Abstract
A 6-year nationwide study of fungemia in Denmark was performed using data from an active fungemia surveillance program and from laboratory information systems in nonparticipating regions. A total of 2,820 episodes of fungemia were recorded. The incidence increased from 2004 to 2007 (7.7 to 9.6/100,000) and decreased slightly from 2008 to 2009 (8.7 to 8.6/100,000). The highest incidences were seen at the extremes of age (i.e., 11.3 and 37.1/100,000 for those <1 and 70 to 79 years old, respectively). The rate was higher for males than for females (10.1 versus 7.6/100,000, P = 0.003), with the largest difference observed for patients >50 years of age. The species distribution varied significantly by both age and gender. Candida species accounted for 98% of the pathogens, and C. albicans was predominant, although the proportion decreased (64.4% to 53.2%, P < 0.0001). C. glabrata ranked second, and the proportion increased (16.5% to 25.9%, P = 0.003). C. glabrata was more common in adults and females than in children and males, whereas C. tropicalis was more common in males (P = 0.020). C. krusei was a rare isolate (4.1%) except at one university hospital. Acquired resistance to amphotericin and echinocandins was rare. However, resistance to fluconazole (MIC of >4 μg/ml) occurred in C. albicans (7/1,183 [0.6%]), C. dubliniensis (2/65 [3.1%]), C. parapsilosis (5/83 [6.0%]), and C. tropicalis (7/104 [6.7%]). Overall, 70.8% of fungemia isolates were fully fluconazole susceptible, but the proportion decreased (79.7% to 68.9%, P = 0.02). The study confirmed an incidence rate of fungemia in Denmark three times higher than those in other Nordic countries and identified marked differences related to age and gender. Decreased susceptibility to fluconazole was frequent and increasing.
A seminational active fungemia surveillance program was initiated in Denmark in 2003 (5) and until 2009 included two- thirds of the Danish population (5, 6). The data from 2004 to 2006 has previously been reported and documented a notably high annual incidence rate of 10 to 11 episodes per 100,000 inhabitants (6). In comparison, in three other Nordic countries the rate of candidemia has been in the range of 2.2 to 3.5/100,000, with 65 to 70% of the cases being caused by Candida albicans (24, 29, 36, 37, 44). In European surveys, rates of 2.5 to 5.0/100,000 have been reported (3, 9, 30, 33, 49), with 56% (43 to 67%) of the cases being caused by C. albicans in a recent European study (49). Finally, in the United States, rates of 6.0 to 8.7/100,000 have been reported in most population-based studies, with 45 to 57% of the cases being caused by C. albicans (18, 20, 22). One major exception is a study reporting a rate as high as 24/100,000 in Baltimore compared to 7.1/100,000 in Connecticut despite the fact that rates calculated per number of admissions were comparable (2).
C. albicans remains the predominant species on both sides of the Atlantic Ocean. However, geographical differences in Candida species distributions exist. Thus, C. glabrata is more common in the northern hemisphere, while C. parapsilosis and C. tropicalis are more common in the southern parts of the world (4). This has significant importance due to the difference in susceptibility patterns, with C. glabrata having reduced susceptibility to fluconazole and C. parapsilosis to the echinocandins; primary antifungal regimens should therefore be adjusted to the local epidemiology. Surveys conducted in Europe have reported C. glabrata proportions from 8 to 22% (3, 6, 23, 24, 29, 30, 37, 38, 45, 47, 48). In this perspective, the first years of surveillance in Denmark revealed an unexpectedly high proportion of blood isolates to be C. glabrata (23%) (6).
However, species distribution and incidence data may have been skewed due to underrepresentation of regions without university hospitals, and therefore, we found it of interest to extend the previous 3-year seminational study to a nationwide study that includes data from all of Denmark during the 6-year period from 2004 to 2009. The objective was to determine population-, gender-, and age-specific incidence rates and species distributions and thereby extend previous seminational observations of trends in a high-incidence country.
MATERIALS AND METHODS
Surveillance and population. (i) Retrospective part.
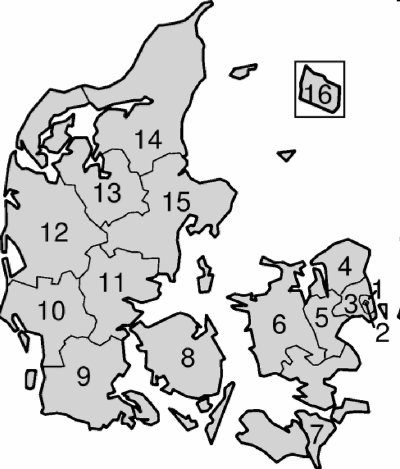
Six departments of clinical microbiology serving one-third of the Danish population participated in the retrospective part of the study; these departments were situated at Slagelse Hospital (center no. 6 and 7), Vejle Hospital (center no. 11), Herning Hospital (center no. 12), Viborg Hospital (center no. 13), Esbjerg Hospital (center no. 10), and Sønderborg Hospital (center no. 9). Characteristics of uptake area, number of admissions, and population sizes are summarized in Table 1, and geographical locations are shown in Fig. 1. Information on total numbers of bloodstream isolates and species diagnosis was retrieved from the departments' laboratory information systems.
TABLE 1.
Characteristics of the 14 participating centersb
| Center no. and name | No. of hospitals (university/ district) | Population (mean 2004-2009) | Blood culture system | No. of bottles/BC (ae/an/myc) | Incubation time (days) | No. of isolates/yr (mean 2004-2009) | Incidence/100,000 populationa (mean 2004-2009) | Incidence/1,000 admissionsa (mean 2004-2009) | Proportion of C. albicans/ C. glabrata (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 Rigshospitalet | 1 (1/0) | NA | Bactec | 2 (1/1/0)^ | 7 | 79 | NA | 1.22 | 51/20 |
| 2 Copenhagen city hospitals | 6 (1/5) | 634,750 | BacT/Alert | 4 (2/2/0) | 5 | 47 | 7.33 | 0.39 | 51/30 |
| 3 Copenhagen county | 4 (3/1) | 619,088 | Bactec | 4 (2/2/0)^ | 5 | 48 | 7.75 | 0.35 | 60/19 |
| 8 Funen | 9 (1/8) | 479,564 | Bactec | 2 (1/1/0) | 6 | 55 | 11.54 | 0.53 | 60/17 |
| 15 Aarhus | 8 (3/5) | 664,413 | BacT/Alert | 2 (1/1/0) | 6 | 67 | 10.01 | 0.49 | 61/20 |
| 14 N-Jutland | 7 (1/6) | 503,174 | BacT/Alert | 3 (2/1/0) | 7 | 45 | 8.94 | 0.42 | 62/21 |
| 4 Frederiksborg | 4 (0/4) | 364,333 | BacT/Alert | 4 (2/2/0) | 5-6 | 26 | 7.23 | 0.36 | 54/25 |
| 5 Roskilde | 2 (1/1) | 243,451 | Bactec | 3 (2/1/0) | 5 | 19 | 7.80 | 0.37 | 55/18 |
| 6 & 7 SW-Sealand* | 9 (0/9) | 576,756 | BacT/Alert | 3 (2/1/0) | 6 | 24 | 4.16 | 0.21 | 54/15 |
| 11 Vejle* | 5 (0/5) | 345,000 | BacT/Alert | 2 (1/1/0) | 5,6 | 25 | 7.39 | 0.37 | 58/23 |
| 12 Herning* | 4 (0/4) | 276,842 | BacT/Alert | 2 (1/1/0) | 6 | 17 | 6.14 | 0.37 | 52/37 |
| 13 Viborg* | 3 (0/3) | 224,789 | Bactec → BacT/Alert** | 2 (1/1/0) or 4 (2/2/0) | 6 | 10 | 4.45 | 0.20 | 73/13 |
| 10 Esbjerg* | 2 (0/2) | 235,863 | Bactec → BacT/Alert** | 3 (2/1/0) | 5 | 13 | 5.44 | 0.30 | 68/16 |
| 9 S-Jutland* | 4 (0/4) | 285,390 | BacT/Alert | 2 (1/1/0) | 7 | 10 | 4.06 | 0.25 | 51/22 |
Incidence calculation based upon number of isolates.
NA, not applicable; center 1 is a tertiary hospital receiving patients referred from other hospitals from the entire country. ^, center 1 (since 1 January 2007) and center 3 (since 1 November 2006) recommend inclusion of a mycosis bottle in blood cultures for risk patients. *, centers participating in the retrospective part of the study. **, center 13 changed the blood culture system from Bactec to BacT/Alert in 2009, and center 10 changed the blood culture system from Bactec to BacT/Alert in 2007. BC, blood culture; ae/an/myc, aerobic/anaerobic/mycosis.
