Abstract
Glucose-6-phosphatase (G6Pase) is a key enzyme that is responsible for the production of glucose in the liver during fasting or in type 2 diabetes mellitus (T2DM). During fasting or in T2DM, peroxisome proliferator-activated receptor α (PPARα) is activated, which may contribute to increased hepatic glucose output. However, the mechanism by which PPARα up-regulates hepatic G6Pase gene expression in these states is not well understood. We evaluated the mechanism by which PPARα up-regulates hepatic G6Pase gene expression in fasting and T2DM states. In PPARα-null mice, both hepatic G6Pase and phosphoenolpyruvate carboxykinase levels were not increased in the fasting state. Moreover, treatment of primary cultured hepatocytes with Wy14,643 or fenofibrate increased the G6Pase mRNA level. In addition, we have localized and characterized a PPAR-responsive element in the promoter region of the G6Pase gene. Chromatin immunoprecipitation (ChIP) assay revealed that PPARα binding to the putative PPAR-responsive element of the G6Pase promoter was increased in fasted wild-type mice and db/db mice. These results indicate that PPARα is responsible for glucose production through the up-regulation of hepatic G6Pase gene expression during fasting or T2DM animal models.
Keywords: Gene Expression, Gluconeogenesis, Liver Metabolism, PPAR, Transcription, Glucose-6-phosphatase, Phosphoenolpyruvate Carboxykinase, Type 2 Diabetes
Introduction
Gluconeogenesis is a metabolic adaptation that occurs during energy deprivation, such as fasting in mammals. During the fasting state, blood glucose levels are maintained by gluconeogenesis in liver. In patients with type 2 diabetes mellitus (T2DM),3 gluconeogenesis contributes in part to the chronically elevated blood glucose levels (1). In this state, free fatty acids are elevated and have been implicated as a cause of insulin resistance (2). In addition, free fatty acids have been shown to increase hepatic glucose production by activating key enzymes of gluconeogenesis like phosphoenolpyruvate carboxykinase (PEPCK), fructose-1,6-bisphosphatase, and glucose-6-phosphatase (G6Pase) (3–6).
G6Pase (EC 3.1.3.9) is expressed mainly in the liver, kidney, and β-cells of the pancreas where the enzyme hydrolyzes glucose 6-phosphate to glucose at the final step of gluconeogenesis (7). It is a multienzyme complex consisting of catalytic and transporter subunits. The G6Pase catalytic subunit gene is positively controlled at the transcriptional level by glucocorticoids, cAMP, glucose, and fatty acids (8–10), whereas its gene expression is inhibited by insulin, tumor necrosis factor-α, and interleukin-6 (11, 12). Studies have shown that G6Pase activity is increased in T2DM-associated hyperglycemia and hyperlipidemia (5, 8), and the G6Pase mRNA level is also elevated in diabetic animal models (13–15). Furthermore, overexpression of the G6Pase gene in rat liver resulted in glucose intolerance and hyperinsulinemia (16).
The peroxisome proliferator-activated receptors (PPARs) are a family of nuclear receptors that act as transcription factors for regulating energy balance in lipid and glucose homeostasis (17). The receptor family is composed of three isotypes: α, γ, and δ. PPARα is expressed mainly in brown adipose tissue and liver, but it is also present in the heart, kidney, and skeletal muscle (18). PPARγ is expressed predominantly in adipose tissue where it plays key roles in adipocyte differentiation and lipogenesis (18, 19). The PPARδ isoform is ubiquitously expressed, but its function is not well defined (18). In relation to their DNA binding, ligand binding, and cofactor binding domains, the PPARs share a high degree of structural homology with other nuclear hormone receptors (20). The PPARs function by forming heterodimers with retinoid X receptor α (RXRα) and transduce intracellular signals when ligands bind to the dimerized complex. The PPARα/RXRα heterodimer activates gene expression by binding to a cis-element, known as peroxisome proliferator response element (PPRE), in the target gene promoter.
Studies from PPARα-null mice showed that PPARα regulates genes involved in lipid metabolism in the liver, including fatty acid uptake (21), β-oxidation occurring in the mitochondria or peroxisome (22), and genes involved in glycerol (23) and lipoprotein metabolism (24, 25). PPARα is responsible for lowering circulating triglyceride levels by increasing fatty acid oxidation and reducing adiposity, which improves insulin sensitivity (26–28). Furthermore, PPARα-null mice show severe hypoglycemia following 24-h fasting, characterized by a 50% drop in blood glucose level, suggesting a potential role of PPARα in glucose homeostasis and metabolism (29). Although the synergistic effect of PPARα with glucocorticoid receptor on PEPCK promoter has been studied in liver (30), and several mechanisms accounting for reduced glucose production in PPARα-null mice were proposed (23), little is known about direct role of PPARα on the regulation of the gene expression of G6Pase.
