qiagen RNeasy Mini purification kit (Qiagen, Hilden, Germany). cDNA was reverse-transcribed using the First-Strand cDNA synthesis kit III (Invitrogen, Karlsruhe, Germany). Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed using the Geneamp 9700 Thermocycler PCR System (Applied Biosystems, Germany) and the following primers: rCCN2/CTGF (forward: 5'-CGTCCATGCTGCACAGG-3'; reverse: 5'-CAAGTTTGAGCTTTCTGG-3') as previously described[3]. The size of the amplified fragment was 144 bp.
Electrophoretic mobility shift assay
Nuclear extracts of rat PC treated with rrIL-6 (10 μg/L, 30 min), were prepared and the electrophoretic mobility shift assay (EMSA) performed slightly modified from that previously described[27]. In brief, for EMSA, 3.4 μg of total nuclear protein were used for each lane. The oligonucleotide sequence used to assess the two proposed STAT binding sites in the CCN2/CTGF promoter (proximal: -418 to -415 bp, distal: -384 to -381 bp)[28] was 5'-AGGAATTCCTGCTGTTTGCCTCTTCAGCTACCTACTTCCT-3'. The mutated proximal STAT binding site (mt, proximal) was analyzed using the following oligonucleotide oligonucleotide sequence: 5'-AAGAATTCCTGCTGTTTGCCTCTTCAGCTACCTACTTCCT-3'. The oligonucleotide sequence of the probe reflective of the mutant distal putative STAT binding site (mt, distal) was: 5'-AGGAATTCCTGCTGTTTGCCTCTTCAGCTACCTACTTCTT-3'. All oligos were synthetized by MWG Biotech (Ebersberg, Germany). The synthetic oligonucleotides were labeled using a T4 polynucleotide kinase (DNA 5'-End Labeling Kit; Promega, Mannheim, Germany) and [γ32P]ATP (Perkin Elmer, Rodgau-Jügesheim, Germany). Following incubation of the radiolabeled probes with nuclear extracts, protein-DNA complexes were resolved on a NOVEX 6% retardation gel (Invitrogen) and detected using a Typhoon 9410 PhosphoImager (Amersham Biosciences, Freiburg, Germany).
Generation of recombinant CCN2/CTGF reporter adenovirus (Ad-hCTGF-Luc)
For generation of the reporter adenovirus Ad-hCTGF-Luc, the approximately 2.5-kbp ClaI fragment of vector pGL3-Basic-hCTGF-Luc[12] harbouring a fusion of human CCN2/CTGF gene promoter and the luciferase reporter gene was cloned into the ClaI site of vector pΔE1sp1A[29] resulting in the generation of pΔE1sp1A-hCTGF-Luc. The integrity of cloning boundaries was verified by restriction analysis and sequencing with the flanking primers 5'-GCGTAACCGAGTAAGAATTTG-3' and 5'-GGCGACCATCAATGCTGGAG-3' that were obtained from MWG-Biotech AG (Ebersberg, Germany).
The integration of the reporter cassette from pΔE1sp1A-hCTGF-Luc into the adenoviral backbone vector pJM17[30] was performed by in vitro homologous recombination in the human embryo kidney cell line 293 using a protocol described before[31]. Successful generation of recombinant viral particles was visualized by viral foci formation. After total infection, the viral particles were released from cells by three rounds of a freeze-thaw cycle and separated from cell debris by centrifugation at 3000 r/min for 10 min. To generate high titer viral stocks, 293 cells were re-infected at a multiplicity of infection (MOI) of 1 and grown for 3-4 d. Amplified viruses were harvested, concentrated through standard CsCl gradient centrifugation and subsequently purified using the BD Adeno-X™ Purification Filter system (BD Biosciences, Clontech, Palo Alto, CA) according to the manufacturer’s instructions.
Luciferase gene reporter assay
Cells were cultured in black 96 well plates and infected with 1 × 108 virons/mL of Ad-hCTGF-luc reporter virus. After specific treatment, the luciferase activity was measured as described previously[3].
Culture of primary human hepatocytes
Primary human hepatocytes from a 15-year-old female donor of Caucasian origin with history of traumatic head injury (BD Gentest™ Cryopreserved Human Hepatocytes, Cat. No. 454551, Lot. No. 208, BD Bioscience, Franklin Lakes, NJ) were cultured according to the distributor’s instructions. Primary human hepatocytes were stored in liquid nitrogen vapor until ready and were gradually thawed for further use[32]. Cell purification was performed using Cryopreserved Hepatocytes Purification Kit (Cat. No. 454500, BD Bioscience). The viability of the final cell suspension, checked by trypan blue exclusion, was around 70% and cell recovery was 9-11 × 106 cells/vial. For the induction assay, primary human hepatocytes were seeded in ISOMs Seeding Media supplemented with 5% FCS, 2 mmol/L L-glutamine, penicillin (100 kIU/L), streptomycin (100 g/L) (all from BioWhittaker Europe) and ascorbic acid (50 mg/L, Merck Biochemicals, Darmstadt, Germany) on type I collagen coated plastic dishes (BD Bioscience) with a density of 19 × 104 cells/cm2 and cultured in a humidified atmosphere (37°C, 5% CO2). The first change of medium was performed with HepatoStim medium (Cat. No. 355056, BD Bioscience) supplemented with L-glutamine, penicillin and streptomycin as described above and rhEGF (Cat. No. 354052, BD Bioscience) 4 h after seeding. The cells were incubated for another 24 h until the medium was changed and supplemented with the indicated concentrations of rhIL-6, gp80 and gp130 for further 24 and 48 h as described in the respective figure legends.
Patients
Patients admitted to the hospital with different severity of an acute phase reaction [n = 36; mean age 47 years, range 1-86 years; 27 males (age 8-86 years) and 9 females (age 1-54 years)] having serum IL-6 concentrations routinely determined as part of their disease-related diagnostics, were included in the study. Patients were divided into two groups according to their serum IL-6 concentrations: Group 1 - IL-6 < 100 ng/L [n = 21; mean age 54 years, range 20-74 years; 18 males (age 18-74 years) and 3 females (age 14-37 years)]; Group 2 - IL-6 > 100 ng/L [n = 15; mean age 42 years, range 1-86 years; 9 males (age 8-86 years) and 6 females (age 1-54 years)]. Peripheral venous blood samples were taken in the morning (6 to 8 AM) upon admission to the hospital. Serum was separated at 4000 g 30 to 60 min after clot-retraction and stored at -80°C. At the time of blood sample collection, the patients did not suffer from liver fibrosis or renal insufficiency, as indicated by normal serum activities of ALT, AST, gamma-GT (GGT), and creatinine concentrations, respectively. All blood samples were used anonymously.