FIG. 1.
Geographical uptake area of the 14 participating centers designated by the following numbers: 1, Rigshospitalet; 2, Copenhagen city hospitals; 3, Copenhagen county; 4, Frederiksborg; 5, Roskilde; 6 and 7, SW-Sealand; 8, Funen; 9, S-Jutland; 10, Esbjerg; 11, Vejle; 12, Herning; 13, Viborg; 14, N-Jutland; and 15, Aarhus. The island Bornholm, 16, was served by Roskilde, 5, in 2004 but in the rest of the period by Copenhagen city hospitals, 2. No other laboratories process blood cultures in Denmark. (Adapted from a map available on Wikipedia under a Creative Commons license.)
(ii) Prospective part.
Eight Danish departments of clinical microbiology at Rigshospitalet (center no. 1), Hvidovre Hospital (center no. 2), Herlev Hospital (center no. 3), Odense University Hospital (center no. 8), Skejby Hospital (center no. 15), Aalborg Hospital (center no. 14), Hillerød Hospital (center no. 4), and Statens Serum Institut (center no. 5) have participated in the prospective seminational surveillance since 2004, covering two-thirds of the Danish population. Isolates were referred to the National Mycology Reference Laboratory for verification of species identification and susceptibility testing (see below). Completeness was ensured through comparison with local laboratory records.
Two blood culture systems were used: the BacT/Alert (bioMérieux, Marcy l'Etoile, France) and the Bactec (Becton Dickinson, Franklin Lakes, NJ) blood culture system (Table 1). In total, 55.5% of the cases were detected using BacT/Alert, and 44.5% using Bactec. For fungemia patients with successive blood culture isolates, separate episodes were included if they occurred at least 21 days apart or were caused by different species.
The size of the Danish population increased marginally during the study period (2.1%, 5,397,640 to 5,511,451; www.statistikbanken.dk). Information on the national number of nonpsychiatric admissions, trauma, violent injuries, and poisoning (classified together) and of hematological and gastrointestinal cancers was retrieved at the website www.sundhedsdata.sst.dk.
Species identification.
Species identification at the reference laboratory was based on colony morphology on chromogenic agar (CHROMagar Co., Paris, France), microscopic morphology on corn meal agar and rice plus Tween agar (SSI Diagnostika, Hillerød, Denmark), growth at 35°C and 43°C, and assimilation profile by use of a commercial system (ATB ID32C; bioMérieux). Rapid tests for the identification of C. dubliniensis and C. glabrata (Bichro-Dubli and Glabrata RTT; Fumouze Diagnostics, Simoco, Denmark) were used increasingly over the study period. If no reliable species diagnosis was obtained, molecular identification was performed as described below.
Susceptibility testing.
Susceptibility testing for amphotericin B, anidulafungin, caspofungin, fluconazole, itraconazole, voriconazole, and posaconazole was done for a total of 2,091 (72.1%), 514 (17.7%), 2,086 (72.0%), 2,103 (72.5%), 2,079 (71.7%), 2,010 (69.3%), and 1,055 (36.4%) isolates, respectively, according to the EUCAST definitive document E7.1 (40); exceptions were amphotericin B (2006 to 2009) and caspofungin (2008 to 2009), for which Etest (AB bioMérieux, Herlev, Denmark) and RPMI 2% glucose agar (SSI Diagnostika, Hillerød, Denmark) were used. Manufacturers and stock solutions were as follows: dimethyl sulfoxide (DMSO), D8779, Sigma-Aldrich, Vallensbæk Strand, Denmark; fluconazole, Pfizer A/S, Ballerup, Denmark, or Sigma-Aldrich (10,000 μg/ml); amphotericin B, A2411, Sigma-Aldrich (5000 μg/ml in DMSO); caspofungin, Merck, Sharp and Dohme, Glostrup, Denmark (5,000 μg/ml in DMSO); itraconazole, Janssen-Cilag, Birkerød, Denmark, or Sigma-Aldrich (5,000 μg/ml in DMSO); and voriconazole, Pfizer A/S, Ballerup, Denmark (5,000 μg/ml in DMSO). Two-fold dilutions in RPMI supplemented to a final concentration of 2% glucose were prepared in microtiter plates and stored at −20°C until use. Microtiter plates were read spectrophotometrically at 490 nm. The MIC was defined as the lowest drug dilution giving 100% growth inhibition for amphotericin B and 50% growth inhibition for the other compounds. C. krusei ATCC 6258 was included as a quality control in each run. For this strain the accepted ranges for amphotericin B, fluconazole, itraconazole, and voriconazole were as published in reference 15 and 0.25 to 2 μg/ml for caspofungin. Susceptibility classification was done according to the following breakpoints (susceptible [S≤]/resistant [R>]) (μg/ml): for amphotericin B, 1/1 (14); for anidulafungin, 0.015/0.015 for C. albicans, 0.064/0.064 for C. glabrata and C. tropicalis, and 0.125/0.125 for C. krusei (8); for caspofungin, 2/2 for Candida species other than C. parapsilosis (8, 14); for fluconazole, 2/4 for Candida species other than C. glabrata and C. krusei (42); for voriconazole, 0.125/0.125 for Candida species other than C. glabrata and C. krusei (41); and for itraconazole, 0.125/0.5 (14). For posaconazole, no breakpoints have yet been proposed, but 0.064/0.064 was applied for Candida species other than C. glabrata and C. krusei, based upon wild-type distributions (data not shown).
Molecular identification and FKS gene sequence analysis.
DNA was released from fungal colonies as previously described (12). Species identification was performed using universal fungal primers (internal transcribed spacer 1 [ITS1] [CGTAGGTGAACCTGCGG] and ITS4 [TCCTCCGCTTATTGATATGC]) (51). These primers amplify the intervening 5.8S ribosomal DNA (rDNA) and the adjacent ITS1 and ITS2 (51). Sequences were subjected to BLAST analyses with the online sequence databases available through NCBI and aligned to reference strains for species identification. Sequence analysis, alignments, and phylogenetics were performed with the bioinformatics software CLC DNA Workbench (CLC bio, Denmark). FKS gene sequence analysis was performed as previously described (8). This gene encodes the target enzyme (glucan synthase) for echinocandins.
Consumption of antifungal compounds.
Information concerning overall use of antifungal agents in defined daily doses (DDD) in Denmark over a 10-year period from 2000 to 2009 both in hospitals and primary health care settings were available from the Danish Medicines Agency at http://dkma.medstat.dk/MedStatDataViewer.php. Similar information for Norway was available from the Norwegian Institute of Public Health at http://www.legemiddelforbruk.no/english/.
Data regarding fluconazole use by gender in the primary health care setting were available for the most recent 5-year period from 2005 to 2009 at http://dkma.medstat.dk/MedStatDataViewer.php. However, gender-specific data are not available for use at hospitals.
Statistics.
Incidences per 100,000 inhabitants are calculated using the population sizes per January 1 each year. The mean is used when incidences for the entire 6-year period are calculated. Numbers on admissions for each geographical region in Denmark were reported by the local study participant.
The chi-square test was used for comparison of changes in species distribution. P values of <0.05 (two-tailed) were considered statistically significant.
RESULTS
Epidemiology.
During the 6-year period from 2004 to 2009, a total of 2,820 episodes of fungemia comprising 2,901 isolates were recorded in 2,694 patients, leading to a mean annual incidence of 8.6/100,000 inhabitants. The number of patients, episodes, and recovered isolates increased by 24.3%%, 26.6%, and 26.2%, respectively, over the first 4-year period (2004 to 2007) and then decreased to an intermediate level in 2008 to 2009 (Table 2). Similarly, the incidence rate increased over the first 4 years from 7.7 to 9.6/100,000 and then stabilized at 8.7 and 8.6/100,000 per year in 2008 and 2009, respectively. The rate of fungemia per 10,000 somatic admissions was 4.1 for the entire study period (rate by year from 2004 to 2009: 3.7, 4.1, 4.1, 4.5, 4.1, and 3.9, respectively). The highest rates were seen at the centers serving university hospitals (Table 1).
TABLE 2.