In this study, we have identified and characterized PPRE in the promoter region of G6Pase gene using luciferase reporter assay and chromatin immunoprecipitation (ChIP) assay. Additionally, we showed that G6Pase gene expression was down-regulated in the PPARα-null mice and up-regulated in livers of fasting and db/db mice. These findings indicate that G6Pase is a direct target of PPARα and that PPARα may be responsible for glucose production through the regulation of hepatic G6Pase gene expression during fasting as well as in T2DM.
EXPERIMENTAL PROCEDURES
Animals and Materials
Mice were housed with a 12-h light/12-h dark cycle. All animals were fed a regular chow diet until the fasting and refeeding treatment started. For the dietary manipulation study, each group of four male C57BL/6J or PPARα-null mice was tested. For fasting group, mice were fasted for 24 h during a light and dark cycle. For the refeeding group, the mice fasted for 24 h were refed with a high carbohydrate meal for 12 h under the dark cycle. All mice were killed at the same time which is just after the finish time of the dark cycle. PPARα-null mice were a generous gift from Frank J. Gonzalez (31). C57BL/6J male mice for wild-type and db/db male mice were purchased from Charles River Laboratory. A standard diet and a high carbohydrate/fat-free diet were purchased from Harlem Teklad Co. (Madison, WI). The animal experiments were approved by Institutional Animal Care and Use Committee of the Yonsei University College of Medicine. Wy14,643 (Sigma-Aldrich) and fenofibrate (Sigma-Aldrich) were used as PPARα ligands. Dexamethasone (Sigma-Aldrich) was used as the glucocorticoid receptor ligand.
Metabolite Measurement
Blood glucose drawn from mouse tail vein was analyzed using a glucose monitor, One Touch Sure Step (Lifescan). Plasma insulin levels were measured by enzyme-linked immunosorbent assay (ELISA) kit (ALPCO, Salem, NH).
Cell Culture
A HepG2 human hepatoma cell line was maintained in high glucose (25 mm) Dulbecco's modified Eagle's medium (DMEM; Hyclone, South Logan, UT) supplemented with 10% (v/v) fetal bovine serum (FBS; Hyclone), 100 units/ml penicillin, and 100 μg/ml streptomycin (Hyclone). Cells were grown at 37 °C/5% CO2 humidified incubator. Primary hepatocytes isolated from C57BL/6J mice liver were plated and cultured for 6 h in DMEM high glucose containing 10% (v/v) FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 nm dexamethasone, and 10 nm insulin. And then, FBS, dexamethasone, and insulin were excluded from the medium and cultured for an additional 16 h in the presence or absence of Wy14,643, fenofibrate, dexamethasone, or cAMP.
Total RNA Isolation and Quantitative Real-time PCR (qPCR)
Total RNA was isolated from the mice liver using the easy spin RNA extraction kit (iNtRON) according to the manufacturer's instructions. Reverse transcription and qPCR analysis were performed as described in our previous study (32). Relative gene expression was determined by the standard curve methods. Ribosomal protein, large, p0 (Rplp0) was used as an internal control for RNA quality and quantity. For qPCR amplification, the following gene-specific PCR primers were used: 5′-TGGTAGCCCTGTCTTTCTTTG-3′ (sense) and 5′-TTCCAGCATTCACACTTTCCT-3′ (antisense) for G6Pase; 5′-ACACACACACATGCTCACAC-3′ (sense) and 5′-ATCACCGCATAGTCTCTGAA-3′ (antisense) for PEPCK; 5′-TGCCAAGGAGTCGAGGATGT-3′ (sense) and 5′-TCGGCACCAGGAACCAA-3′ (antisense) for PPARα; 5′-CTGTTAGCAGGATGGCAGCTT-3′ (sense) and 5′-TTTCCTGGAGAGATGCTGTGG-3′ (antisense) for glucokinase (Gck); 5′-ATCTGGTGATTGTG GTGACAGG-3′ (sense) and 5′-GGGGTGTGGGTTGAAAGAAA-3′ (antisense) for liver-type pyruvate kinase (L-PK); 5′-ACAAACGATGACCCTCCTCA-3′ (sense) and 5′-TCTGGGGTCAGAGGAAGAG-3′ (antisense) for PGC-1α; 5′-GCAGGTGTTTGACAACGGCAG-3′ (sense) and 5′-GATGATGGAGTGTGGCACCGA-3′ (antisense) for Rplp0.
Western Blot Analysis
Proteins isolated from the mice liver using the radioimmuneprecipitation assay buffer (50 mm Tris-Cl (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 0.1% SDS, 1% deoxycholic acid, 0.5 mm DTT, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin) were separated by SDS-PAGE and transferred onto nitrocellulose transfer membrane (Whatman). The membrane was blocked with nonfat skim milk and agitated with PPARα, G6Pase, and GAPDH antibodies that were purchased from Millipore (mAb 3890), Santa Cruz Biotechnology (sc-27198), and Cell Signaling (2118), respectively. The signals were detected with LAS-3000 (Fuji).