Determination of human serum IL-6 and CCN2/CTGF concentrations
Quantitative determination of human IL-6 was performed using a chemiluminescence assay on the Immulite 2000 autoanalyzer (Siemens Medical Healthcare, Erlangen Germany). Serum levels of the CCN2/CTGF were determined in replicates using a human CCN2/CTGF sandwich enzyme-linked immunosorbent assay provided by DRG, Mountainside, NJ, USA (Cat. No. 090731). CCN2/CTGF protein standards were obtained from BioVendor, Heidelberg, Germany (Cat. No. RD172035100).
Statistical analysis
For statistical analysis, SPSS 16.0 (SPSS, Chicago, IL) was used, applying two-tailed unpaired Student’s t tests with a P value for significance set at least at 0.05. The correlations between variables were analyzed with the Pearson correlation tests. Values of P < 0.05 were considered statistically significant.
RESULTS
IL-6 inhibits CCN2/CTGF expression in cultured rat hepatocytes
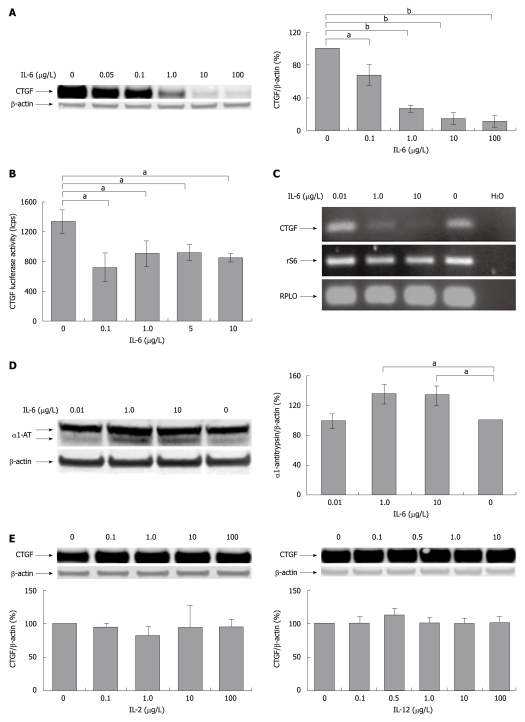
We tested the effects of cytokines produced by liver residing immunocompetent cells on CCN2/CTGF expression in PC (Figure 1). Application of rrIL-6 (as representative of the IL-6 family of interleukins) strikingly reduced hepatocellular CCN2/CTGF protein expression in a dose dependent manner as seen in Western blotting analysis (Figure 1A) and also inhibited the transcriptional activation of the pGL3-hCTGF-luc reporter (Figure 1B). This finding is supported by the observation that rrIL-6 suppressed mRNA level of CCN2/CTGF in PC (Figure 1C). The difference in the intensity of reduction in Western blotting analysis and reporter gene assay may be explained by the fact that consistent reduction of CCN2/CTGF de novo synthesis, as seen by the moderate reduction of CCN2/CTGF promoter activity, eventually results in a strong reduction of overall availability of CCN2/CTGF within the cell, as seen by an even stronger decrease of CCN2/CTGF protein expression in Western blotting analysis. As expected the stimulation with IL-6 induced synthesis of α1-antitrypsin, an acute phase protein in cultured rat PC (Figure 1D).
Figure 1.
Interleukin-6 inhibits CYR61/CTGF/NOV 2/connective tissue growth factor expression in cultured rat hepatocytes. A: Western blotting of CYR61-CTGF-NOV (CCN) 2/connective tissue growth factor (CTGF) of rat hepatocytes (PC) cultured for 24 h under serum-free conditions with or without addition of indicated concentrations of rr interleukin (rrIL)-6. β-actin served as loading control. A representative blot is shown. Blots were quantified relative to β-actin using the Lumi Imager System. Quantifications represent the mean ± SD of 3 independent cultures. aP < 0.05, bP < 0.0001 vs untreated control; B: CCN2/CTGF reporter gene activation. Rat PC were cultured in serum-free medium for 24 h and transfected with Ad-hCTGF-Luc then subjected to the indicated concentrations of rrIL-6 16 h after transfection and cultured for another 24 h before harvest. Mean values (± SD from 3 cultures) are shown. aP < 0.05 vs untreated control; C: Reverse-transcriptase polymerase chain reaction (RT-PCR) of CCN2/CTGF of rat PC. Rat PC were cultured in serum-free medium and treated with rrIL-6 at indicated concentrations for 24 h. RT-PCR was performed using primers for rat CCN2/CTGF as described in Materials and Methods. rS6 and RPLO served as internal control. A representative experiment of 3 independent cultures is shown; D: Western blotting of α1-AT of rat PC cultured for 24 h under serum-free conditions with or without addition of indicated concentrations of rrIL-6. A representative blot is shown. Blots were quantified as described in (A). aP < 0.05 vs untreated control; E: Western blottings of CCN2/CTGF of PC cultured for 24 h in serum-free medium with or without addition of indicated concentrations of IL-2 or IL-12. β-actin served as loading control. Representative blots of 3 independent experiments are shown. Blots were quantified as described in (A).
In contrast, treatment with IL-2 (as representative of the common γ chain family of interleukins), and IL-12 (as representative of the IL-12 family of interleukins), showed no change in either CCN2/CTGF protein expression (Figure 1E) or transcriptional activation of the pGL3-hCTGF-luc reporter gene (unpublished data) in cultured PC.
TGFβ1 fails to induce CCN2/CTGF expression in cells pretreated with IL-6
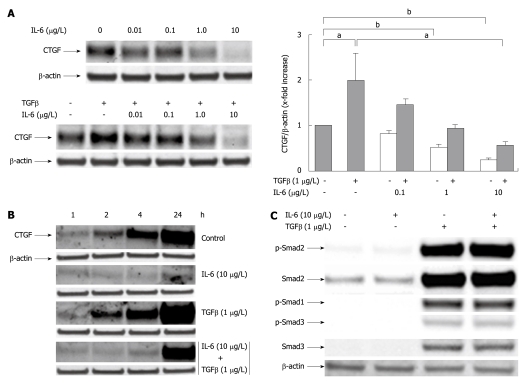
As previously reported[12] rhTGFβ1 induced CCN2/CTGF protein expression in PC (Figure 2). Based on this, we tested whether rrIL-6 was able to reduce not just spontaneous, but also TGFβ1-driven CCN2/CTGF protein expression. Indeed TGFβ1 largely failed to induce CCN2/CTGF expression in cells pretreated with different dosages of rrIL-6 (Figure 2A), an observation particularly prominent at earlier time points (2, 4 h) (Figure 2B). The inhibition of TGFβ1 driven CCN2/CTGF expression by rrIL-6 did not involve a modulation of TGFβ1 induced Smad signalling as rrIL-6 had no negative effect on phosphorylation of both Smad-2 and -3 proteins (Figure 2C). However, the observed immediate suppression of CCN2/CTGF synthesis, already within the first 2 h after stimulation with IL-6, suggests a direct interaction between IL-6 induced STAT3 signalling and transcriptional activation of the CCN2/CTGF promoter.