Number of cases, episodes, and isolates each year and distribution of fungal isolates according to species and yeara
| Parameterb | 2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Patients | 403 | NA | 436 | +8.2 | 455 | +4.4 | 501 | +10.1 | 455 | −9.2 | 444 | −2.4 | 2694 | NA |
| Cases | 414 | NA | 462 | +11.6 | 474 | +2.6 | 524 | +10.5 | 474 | −9.5 | 472 | −0.4 | 2820 | NA |
| Isolates | 424 | NA | 482 | +13.7 | 492 | +2.1 | 534 | +8.7 | 484 | −9.5 | 485 | +0.2 | 2901 | NA |
| C. albicans | 273 | 64.4 | 299 | 62.0 | 276 | 56.1 | 279 | 52.2 | 272 | 56.2 | 258 | 53.2 | 1657 | 57.1 |
| C. dubliniensis | 5 | 1.2 | 11 | 2.3 | 14 | 2.8 | 15 | 2.8 | 15 | 3.1 | 14 | 2.9 | 74 | 2.6 |
| C. glabrata | 70 | 16.5 | 96 | 19.9 | 104 | 21.1 | 119 | 22.3 | 98 | 20.2 | 125 | 25.8 | 612 | 21.1 |
| C. krusei | 12 | 2.8 | 10 | 2.1 | 28 | 5.7 | 22 | 4.1 | 28 | 5.8 | 19 | 3.9 | 119 | 4.1 |
| C. parapsilosis | 17 | 4.0 | 11 | 2.3 | 22 | 4.5 | 21 | 3.9 | 18 | 3.7 | 19 | 3.9 | 108 | 3.7 |
| C. tropicalis | 17 | 4.0 | 24 | 5.0 | 20 | 4.1 | 29 | 5.4 | 27 | 5.6 | 21 | 4.3 | 138 | 4.8 |
| Candida species* | 12 | 2.8 | 7 | 1.5 | 11 | 2.2 | 22 | 4.1 | 11 | 2.3 | 16 | 3.3 | 79 | 2.7 |
| Non-Candida albicans** | 13 | 3.1 | 16 | 3.3 | 10 | 2.0 | 13 | 2.4 | 6 | 1.2 | 11 | 2.3 | 69 | 2.4 |
| Other fungi*** | 5 | 1.2 | 8 | 1.7 | 7 | 1.4 | 14 | 2.6 | 9 | 1.9 | 2 | 0.4 | 45 | 1.6 |
The percentages for the numbers of patients and cases and total number of isolates indicate change in number compared to the previous year, whereas for each individual species, percentages indicate the species distribution that particular year. NA, not applicable.
*, Candida spp. include indicated number of the following isolates: C. ciferrii, 1; C. colliculosa, 1; C. fermentati, 3; C. guilliermondii, 14; C. holmii, 1; C. inconspicua, 1; C. inconspicua/norvegensis, 1; C. intermedia, 2; C. kefyr, 11; C. lipolytica, 2; C. lusitaniae, 22; C. nivariensis, 1; C. norvegensis, 1; C. palmioleophila, 9; C. pelliculosa, 5; C. pulcherrima, 1; C. rugosa, 1; C. utilis, 1; and C. valida, 1. **, non-C. albicans denotes isolates that were not C. albicans but not referred to the mycology reference laboratory for species identification. ***, other fungi include indicated number of the following isolates: Cryptococcus neoformans, 9; Cryptococcus sp., 1; F. dimerum, 2; F. solani, 5; Fusarium sp., 4; Geotrichum capitatum, 1; Pichia fabii, 1; Pichia carribica, 1; Rhodotorula glutinis/Rhodotorula mucilaginosa, 1; Rhodotorula mucilaginosa, 1; Rhodotorula sp., 1; S. cerevisiae, 15; Trichosporon inkin, 1; Trichosporon mucoides, 1; and Williopsis sp., 1.
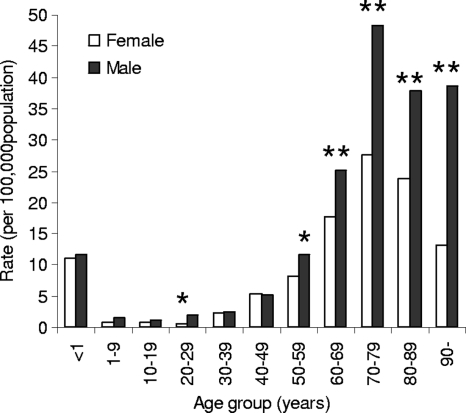
The median age (66 years, range of 0 to 98 years, interquartile range of 55 to 74 years) and the proportion of males (56.5%) remained constant. The highest incidence rates were seen at the extremes of age (Table 3). Only 1.5% of the patients were below the age of 1 year (rate, 11.3/100,000), 2.9% were 1 to 20 years of age (rate, 1.1/100,000), 65.1% were older than 60 years (rate, 27.0/100,000), and 27.4% were older than 70 years of age (rate, 33.2/100,000). The rate was significantly higher for males than for females (10.1 versus 7.6/100,000, P = 0.003), with the largest gender difference for patients 20 to 29 years and older than 50 years (Fig. 2).
TABLE 3.
Age-specific incidence of fungemia by year
| Yr studied | No. of isolates per 100,000 population for indicated age (yr) group |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1-9 | 10-19 | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | 80-89 | 90+ | ||
| 2004 | 12.3 | 0.8 | 1.3 | 1.1 | 2.7 | 4.0 | 9.1 | 20.7 | 33.7 | 19.5 | 29.5 | 7.9 |
| 2005 | 9.3 | 0.8 | 0.9 | 0.9 | 2.3 | 5.3 | 11.1 | 20.9 | 36.0 | 38.7 | 5.8 | 8.9 |
| 2006 | 12.4 | 0.8 | 0.8 | 1.4 | 1.5 | 5.4 | 8.9 | 23.1 | 44.3 | 25.0 | 17.2 | 9.1 |
| 2007 | 10.7 | 1.7 | 1.5 | 1.1 | 4.0 | 5.9 | 12.1 | 21.7 | 36.0 | 34.4 | 22.6 | 9.8 |
| 2008 | 9.3 | 1.2 | 1.2 | 1.4 | 2.4 | 5.7 | 9.3 | 20.6 | 36.2 | 27.0 | 25.1 | 8.8 |
| 2009 | 13.8 | 1.2 | 1.0 | 1.3 | 1.5 | 5.3 | 9.1 | 21.3 | 36.6 | 28.4 | 16.5 | 8.8 |
| Total | 11.3 | 1.1 | 1.1 | 1.2 | 2.4 | 5.3 | 10.0 | 21.4 | 37.1 | 28.8 | 19.4 | |
FIG. 2.
Annual incidence of fungemia, by age and gender, in Denmark from 2004 to 2009. *, P = 0.0003; **, P < 0.0001.
The overall species distribution is shown in Table 2, and the ratio of the two most common species by center in Table 1. Candida species accounted for 98.4% of the fungal isolates, and C. albicans was the predominant species (in total, 57.1%). However, the proportion of C. albicans isolates varied considerably among the participating hospitals (range of 51 to 73%) (Table 1), and there was an overall decline throughout the study period (64% to 53%, P < 0.0001) (Table 2). C. glabrata was the second most frequent species (21%), again with considerable variation in frequency according to center (13% to 37%) and with an increase over the study period (17% to 26%, P = 0.003). The variation in distribution of C. glabrata between centers using Bactec (17.9%, 231/1,289) and those using BacT/Alert (23.6%, 381/1,612) was statistically significant (P = 0.0002). This was also the case if the analysis was performed separately for the group of 60 to 79 years of age (Bactec, 19.2% [131/684]; BacT/Alert, 24.9% [219/880], P = 0.0071). C. krusei, C. tropicalis, and C. parapsilosis were rare isolates (3.7% to 4.8%), and their occurrence remained stable. Thirty-seven percent (44/119) of the C. krusei isolates were from center 1 (Rigshospitalet, the major tertiary center in Denmark), where this species accounted for 9.3% of the fungal isolates.
The distribution of species varied by gender and by age group. Thus, C. glabrata was significantly more common in females (23.1% versus 19.7%, P = 0.03), whereas C. tropicalis was more common in males (5.6% versus 3.7%, P = 0.02) (Table 4). Overall, C. albicans and C. parapsilosis accounted for 90% of the infections in patients less than 10 years old, and notably, C. glabrata and C. krusei were not recovered in any patients younger than 20 years of age, with the exception of five C. krusei isolates (all from center 1) and four C. glabrata isolates (two from center 1) (Table 5). On the contrary, 28% of the fungemia isolates were either C. glabrata or C. krusei in patients older than 60 years of age.
TABLE 4.