Transient Transfection and Luciferase Assays
Promoter-luciferase reporter constructs were constructed by amplifying the promoter region of pmG6Pase (−1188/+66) from mouse genomic DNA by PCR and inserting them into the pGL4.14 vector (pGL4.14; Promega). Serial deletion constructs from G6Pase promoter construct were prepared by amplifying the indicated regions and subcloned in the pGL4.14 vector. The PPRE point mutation was introduced into the putative PPARα binding site in pmG6Pase (−1188/+66) construct by displacing PPRE with EcoRV restriction sites to generate mutant PPRE. To exclude the length effects of individual promoters, luciferase activities of each promoter reporter which were co-transfected with pcDNA3 in the absence of ligand were artificially defined as 1. HepG2 cells were plated in 12-well tissue culture dishes at a density of 2 × 105 cells/well in 1 ml of medium. Transient transfections were performed as described previously (32). HepG2 cells were transiently co-transfected with firefly luciferase fusion genes containing each serially deleted mouse G6Pase promoter sequence from −1188 to +66 and expression vectors encoding Renilla luciferase and either empty pcDNA3 vector or the same vector encoding the murine form of Pparα. After 24 h, media were changed to medium with or without 20 μm Wy14,643. After ligand treatment, cells were incubated for 24 h and then harvested for luciferase activity measurements. The following primers were used for making PPRE mutation: sense, 5′-GTCGATATCTTTGGGCTGGATTGACCTACAG-3′; antisense, 5′-TGAGATATCGACTCAAAAAACCACTTTTGTC-3′. Human PEPCK (−599/+61) and Gck (−1000/+158) promoters were amplified from human genomic DNA, respectively.
ChIP Assay
For the mouse ChIP assay, equal amounts of freshly isolated livers from mice were pooled and washed with ice-cold phosphate-buffered saline containing protease inhibitors (1 μg/ml leupeptin, 1.4 μg/ml pepstatin, 2.0 μg/ml phenylmethylsulfonyl fluoride, 1 mm EDTA, and 1 mm EGTA). The tissue was disrupted in IKA T10 basic homogenizer (WAG) at a low setting. Formaldehyde was added to a final concentration of 1% (v/v), and samples were rotated for 8 min. A ChIP assay was performed on cells collected by centrifugation at 400 × g with an Upstate assay kit according to the manufacturer's instructions. The DNA was recovered from eluted DNA-protein complex and purified by using Qiagen PCR purification kit. The purified DNA was amplified by qPCR and determined by comparative ΔΔCT method in using primers appropriated for each set as follows: 5′-GCTGTTTTTGTGTGCCTGTT-3′ (sense) and 5′-TGCTATCAGTCTGTGCCTTG-3′ (antisense) for G6Pase PPRE-ChIP; 5′-GATCGCTGACCTCAGAGACA-3′ (sense) and 5′-GCCACCAATGCACATGAGAT-3′ (antisense) for G6Pase outside PPRE-ChIP in mouse. The relative amount of the precipitated target sequence was determined via normalization to the 1% of purified total genomic DNA input.
Statistical Analysis
Three to five experiments of all studies were performed, using triplicate replications. The data are represented as mean ± S.E. All datasets were analyzed for statistical significance using a two-tailed unpaired Student's t test. All p values below 0.05 were considered significant. Statistical analysis was carried out using SPSS (Version 11.5; SPSS Inc., Chicago, IL).