Figure 2.
Interleukin-6 acts as an inhibitor of transforming growth factor β1 induced CYR61/CTGF/NOV 2/connective tissue growth factor protein expression in cultured rat hepatocytes. A: Western blottings of CYR61/CTGF/NOV (CCN) 2/connective tissue growth factor (CTGF) of rat hepatocytes (PC) cultured under serum-free conditions with or without addition of rr interleukin (rrIL)-6 at indicated concentrations 30 min prior addition of rhTGFβ1 (1 μg/L). The cell culture only with IL-6 at indicated concentrations served as internal control. Cells were harvested after another 24 h. β-actin served as loading control. Representative blots are shown. Blots were quantified relative to β-actin using the Lumi Imager System. Quantifications represent the mean ± SD of 3 independent cultures. aP < 0.05, bP < 0.0001 vs untreated control; B: Western blottings of CCN2/CTGF of rat PC cultured as stated in (A) under serum-free conditions with or without addition of rrIL-6 (10 μg/L) 30 min prior addition of rhTGFβ1 (1 μg/L) where indicated. The cells were harvested after 1, 2, 4 and 24 h. β-actin served as loading control. A representative blot of 3 independent experiments is shown; C: Western blottings of phosphorylated and total Smad2 and Smad3, the latter antibody cross-reacting with Smad1. Rat PC were cultured for 24 h under serum-free conditions with or without addition of rh transforming growth factor (TGF) β1 (1 μg/L) and indicated concentrations of rrIL-6. Representative blots are shown.
Complexation of IL-6 and sgp80 enhances the inhibitory effect of this cytokine on CCN2/CTGF protein expression in primary human hepatocytes
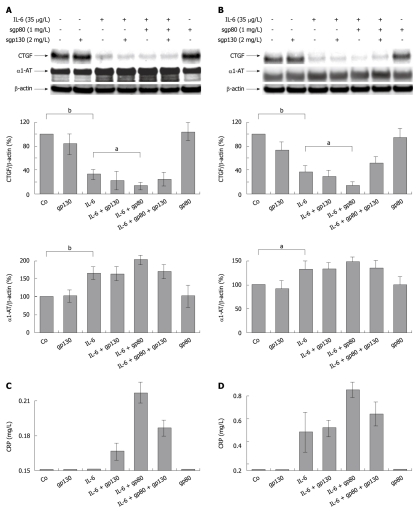
In order to avoid possible species specific phenomena, we therefore used primary human hepatocytes for ongoing studies in this direction. Our aim was to investigate whether the enhancing effect of sgp80 on IL-6 signaling, as previously described by Rose-John et al[18] for other cellular systems, was also transferrable to IL-6 dependent repression of hepatocellular CCN2/CTGF protein expression.
As observed in rat PC, application of rhIL-6 strikingly reduced CCN2/CTGF protein expression in primary human hepatocytes cultured for another 24 h and 48 h after stimulation (Figure 3A and B). This effect was enhanced by pre-incubation of IL-6 with sgp80 and attenuated by co-incubation with recombinant human soluble gp130 (sgp130) complexing with IL-6, and sgp80 (Figure 3B). As expected, synthesis of classical acute phase proteins such as α1-AT and C-reactive protein (CRP) was increased in both cell fraction (Figure 3A and B) and conditioned culture medium of primary human hepatocytes following pre-incubation of IL-6 with sgp80 (Figure 3C and D).
Figure 3.
Soluble gp80 receptor enhances the inhibitory effect of interleukin-6 on hepatocellular CYR61/CTGF/NOV 2/connective tissue growth factor expression in primary human hepatocytes. A: Western blottings of CYR61/CTGF/NOV (CCN) 2/Connective tissue growth factor (CTGF) and α1-AT of primary human hepatocytes cultured under serum-free conditions for 24 h and stimulated with rh interleukin (rhIL)-6 (35 μg/L), soluble gp80 receptor (sgp80, 1 mg/L) and soluble gp130 receptor (sgp130, 2 mg/L) or a complex of both. Cells were harvested after 24 h. β-actin served as loading control. Blots were quantified relative to β-actin using the Lumi Imager System. Representative blots are shown. aP < 0.005, bP < 0.0001 vs untreated or IL-6 treated control; B: Primary human hepatocytes were cultured and stimulated as described in (A). Cells were harvested after 48 h. β-actin served as loading control. Blots were quantified as described in (A). Representative blots are shown. aP < 0.005, bP < 0.0001 vs untreated or IL-6 treated control; C, D: Ultrasensitive C-reactive Protein as determined using a particle enhanced ultra sensitive assay on the Siemens BN2 nephelometer in supernatants from primary human hepatocytes cultures harvested after 24 h (C) and 48 h (D). The baseline indicates the lower detection limit of the assay at 0.15 mg/L.
Inhibition of STAT3 counteracts the IL-6 induced suppression of CCN2/CTGF expression in cultured rat hepatocytes
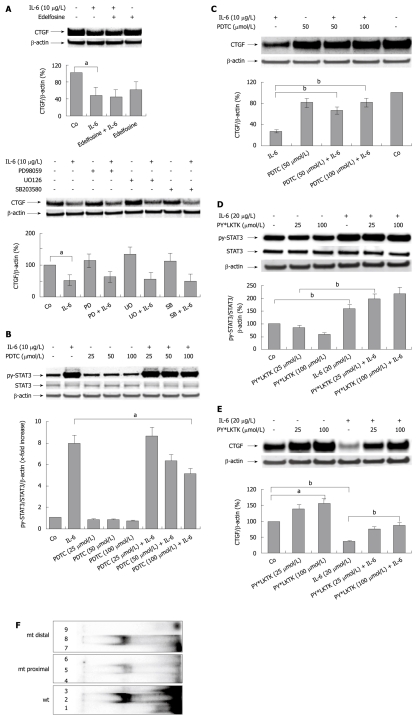
To study the mechanism of IL-6 induced suppression of CCN2/CTGF, the signal transduction pathway known to mediate IL-6 specific effects to the nucleus was interrupted at different levels using specific inhibitors (Figure 4). Inhibition of MAP-kinase signaling by the specific inhibitors (PD98059, SB203580, and UO126) did not abrogate the inhibitory effects of IL-6 on hepatocellular CCN2/CTGF synthesis (Figure 4A). Also, blocking of phosphatidylinositol phospholipase C signaling by administration of edelfosine did not interfere with IL-6 signaling to the CCN2/CTGF promoter (Figure 4A).
