Abstract
Purpose
Immunologic targeting of tumor-specific gene mutations may allow precise eradication of neoplastic cells without toxicity. Epidermal growth factor receptor variant III (EGFRvIII) is a constitutively activated and immunogenic mutation not expressed in normal tissues but widely expressed in glioblastoma multiforme (GBM) and other neoplasms.
Patients and Methods
A phase II, multicenter trial was undertaken to assess the immunogenicity of an EGFRvIII-targeted peptide vaccine and to estimate the progression-free survival (PFS) and overall survival (OS) of vaccinated patients with newly diagnosed EGFRvIII-expressing GBM with minimal residual disease. Intradermal vaccinations were given until toxicity or tumor progression was observed. Sample size was calculated to differentiate between PFS rates of 20% and 40% 6 months after vaccination.
Results
There were no symptomatic autoimmune reactions. The 6-month PFS rate after vaccination was 67% (95% CI, 40% to 83%) and after diagnosis was 94% (95% CI, 67% to 99%; n = 18). The median OS was 26.0 months (95% CI, 21.0 to 47.7 months). After adjustment for age and Karnofsky performance status, the OS of vaccinated patients was greater than that observed in a control group matched for eligibility criteria, prognostic factors, and temozolomide treatment (hazard ratio, 5.3; P = .0013; n = 17). The development of specific antibody (P = .025) or delayed-type hypersensitivity (P = .03) responses to EGFRvIII had a significant effect on OS. At recurrence, 82% (95% CI, 48% to 97%) of patients had lost EGFRvIII expression (P < .001).
Conclusion
EGFRvIII-targeted vaccination in patients with GBM warrants investigation in a phase III, randomized trial.
INTRODUCTION
Glioblastoma multiforme (GBM), the most common primary malignant neoplasm of the CNS, remains universally fatal. Patients with newly diagnosed GBM have a median overall survival (OS) of only 14.6 months despite maximal surgical resection, conformal radiation,1 and systemic chemotherapy.2 These conventional modalities lack specificity and, as a result, are limited by damage to normal tissue.1 Immunologic recognition of tumor-specific mutations, however, holds the promise of more precisely eliminating neoplastic cells.
The mutated epidermal growth factor receptor variant III (EGFRvIII) is a cell surface protein containing a tumor-specific epitope reportedly expressed on approximately one third of GBMs3,4 and a broad array of neoplasms in other tissues, including the breast, lung, and head and neck.5–8 It is not found in any normal tissues.9 EGFRvIII is characterized by a consistent in-frame deletion of 801 base pairs from the extracellular domain that splits a codon and produces a novel glycine at the fusion junction and approximates two normally distant parts of the protein. This mutation encodes a protein with a constitutively active tyrosine kinase10 that enhances tumorigenicity11,12 and tumor cell migration13 and confers radiation and chemotherapeutic resistance to tumor cells.14–16 For patients with GBM who survive 1 year or longer after diagnosis, the expression of EGFRvIII is also an independent negative prognostic indicator of survival.4 Thus, several factors make EGFRvIII an ideal target for antitumor immunotherapy.
On the basis of preclinical data supporting the safety and potential efficacy of an EGFRvIII-targeted vaccine,17,18 a phase II, multicenter, prospective trial was undertaken to assess the immunogenicity of an EGFRvIII-targeted peptide vaccine and to estimate progression-free survival (PFS) from vaccination and histologic diagnosis in patients newly diagnosed with GBM who expressed EGFRvIII.
PATIENTS AND METHODS
Vaccine Product
PEPvIII (LEEKKGNYVVTDHC) is a 13-amino-acid peptide with an additional terminal cysteine that spans the EGFRvIII mutation (AnaSpec, San Jose, CA). The peptide preparation was > 95% pure as assessed by high-pressure liquid chromatography and was conjugated to keyhole limpet hemocyanin (KLH; biosyn, Carlsbad, CA) at a 1:1 ratio (w/w) (PEPvIII-KLH) using the heterobifunctional cross-linker sulfosuccinimidyl 6-[3′(2-pyridyldithio)-propionamido]hexanoate (Pierce, Rockford, IL).
Patient Selection
Adults with newly diagnosed EGFRvIII-expressing GBM with a gross total resection (> 95%) and a Karnofsky performance status (KPS) ≥ 80% who had no radiographic evidence of progression after standard of care external beam radiation therapy and concurrent temozolomide (TMZ) and were willing to sign an informed consent were eligible for vaccination. The trial was approved by the US Food and Drug Administration (BB-IND-9944) and the local institutional review boards at Duke University Medical Center and M. D. Anderson Cancer Center.
Clinical Protocol
The initial three vaccinations of PEPvIII-KLH were given every two weeks starting 4 weeks after the completion of radiation. Subsequent vaccines were given once a month until radiographic evidence of tumor progression or death. All vaccines were given intradermally in the inguinal region within 10 cm of the inguinal ligament on alternating sides.