Distribution of fungal isolates according to species and gendera
| Female |
Male |
P* | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| C. albicans | 708 | 56.3 | 937 | 57.4 | NS |
| C. dubliniensis | 29 | 2.3 | 45 | 2.8 | NS |
| C. glabrata | 290 | 23.1 | 322 | 19.7 | 0.03 |
| C. krusei | 51 | 4.1 | 68 | 4.2 | NS |
| C. parapsilosis | 51 | 4.1 | 56 | 3.4 | NS |
| C. tropicalis | 46 | 3.7 | 91 | 5.6 | 0.02 |
| Candida spp. | 29 | 2.3 | 50 | 3.1 | NS |
| Non-C. albicans | 33 | 2.6 | 36 | 2.2 | NS |
| Other fungi | 20 | 1.6 | 25 | 1.5 | NS |
| Total | 1,257 | 43.5 | 1,630 | 56.5 | <0.0001 |
*, chi-square test, P < 0.05 was regarded statistically significant. For 14 patients, the gender was unknown. NS, not significant.
TABLE 5.
Distribution of fungal isolates according to species and age group
| Speciesa | No. (%) of isolates found for indicated age (yr) group |
Total no. (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1-9 | 10-19 | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | 80-89 | 90+ | ||
| C. albicans | 32 (73) | 32 (73) | 29 (74) | 24 (52) | 65 (58) | 143 (57) | 252 (58) | 417 (54) | 474 (60) | 167 (51) | 22 (54) | 1,657 (57) |
| C. dubliniensis | 3 (7) | 6 (5) | 9 (4) | 17 (4) | 20 (3) | 15 (2) | 3 (1) | 1 (2) | 74 (3) | |||
| C. glabrata | 1 (2) | 3 (8) | 9 (20) | 15 (13) | 49 (20) | 79 (18) | 177 (23) | 174 (22) | 93 (29) | 12 (29) | 612 (21) | |
| C. krusei | 3 (7) | 2 (5) | 2 (4) | 1 (1) | 7 (3) | 23 (5) | 55 (7) | 17 (2) | 8 (2) | 1 (2) | 119 (4) | |
| C. parapsilosis | 8 (18) | 7 (16) | 2 (5) | 2 (4) | 6 (5) | 7 (3) | 16 (4) | 27 (4) | 23 (3) | 10 (3) | 108 (4) | |
| C. tropicalis | 3 (7) | 5 (4) | 17 (7) | 12 (3) | 33 (4) | 45 (6) | 22 (7) | 1 (2) | 138 (5) | |||
| Candida spp.* | 2 (5) | 2 (5) | 2 (4) | 3 (3) | 13 (5) | 19 (4) | 14 (2) | 18 (2) | 6 (2) | 79 (3) | ||
| Non-C. albicans** | 1 (2) | 3 (3) | 1 (0) | 10 (2) | 17 (2) | 20 (3) | 14 (4) | 3 (7) | 69 (2) | |||
| Other fungi | 1 (2) | 1 (2) | 1 (3) | 1 (2) | 8 (7) | 3 (1) | 9 (2) | 10 (1) | 8 (1) | 2 (1) | 1 (2) | 45 (2) |
| Total | 44 | 44 | 39 | 46 | 112 | 249 | 437 | 770 | 794 | 325 | 41 | 2,901 |
*, Candida spp. includes indicated number of the following isolates: C. ciferrii, 1; C. colliculosa, 1; C. fermentati, 3; C. guilliermondii, 14; C. holmii, 1; C. inconspicua, 1; C. inconspicua/norvegensis, 1; C. intermedia, 2; C. kefyr, 11; C. lipolytica, 2; C. lusitaniae, 22; C. nivariensis, 1; C. norvegensis, 1; C. palmioleophila, 9; C. pelliculosa, 5; C. pulcherima, 1; C. rugosa, 1; C. utilis, 1; and C. valida, 1. **, non-C. albicans denotes isolates that were not C. albicans but not referred to the mycology reference laboratory for species identification. ***, other fungi include indicated number of the following isolates: Cryptococcus neoformans, 9; Cryptococcus sp., 1; F. dimerum, 2; F. solani, 5; Fusarium sp., 4; Geotrichum capitatum, 1; Pichia fabii, 1; Pichia carribica, 1; Rhodotorula glutinis/Rhodotorula mucilaginosa, 1; Rhodotorula mucilaginosa, 1; Rhodotorula sp., 1; S. cerevisiae, 15; Trichosporon inkin, 1; Trichosporon mucoides, 1; and Williopsis sp., 1.
Polyfungal infections occurred in 82 cases (2.9%, range of 10 to 20 per year). Seventy-two of these involved two species, whereas 10 involved three species. In 57 (69.5%) of these cases, C. albicans was isolated in combination with another yeast, with C. glabrata accounting for 48 cases. The majority (70/82, 85.4%) of the polyfungal infections included at least one species with intrinsically decreased susceptibility to fluconazole (C. glabrata [in 60 cases], Saccharomyces cerevisiae [in 5 cases], C. krusei [in 9 cases], C. guilliermondii [in 1 case], and Rhodotorula sp. [in 1 case]).
Antifungal susceptibility.
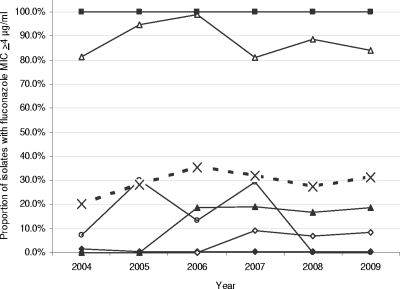
Overall, MICs below the susceptibility breakpoints were found for 99.0% of the blood isolates for amphotericin B, 98.5% for caspofungin, 93.4% for anidulafungin, 70.8% for fluconazole, 77.4% for itraconazole, 80.4% for voriconazole, and 72.1% for posaconazole. During the study period there was a significant increase in the proportion of isolates with decreased susceptibility to fluconazole (defined as an MIC of ≥4 μg/ml), with 20.3% (65/320) in 2004, 28.1% (96/342) in 2005, 35.3% (128/363) in 2006, 32.1% (127/396) in 2007, 27.5% (97/353) in 2008, and 31.1% (103/331) in 2009 (chi-square for trend, P = 0.02) (Fig. 3). This was due mainly to a change in species distribution; the proportion of C. tropicalis isolates with a fluconazole MIC of >2 μg/ml did, however, increase significantly over the 6-year period (P = 0.0354) (Fig. 3).
FIG. 3.
Proportion of isolates for which fluconazole MIC was >2 μg/ml, by species and year. C. albicans, solid line with solid diamonds; C. dubliniensis, solid line with open diamonds; C. glabrata, solid line with open triangles; C. krusei, solid line with solid squares; C. parapsilosis, solid line with open circles; C. tropicalis, solid line with solid triangles; and all isolates, dotted line with crosses.
Nearly all Candida isolates were susceptible to amphotericin B. Exceptions were seven C. glabrata isolates (1.6%), five C. krusei isolates (4.7%), one C. tropicalis (1%) isolate, and one C. norvegensis isolate (1/1) (Table 6). The echinocandins displayed activity against the majority of Candida isolates. One C. dubliniensis isolate with a caspofungin MIC of >32 μg/ml and an anidulafungin MIC of 0.5 μg/ml was found to possess an S645P mutation in the FKS1 gene. Moreover, the anidulafungin MIC was above the applied susceptibility breakpoint for nine additional Candida isolates, including one C. albicans (0.4%, anidulafungin MIC of 0.06 μg/ml, caspofungin MIC of 0.25), one C. dubliniensis isolate (8.7%, anidulafungin MIC of 0.125 μg/ml, caspofungin MIC of 1 μg/ml), two C. glabrata isolates (2%, anidulafungin MICs of 0.5 and 1 μg/ml, caspofungin MICs both of 0.5 μg/ml), and one C. tropicalis isolate (3.6%, anidulafungin MIC of 1 μg/ml, caspofungin MIC of 0.125 μg/ml). Among other Candida species, only C. guilliermondii was classified as anidulafungin resistant (MICs of 0.5 to 4 μg/ml), whereas isolates belonging to the closely related species C. palmioleophila were susceptible (MICs of <0.03 mg/ml, 8/8 isolates).
TABLE 6.