Figure 4.
Interleukin-6 mediates its inhibitory effect on hepatocellular CYR61/CTGF/NOV 2/connective tissue growth factor expression through activation of the STAT3 pathway. A: Western blottings ofCYR61/CTGF/NOV (CCN) 2/connective tissue growth factor (CTGF) of rat hepatocytes (PC) cultured under serum-free conditions with or without addition of the phosphatidylinositol phospholipase C inhibitor edelfosine (10 μmol/L, above) or specific MAP-Kinase inhibitors PD98059 (30 μmol/L), UO126, (10 μmol/L), as well as SB203580, (30 μmol/L) (below) administered to the culture medium 30 min before the addition of rr interleukin (IL)-6 (10 μg/L). Cells were harvested after 24 h. β-actin served as loading control. Blots were quantified relative to β-actin using the Lumi Imager System. A representative blot of 3 independent experiments is shown. aP < 0.005. PD: PD98059; SB: SB203580; UO: UO126; B: Western blottings of phosphorylated and total STAT3 of rat PC cultured under serum-free conditions with or without addition of PDTC at indicated concentrations 2 h prior addition of rrIL-6 (10 μg/L). Cells were harvested after 30 min. β-actin served as loading control. Representative blots of 3 independent cultures are shown. Blots were quantified relative to β-actin using the Lumi Imager System. Quantifications represent the mean ± SD of 3 independent cultures. aP < 0.05 vs IL-6 treated (PDTC untreated) control; C: Western blottings of CCN2/CTGF of rat PC cultured under serum-free conditions with or without addition of PDTC at indicated concentrations 2 h prior addition of rrIL-6 (10 μg/L). Cells were harvested after another 2 h. β-actin served as loading control. A representative blot out of 3 is shown. Blots were quantified as described in (A). bP < 0.0001 vs IL-6 treated (PDTC untreated) control; D: Western blottings of PY-STAT3 and STAT3 of rat PC cultured under serum-free conditions with or without addition of PY*LKTK at indicated concentrations 1 h prior addition of rrIL-6 (20 μg/L). Cells were harvested after another 30 min. β-actin served as loading control. Blots were quantified relative to β-actin using the Lumi Imager System. A representative blot out of 3 is shown. bP < 0.0001; E: Western blottings of CCN2/CTGF of rat PC cultured under serum-free conditions with or without addition of PY*LKTK at indicated concentrations 1 h prior addition of rrIL-6 (20 μg/L). Cells were harvested after another 24 h. β-actin served as loading control. Blots were quantified relative to β-actin using the Lumi Imager System. A representative blot out of 3 is shown. aP < 0.005, bP < 0.0001; F: EMSA using nuclear lysates of PC treated with rrIL-6 (10 μg/L; 30 min) and 32P-labeled double-stranded oligonucleotide probes containing the two proposed wild-type (wt) STAT binding sites as well as the mutated (mt) proximal and distal STAT binding sites in the CTGF promoter. Lane 1: Labeled probe containing both proposed STAT binding sites; lane 2: Nuclear extract and labeled probe containing both proposed STAT binding sites (wt); lane 3: Nuclear extract, labeled probe and 100-fold molar excess of unlabeled probe containing both proposed STAT binding sites (wt); lane 4: Labeled mutated (mt, proximal) probe; lane 5: Nuclear extract and labeled mutated (mt, proximal) probe; lane 6: Nuclear extract, labeled mutated (mt, proximal) probe and 100-fold molar excess of unlabeled mutated (mt, proximal) probe; lane 7: Labeled mutated (mt, distal) probe; lane 8: Nuclear extract and labeled mutated (mt, distal) probe; lane 9: Nuclear extract, labeled mutated (mt, distal) probe and 100-fold molar excess of unlabeled mutated (mt, proximal) probe. The following specific activities were determined using scintillation counting: wt double strand oligonucleotide, 5.23 × 107 cpm/μg DNA; mt proximal oligonucleotide, 3.70 × 107 cpm/μg DNA; mt distal oligonucleotide, 3.73 × 107 cpm/μg DNA. The activities put on the gel were: wt double strand oligonucleotide, 33090 cpm; mt proximal oligonucleotide, 45 844 cpm; mt distal oligonucleotide, 31 556 cpm.
However, exposure of cells to rrIL-6 leads to an activation of the JAK/STAT3 pathway by inducing STAT3 phosphorylation in primary rat PC[33]. PC were pre-treated with pyrrolidine dithiocarbamate (PDTC) for 2 h and subsequently stimulated with rrIL-6. PDTC was previously proven to primarily impair STAT3 phosphorylation in PC[34]. The extent of the phosphorylation of the tyrosine residue (PY705) of STAT3, important for STAT3 dimerisation and DNA binding activities, was analyzed by Western blotting analysis using a phospho-specific antibody in cells treated with rrIL-6 for up to 30 min. PDTC reduced STAT3 phosphorylation in a dose dependent manner (Figure 4B), while CCN2/CTGF protein expression, reduced by rrIL-6, could be restored up to 80%-90% of the untreated control 2 h after pre-application of PDTC (Figure 4C). One hour pre-incubation of rat hepatocytes with the cell permeable STAT3 SH2 domain binding peptide (PY*LKTK), known to be a highly selective inhibitor of STAT3 DNA binding activity[35], in contrast to PDTC, did not impair STAT3 (PY705) phosphorylation, as expected (Figure 4D), but had a strong counteracting effect on IL-6 induced inhibition of CCN2/CTGF expression (Figure 4E). These results suggest that IL-6 mediates its repressive effect on CCN2/CTGF synthesis via direct interaction of activated (phosphorylated) STAT3 with the CCN2/CTGF promoter. An EMSA demonstrated that the synthetic double-stranded oligonucleotide containing the two putative STAT binding sites forms a major protein−DNA complex with nuclear extracts from rat PC treated with 10 μg/L rrIL-6 (Figure 4F; wt, labeled). Whereas application of a mutated proximal binding site (Figure 4F; mt proximal, labeled) did affect binding of STAT3 to the CTGF promoter sequence, the mutated distal binding site (Figure 4F; mt distal, labeled) did not. These findings suggest that the distal binding site has a lower affinity for STAT3 than the proximal site.