Patients were monitored once a month by physical examination and every two months by magnetic resonance imaging. Progressive disease was defined radiographically according to the MacDonald criteria19 or by the development of a new contrast-enhancing lesion of > 1 cm at the discretion of the treating neurooncologist. On tumor progression, further treatment was at the discretion of the patient's treating neurooncologist.
Immunologic Monitoring and Immunohistochemical Analysis
Delayed-type hypersensitivity (DTH) testing for cellular immune responses to PEPvIII (1 mg/mL), as well as to the recall antigens tetanus toxoid (NDC 49281-800-83 undiluted; Aventis Pasteur, Swiftwater, PA), Candida (#M15 1:1000; Greer Laboratories, Lenoir, NC), and Trichophyton (#M26, 1:1000; Greer), was performed by using standard intradermal injections in a volume of 100 μL. A positive skin test for all antigens was defined as > 5 mm induration within 48 to 72 hours.
Serum for assessing humoral responses was stored at −20°C before analysis in an enzyme-linked immunosorbent assay or a PEPvIII-Dynabead (Invitrogen, Carlsbad, CA) assay, as described previously.20
Immunohistochemistry (IHC) for EGFRvIII was performed on paraffin-embedded tissue, as previously described.7,21 IHC was performed in a Clinical Laboratory Improvement Amendments–certified laboratory using compliant techniques.
Matched Cohort
Patients in the matched cohort selected for PFS and OS comparisons were all treated contemporaneously at the University of Texas M. D. Anderson Cancer Center. In the matched control cohort (n = 17), all patients were adults; had EGFRvIII-expressing primary GBMs, a KPS ≥ 80%, and a resection of > 95% of the original tumor volume; and had been treated with radiation and TMZ. Patients with tumor progression within 4 weeks of completing radiation therapy were also excluded from this matched control cohort.
Methylguanine Methyltransferase Promoter Methylation Status
Samples were isolated from five sections of a representative tissue block. Following deparaffinization and proteinase K treatment, DNA was isolated from tumor tissue using a kit from Epicenter (Madison, WI). After sodium bisulfite treatment, methyl-specific polymerase chain reaction was performed as previously described.22 A ratio of methylated-to-unmethylated peaks of ≥ 1.0 was scored as methylated.
Statistical Analysis
A two-stage clinical trial design was used to differentiate between a 6-month PFS rate of 20% and 40% from the time of vaccination with α and β = .1 on the basis of data derived from the matched cohort. If five or more of the 22 patients accrued in stage 1 were alive and free from disease progression at 6 months after vaccination, then an additional 22 patients were to be accrued during a second stage. If 11 or more of the 44 patients survived 6 months progression free from the time of vaccination, the treatment regimen was to be considered worthy of additional investigation. PFS and OS were estimated using Kaplan-Meier methods. The Cox proportional hazards model was used to compare PFS and OS of the vaccinated patients with the matched cohort; adjustments for known prognostic factors and covariates were made in the model. The study was not initially powered for these comparisons, however. Adverse events were defined according to the National Cancer Institute's Common Toxicity Criteria (Version 2.0). Frequencies were used to describe the EGFRvIII-specific antibody responses and DTH reactions. A binomial test was used to assess whether the proportion of patients with EGFRvIII IHC staining changes due to vaccination was significantly different from zero.
RESULTS
Study Population
At both centers, all known patients with EGFRvIII-expressing newly diagnosed GBM were screened for study eligibility. Only two patients at each site refused participation despite being eligible. Twenty-one patients were enrolled and vaccinated before accrual was suspended at the end of stage 1. On retrospective quality control review, three patients were found not to meet eligibility criteria because < 95% of the tumor volume had been resected. Therefore, the primary focus of analyses reported here will involve the 18 eligible patients. Follow-up through October 2008 is reflected in this article. All but one of the patients who were vaccinated received TMZ delivered concurrently with radiotherapy2 (Table 1). TMZ therapy after radiation was not used in conjunction with the vaccinations. Study data are summarized in Table 2.
Table 1.
Demographic Characteristics of Patients With Primary Glioblastoma Treated With EGFRvIII Peptide Vaccination and the Matched Cohort
| Parameter |
EGFRvIII Vaccine Group |
Matched Cohort |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total No. of Patients | 18 | 17 | ||
| Age, years | ||||
| Median | 52 | 59 | ||
| Range | 29-67 | 37-71* | ||
| Sex (male) | 13 | 72 | 8 | 47.1 |
| KPS | ||||
| 100 | 7 | 39 | 1 | 6 |
| 90 | 7 | 39 | 9 | 53 |
| 80 | 4 | 22 | 7 | 41† |
| EGFRvIII expression | 18 | 100 | 17 | 100 |
| Extent of surgical resection, % | ||||
| Median | 100 | 100 | ||
| Range | 95-100 | 95-100 | ||
| Radiation | 18 | 100 | 17 | 100 |
| TMZ treatment | 17 | 94 | 17 | 100 |
Abbreviations: EGFRvIII, epidermal growth factor receptor variant III; KPS, Karnofsky performance status; TMZ, temozolomide.