RESULTS
Effect of Fasting on mRNA Levels of PPARα and Gluconeogenic Genes in Livers of Wild-type and PPARα-null Mice
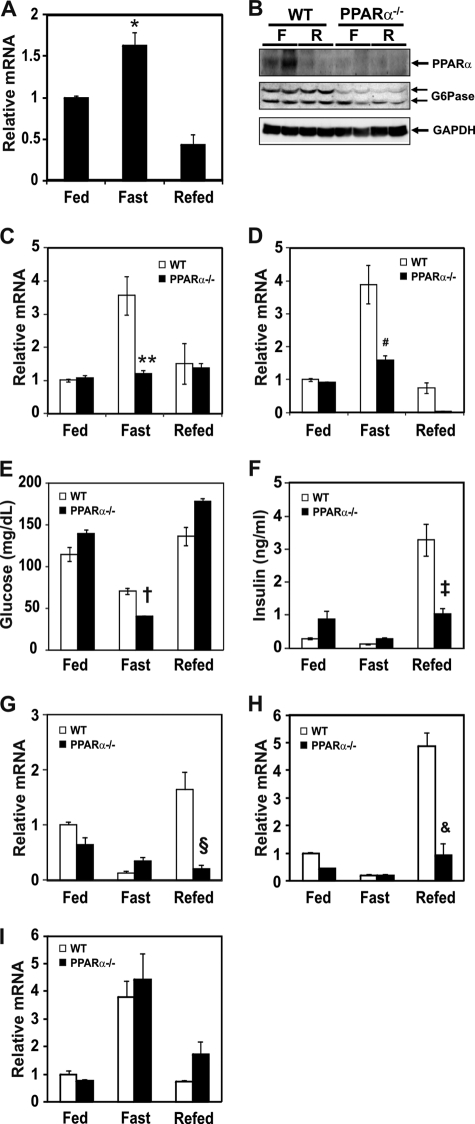
PPARα-null mice are characterized by defects in fatty acid oxidation and hypoglycemia during fasting (29, 31, 33). To explore a molecular mechanism governing the PPARα and gluconeogenic gene expression, mRNA levels of PPARα, G6Pase, and PEPCK were measured in the mice subjected to fasting and/or refeeding. During the fasting period, PPARα mRNA levels were increased in the liver of wild-type mice and decreased by refeeding (Fig. 1A). PPARα protein was also increased by fasting and decreased by refeeding (Fig. 1B). PPARα protein was not increased in the liver of PPARα-null mice regardless of dietary condition. mRNA levels of G6Pase (Fig. 1C) and PEPCK (Fig. 1D) were elevated during fasting and returned to control levels in the livers of refed wild-type mice. However, mRNA levels of G6Pase and PEPCK were not increased by fasting in the PPARα-null mice, suggesting that PPARα could be involved in the up-regulation of G6Pase and PEPCK gene expression. Because PPARα is shown to mediate adaptive response to fasting (29), the serum blood glucose level may be lower in PPARα-null mice during fasting due to decreased gluconeogenesis in liver. Indeed, the serum glucose level was decreased in fasted PPARα-null mice compared with wild-type control mice (Fig. 1E). Interestingly, the serum insulin level was not increased in PPARα-null mice by refeeding (Fig. 1F), and this result is consistent with the report that PPARα plays a role in the insulin secretion in pancreas (34). Analysis of diet-sensitive gene expression levels showed that Gck and L-PK were not affected in the livers of fasting wild-type and PPARα-null mice (Fig. 1, G and H). However, Gck and L-PK expression was not induced by refeeding due to decreased insulin secretion probably in PPARα-null mice (Fig. 1, G and H). This phenomenon might be closely associated with decreased plasma insulin level in the refed PPARα-null mice. PGC-1α, which acts as a co-activator of several transcription factors, is induced in fasting liver as well (35). To test whether a deficiency of PPARα could affect expression of the PGC-1α gene, we measured PGC-1α mRNA levels in each dietary condition. The mRNA level of PGC-1α in PPARα-null mice was similar to that of wild-type mice (Fig. 1I).
FIGURE 1.
Effect of fasting and refeeding on PPARα and gluconeogenic mRNA levels in liver of wild-type and PPARα-null mice. A, mRNA level of PPARα from wild-type mice was measured by qPCR in the fed, fasted (for 24 h), and refed groups (fasted for 24 h followed by refeeding a high carbohydrate/low fat diet for 12 h in the dark cycle). qPCR data were normalized to Rplp0 mRNA level. Each value represents the amounts of mRNA relative to that of the feeding group, which is arbitrarily defined as 1. B, PPARα and G6Pase protein levels of fasted and refed groups as above are shown. C and D, mRNA levels of G6Pase (C) and PEPCK (D) in liver from wild-type (open bars) and PPARα-null (filled bars) mice were measured by qPCR in the fed, fasted, and refed groups as above. Each value represents the amounts of mRNA relative to that of the feeding group of wild-type mice, which is arbitrarily defined as 1. E and F, plasma glucose (E) and insulin (F) levels of fed, fasted, and refed group of wild-type and PPARα-null mice are shown. G–I, mRNA levels of Gck (G), L-PK (H), and PGC-1α (I) in liver from wild-type (open bars) and PPARα-null (filled bars) mice were measured as above. Bars represent means ± S.E. (error bars) for four animals per group. *, p < 0.004, fasting versus refed; **, p < 0.002, wild-type versus PPARα-null mice in the fasting state; #, p < 0.002, wild-type versus PPARα-null mice in the fasting state; †, p < 0.02, wild-type versus PPARα-null mice in the fasting state; ‡, p < 0.004, wild-type versus PPARα-null mice in the refed state; §, p < 0.003, wild-type versus PPARα-null mice in the refed state; &, p < 0.002, wild-type versus PPARα-null mice in the refed state.