Inverse association between IL-6 and CCN2/CTGF serum concentrations in patients with different severity of an acute phase reaction
All the findings discussed above were based exclusively on results of in vitro experiments. However, results obtained from in vitro studies are frequently not directly transferable to the in vivo situation. Therefore, we investigated serum concentrations of CCN2/CTGF and IL-6 in Caucasian patients with different extent of an acute phase reaction, hypothesizing that a change in serum concentrations of IL-6 influences CCN2/CTGF serum concentrations in the respective patients.
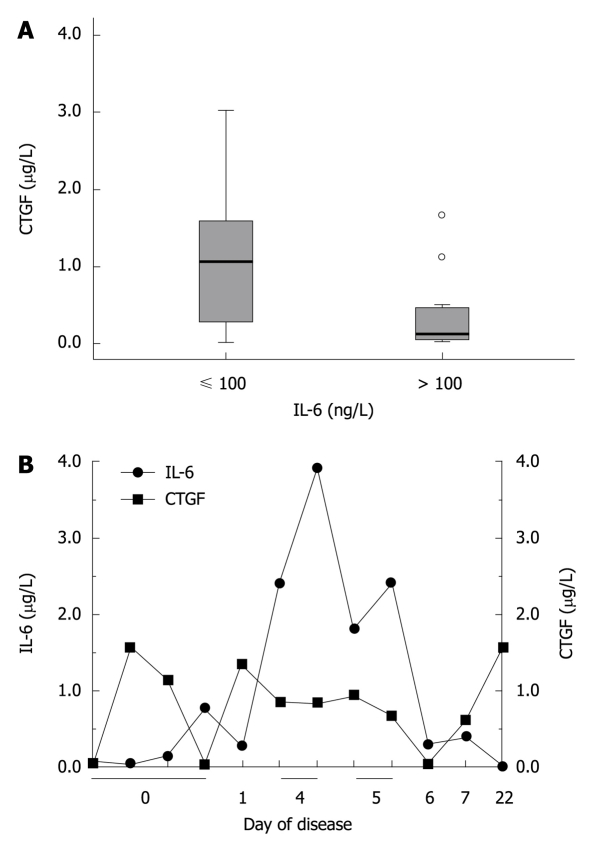
In order to further corroborate this hypothesis, we clustered sepsis patients into two groups with defined ranges of IL-6 serum concentrations [2-99 ng/L (< 100 ng/L), 104-6100 ng/L (> 100 ng/L)], and compared CCN2/CTGF serum concentrations between both groups (Figure 5A). Results impressively demonstrate an inverse association between IL-6 and CCN2/CTGF serum concentrations with highly significant differences (P < 0.0001) between patients with low IL-6 concentrations and those with IL-6 concentrations > 100 ng/L. Data are shown in Tables 1, 2 and 3.
Figure 5.
Inverse relation of interleukin-6 and CYR61/CTGF/NOV 2/connective tissue growth factor serum concentrations in patients with different severity of an acute phase reaction. A: Patients (n = 36) were categorized according to their interleukin (IL)-6 serum concentrations. Corresponding CYR61/CTGF/NOV (CCN) 2/connective tissue growth factor (CTGF) concentrations were significantly lower in patients with high IL-6 serum concentrations (IL-6 > 100 ng/L, n = 15) compared to those with low IL-6 serum concentrations (IL-6 ≤ 100 ng/L, n = 21). Box plot are displayed, where the dotted line indicates the median per group, the box represents 50% of the values, and horizontal lines show minimum and maximum values of the calculated non-outlier values; open circles indicate outlier values; B: Longitudinal development of CCN2/CTGF and IL-6 serum concentrations in one representative individual patient (20 years old, male) over a time period of 22 d.
Table 1.
Mean and 95% CI for mean (in braces) of serum concentrations of interleukin-6 and connective tissue growth factor in two groups of patients with different severity of an acute phase reaction
| IL-6 (ng/L) | CTGF (μg/L) | |
| Group 1: IL-6 < 100 ng/L (n = 21) | 36 (1.7-54.3) | 880.7 (344.0-1417.4) |
| Group 2: IL-6 ≥ 100 ng/L (n = 15) | 1028.3 (46.6-2010.0) | 391.2 (109.2-673.3) |
IL-6: Interleukin-6; CTGF: Connective tissue growth factor.
Table 2.
One sample statistics
| n | Mean CTGF (μg/L) | Std. deviation | Std. error mean | |
| IL-6 < 100 | 21 | 851.18 | 900.50 | 196.51 |
| IL-6 ≥ 100 | 15 | 391.23 | 509.36 | 131.52 |
IL-6: Interleukin-6; CTGF: Connective tissue growth factor.
Table 3.
One-sample test
|
Test value = 0 |
||||
| t | Mean difference CTGF (μg/L) |
95% CI of the difference |
||
| Lower | Upper | |||
| IL-6 < 100 | 4.332 | 851.18 | 441.27 | 1261.08 |
| IL-6 ≥ 100 | 2.975 | 391.23 | 109.15 | 673.30 |
IL-6: Interleukin-6; CTGF: Connective tissue growth factor.
We next observed the longitudinal development of CCN2/CTGF and IL-6 serum concentrations in one representative individual patient over a time period of 22 d. Figure 5B demonstrates an inverse relation between IL-6 and CCN2/CTGF concentrations, suggesting an indirect response of serum CCN2/CTGF concentrations to the individual’s acute phase status.
DISCUSSION
It is firmly established that the fibrogenic process in the liver is prominently regulated by TGFβ1[36-38]. However, TGFβ1 has not only multiple profibrogenic, but also immunosuppressive effects[39-41]. Vice-versa, immunosuppressive agents, such as glucocorticoids, are capable of enhancing TGFβ1 induced target gene expression, in particular of CCN2/CTGF, in rat PC[42]. We and others have previously reported that PC substantially synthesize CCN2/CTGF during culture and in injured liver, that CCN2/CTGF is sensitively up-regulated by TGFβ1[10,11] in a Smad2 dependent mechanism and that PC are likely to be the major cellular source of CCN2/CTGF in the liver[12]. In turn, CCN2/CTGF acts as a downstream sensitizer of TGFβ1 actions in PC[3].
Inflammation and injury to tissue results in a variety of local and systemic events. However, although the local events of edema formation and cellular infiltration have received considerably more attention, the systemic response to inflammation is no less profound. The particular systemic event which forms the substance of this communication is the change in the circulating levels of plasma proteins which occurs after inflammatory injury, and the manner in which these changes in plasma concentration are controlled by changes in the rate of synthesis. The changes which occur are regulated at the liver by alteration of the rate of synthesis of the individual protein[43,44].