P = .055.
P = .016.
Table 2.
Patient Summary
| Patient | Age (years) | Sex | KPS | MGMT Methylation | Humoral Response |
DTH Response |
PFS (months) | OS (months) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Vaccination | After Vaccination | Before Vaccination | After Vaccination | |||||||
| 1 | 62 | M | 90 | ME | N/A | N/A | — | — | 6.7 | 16.2 |
| 2 | 49 | M | 80 | N/A | N/A | N/A | — | — | 6.4 | 18.0 |
| 3 | 51 | M | 100 | N/A | N/A | N/A | — | — | 9.9 | 21.0 |
| 4 | 48 | M | 100 | U | — | +* | — | + | 58.5† | 58.5‡ |
| 5 | 52 | M | 100 | ME | — | — | — | — | 13.5 | 22.6 |
| 6 | 42 | M | 100 | U | — | — | — | + | 14.9 | 35.8 |
| 7 | 51 | M | 90 | U | — | — | — | + | 54.6† | 54.6‡ |
| 8 | 33 | M | 100 | N/A | — | — | — | — | 53.7† | 53.7‡ |
| 9 | 46 | F | 90 | ME | — | — | — | — | 15.4 | 34.9 |
| 10 | 29 | M | 90 | N/A | — | — | — | N/A | 6.4 | 11.2 |
| 11 | 64 | M | 90 | U | N/A | N/A | — | — | 12.0 | 21.6 |
| 12 | 64 | M | 80 | U | — | + | — | — | 27.6 | 44.1 |
| 13 | 54 | F | 90 | N/A | — | — | — | — | 17.6 | 26.0 |
| 14 | 63 | M | 80 | ME | — | + | — | — | 10.9 | 20.8 |
| 15 | 67 | F | 80 | ME | — | + | — | — | 6.5 | 13.4 |
| 16 | 53 | F | 100 | ME | — | + | — | — | 30.0 | 47.7 |
| 17 | 52 | M | 100 | ME | — | + | — | — | 5.4 | 47.4‡ |
| 18 | 64 | F | 90 | U | — | — | — | — | 16.4 | 23.1 |
Abbreviations: KPS, Karnofsky performance status; MGMT, methylguanine methyltransferase; DTH, delayed-type hypersensitivity; PFS, progression-free survival; OS, overall survival; M, male; ME, methylated; N/A, sample not available; U, unmethylated; F, female; (—), no response; (+), positive response.
Positive humoral response in CSF.
No progression.
Alive.
Toxicity and Adverse Events
Toxicity was generally minimal (Appendix Table A1, online only) and mostly related to injection site reactions, which never produced grade ≥ 2 toxicity. However, one patient was removed from the study when a presumed severe allergic reaction (numbness and tingling in the perioral area) to the vaccine components was suspected, although a similar event occurred several weeks later when the patient had an unrelated procedure. This was still considered a serious adverse event. In addition, one patient developed asymptomatic areas of T2-signal hyperintensity that on subsequent magnetic resonance imaging scans demonstrated contrast enhancement that eventually resolved. These lesions remained hypometabolic on positron emission tomography. This reaction was deemed a grade 1 leukoencephalopathy toxicity (Appendix Table A1 and Fig 1) and resolved without treatment.
Fig 1.
Axial magnetic resonance images showing progression of multiple lesions surrounding the corpus callosum. Left: T1-weighted, contrast-enhanced image 8 months from first vaccination showing new enhancing lesions distant from the right frontal tumor cavity. Right: T2-weighted image showing hyperintense lesion in similar distribution. Areas of contrast-enhancement have resolved, but areas of T2 hyperintensity have persisted.
PFS
The median PFS from time of histologic diagnosis for the 18 eligible patients was 14.2 months (95% CI, 9.9 to 17.6 months; Fig 2A and Appendix Table A2, online only). Among the 18 eligible patients, 12 patients (67%; 95% CI, 40% to 83%) were alive and without radiographic evidence of progression 6 months after vaccination, and 17 patients (94%; 95% CI, 67% to 99%) were alive and without radiographic evidence of progression 6 months after histologic diagnosis (Appendix Table A2). After considering these data, we rejected the null hypothesis for the primary end point. Accrual was terminated after this interim analysis because the number of patients living progression free for more than 6 months after vaccination (n = 12) exceeded the critical value associated with the hypothesis test to be conducted at the end of the second stage of the study (n ≥11).