PPARα Ligands Increase G6Pase mRNA Levels in Primary Cultured Hepatocytes
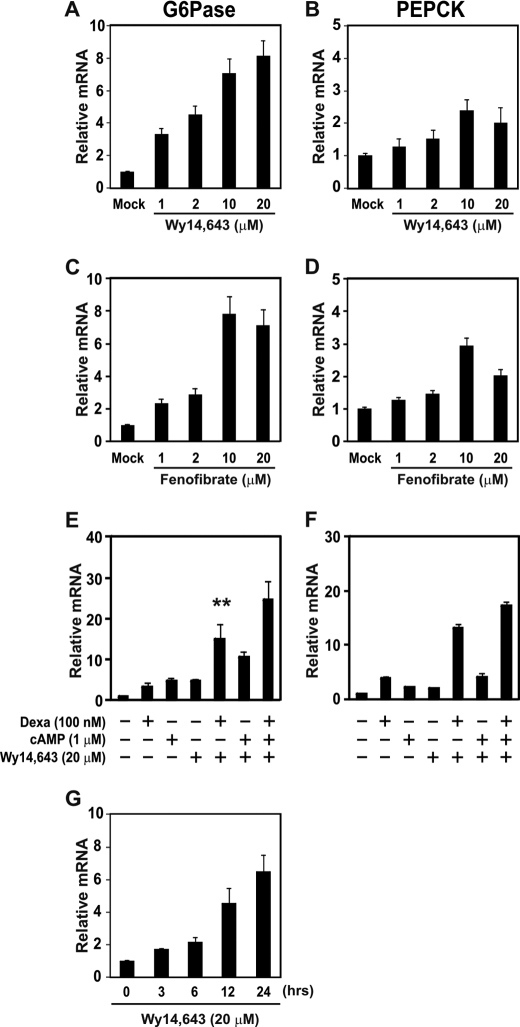
To observe the effects of PPARα agonists on the gluconeogenic gene expression, primary cultured hepatocytes isolated from C57BL/6J mice were treated with Wy14,643 or fenofibrate. G6Pase mRNA levels were increased by Wy14,643 and fenofibrate treatment in a dose-dependent manner (Fig. 2, A and C). However, both PPARα agonists slightly increased the PEPCK mRNA level (Fig. 2, B and D), and mRNA levels of PPARα and PGC-1α were not affected by Wy14,643 and fenofibrate treatment (supplemental Fig. 1, A–D).
FIGURE 2.
Effect of PPARα ligands on the mRNA level of gluconeogenic genes in the primary cultured hepatocytes. A–D, hepatocytes isolated from mice were treated with 1, 2, 10, or 20 μm Wy14,643 (A and B) or fenofibrate (C and D), respectively, for 24 h. G6Pase (A and C) and PEPCK (B and D) mRNA levels were measured by qPCR. E and F, effects of dexamethasone, cAMP, and Wy14,643 on the mRNA levels of G6Pase (E) and PEPCK (F) are shown. Primary cultured hepatocytes were treated with 100 nm dexamethasone, 1 μm cAMP, and/or 20 μm Wy14,643 for 24 h as indicated. mRNA levels were measured by qPCR. All mRNA levels were normalized to Rplp0 mRNA levels. G, time course measurement of G6Pase mRNA level by qPCR in primary cultured hepatocytes is shown. Cells were treated with 20 μm Wy14,643 as indicated. **, p < 0.02, Wy14,643 versus Wy14,643 and dexamethasone treatment.
Because glucocorticoid is known to up-regulate gluconeogenic genes (36, 37), we evaluated the effects of dexamethasone, cAMP, and/or Wy14.643 on G6Pase gene expression in the primary cultured hepatocytes (Fig. 2, E and F). Dexamethasone and Wy14,643 synergistically increased G6Pase and PEPCK expression. Time course experiments showed that the Wy14,643 effect on G6Pase gene expression was maximum at the 24-h incubation time (Fig. 2E).