In this study, we provide evidence that IL-6 strongly down-regulates spontaneous as well as TGFβ1-induced CCN2/CTGF protein and mRNA expression in PC, an effect enhanced by the extracellular presence of the soluble IL-6 receptor gp80. These data were confirmed by an inverse relation between IL-6 and CCN2/CTGF serum concentration in patients with different extent of an acute phase reaction. The inhibition of TGFβ1 driven CCN2/CTGF expression by IL-6 did not involve modulation of TGFβ1 induced Smad2 (and Smad1/3), as well as MAP-kinase or phosphoinositide 3-kinase signaling, but required activation of the STAT3 pathway. Furthermore, the difference between the potency of pyrrolidine dithiocarbamate (PDTC), an inhibitor primarily of STAT3 phosphorylation, and PY*LKTK, a highly selective inhibitor of STAT3 DNA binding activity, as counteractors of IL-6 induced repression of CCN2/CTGF expression in PC imply that downregulation of CCN2/CTGF synthesis by IL-6 is mediated through direct interaction of activated (phosphorylated) STAT3 with the CCN2/CTGF promoter.
In the 600 bp fragment upstream of the transcription start site in the CCN2/CTGF promoter sequence putative STAT binding sites have been described (proximal: -418 to -415 bp, distal: -384 to -381 bp)[28], but their functional relevance has not been verified yet. The immediate suppression of CCN2/CTGF synthesis within the first 30 min after stimulation with IL-6, suggest a direct interaction between IL-6 induced STAT3 signalling and transcriptional activation of the CCN2/CTGF promoter, a hypothesis herein confirmed by EMSA showing that the distal binding site seems to have a lower affinity for STAT3 than the proximal one.
Based on these results, it may be suggested that CCN2/CTGF belongs to the family of negative acute-phase-reactants, displaying a decrease of synthesis during the acute inflammatory process. Several negative acute-phase-proteins have been identified so far, e.g. albumin, transferrin, transthyretin and transcortin[45]. But apart from transcortin, whose reduced bioavailability results in decreased glucocorticoid binding and, thus, in an enhancement of the inflammatory response, little is known of the biological function of these proteins.
Earlier studies by van Gool et al[46,47] investigated the clinical significance of the depressed acute-phase reaction in 14 patients with acute hepatitis B, and found that the acute-phase-reactant α2-macroglobulin (α2M) was negatively correlated to the subsequent course of hepatitis, and to the duration of the illness. This was most likely due to inflammatory inhibiting effects of α2M.
Therefore, it may be suggested that the observed downregulation of negative acute-phase-proteins should have similar effects on the pathogenesis of liver fibrosis as does the upregulation of acute-phase-proteins, i.e. attenuation of the inflammatory state through an enhancement of immune stimulation, decreased tissue injury, and, thus, in an retardation of the fibrogenic process. The opposite effects may be observed in conditions of immune suppression. In other terms, CCN2/CTGF would per se act as an immunosuppressive and profibrogenic which is antagonized by the acute-phase-reaction as an unspecific immune response. On the contrary, recent results of Karger et al[22] show that CCN2/CTGF induces IL-6 gene expression in pancreatic stellate cells, thus, in turn, enhancing the local inflammatory reactions in the pancreas. Little is known of the immunomodulatory capacities of CCN2/CTGF, but its crucial role in fibrogenesis is documented by strong upregulation in fibrotic liver tissue[5,6,48] and even more importantly by recent studies in which knock-down of CCN2/CTGF by siRNA leads to substantial attenuation of experimental liver fibrosis[8,9].
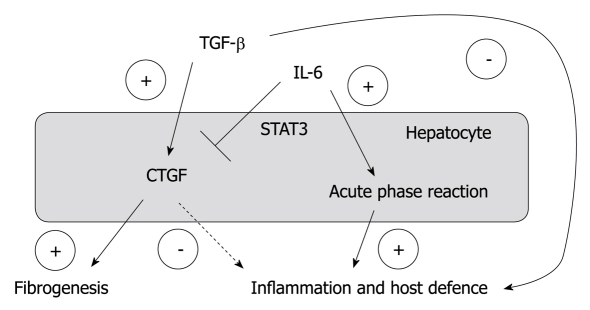
In summary, our results of an inhibitory effect of IL-6 on hepatocellular CCN2/CTGF expression suggest a role of CCN2/CTGF as “negative” acute-phase-protein, whose decreased synthesis during the acute-phase-reaction results in an, at least temporary, interruption of (TGFβ1 mediated) immune suppression and fibrogenesis, amplified by CCN2/CTGF (Figure 6). However, for an appropriate assessment of this phenomenon, a detailed understanding of a possible immunomodulatory role of CCN2/CTGF is needed. Our results hopefully initiate further studies in this direction.
Figure 6.
A simplified and schematic overview of the proposed interplay of interleukin-6 and tissue growth factor β1 on the regulation of CYR61/CTGF/NOV 2/connective tissue growth factor expression in hepatocytes and its relevance for inflammation, host defence and fibrogenesis in chronic liver disease. CTGF: Connective tissue growth factor; IL-6: Interleukin-6; TGF: Transforming growth factor.
COMMENTS
Background
Hepatocytes (PC) are a major cellular source of connective tissue growth factor (CCN2/CTGF), a downstream amplifier of profibrogenic transforming growth factor (TGF)-β1. Earlier reports identified the rat acute phase reactant α2-macrofetoprotein (α2M) as an inhibitor of experimental hepatitis and fibrosis; however, the impact of acute phase reaction initiating interleukins such as interleukin (IL)-6 on CCN2/CTGF synthesis in PC is still unknown. This paper investigates the mechanisms involved in a possible modulator role of IL-6 signalling on CCN2/CTGF expression in PC and looks for a possible relation between serum concentrations of these two parameters in patients with acute inflammation.
Research frontiers
CTGF=CCN2, one of six members of cysteine-rich, secreted, heparin-binding proteins with a modular structure, is widely recognized as an important player in hepatic and non-hepatic fibrogenic pathways. Its expression is strongly increased in fibrotic tissues and TGF-β, the profibrogenic master cytokine, is a strong stimulator of CTGF synthesis in hepatocytes, biliary epithelial cells and stellate cells. Functional activity as a mediator of fibre-fibre, fibre-matrix and matrix-matrix interactions, as an enhancer of profibrogenic TGF-β and several secondary effects owing to TGF-β enhancement, and as a down-modulator of the bioactivity of bone morphogenetic protein-7 have been shown or at least proposed. Consequently, knockdown of CTGF considerably attenuates experimental liver fibrosis. The spill-over of CTGF from the liver into the blood stream proposes this protein as a non-invasive reporter of TGF-β bioactivity in this organ. Indeed, it was shown that CTGF levels in sera correlate significantly with fibrogenic activity.