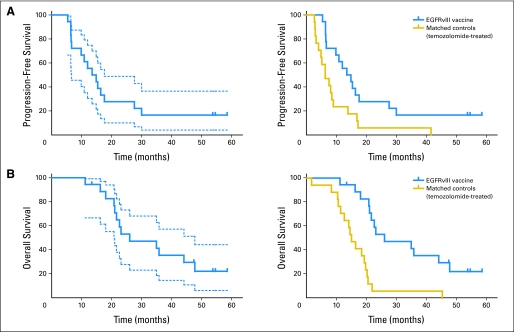
Fig 2.
(A) Progression-free survival (PFS). Left: The median PFS from histologic diagnosis in the patients who had been vaccinated (n = 18; solid blue line) was 14.2 months (95% CI, 9.9 to 17.6 months). Dotted blue lines show 95% CIs. Right: In the temozolomide (TMZ) -treated historical cohort (n = 17; gold line), the median PFS was 6.3 months (95% CI, 4.1 to 9.0 months). The PFS of patients who had been vaccinated compares favorably with that of the TMZ-treated cohort before (P = .013) and after (P = .041) adjustment for age and Karnofsky performance status (KPS). (B) Overall survival (OS). Left: The median survival of patients who had been vaccinated (n = 18; solid blue line) was 26.0 months (95% CI, 21.0 to 47.7 months;). Dotted blue lines show 95% CIs. Right: In the TMZ-treated historical cohort (n = 17; gold line), the median survival was 15.0 months (95% CI, 11.4 to 19.7 months). The OS of patients who had been vaccinated compares favorably with that of the TMZ-treated cohort before (P = .002) and after (P = .001) adjustment for age and KPS. EGFRVIII, epidermal growth factor receptor variant III.
In the matched cohort (n = 17), the median PFS from histologic diagnosis was 6.3 months (95% CI, 4.1 to 9.0 months). The PFS hazard ratio (HR) comparing the matched cohort with our patients was 2.4 (95% CI, 1.2 to 5.0; P = .013; Fig 2A). Even after adjustment for age and KPS, the PFS of vaccinated patients remained significantly greater than that observed in the TMZ-treated matched control group (HR, 2.2; 95% CI, 1.0 to 4.8; P = .041). Inferences were not affected by the exclusion of the three ineligible patients.
Survival Time
The median OS from time of histologic diagnosis for the 18 eligible patients was 26.0 months (95% CI, 21.0 to 47.7 months). Among the 18 eligible patients, 94% (95% CI, 67% to 99%) were alive 12 months after vaccination and 94% (95% CI, 67% to 99%) were alive at 12 months after histologic diagnosis (Fig 2B and Appendix Table A2). In the matched cohort (n = 17), time from histologic diagnosis to the median OS was 15.0 months (95% CI, 11.4 to 19.7 months). After adjustment for age and KPS, the survival of vaccinated patients was significantly better than that observed in a TMZ-treated matched control group (HR, 5.1; 95% CI, 1.9 to 13.9; P = .001). Inferences were not affected by the exclusion of the three ineligible patients.
IHC Analysis of EGFRvIII Expression
Among recurrent tumors from which pathologic material was obtained (n = 11), all were evaluated by IHC for EGFRvIII expression. Of these 11 samples, 82% (95% CI, 48% to 97%) had lost EGFRvIII expression at recurrence (binomial test P < .001; Table 3; Fig 3). Of the two patients who had positive EGFRvIII staining at recurrence, one had < 1% of cells staining for EGFRvIII.
Table 3.
EGFRvIII Immunohistochemistry Before and After Vaccination
| Before Vaccination | At Recurrence |
|---|---|
| Positive | Negative |
| Positive | Negative |
| Positive | Negative |
| Positive | Positive (< 1%) |
| Positive | Negative |
| Positive | Negative |
| Positive | Negative |
| Positive | Negative |
| Positive | Negative |
| Positive | Positive |
| Positive | Negative |
NOTE. Percent negative after vaccine is 82% (95% CI, 48% to 97%) or nine of 11; binomial test P < .001.
Abbreviation: EGFRvIII, epidermal growth factor receptor variant III.
Fig 3.
Epidermal growth factor receptor (EGFR) and EGFR variant III (EGFRvIII) immunohistochemistry of a patient with glioblastoma multiforme (GBM). Staining with (A) EGFR and (B) EGFRvIII before vaccine. (C) Preservation of EGFR staining but (D) specific loss of EGFRvIII staining at recurrence after vaccination.
Immune Responses
At the interim analysis when the study was terminated, 14 patients had serum samples that had been analyzed for EGFRvIII-specific humoral responses. Six (43%; 95% CI, 18% to 71%; P < .001) had evidence of a humoral response against PEPvIII. The maximum concentration of antibody that reacted against EGFRvIII, as estimated by comparison with an EGFRvIII-specific monoclonal antibody,20 was 910 ng/mL. In one patient with a positive serum titer, CSF was obtained and it demonstrated a concentration of antibody against EGFRvIII of 16.2 ng/mL.