Effect of PPARα on the G6Pase Promoter Reporter Activities in HepG2 Cells
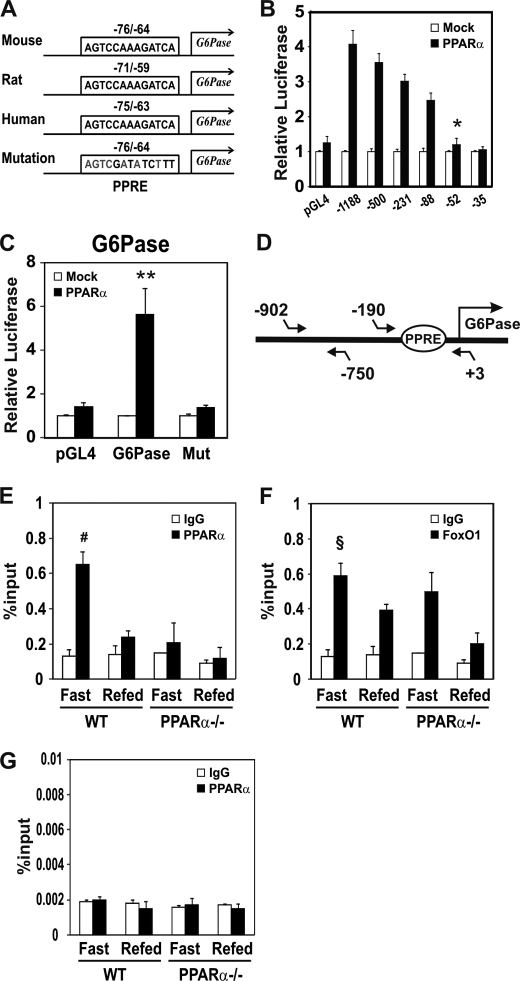
A computer search for putative PPRE in the G6Pase gene suggested that the cis-element could be located in the −76 bp/−64 bp region and the sequence is evolutionally conserved in human, mouse, and rat (Fig. 3A). To localize the putative PPRE in the G6Pase gene, serial deletion constructs were prepared, and their responsiveness to PPARα was tested. As shown in Fig. 3B, PPARα increased G6Pase promoter activity (pmG6Pase pro, −1188/+66) by 4.5-fold. The promoter activity was significantly decreased when the promoter was deleted down to −52 bp, suggesting that a putative PPRE could be present between −88 bp and −52 bp. To identify the PPRE site on the promoter, a PPRE mutation was introduced in the G6Pase promoter (−1188/+66) using site-directed mutagenesis. The promoter activity of mG6Pase-PPREmut by PPARα was significantly decreased (Fig. 3C), suggesting a direct participation of PPARα in the up-regulation of the G6Pase gene. In addition, PPARα also induced PEPCK promoter activity. However, Gck promoter activity was not affected by PPARα (supplemental Fig. 2A).
FIGURE 3.
Identification of PPRE in the 5′-flanking region of the G6Pase gene. A, comparison of PPRE sequence among mouse, rat, and human G6Pase 5′-flanking regions and a mutated PPRE mouse promoter sequence used for luciferase reporter assays. The numbers indicate the distance in nucleotides from the transcription start site (+1) of mouse, rat, and human G6Pase genes. B, effects of PPARα on the promoter reporter activities of serial deletion constructs of the G6Pase gene. Each serial deletion construct of the mouse G6Pase promoter was transiently co-transfected with pcDNA3 (open bars) or PPARα expression vector (filled bars) in HepG2 cells. After 24 h, media were changed with 20 μm Wy14,643. Luciferase activities were normalized to Renilla activity. C, effect of PPRE mutation on the G6Pase −1188/+66 promoter reporter construct. HepG2 cells were transiently co-transfected with pGL4, G6Pase promoter reporter (−1188/+66), or G6Pase PPRE mutant promoter reporter (Mut), respectively, with pcDNA3 (open bars) or PPARα expression vector (filled bars) as above. D, schematics of the PCR amplification region containing PPRE in the G6Pase promoter. The region between −190 and +4 was amplified. E–G, ChIP assay for PPARα binding to PPRE of the G6Pase gene. For the ChIP assay, chromatin was extracted from the livers of mice fasted for 24 h or refed for 12 h after 24-h fasting (n = 3 per group). The chromatin was immunoprecipitated with antibody against PPARα (E and G) and FoxO1 (F). The quantity of DNA in the precipitation was normalized to chromatin input (1/100 of chromosomal DNA used for precipitation). The amplified region from the G6Pase gene promoter was −190/+3 (E and F) and −902/−750 (G). FoxO1 binding to PPRE of the G6Pase promoter was used shown as a positive control (F), and the nonspecific region between −902 bp and −750 bp, which is outside the PPRE in the G6Pase promoter, was used as a negative control (G). Results represent the mean ± S.E. (error bars) for three experiments with each sample assayed in triplicate.*, p < 0.004, −88 bp versus −52 bp; **, p < 0.003, mock versus PPARα; #, p < 0.02, IgG versus PPARα; §, p < 0.02 IgG versus FoxO1.
Binding of PPARα to Putative G6Pase-PPRE in the Liver of Fasting/Refeeding Mice
To confirm that PPARα binds directly to the mouse G6Pase gene promoter region in vivo, a ChIP assay was performed. Fragmented chromatin was immunoprecipitated by anti-PPARα antibodies followed by qPCR amplifications, containing G6Pase-PPRE (Fig. 3D). The region outside the PPRE target region was chosen as a negative control (Fig. 3G). As shown in Fig. 3E, the binding of PPARα to the putative PPRE of the G6Pase promoter was increased in liver of the fasting group, consistent with data shown in Fig. 1C. In contrast, PPARα protein was not recruited in the fasted PPARα-null mice. These results indicate that PPARα binding to the putative PPRE of the G6Pase promoter is active under fasting condition. FoxO1 binding to G6Pase promoter, which is used as a positive control of transcription factor binding, was increased during the fasting state of both wild-type and PPARα-null mice (Fig. 3F).