In vitro activity of seven antifungal compounds against Danish fungemia isolatesa
| Species | Antifungal agent (no. of isolates) | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | BP (S≤/R>) | % R |
|---|---|---|---|---|---|---|
| C. albicans | Amphotericin B (1,179) | 0.016-1 | 0.25 | 0.5 | 1/1 | 0 |
| Anidulafungin (282) | ≤0.03-0.06 | ≤0.03 | ≤0.03 | 0.015/0.015 | 0.4 | |
| Caspofungin (1,174) | 0.016-16 | 0.125 | 0.5 | 2/2 | 0.1 | |
| Fluconazole (1,183) | ≤0.125->16 | ≤0.125 | 0.5 | 2/4 | 0.6 | |
| Itraconazole (1,167) | ≤0.03->4 | 0.03 | 0.03 | 0.125/0.5 | 0.1 | |
| Voriconazole (1,121) | ≤0.03->4 | ≤0.03 | 0.06 | 0.125/0.125 | 0.3 | |
| Posaconazole (563) | ≤0.03->4 | ≤0.03 | 0.03 | 0.064/0.064 | 0.4 | |
| C. dubliniensis | Amphotericin B (65) | 0.008-0.5 | 0.06 | 0.25 | 1/1 | 0 |
| Anidulafungin (23) | ≤0.03-0.5 | ≤0.03 | ≤0.03 | 0.015/0.015 | 8.7 | |
| Caspofungin (65) | 0.03->32 | 0.25 | 1 | 2/2 | 3.1 | |
| Fluconazole (65) | ≤0.125->16 | 0.25 | 1 | 2/4 | 3.1 | |
| Itraconazole (65) | ≤0.03-0.125 | 0.03 | 0.03 | 0.125/0.5 | 0 | |
| Voriconazole (65) | ≤0.03-0.5 | ≤0.03 | ≤0.03 | 0.125/0.125 | 1.5 | |
| Posaconazole (38) | ≤0.03-0.06 | ≤0.03 | 0.06 | 0.064/0.064 | 0 | |
| C. glabrata | Amphotericin B (449) | 0.06-4 | 0.5 | 1 | 1/1 | 1.6 |
| Anidulafungin (104) | ≤0.03-1 | ≤0.03 | ≤0.06 | 0.064/0.064 | 2 | |
| Caspofungin (449) | 0.016-2 | 0.5 | 1 | 2/2 | 0 | |
| Fluconazole (455) | 0.5->16 | 8 | >16 | NA | NA | |
| Itraconazole (448) | ≤0.03->4 | 0.25 | 2 | 0.125/0.5 | 34.8 | |
| Voriconazole (436) | ≤0.03->4 | 0.25 | 2 | NA | NA | |
| Posaconazole (231) | ≤0.03->4 | 0.25 | 2 | NA | NA | |
| C. krusei | Amphotericin B (106) | 0.06-4 | 1 | 1 | 1/1 | 4.7 |
| Anidulafungin (28) | ≤0.03-0.06 | ≤0.03 | ≤0.03 | 0.125/0.125 | 0 | |
| Caspofungin (106) | 0.06-2 | 0.5 | 1 | 2/2 | 0 | |
| Fluconazole (106) | 8->16 | >16 | >16 | NA | NA | |
| Itraconazole (106) | 0.03-1 | 0.25 | 0.5 | 0.125/0.5 | 1.9 | |
| Voriconazole (104) | 0.06-2 | 0.25 | 0.25 | NA | NA | |
| Posaconazole (62) | 0.03-0.5 | 0.125 | 0.5 | NA | NA | |
| C. parapsilosis | Amphotericin B (82) | 0.06-1 | 0.5 | 1 | 1/1 | 0 |
| Anidulafungin (22) | 0.5-2 | 1 | 2 | NA | NA | |
| Caspofungin (83) | 0.25-16 | 2 | 4 | NA | NA | |
| Fluconazole (83) | ≤0.125->16 | 1 | 4 | 2/4 | 6.0 | |
| Itraconazole (83) | ≤0.03-0.25 | 0.06 | 0.125 | 0.125/0.5 | 0 | |
| Voriconazole (79) | ≤0.03-2 | ≤0.03 | 0.06 | 0.125/0.125 | 1.3 | |
| Posaconazole (44) | ≤0.03-0.25 | 0.03 | 0.06 | 0.064/0.064 | 2.3 | |
| C. tropicalis | Amphotericin B (103) | 0.016-2 | 0.5 | 1 | 1/1 | 1 |
| Anidulafungin (28) | ≤0.03-1 | ≤0.03 | ≤0.03 | 0.064/0.064 | 3.6 | |
| Caspofungin (104) | 0.03-2 | 0.25 | 1 | 2/2 | 0 | |
| Fluconazole (104) | ≤0.125->16 | 0.5 | 4 | 2/4 | 6.7 | |
| Itraconazole (104) | ≤0.03->4 | 0.03 | 0.25 | 0.125/0.5 | 3.8 | |
| Voriconazole (102) | ≤0.03->4 | ≤0.03 | 0.25 | 0.125/0.125 | 6.0 | |
| Posaconazole (55) | ≤0.03->4 | 0.03 | 0.125 | 0.064/0.064 | 18.2 | |
| Candida spp. | Amphotericin B (64) | 0.016-2 | 0.25 | 0.5 | 1/1 | 1 |
| Anidulafungin (20) | ≤0.03-1 | ≤0.03 | 1 | NA | NA | |
| Caspofungin (64) | 0.125-8 | 0.5 | 2 | 2/2 | 3.1 | |
| Fluconazole (64) | ≤0.125->16 | 4 | >16 | 2/4 | 45.3 | |
| Itraconazole (63) | ≤0.03->4 | 0.125 | 1 | 0.125/0.5 | 11.1 | |
| Voriconazole (61) | ≤0.03-4 | 0.125 | 0.5 | 0.125/0.125 | 37.7 | |
| Posaconazole (38) | ≤0.03-2 | 0.06 | 0.25 | 0.064/0.064 | 39.5 | |
| Other fungi | Amphotericin B (43) | 0.06->16 | 0.5 | 2 | 1/1 | 16.3 |
| Anidulafungin (7) | ≤0.03->4 | 0.125 | >4 | NA | NA | |
| Caspofungin (41) | 0.25->32 | 2 | 16 | NA | NA | |
| Fluconazole (43) | ≤0.125->16 | 8 | >16 | NA | NA | |
| Itraconazole (43) | 0.03->4 | 0.5 | >4 | 0.125/0.5 | 41.9 | |
| Voriconazole (42) | ≤0.03->4 | 0.25 | 2 | NA | NA | |
| Posaconazole (24) | 0.03->4 | 0.5 | >4 | NA | NA |
BP, breakpoint; S, sensitivity; R, resistance.
The susceptibility pattern for the azoles was more complex. The majority of Candida isolates were susceptible to azoles, with the exception of those belonging to the intrinsically less susceptible or resistant species, like C. glabrata and C. krusei (Table 6). However, 21 Candida isolates belonging to species normally susceptible were fluconazole resistant (fluconazole MIC of >4 μg/ml) and included seven C. albicans (0.6%), two C. dubliniensis (3.1%), five C. parapsilosis (6.0%), and seven C. tropicalis (6.7%) isolates. Moreover, for 29 additional isolates from other Candida species (45.3%), the fluconazole MIC was greater than 4 μg/ml (C. palmioleophila, 8/8; C. inconspicua/C. norvegensis, 3/3; C. lusitaniae, 5/17; C. guilliermondii, 5/15; C. pelliculosa, 2/5; C. kefyr, 1/6; and 1/1 of the following species: C. ciferrii, C. holmii, C. rugosa, C. valida, and C. utilis).
In comparison, the voriconazole MIC of >0.125 μg/ml was, with few exceptions, seen only for isolates belonging to species with intrinsically reduced azole susceptibility. However, two C. albicans (0.3%, MICs of 0.5 and 8 μg/ml), one C. dubliniensis (1.5%, MIC of 0.5 μg/ml), one C. parapsilosis (1.3%, MIC of 2 μg/ml), and six C. tropicalis (6%, MICs of 2, 4, 4, 8, 8, and 8 μg/ml) isolates had voriconazole MICs of >0.25 μg/ml. None of these isolates was susceptible to fluconazole, itraconazole, or posaconazole.
Susceptibility to itraconazole was closely related to fluconazole susceptibility and the species. Thus, 81.1% of the isolates had identical susceptibility classifications for fluconazole and itraconazole. Seventy-one isolates (3.5%) were fluconazole resistant but itraconazole susceptible, and two isolates were categorized as fluconazole susceptible but itraconazole resistant (0.1%). Similarly, for posaconazole, the susceptibility pattern was again closely related to the species (Table 6). Notably, better activity was observed against fluconazole-resistant Candida species, though resistance (MIC of >0.125 μg/ml) was seen for 1/1 C. ciferrii, 4/9 C. guilliermondii, 1/2 C. inconspicua/C. norvegensis, 5/8 C. palmioleophila, and 1/2 C. pelliculosa isolates.