The median OS from histologic diagnosis for the six patients who developed EGFRvIII-specific antibody responses was 47.7 months (95% CI, 20.8 to ∞ months; Fig 4). For the eight patients who did not develop antibody responses, the OS was only 22.8 months (95% CI, 21.0 to 34.9 months). After adjustment for age, KPS, and methylguanine methyltransferase (MGMT) methylation, the OS from vaccination (P = .025) and histologic diagnosis (P = .025) of the patients who developed antibody responses was found to be greater than the OS for those who had not. However, these findings need to be validated prospectively in a model with a larger number of patients.
Fig 4.
Immune response correlates. Overall survival (OS) from histologic diagnosis for all patients for whom serum was available to test for epidermal growth factor receptor variant III (EGFRvIII) –specific antibody titers (n = 14; left) and delayed-type hypersensitivity (DTH; n = 17; right). The blue line shows patients with EGFRvIII-specific immune responses, and the gold line shows patients without EGFRvIII-specific responses. Left: The median OS for the six patients who developed EGFRvIII-specific antibody responses was 47.7 months (95% CI, 20.8 to ∞ months). For the eight patients who did not develop antibody responses, the OS was only 22.8 months (95% CI, 21.0 to 34.9 months). After adjustment for age, Karnofsky performance status, and methylguanine methyltransferase methylation, the OS from vaccination of the patients who developed antibody responses was found to be greater (hazard ratio, 0.08; 95% CI, 0.007 to 0.93; P = .043). Right: The median OS for the three patients who developed DTH responses specific for PEPvIII (a 13-amino-acid peptide with an additional terminal cysteine that spans the EGFRvIII mutation) has not been reached at 50 months. For the 14 patients who did not develop DTH responses to PEPvIII, the OS was 23.1 months (95% CI, 21.0 to 44.1 months). Patients who developed PEPvIII DTH responses had a significantly longer OS (P = .03).
To assess patient cellular immune responses to the vaccinating antigen and endogenous antigens in vivo, DTH skin tests were performed with PEPvIII and recall antigens. All patients showed no response to PEPvIII before vaccination. After vaccination, three (18%; 95% CI, 4% to 43%; binomial proportions P < .001) of 17 showed a positive DTH response to PEPvIII after vaccination.
The median OS from histologic diagnosis for the three patients who developed PEPvIII-specific DTH responses had not been reached at 50 months follow-up. For the 14 patients who did not develop DTH responses to PEPvIII, the OS was 23.1 months (95% CI, 21.0 to 44.1 months). Although the number of patients who developed PEPvIII DTH responses was small, the patients did have a significantly longer PFS and OS from vaccination and histologic diagnosis (P = .03). Conversely, DTH responses to recall antigens did not have a significant effect on PFS from vaccination (P = .81) or histologic diagnosis (P = .88) or on OS from vaccination (P = .58) or histologic diagnosis (P = .61). These small patient numbers, however, preclude meaningful adjustment for known prognostic factors such as age, KPS, and MGMT methylation.
MGMT Methylation
Because almost all of our study patients were treated with concurrent TMZ during radiotherapy, an unintentional study bias may have been introduced by selecting patients with GBM that possessed the capacity for MGMT methylation, which compromises DNA repair and is associated with longer survival in patients with GBM who receive alkylating agents such as TMZ.22 Of the patients from whom tumor tissue was obtained for MGMT testing (n = 13), seven (54%) had MGMT methylation, which is a slightly higher proportion than previously reported.22 However, the PFS and OS from either histologic diagnosis or vaccination is actually unexpectedly longer in our study for patients with unmethylated MGMT. This does not reach statistical significance in unadjusted univariate analyses but when adjusted for age and KPS, patients with unmethylated MGMT had a significantly longer PFS from vaccination (HR, 0.17; 95% CI, 0.032 to 0.90; P = .037) and histologic diagnosis (HR, 0.17; 95% CI, 0.032 to 0.90; P = .037) than patients with methylated MGMT who were treated with TMZ. There was also a trend for these patients to have a longer OS from vaccination (P = .062) and histologic diagnosis (P = .062). These data are summarized in Appendix Figure A1 and Appendix Table A3 (online only). A larger number of patients is needed, however, to validate these observations.