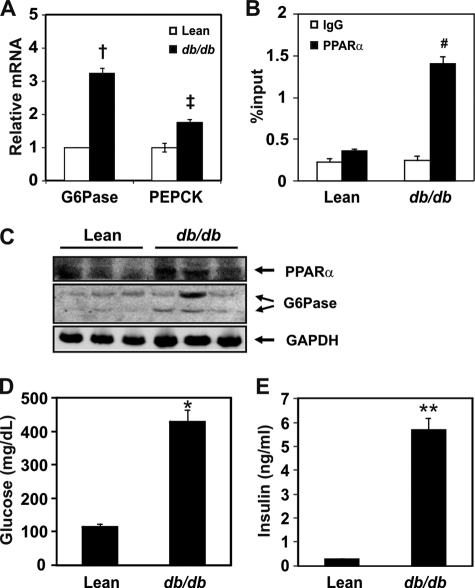
Binding of PPARα to the Putative G6Pase-PPRE Site Is Increased in db/db Mice
Gluconeogenesis occurs actively in the livers of patients with T2DM and in T2DM animal models (38). Moreover, PPARα expression is known to be increased in the liver of diabetic animals (39). Because our data suggested that G6Pase might be a target of PPARα, we measured the mRNA levels of G6Pase and PEPCK in db/db mice. As shown in Fig. 4A, mRNA levels of G6Pase and PEPCK were significantly increased by 3.2-fold and 1.8-fold, respectively, in db/db mice compared with the control mice. To confirm that PPARα binding to the putative PPRE in the G6Pase promoter was increased in the diabetic db/db animal model, we performed a ChIP assay using an anti-PPARα antibody. PPARα binding to the putative PPRE is increased compared with control mice (Fig. 4B). To check PPARα and G6Pase protein levels in the diabetic mice model, an immunoblotting assay was performed using anti-PPARα and anti-G6Pase antibodies. PPARα as well as G6Pase protein levels were increased in liver of db/db mice (Fig. 4C). Glucose and insulin levels of db/db mice showed hyperglycemia and hyperinsulinemia (Fig. 4, D and E). These results indicated that PPARα-mediated activation of G6Pase expression may contribute to the increased hepatic glucose production in db/db mice.
FIGURE 4.
Binding of PPARα to the putative PPRE in the G6Pase gene in the liver of db/db mice. A, mRNA levels of G6Pase and PEPCK in lean and db/db mice. All mRNA levels were measured by qPCR and were normalized to that of Rplp0. B, ChIP assay for PPARα binding to PPRE of the G6Pase gene in db/db mice. The region between −190/+4 was amplified. Chromatins extracted from the livers of lean or db/db mice were precipitated by PPARα antibodies. The DNA fragments were amplified by qPCR using primers containing PPRE in the G6Pase promoter. Details are described under “Experimental Procedures.” C, protein levels of PPARα and G6Pase in lean and db/db mice. D and E, plasma glucose (D) and insulin (E) levels of lean versus db/db mice. †, p < 0.004, lean versus db/db mice; ‡, p < 0.003, lean versus db/db mice; #, p < 0.021, IgG versus PPARα precipitation; *, p < 0.021; **, p < 0.001.
DISCUSSION
Gluconeogenesis occurs in the liver of animals when they are subjected to long term fasting (40) or in a diabetic state (41). Because PPARα plays an important role as a lipid sensor and regulator of cellular energy metabolism, fatty acid-mediated PPARα activation is critical in fatty acid and lipid metabolism (42, 43). Although human inborn errors of the mitochondrial fatty acid oxidation are asymptomatic in a normal feeding condition, these patients show hypoketotic hypoglycemia, liver dysfunction, cardiomyopathy, intracellular accumulation of neutral lipid in liver and heart, and sudden death in a short term fasting condition (44). The molecular mechanism of the symptoms is not well understood, however, possible causes include: 1) an inadequate energy supply in the heart, which uses mitochondrial β-oxidation of fatty acids as the main source of energy, and 2) toxic effects of elevated intracellular concentrations of the intermediary metabolites of fatty acids (45). These characteristics are partially similar to PPARα-deficient mice subjected to dietary fasting (29, 31, 33). PPARα-null mice demonstrate increased levels of plasma free fatty acid due to defects in fatty acid oxidation in the fasting state. Additionally, the plasma glucose levels of PPARα-null mice are lower than those of wild-type mice under fasting conditions (Fig. 1E) (29), suggesting a potential link between PPARα and gluconeogenesis. Although increased free fatty acids could be responsible for enhanced gluconeogenesis and hepatic glucose production (3, 46), a direct mechanism of how PPARα increases glucose production is not well addressed.