Isolates belonging to other fungi were less susceptible (Table 6). Amphotericin B displayed the broadest activity against this group and was the only agent with activity against Rhodotorula spp. However, for seven other fungal isolates (16.3%), all of which were Fusarium species, amphotericin B MICs were 2 to >16 μg/ml (2/2 F. dimerum, 3/5 F. solani, and 2/4 Fusarium species isolates). Caspofungin showed in vitro activity against Saccharomyces, Pichia, and Williopsis isolates (MIC50 of 1 μg/ml, range of 0.25 to 2 μg/ml), but Fusarium, Cryptococcus, Geotrichum, Trichosporon, and Rhodotorula isolates were resistant (MIC50 of 16 μg/ml, range of 4 to >32 μg/ml). The majority of other fungi were fluconazole resistant (65.1%), whereas voriconazole MICs against Fusarium species were 1 to 4 μg/ml (MIC50 of 1 μg/ml), and low MICs (≤0.5 μg/ml) were found for Saccharomyces, Pichia, and Geotrichum isolates. Posaconazole MICs for Fusarium species isolates were 2 to ≥8 μg/ml.
Consumption of antifungals.
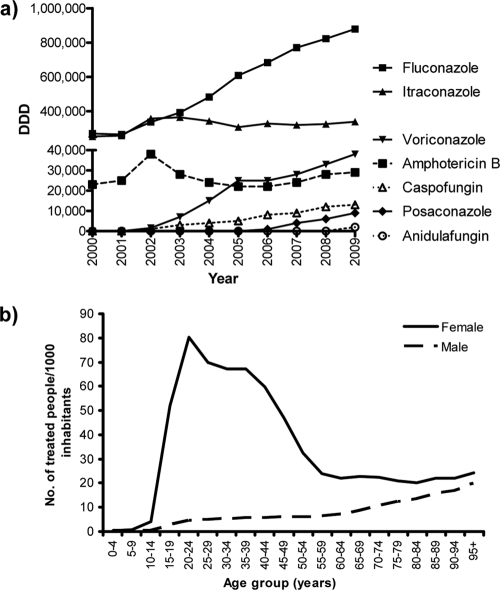
The total consumption of antifungals in Denmark is shown in Fig. 4a. The total annual usage of systemic antifungals increased 140% from 546,000 DDD (102.4 DDD/1,000 inhabitants) to 1,309,000 DDD (249.1 DDD/1,000 inhabitants) over a 10-year period from 2000 to 2009. For the systemic azoles, 76% were used in the primary health care sector, and for fluconazole specifically this was true for 67%, a proportion that remained stable over the observation period. Fluconazole was prescribed more often to females than to males in the primary health care sector (Fig. 4b). Thus, the annual number of fluconazole-treated people per 1,000 inhabitants was 36.6 for females versus 5.2 for males, and the dispensed volume of fluconazole per 1,000 inhabitants per day was 0.38 for females versus 0.12 for males in this setting (http://dkma.medstat.dk/MedStatDataViewer.php).
FIG. 4.
Consumption of systemic antifungal compounds in Denmark between 2000 and 2009 (in DDD) (a) and for fluconazole by gender and age group (mean no. of treated people per 1,000 inhabitants, 2005 to 2009) (b).
DISCUSSION
Almost 3,000 cases were included in this first study of the incidence rate of fungemia in all of Denmark, and to our knowledge it is the largest population-based nationwide fungemia study published so far. A seminational fungemia surveillance program established in 2004 covered two-thirds of the Danish population, with participation of all university hospitals plus all stem cell and organ transplantations centers; it estimated the incidence rate to be 10.4/100,000 inhabitants (6). In Norway the incidence rate of fungemia at county hospitals was half the rate at university hospitals (43), a trend that was also observed in our study. Thus, the observed Danish national incidence rate of 8.6/100,000 is not surprising. The rate increased over the years 2004 to 2007 and then stabilized at an intermediate level in 2008 to 2009. Nationwide studies in the other Nordic countries have reported lower but increasing rates: in Norway, 2.4 to 4 (comparing 1991 to 2003 and 2008) (29, 44); in Iceland, 1.4 to 4.9 (comparing 1980 to 1984 and 1995 to 1999) (9); and in Finland, 1.9 to 3 per 100,000 inhabitants (comparing 1995 to 1999 and 2007) (36, 37). In this context the rate in Denmark appears notably high. The age-specific rate is, however, similar to the rate for the <1-year age group (Denmark, 11.3; Iceland, around 11; Norway, 10.3; and Finland, 9.4 per 100,000) and lower than those in several other surveys from Europe, Australia, and the United States (24.8 to 38.8 and even >140/100,000 in black children below the age of 1 year) (3, 13, 20). In contrast, remarkable differences have been seen with the elderly population in Nordic countries (e.g., for 70 to 79 years of age, Denmark, 37.1; Iceland, around 15; Norway, 7.4; and Finland, 8.8 per 100,000), and this Danish rate is also higher than those reported from both Spain and Australia (3, 13). Thus, the high overall rate in Denmark seems to be driven mainly by a high rate in the elderly population.
The incidence of fungemia was higher for males than for females, with the most marked gender difference in the age groups 20 to 29 and >50 years. Furthermore, C. tropicalis was significantly more common in males. The reasons for these differences are not clear. Significantly higher rates of hematological (56%) and gastrointestinal (54%) cancers that are risk factors for candidemia are seen with males than with females (the National Board of Health, Denmark; http://www.sst.dk). In agreement with this, C. tropicalis, which has been associated with hematological malignancy (1, 25, 46, 52), was more commonly isolated from males. It is also likely that the higher rate in males in their twenties is related to a twice-as-high frequency of trauma, violent injury, and poisoning in males compared to females in this age group (the National Board of Health, Denmark http://www.sst.dk). However, these gender differences should be explored further, as they seem not to have been ascertained previously in population-based surveys.
We observed a significant change in species distribution over the 6-year period, with a decline in C. albicans and an increase in C. glabrata. The same trend is well documented in the other Nordic countries over the last decades (9, 29, 36, 37, 44), and C. glabrata has become the second most common pathogen in the northern hemisphere (4). This shift has been associated with increased fluconazole use overall (32) and especially in intensive care and hematological and pediatric settings (19, 27, 28, 50). Invasive disease originates from the colonizing flora (11, 16, 26, 39), and thus, prior antifungal therapy in primary health care or hospital settings may affect species distribution of subsequent invasive infections. Over the last 10 years the consumption of antifungals in Denmark has increased by 140% and reached 249 DDD/1,000 inhabitants in 2009; for comparison, the total annual consumption in Norway was 78.4 DDD/1,000 inhabitants (http://www.legemiddelforbruk.no/english/). During the same period, fluconazole use in Denmark increased to a level 2.7 times higher per inhabitant than that in Norway (159.5 versus 58.8 DDD/1,000 inhabitants). In Denmark the majority of fluconazole is prescribed in the primary health care setting and far more often to women than men. These differences may in part explain the prominence of C. glabrata in Denmark compared with other Nordic countries, the continuing rise, and the difference among genders. However, as reported previously, C. glabrata and C. krusei are still remarkably infrequent in the pediatric setting, and hence, fluconazole is still a reasonable option for the treatment of candidemia before species identification in this particular setting, except in children with prior azole exposure.
Recent reports have suggested that the choice of blood culture system may influence the recovery of C. glabrata and that the Bactec system may be inferior to the BacT/Alert system, in this respect (6, 44). The present data support this observation, as the recovery rate of C. glabrata was significantly lower at centers using the Bactec system than at centers using the BacT/Alert system. A theoretical bias for this observation could be that the age distribution varied between centers using the two blood culture systems, and therefore, the analysis was repeated for the 60- to 79-year-old age group with the same result. It is consequently suggested that the mycosis medium be included in blood cultures at centers using the Bactec system when a patient is at risk for candidemia.
To our knowledge, this is the first surveillance study reporting the emergence of C. palmioleophila with one isolate in 2006, three isolates in 2007, and five isolates in 2009. This species is phenotypically easily confused with C. guilliermondii, but in contrast to C. guilliermondii, it is highly susceptible to the echinocandin class of drugs (17, 21). As for many other emerging Candida species, susceptibility to fluconazole is low (35), and thus, susceptibility testing and correct species identification is necessary to guide appropriate treatment.