DISCUSSION
Our study results demonstrate that vaccinating patients who have newly diagnosed EGFRvIII-positive GBM with a peptide containing an EGFRvIII-specific epitope is safe, induces specific immunity against EGFRvIII, and is associated with the elimination of EGFRvIII-expressing cells at recurrence. This observation that an EGFRvIII-targeted vaccine is capable of potentially eliminating EGFRvIII-expressing tumor cells in the majority of patients is an intriguing finding and consistent with what we found in our preclinical studies in mice.18 It suggests that the immunologic privilege of the brain may not be absolute in this context as well. It is difficult to differentiate between elimination of a specific population of tumor cells expressing EGFRvIII and a downregulation of the expression of the mutated tyrosine kinase, however. Recurrent tumors in these patients continue to express the wild-type EGFR protein. This raises the possibility that intramolecular cross-priming that might induce immune responses against wild-type EGFR may be attenuated.
We also found that patients with GBM who had received the PEPvIII-KLH vaccine had significantly longer OS than a contemporaneously treated cohort matched for eligibility criteria and treatment, and they compare favorably with recently published trials evaluating other patient cohorts treated with carmustine polymers (13.7 months)23 or serial TMZ cycles (14.6 months).2 Although the median OS time observed in this study is encouraging, it may not be different from an untreated population or from that reported in other recent studies using different immunotherapy approaches.24–28 Although nearly consecutive patients with EGFRvIII-expressing GBM were enrolled at two different centers during this trial, the small sample size and restricted eligibility criteria, although chosen to optimize the identification of an immune response, led to some bias that cannot be adequately addressed despite our attempts to select an adequate matched control population. Thus, although these data suggest that vaccination with PEPvIII-KLH may improve PFS and OS in this population, definitive evidence of efficacy will require a blinded, placebo-controlled, randomized phase III study, which is currently in the planning stages.
Our finding that vaccinated patients with an unmethylated MGMT promoter, which usually confers resistance to TMZ,22 actually had a longer PFS and OS than those patients with methylated MGMT raises the possibility that EGFRvIII-targeted vaccination may be an effective alternative for those patients and mandates that phase III trials of this vaccine approach will need to stratify patients for this variable. Although unexpected, this finding is consistent with the observation by Murat et al29 that EGFR signaling, which would be induced in EGFRvIII-expressing tumor cells, confers resistance to TMZ and predicts a poor outcome after standard therapy.
Although this study demonstrates the possible benefits of vaccination with a peptide that contains a tumor-specific epitope, there remain a number of issues that must be addressed to optimize this therapeutic modality. While one distinct advantage of our approach is that the vaccine is available off-the-shelf and does not involve labor-intensive and expensive cell preparation techniques, a notable disadvantage is that only a subset of patients with GBM expresses the targeted antigen, EGFRvIII. Thus, although a tumor-specific vaccine may have the advantage of minimizing autoimmune complications, the heterogeneity of malignant brain tumors may limit the effectiveness of vaccinations that target only one tumor-specific antigen. Vaccines that target only one antigen may not target all tumors or all cells comprising a tumor and may therefore select for the survival and proliferation of those cells that do not express the targeted antigen. This may ultimately limit this potentially promising approach. Multiantigenic vaccines may serve as an alternative, but they risk the induction of autoimmunity. Using such an approach, however, several investigators have demonstrated robust immunologic responses and encouraging clinical results without catastrophic autoimmune responses.26–28,30,31
Supplementary Material
Acknowledgment
We thank the research staff who supported this study, including Denise Lally-Goss, Sharon McGehee-Norman, Beth Perry, Lammone Crutcher, Susan Graham, and Roxana Gomez. Editorial assistance was provided by Elizabeth Hess. This article is dedicated to Samuel Hassenbusch, MD.
Appendix
Fig A1.
Progression-free survival (PFS) from histologic diagnosis for all patients for whom methylguanine methyltransferase (MGMT) methylation status could be assessed is shown (n = 13). The blue line shows patients with unmethylated MGMT (n = 7) who had a median PFS of 22.0 months (95% CI, 14.9 to ∞ months), and the gold line shows patients with methylated MGMT (n = 6) who had a median survival of 10.9 months (95% CI, 6.5 to 15.4 months). When adjusted for age and Karnofsky performance status, patients who had been vaccinated with unmethylated MGMT had an unexpectedly significantly longer PFS (hazard ratio, 0.17; 95% CI, 0.032 to 0.90; P = .037).
Table A1.
Adverse Events and Attribution
| Adverse Event | No. of Patients | Severity | Relationship to Study |
|---|---|---|---|
| Neurology | 2 | 2 | Unlikely |
| 1 | Probable* | ||
| Constitutional | 2 | 1 | Possible |
| 1 | Unlikely | ||
| GI | 3 | 1 | Unlikely |
| 1 | Unlikely | ||
| 1 | Unlikely | ||
| Pain | 2 | 2 | Unlikely |
| 1 | Unlikely | ||
| Dermatology/skin | 1 | 1 | Probable |
| Allergy | 2 | 3 | Possible |
| 2 | Possible |
Same patient as noted in Fig 1.
Table A2.