Innovations and breakthroughs
Fibrogenic restructuring of the liver is commonly caused by chronic inflammatory processes. Upon perpetuation of the initial inflammatory attack, a rapid synthesis of several proteins, which is stimulated by cytokines such as tumor necrosis factor (TNF)-α, IL-1, and particularly IL-6, takes place in order to restore homeostasis. This process is widely known as the hepatocellular acute phase reaction upon the initial tissue injury, infection or inflammation CTGF has been implicated in the pathogenesis of hepatic fibrosis and is currently suggested to be an important downstream amplifier of the effects of the profibrogenic master cytokine TGF-β which explains why experimental knockdown of CTGF considerably attenuates experimental liver fibrosis. Earlier reports gave evidence that the stereotypical rat acute phase reactant α2M acts as an inhibitor of experimental hepatitis; however, the impact of this or other acute phase proteins such as IL-6 on CCN2/CTGF production in PC and the molecular basis of CCN2/CTGF involvement in the acute phase reaction was long unknown, thereby launching the present study, whose results identify CTGF as a hepatic negative acute phase protein.
Applications
The results of the present study, showing an inhibitory effect of IL-6 on hepatocellular CCN2/CTGF expression, suggest a role of CCN2/CTGF as “negative” acute-phase-protein, whose decreased synthesis during the acute-phase-reaction results in an, at least temporary, interruption of (TGFβ1 mediated) immune suppression and fibrogenesis, amplified by CCN2/CTGF. However, for an appropriate assessment of this phenomenon, a detailed understanding of a possible immunomodulatory role of CCN2/CTGF is needed. The results hopefully initiate further studies in this direction.
Terminology
Acute Phase Reaction: The term acute phase response summarizes the endocrine or metabolic changes observed in an organism, either locally or systemically, a short time after injuries or the onset of infections, immunological reactions, and inflammatory processes. The acute phase reaction is initiated and mediated by a number of cytokines with inflammatory activities secreted by a variety of cell types (i.e. granulocytes, monocytes, lymphocytes, etc.) in response to the inflammatory stimuli. Acute Phase Protein: Acute-phase proteins are a class of proteins whose plasma concentrations increase (positive acute-phase proteins) or decrease (negative acute-phase proteins) during the acute phase reaction. CTGF=CCN2: CTGF is a 38 kDa, cysteine-rich, secreted peptide and a classical downstream target of TGF-β. Among the many functions of the CTGF gene family are embryogenesis, wound healing and regulation of extracellular matrix production. Liver Fibrosis: Hepatic fibrosis is overly exuberant wound healing in which excessive connective tissue builds up in the liver. The extracellular matrix is either overproduced, degraded deficiently, or both. The trigger is chronic injury, especially if there is an inflammatory component. TGF-β: TGF-β is a multifunctional cytokine that regulates tissue morphogenesis and differentiation through effects on cell proliferation, differentiation, apoptosis, and extracellular matrix production. TGF-β has been implicated as a “master switch” in induction of fibrosis in many tissues including the liver.
Peer review
This work identifies CTGF as a hepatocellular negative acute phase protein which is down-regulated by IL-6 via the STAT3 pathway through interaction on the DNA binding level. The paper is well written, the biochemical documentation excellent, and the results clearly show a significant implication of CTGF in the inflammatory response of the liver.
Acknowledgments
We thank Henkel C, Department of Pathology, University Hospital Aachen, for support when using the Typhoon 9410 PhosphoImager. Ad-hCTGF-Luc was kindly provided by van de Leur E, Institute of Clinical Chemistry and Pathobiochemistry, University Hospital Aachen.
Footnotes
Peer reviewer: Dr. T Choli-Papadopoulou, Associate Professor, Department of Biochemistry, Aristotle University of Thessaloniki, School of Chemistry, Thessaloniki 55124, Greece
S- Editor Sun H L- Editor O’Neill M E- Editor Ma WH
References
- 1.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 3.Gressner OA, Lahme B, Siluschek M, Rehbein K, Weiskirchen R, Gressner AM. Connective tissue growth factor is a Smad2 regulated amplifier of transforming growth factor beta actions in hepatocytes--but without modulating bone morphogenetic protein 7 signaling. Hepatology. 2009;49:2021–2030. doi: 10.1002/hep.22850. [DOI] [PubMed] [Google Scholar]
- 4.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi N, Kakimuma T, Soma Y, Grotendorst GR, Tamaki K, Harada M, Igarashi A. Connective tissue growth factor is directly related to liver fibrosis. Hepatogastroenterology. 2002;49:133–135. [PubMed] [Google Scholar]
- 6.Paradis V, Dargere D, Vidaud M, De Gouville AC, Huet S, Martinez V, Gauthier JM, Ba N, Sobesky R, Ratziu V, et al. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology. 1999;30:968–976. doi: 10.1002/hep.510300425. [DOI] [PubMed] [Google Scholar]
- 7.Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26:1–9. doi: 10.1016/s1386-6346(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 8.George J, Tsutsumi M. siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther. 2007;14:790–803. doi: 10.1038/sj.gt.3302929. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Xie Q, Shi Y, Li D, Zhang M, Jiang S, Zhou H, Lu H, Jin Y. Inhibition of connective tissue growth factor by siRNA prevents liver fibrosis in rats. J Gene Med. 2006;8:889–900. doi: 10.1002/jgm.894. [DOI] [PubMed] [Google Scholar]
- 10.Gressner OA, Lahme B, Siluschek M, Rehbein K, Herrmann J, Weiskirchen R, Gressner AM. Activation of TGF-beta within cultured hepatocytes and in liver injury leads to intracrine signaling with expression of connective tissue growth factor. J Cell Mol Med. 2008;12:2717–2730. doi: 10.1111/j.1582-4934.2008.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weng HL, Ciuclan L, Liu Y, Hamzavi J, Godoy P, Gaitantzi H, Kanzler S, Heuchel R, Ueberham U, Gebhardt R, et al. Profibrogenic transforming growth factor-beta/activin receptor-like kinase 5 signaling via connective tissue growth factor expression in hepatocytes. Hepatology. 2007;46:1257–1270. doi: 10.1002/hep.21806. [DOI] [PubMed] [Google Scholar]