Susceptibility testing confirmed the broad activity of amphotericin B and echinocandins. Thus, 99% were susceptible to amphotericin B, with exceptions being C. norvegensis, C. krusei, C. glabrata, and the majority of Fusarium isolates. Amphotericin B MICs were below 1 μg/ml for all 20 C. lusitaniae isolates. However, this species is known for its reduced clinical susceptibility to amphotericin B and higher mutational rate and thus should be regarded as having intrinsically reduced amphotericin B susceptibility despite being classified as susceptible based on MIC determinations (10). Also, the echinocandins caspofungin and anidulafungin were active against the vast majority of the isolates. The current CLSI breakpoint of ≤2 μg/ml for anidulafungin and caspofungin and Candida species is undergoing revision: ≤0.25 μg/ml has been proposed for C. albicans, C. glabrata, C. krusei, and C. tropicalis, and ≤2 μg/ml for C. parapsilosis and C. guilliermondii (8, 34). Breakpoints for the echinocandins have not yet been established by EUCAST. However, the EUCAST and CLSI methods have been compared using the same set of wild-type and resistant mutants, and the species-specific breakpoints suggested by these data were applied in the present study (8). One limitation is that caspofungin MIC distributions are variable due to unknown factors, and thus, the breakpoint of ≤2 μg/ml may be too high (7). We found more isolates classified as susceptible to caspofungin than to anidulafungin and believe this is an artifact due to this issue. One isolate was, however, resistant with an anidulafungin MIC of 0.5 μg/ml, a caspofungin MIC of >32 μg/ml, and a well-characterized hot spot mutation in the FKS1 target gene. Furthermore, caspofungin MICs were above the proposed revised breakpoint for three of the other five Candida isolates with an increased anidulafungin MIC, suggesting that these unrelated isolates may have acquired resistance mechanisms for echinocandins. Thus, although still rare, acquired echinocandin resistance is detected among Danish blood isolates.
We found that 29% of the blood isolates were not fully fluconazole susceptible, which confirms our previous finding that reduced fluconazole susceptibility is prevalent in Denmark (5, 6). Acquired fluconazole resistance was investigated in two recent surveys, one global and one including patients at two tertiary centers in the United States (31, 35). Among more than 250,000 isolates from 41 countries, fluconazole resistance was found in 2% of C. albicans, 3.9% of C. dubliniensis, 9% of C. tropicalis, and 6.8% of C. parapsilosis isolates (35). Notably, at the U.S. tertiary centers, acquired resistance accounted for a third of the resistant isolates (31). In our country, acquired resistance was seen at similar rates among the listed species. But in contrast to the United States, acquired resistance accounted for less than 2.7% of the resistance in candidemia cases, and thus, species identification is still a reliable predictor of susceptibility in Denmark.
In conclusion, this first 6-year nationwide population-based study of fungemia in Denmark has revealed several significant findings. First, the previously reported high rate of fungemia in Denmark was confirmed, and it still stands out in both a Nordic and a global perspective. Also, the high proportion of isolates not fully susceptible to fluconazole remained unchanged after inclusion of fungemias from all district and county hospitals. Second, the species distribution has been changing, with fewer C. albicans and more C. glabrata isolates, and of note, the newly reported species C. palmioleophila has emerged as a bloodstream pathogen. Third, acquired resistance continued to be low, particularly for amphotericin B and the echinocandins, but fluconazole resistance in 2009 exceeded 5% for C. tropicalis and C. parapsilosis. Finally, substantial age- and gender-related differences in incidence rates and species distribution were shown in a population-based setting.
Acknowledgments
This study was supported financially by an unrestricted grant from Gilead.
Footnotes
Published ahead of print on 27 October 2010.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Ahlquist, A., M. M. Farley, L. H. Harrison, W. S. Baughman, S. S. Magill, and T. Chiller. 2009. Epidemiology of candidemia in metropolitan Atlanta and Baltimore City and County: preliminary results of population-based active, laboratory surveillance—2008-2009, abstr. M-1241. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother.
- 3.Almirante, B., D. Rodriguez, B. J. Park, M. Cuenca-Estrella, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, P. Saballs, S. K. Fridkin, J. Morgan, J. L. Rodriguez-Tudela, D. W. Warnock, and A. Pahissa. 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43:1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arendrup, M. C. 2010. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 16:445-452. [DOI] [PubMed] [Google Scholar]
- 5.Arendrup, M. C., K. Fuursted, B. Gahrn-Hansen, I. M. Jensen, J. D. Knudsen, B. Lundgren, H. C. Schonheyder, and M. Tvede. 2005. Seminational surveillance of fungemia in Denmark: notably high rates of fungemia and numbers of isolates with reduced azole susceptibility. J. Clin. Microbiol. 43:4434-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arendrup, M. C., K. Fuursted, B. Gahrn-Hansen, H. C. Schonheyder, J. D. Knudsen, I. M. Jensen, B. Bruun, J. J. Christensen, and H. K. Johansen. 2008. Semi-national surveillance of fungemia in Denmark 2004-2006: increasing incidence of fungemia and numbers of isolates with reduced azole susceptibility. Clin. Microbiol. Infect. 14:487-494. [DOI] [PubMed] [Google Scholar]
- 7.Arendrup, M. C., G. Garcia-Effron, W. Buzina, K. L. Mortensen, N. Reiter, C. Lundin, H. E. Jensen, C. Lass-Florl, D. S. Perlin, and B. Bruun. 2009. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 53:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arendrup, M. C., G. Garcia-Effron, C. Lass-Florl, A. G. Lopez, J. L. Rodriguez-Tudela, M. Cuenca-Estrella, and D. S. Perlin. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and IsoSensitest media. Antimicrob. Agents Chemother. 54:426-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asmundsdóttir, L. R., H. Erlendsdottir, and M. Gottfredsson. 2002. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J. Clin. Microbiol. 40:3489-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson, B. J., R. E. Lewis, and D. P. Kontoyiannis. 2008. Candida lusitaniae fungemia in cancer patients: risk factors for amphotericin B failure and outcome. Med. Mycol. 46:541-546. [DOI] [PubMed] [Google Scholar]
- 11.Brillowska-Dabrowska, A., O. Bergmann, I. M. Jensen, J. O. Jarløv, and M. C. Arendrup. 2010. Typing of Candida isolates from patients with invasive infection and concomitant colonization. Scand. J. Infect. Dis. 42:109-113. [DOI] [PubMed] [Google Scholar]
- 12.Brillowska-Dabrowska, A., D. M. Saunte, and M. C. Arendrup. 2007. Five-hour diagnosis of dermatophyte nail infections with specific detection of Trichophyton rubrum. J. Clin. Microbiol. 45:1200-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, S., M. Slavin, Q. Nguyen, D. Marriott, E. G. Playford, D. Ellis, and T. Sorrell. 2006. Active surveillance for candidemia, Australia. Emerg. Infect. Dis. 12:1508-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed, vol. 28. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 15.Cuenca-Estrella, M., M. C. Arendrup, E. Chryssanthou, E. Dannaoui, C. Lass-Florl, P. Sandven, A. Velegraki, and J. L. Rodriguez-Tudela. 2007. Multicentre determination of quality control strains and quality control ranges for antifungal susceptibility testing of yeasts and filamentous fungi using the methods of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin. Microbiol. Infect. 13:1018-1022. [DOI] [PubMed] [Google Scholar]
- 16.Dalle, F., N. Franco, J. Lopez, O. Vagner, D. Caillot, P. Chavanet, B. Cuisenier, S. Aho, S. Lizard, and A. Bonnin. 2000. Comparative genotyping of Candida albicans bloodstream and nonbloodstream isolates at a polymorphic microsatellite locus. J. Clin. Microbiol. 38:4554-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desnos-Ollivier, M., M. Ragon, V. Robert, D. Raoux, J. C. Gantier, and F. Dromer. 2008. Debaryomyces hansenii (Candida famata), a rare human fungal pathogen often misidentified as Pichia guilliermondii (Candida guilliermondii). J. Clin. Microbiol. 46:3237-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hachem, R., H. Hanna, D. Kontoyiannis, Y. Jiang, and I. Raad. 2008. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 112:2493-2499. [DOI] [PubMed] [Google Scholar]
- 20.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen, R. H., and M. C. Arendrup. 2010. Candida palmioleophila: characterisation of the emerging pathogen and its unique susceptibility profile in comparison with five related species, abstr. M-363. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother. [DOI] [PMC free article] [PubMed]
- 22.Kao, A. S., M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stephens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjeh. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis. 29:1164-1170. [DOI] [PubMed] [Google Scholar]
- 23.Kibbler, C. C., S. Seaton, R. A. Barnes, W. R. Gransden, R. E. Holliman, E. M. Johnson, J. D. Perry, D. J. Sullivan, and J. A. Wilson. 2003. Management and outcome of bloodstream infections due to Candida species in England and Wales. J. Hosp. Infect. 54:18-24. [DOI] [PubMed] [Google Scholar]
- 24.Klingspor, L., E. Tornqvist, A. Johansson, B. Petrini, U. Forsum, and G. Hedin. 2004. A prospective epidemiological survey of candidemia in Sweden. Scand. J. Infect. Dis. 36:52-55. [DOI] [PubMed] [Google Scholar]
- 25.Kontoyiannis, D. P., I. Vaziri, H. A. Hanna, M. Boktour, J. Thornby, R. Hachem, G. P. Bodey, and I. I. Raad. 2001. Risk factors for Candida tropicalis fungemia in patients with cancer. Clin. Infect. Dis. 33:1676-1681. [DOI] [PubMed] [Google Scholar]
- 26.Lunel, F. V., L. Licciardello, S. Stefani, H. A. Verbrugh, W. J. Melchers, J. F. Meis, S. Scherer, and A. van Belkum. 1998. Lack of consistent short sequence repeat polymorphisms in genetically homologous colonizing and invasive Candida albicans strains. J. Bacteriol. 180:3771-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manzoni, P., M. Leonessa, P. Galletto, M. A. Latino, R. Arisio, M. Maule, G. Agriesti, L. Gastaldo, E. Gallo, M. Mostert, and D. Farina. 2008. Routine use of fluconazole prophylaxis in a neonatal intensive care unit does not select natively fluconazole-resistant Candida subspecies. Pediatr. Infect. Dis. J. 27:731-737. [DOI] [PubMed] [Google Scholar]
- 28.Neu, N., M. Malik, A. Lunding, S. Whittier, L. Alba, C. Kubin, and L. Saiman. 2009. Epidemiology of candidemia at a children's hospital, 2002 to 2006. Pediatr. Infect. Dis. J. 28:806-809. [DOI] [PubMed] [Google Scholar]
- 29.Nordøy, I., P. Gaustad, P. Sandven, and the Norwegian Yeast Study Group. 2009. Candidemia in Norway, p. 43, poster P105. Abstr. 4th Meet. Trends Med. Mycol.