PFS and OS for Patients Who Met Eligibility Criteria (n = 18)
| Statistic | Experimental Group (n = 18) |
Historical Cohort (n = 17) |
||||
|---|---|---|---|---|---|---|
| Postvaccination |
Postsurgery |
Postsurgery |
||||
| No. | 95% CI | No. | 95% CI | No. | 95% CI | |
| PFS, months | ||||||
| Median | 10.4 | 5.4 to 12.9 | 14.2 | 9.9 to 17.6 | 6.3 | 4.1 to 9.0 |
| 6-month, % | 0.67 | 0.40 to 0.83 | 0.94 | 0.67 to 0.99 | 0.59 | 0.33 to 0.78 |
| 12-month, % | 0.44 | 0.22 to 0.65 | 0.28 | 0.10 to 0.48 | 0.24 | 0.07 to 0.45 |
| 24-month, % | 0.28 | 0.10 to 0.49 | 0.28 | 0.10 to 0.49 | 0.06 | 0.02 to 0.31 |
| OS, months | ||||||
| Median | 21.2 | 17.0 to 44.9 | 26.0 | 21.0 to 47.7 | 15.0 | 11.4 to 19.7 |
| 6-month, % | 1.00 | 1.00 | 0.94 | 0.65 to 0.99 | ||
| 12-month, % | 0.94 | 0.67 to 0.99 | 0.94 | 0.67 to 0.99 | 0.71 | 0.43 to 0.86 |
| 24-month, % | 0.47 | 0.23 to 0.68 | 0.53 | 0.28 to 0.73 | 0.06 | 0.004 to 0.24 |
Abbreviations: PFS, progression-free survival; OS, overall survival.
Table A3.
PFS and OS for Patients Who Had Been Vaccinated by MGMT Methylation Status
| Variable | Unmethylated MGMT (n = 6) |
Methylated MGMT (n = 7) |
||
|---|---|---|---|---|
| No. of Months | 95% CI | No. of Months | 95% CI | |
| PFS from vaccine | 18.9 | 11.0 to ∞ | 7.1 | 3.2 to 12.4 |
| PFS from histologic diagnosis | 22.0 | 14.9 to ∞ | 10.9 | 6.5 to 15.4 |
| OS from vaccine | 36.6 | 19.5 to ∞ | 25.4 | 17.0 to 44.9 |
| OS from histologic diagnosis | 39.9 | 24.1 to ∞ | 28.8 | 20.8 to 47.7 |
Abbreviations: PFS, progression-free survival; OS, overall survival; MGMT, methylguanine methyltransferase.
Footnotes
See accompanying editorial on page 4670
Supported by National Institutes of Health Grants No. R01-CA97222-05 (J.H.S.), 5P50 NS20023 and R37 CA 011898 (D.D.B.), and 5P50 CA108786 (D.D.B., J.H.S.); by grants from the American Brain Tumor Association, Accelerate Brain Cancer Cure, and the Brain Tumor Society (J.H.S.); and Commonwealth Cancer Foundation, the Adam Sliger Foundation, Dr. Marnie Rose Foundation, Anthony Bullock III Foundation, and Golfers Against Cancer (A.B.H.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00643097.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: John H. Sampson, Celldex Therapeutics (C); Amy B. Heimberger, Celldex Therapeutics (C); Mark R. Gilbert, Genentech (C), Merck (C), Pfizer (C); Darell D. Bigner, Celldex Therapeutics (C) Stock Ownership: None Honoraria: John H. Sampson, Celldex Therapeutics; Mark R. Gilbert, Merck, Genentech Research Funding: John H. Sampson, Celldex Therapeutics; Mark R. Gilbert, Schering-Plough, Genentech Expert Testimony: None Other Remuneration: John H. Sampson and Darell D. Bigner received funding under the Duke University Faculty Plan from license fees paid to Duke University by Celldex Therapeutics.