- 12.Gressner OA, Lahme B, Demirci I, Gressner AM, Weiskirchen R. Differential effects of TGF-beta on connective tissue growth factor (CTGF/CCN2) expression in hepatic stellate cells and hepatocytes. J Hepatol. 2007;47:699–710. doi: 10.1016/j.jhep.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Muraguchi A, Kishimoto T, Miki Y, Kuritani T, Kaieda T, Yoshizaki K, Yamamura Y. T cell-replacing factor- (TRF) induced IgG secretion in a human B blastoid cell line and demonstration of acceptors for TRF. J Immunol. 1981;127:412–416. [PubMed] [Google Scholar]
- 14.Feder LS, Todaro JA, Laskin DL. Characterization of interleukin-1 and interleukin-6 production by hepatic endothelial cells and macrophages. J Leukoc Biol. 1993;53:126–132. doi: 10.1002/jlb.53.2.126. [DOI] [PubMed] [Google Scholar]
- 15.Gregory SH, Wing EJ, Danowski KL, van Rooijen N, Dyer KF, Tweardy DJ. IL-6 produced by Kupffer cells induces STAT protein activation in hepatocytes early during the course of systemic listerial infections. J Immunol. 1998;160:6056–6061. [PubMed] [Google Scholar]
- 16.Invernizzi P, Bianchi I, Locati M, Bonecchi R, Selmi C. Cytokines in liver health and disease. In: Gershwin ME, Vierling JM, Manns MP, editors. Liver immunology. New Jersey: Humana Press; 2007. pp. 83–93. [Google Scholar]
- 17.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 18.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weng H, Mertens PR, Gressner AM, Dooley S. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J Hepatol. 2007;46:295–303. doi: 10.1016/j.jhep.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Karger A, Fitzner B, Brock P, Sparmann G, Emmrich J, Liebe S, Jaster R. Molecular insights into connective tissue growth factor action in rat pancreatic stellate cells. Cell Signal. 2008;20:1865–1872. doi: 10.1016/j.cellsig.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 22.van Gool J, Boers W, de Nie I. Inhibitory effects of rat alpha2 macroferoprotein (alphaMFP), an acute phase globulin, on galactosamine hepatitis. Exp Mol Pathol. 1978;29:228–240. doi: 10.1016/0014-4800(78)90041-2. [DOI] [PubMed] [Google Scholar]
- 23.Weiergräber O, Hemmann U, Küster A, Müller-Newen G, Schneider J, Rose-John S, Kurschat P, Brakenhoff JP, Hart MH, Stabel S. Soluble human interleukin-6 receptor. Expression in insect cells, purification and characterization. Eur J Biochem. 1995;234:661–669. doi: 10.1111/j.1432-1033.1995.661_b.x. [DOI] [PubMed] [Google Scholar]
- 24.Seglen P. Preparation of isolated rat liver cells. In: Prescott DM, editor. Methods in cell biology. New York: Academic Press; 1975. pp. 29–83. [DOI] [PubMed] [Google Scholar]
- 25.Gressner AM, Pfeiffer T. Preventive effects of acute inflammation on liver cell necrosis and inhibition of heparan sulphate synthesis in hepatocytes. J Clin Chem Clin Biochem. 1986;24:821–829. doi: 10.1515/cclm.1986.24.11.821. [DOI] [PubMed] [Google Scholar]
- 26.Gressner AM, Wulbrand U. Variation of immunocytochemical expression of transforming growth factor (TGF)-beta in hepatocytes in culture and liver slices. Cell Tissue Res. 1997;287:143–152. doi: 10.1007/s004410050740. [DOI] [PubMed] [Google Scholar]
- 27.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blom IE, van Dijk AJ, de Weger RA, Tilanus MG, Goldschmeding R. Identification of human ccn2 (connective tissue growth factor) promoter polymorphisms. Mol Pathol. 2001;54:192–196. doi: 10.1136/mp.54.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bett AJ, Haddara W, Prevec L, Graham FL. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrory WJ, Bautista DS, Graham FL. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 31.Weiskirchen R, Kneifel J, Weiskirchen S, van de Leur E, Kunz D, Gressner AM. Comparative evaluation of gene delivery devices in primary cultures of rat hepatic stellate cells and rat myofibroblasts. BMC Cell Biol. 2000;1:4. doi: 10.1186/1471-2121-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terry C, Hughes RD. An optimised method for cryopreservation of human hepatocytes. Methods Mol Biol. 2009;481:25–34. doi: 10.1007/978-1-59745-201-4_3. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Kunos G, Gao B. Ethanol rapidly inhibits IL-6-activated STAT3 and C/EBP mRNA expression in freshly isolated rat hepatocytes. FEBS Lett. 1999;457:162–168. doi: 10.1016/s0014-5793(99)01031-5. [DOI] [PubMed] [Google Scholar]
- 34.He HJ, Zhu TN, Xie Y, Fan J, Kole S, Saxena S, Bernier M. Pyrrolidine dithiocarbamate inhibits interleukin-6 signaling through impaired STAT3 activation and association with transcriptional coactivators in hepatocytes. J Biol Chem. 2006;281:31369–31379. doi: 10.1074/jbc.M603762200. [DOI] [PubMed] [Google Scholar]
- 35.Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, Laudano A, Sebti S, Hamilton AD, Jove R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–45455. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 36.Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–d807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 37.Wells RG. Fibrogenesis. V. TGF-beta signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2000;279:G845–G850. doi: 10.1152/ajpgi.2000.279.5.G845. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 39.Bissell DM, Roulot D, George J. Transforming growth factor beta and the liver. Hepatology. 2001;34:859–867. doi: 10.1053/jhep.2001.28457. [DOI] [PubMed] [Google Scholar]
- 40.Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- 41.Moussad EE, Brigstock DR. Connective tissue growth factor: what’s in a name? Mol Genet Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- 42.Wickert L, Chatain N, Kruschinsky K, Gressner AM. Glucocorticoids activate TGF-beta induced PAI-1 and CTGF expression in rat hepatocytes. Comp Hepatol. 2007;6:5. doi: 10.1186/1476-5926-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Billingham ME, Gordon AH. The role of the acute phase reaction in inflammation. Agents Actions. 1976;6:195–200. doi: 10.1007/BF01972208. [DOI] [PubMed] [Google Scholar]
- 44.Billingham ME, Gordon AH, Robinson BV. Role of the liver in inflammation. Nat New Biol. 1971;231:26–27. doi: 10.1038/newbio231026a0. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13:273–279. doi: 10.1002/(SICI)1098-2825(1999)13:6<273::AID-JCLA4>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Gool J. Profiles of acute-phase reactants and clinical significance of alpha 2-macroglobulin in acute hepatitis B. Inflammation. 1983;7:277–289. doi: 10.1007/BF00917265. [DOI] [PubMed] [Google Scholar]
- 47.van Gool J, Ladiges NC, de Nie I, Boers W. Inflammation inhibiting properties of rat alphaM foetoprotein (rat-alpha2 macroglobulin), an acute phase reactant. Agents Actions Suppl. 1977;2:149–161. doi: 10.1007/978-3-0348-7177-8_13. [DOI] [PubMed] [Google Scholar]
- 48.Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26:1–9. doi: 10.1016/s1386-6346(03)00115-3. [DOI] [PubMed] [Google Scholar]