- 30.Odds, F. C., M. F. Hanson, A. D. Davidson, M. D. Jacobsen, P. Wright, J. A. Whyte, N. A. Gow, and B. L. Jones. 2007. One year prospective survey of Candida bloodstream infections in Scotland. J. Med. Microbiol. 56:1066-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oxman, D. A., J. K. Chow, G. Frendl, S. Hadley, S. Hershkovitz, P. Ireland, L. A. McDermott, K. Tsai, F. M. Marty, D. P. Kontoyiannis, and Y. Golan. 2010. Candidemia associated with decreased in vitro fluconazole susceptibility: is Candida speciation predictive of the susceptibility pattern? J. Antimicrob. Chemother. 65:1460-1465. [DOI] [PubMed] [Google Scholar]
- 32.Pasqualotto, A. C., R. A. Zimerman, S. H. Alves, V. R. Aquino, D. Branco, D. Wiltgen, A. do Amaral, R. Cechinel, S. M. Colares, I. G. da Rocha, L. C. Severo, and T. C. Sukiennik. 2008. Take control over your fluconazole prescriptions: the growing importance of Candida glabrata as an agent of candidemia in Brazil. Infect. Control Hosp. Epidemiol. 29:898-899. [DOI] [PubMed] [Google Scholar]
- 33.Pemán, J., E. Canton, and M. Gobernado. 2005. Epidemiology and antifungal susceptibility of Candida species isolated from blood: results of a 2-year multicentre study in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 24:23-30. [DOI] [PubMed] [Google Scholar]
- 34.Pfaller, M. A., D. J. Diekema, D. Andes, M. C. Arendrup, S. D. Brown, M. Motyl, and D. S. Perlin. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat., in press. [DOI] [PubMed]
- 35.Pfaller, M. A., D. J. Diekema, D. L. Gibbs, V. A. Newell, D. Ellis, V. Tullio, A. Rodloff, W. Fu, T. A. Ling, and the Global Antifungal Surveillance Group. 2010. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 48:1366-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poikonen, E., O. Lyytikainen, V. J. Anttila, and P. Ruutu. 2003. Candidemia in Finland, 1995-1999. Emerg. Infect. Dis. 9:985-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poikonen, E., O. Lyytikainen, and P. Ruutu. 2009. Candidemia in Finland, 1995-1999 vs 2004-2007, abstr. P-1961. Abstr. 20th Eur. Conf. Clin. Microbiol. Infect. Dis.
- 38.Presterl, E., F. Daxbock, W. Graninger, and B. Willinger. 2007. Changing pattern of candidemia 2001-2006 and use of antifungal therapy at the University Hospital of Vienna, Austria. Clin. Microbiol. Infect. 13:1072-1076. [DOI] [PubMed] [Google Scholar]
- 39.Reagan, D. R., M. A. Pfaller, R. J. Hollis, and R. P. Wenzel. 1990. Characterization of the sequence of colonization and nosocomial candidemia using DNA fingerprinting and a DNA probe. J. Clin. Microbiol. 28:2733-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Tudela, J. L., M. C. Arendrup, F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, E. Dannaoui, D. W. Denning, J. P. Donnelly, F. Dromer, W. Fegeler, C. Lass-Florl, C. B. Moore, M. Richardson, P. Sandven, A. Velegraki, and P. E. Verweij. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398-405. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Tudela, J. L., J. P. Donnelly, M. C. Arendrup, S. Arikan, F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, E. Dannaoui, and D. W. Denning. 2008. EUCAST Technical Note on voriconazole. Clin. Microbiol. Infect. 14:985-987. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez Tudela, J. L., J. P. Donnelly, M. C. Arendrup, S. Arikan, F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, E. Dannaoui, D. Denning, W. Fegeler, P. Gaustad, N. Klimko, C. Lass-Flörl, C. Moore, M. Richardson, A. Schmalreck, J. Stenderup, A. Velegraki, and P. Verweij. 2008. EUCAST Technical Note on fluconazole. Clin. Microbiol. Infect. 14:193-195. [DOI] [PubMed] [Google Scholar]
- 43.Sandven, P., L. Bevanger, A. Digranes, P. Gaustad, H. H. Haukland, M. Steinbakk, and the Norwegian Yeast Study Group. 1998. Constant low rate of fungemia in Norway, 1991 to 1996. J. Clin. Microbiol. 36:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandven, P., L. Bevanger, A. Digranes, H. H. Haukland, T. Mannsaker, and P. Gaustad. 2006. Candidemia in Norway (1991 to 2003): results from a nationwide study. J. Clin. Microbiol. 44:1977-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sendid, B., A. Cotteau, N. Francois, A. D'Haveloose, A. Standaert, D. Camus, and D. Poulain. 2006. Candidemia and antifungal therapy in a French University Hospital: rough trends over a decade and possible links. BMC Infect. Dis. 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sipsas, N. V., R. E. Lewis, J. Tarrand, R. Hachem, K. V. Rolston, I. I. Raad, and D. P. Kontoyiannis. 2009. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001-2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 115:4745-4752. [DOI] [PubMed] [Google Scholar]
- 47.Swinne, D., M. Watelle, C. Suetens, K. Mertens, P. A. Fonteyne, and N. Nolard. 2004. A one-year survey of candidemia in Belgium in 2002. Epidemiol. Infect. 132:1175-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tortorano, A. M., E. Biraghi, A. Astolfi, C. Ossi, M. Tejada, C. Farina, S. Perin, C. Bonaccorso, C. Cavanna, A. Raballo, and A. Grossi. 2002. European Confederation of Medical Mycology (ECMM) prospective survey of candidemia: report from one Italian region. J. Hosp. Infect. 51:297-304. [DOI] [PubMed] [Google Scholar]
- 49.Tortorano, A. M., J. Peman, H. Bernhardt, L. Klingspor, C. C. Kibbler, O. Faure, E. Biraghi, E. Canton, K. Zimmermann, S. Seaton, and R. Grillot. 2004. Epidemiology of candidemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 23:317-322. [DOI] [PubMed] [Google Scholar]
- 50.Trick, W. E., S. K. Fridkin, J. R. Edwards, R. A. Hajjeh, and R. P. Gaynes. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 51.White, T. J., T. D. Bruns, S. B. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, CA.
- 52.Wingard, J. R. 1995. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin. Infect. Dis. 20:115-125. [DOI] [PubMed] [Google Scholar]