AUTHOR CONTRIBUTIONS
Conception and design: John H. Sampson, Amy B. Heimberger, Gary E. Archer, Kenneth D. Aldape, Allan H. Friedman, Henry S. Friedman, Mark R. Gilbert, Roger E. McLendon, Duane A. Mitchell, David A. Reardon, Raymond Sawaya, Weiming Shi, James J. Vredenburgh,Darell D. Bigner
Financial support: John H. Sampson, Amy B. Heimberger
Administrative support: John H. Sampson, Amy B. Heimberger
Provision of study materials or patients: John H. Sampson, Amy B. Heimberger, Gary E. Archer, Kenneth D. Aldape, Allan H. Friedman, Henry S. Friedman, Mark R. Gilbert, Roger E. McLendon, David A. Reardon, Raymond Sawaya, Weiming Shi, James J. Vredenburgh,Darell D. Bigner
Collection and assembly of data: John H. Sampson, Amy B. Heimberger, Gary E. Archer, Kenneth D. Aldape, Mark R. Gilbert, Roger E. McLendon, Duane A. Mitchell, Raymond Sawaya, Robert J. Schmittling, Weiming Shi, James J. Vredenburgh, Darell D. Bigner
Data analysis and interpretation: John H. Sampson, Amy B. Heimberger, Gary E. Archer, Kenneth D. Aldape, Mark R. Gilbert, James E. Herndon II, Roger E. McLendon, Duane A. Mitchell, Raymond Sawaya, Robert J. Schmittling, Weiming Shi, Darell D. Bigner
Manuscript writing: John H. Sampson, Amy B. Heimberger, Gary E. Archer, Kenneth D. Aldape, Allan H. Friedman, Henry S. Friedman, Mark R. Gilbert, James E. Herndon II, Roger E. McLendon, Duane A. Mitchell, David A. Reardon, Raymond Sawaya, Robert J. Schmittling, Weiming Shi, James J. Vredenburgh, Darell D. Bigner
Final approval of manuscript: John H. Sampson, Amy B. Heimberger, Gary E. Archer, Kenneth D. Aldape, Allan H. Friedman, Henry S. Friedman, Mark R. Gilbert, James E. Herndon II, Roger E. McLendon, Duane A. Mitchell, David A. Reardon, Raymond Sawaya, Robert J. Schmittling, Weiming Shi, James J. Vredenburgh, Darell D. Bigner
REFERENCES
- 1.Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–822. doi: 10.1002/ana.410280614. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci U S A. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 5.Ge H, Gong X, Tang CK. Evidence of high incidence of EGFRvIII expression and coexpression with EGFR in human invasive breast cancer by laser capture microdissection and immunohistochemical analysis. Int J Cancer. 2002;98:357–361. doi: 10.1002/ijc.10224. [DOI] [PubMed] [Google Scholar]
- 6.Sok JC, Coppelli FM, Thomas SM, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 7.Wikstrand CJ, Hale LP, Batra SK, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55:3140–3148. [PubMed] [Google Scholar]
- 8.Purev E, Cai D, Miller E, et al. Immune responses of breast cancer patients to mutated epidermal growth factor receptor (EGF-RvIII, Delta EGF-R, and de2-7 EGF-R) J Immunol. 2004;173:6472–6480. doi: 10.4049/jimmunol.173.10.6472. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey PA, Wong AJ, Vogelstein B, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A. 1990;87:4207–4211. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu CT, Everiss KD, Wikstrand CJ, et al. Receptor dimerization is not a factor in the signalling activity of a transforming variant epidermal growth factor receptor (EGFRvIII) Biochem J. 1997;324:855–861. doi: 10.1042/bj3240855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batra SK, Castelino-Prabhu S, Wikstrand CJ, et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ. 1995;6:1251–1259. [PubMed] [Google Scholar]
- 12.Lal A, Glazer CA, Martinson HM, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–3339. [PubMed] [Google Scholar]
- 13.Boockvar JA, Kapitonov D, Kapoor G, et al. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol Cell Neurosci. 2003;24:1116–1130. doi: 10.1016/j.mcn.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Nagane M, Coufal F, Lin H, et al. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 15.Lammering G, Valerie K, Lin PS, et al. Radiation-induced activation of a common variant of EGFR confers enhanced radioresistance. Radiother Oncol. 2004;72:267–273. doi: 10.1016/j.radonc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery RB, Guzman J, O'Rourke DM, et al. Expression of oncogenic epidermal growth factor receptor family kinases induces paclitaxel resistance and alters beta-tubulin isotype expression. J Biol Chem. 2000;275:17358–17363. doi: 10.1074/jbc.M000966200. [DOI] [PubMed] [Google Scholar]
- 17.Heimberger AB, Archer GE, Crotty LE, et al. Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery. 2002;50:158–166. doi: 10.1097/00006123-200201000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Heimberger AB, Crotty LE, Archer GE, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9:4247–4254. [PubMed] [Google Scholar]
- 19.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 20.Schmittling RJ, Archer GE, Mitchell DA, et al. Detection of humoral response in patients with glioblastoma receiving EGFRvIII-KLH vaccines. J Immunol Methods. 2008;339:74–81. doi: 10.1016/j.jim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007;25:2288–2294. doi: 10.1200/JCO.2006.08.0705. [DOI] [PubMed] [Google Scholar]
- 22.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 23.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955–5964. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 25.De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 26.Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 27.Yu JS, Wheeler CJ, Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 28.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 29.Murat A, Migliavacca E, Gorlia T, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26:3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi T, Akasaki Y, Abe T, et al. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27:452–459. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Rutkowski S, De Vleeschouwer S, Kaempgen E, et al. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91:1656–1662. doi: 10.1038/sj.bjc.6602195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.