In PPARα-null mice, hepatic glucose metabolism is directed toward synthesis of glycogen (47). Indeed, PPARα up-regulates glycerol-3-phosphate dehydrogenase and glycerol kinase gene expression, which are important enzymes that convert glycerol to glyceraldehyde 3-phosphate for inducing gluconeogenic flux (47). In addition, glycogen and UDP-glucose levels were increased in the liver of fasted PPARα-null mice (47) despite gluconeogenic flux and concentration of glucose 6-phosphate unchanged compared with wild-type mice.
Thus, we hypothesized that there is a direct correlation between PPARα and G6Pase gene expression. To this end, we cloned the promoter region of G6Pase, which is one of the critical enzymes responsible for gluconeogenesis, and showed that PPARα binds directly to a putative PPRE in the promoter of G6Pase and increases the transcription of G6Pase gene. In addition, the PPARα protein level was increased by fasting state of both wild-type and db/db mice.
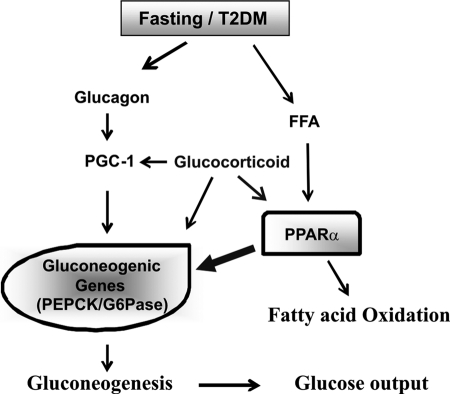
Moreover, our data showed that G6Pase and PEPCK mRNA levels were significantly decreased in fasted PPARα-null mice. In contrast, G6Pase mRNA levels were not altered by a fasting/refeeding regimen in PPARα-null mice, indicating that G6Pase could be a direct target gene of PPARα. We also demonstrated that serum glucose levels in fasted PPARα-null mice were reduced compared with wild-type mice. This mechanism might explain the increase in hepatic gluconeogenesis during fasting in wild-type or a diabetic mice. Interestingly, in the refed animals, the plasma insulin level was not increased in PPARα-null mice. Previous studies showed that PPARα plays a role in glucose-stimulated insulin secretion in pancreatic β-cells (48). Therefore, insulin levels in serum should be decreased in PPARα-null mice during refeeding (Fig. 1F), and insulin target genes like Gck and L-PK in the liver should be also down-regulated (Fig. 1, G and H), causing an increased glucose level in the refed state of PPARα-null mice. Based on previous studies and our data, we suggest a model that PPARα enhances glucose output through gluconeogenic gene regulation in fasting or diabetes state (Fig. 5).
FIGURE 5.
Regulation of gluconeogenic gene expression. A proposed model of the role of PPARα in the regulation of G6Pase/PEPCK gene expression in the fasting state or T2DM (db/db) mice is shown. PPARα could be activated either by free fatty acids (FFA) or glucocorticoid, causing up-regulation of gluconeogenic genes.
Increased free fatty acids have been implicated in hepatic gluconeogenesis in fasting and in T2DM animal models (3, 49). Several putative mechanisms have been proposed to explain the link between free fatty acids and hepatic glucose production (3). Among these mechanisms, free fatty acids up-regulate expression of genes involved in gluconeogenesis through activating transcription factors such as PPARα (50) and HNF-4α (3, 51). Consensus sequence analysis indicated the presence of seven putative HNF-4α binding sites in the G6Pase gene promoter (−751/−466 bp), and these sites were shown to be responsible for free fatty acid-mediated activation of the G6Pase gene (52). Thus, there are possibilities that PPARα could be involved in the free fatty acid-induced activation of G6Pase gene expression through these regions. In addition, it was reported that HNF-4α activated the G6Pase gene expression by binding to the −76 bp/−64 bp region of the promoter (53) in H4IIE cells, but we were not able to observe an increase in the G6Pase promoter activity by HNF-4α or co-transfection with PPARα in HepG2 cells (supplemental Fig. 2B). A possible explanation for the discrepancies between HNF-4α and PPARα activation of G6Pase could be the difference in the cell line characteristics. Because PPARα is expressed at a low level in the H4IIE cell line, the PPARα effect on G6Pase gene expression would not be observed (53). In this study, we demonstrated the binding of PPARα to the G6Pase promoter region using an in vivo ChIP assay (Figs. 3 and 4).
The PPRE of the PEPCK gene has an intermediate affinity (54). Because the PPRE in the PEPCK promoter overlaps with a potential HNF-4 binding region of −433/−457 bp (55), it is assumed that HNF-4α may compete with PPARα in binding to the putative PPRE. Indeed, in the primary cultured hepatocytes, the PPARα ligand did not increase the PEPCK mRNA level (Fig. 2B), probably due to competition between other transcription factors such as HNF-4α and RXRα/RARα (56). However Wy14,643 activated the PEPCK promoter reporter activity (supplemental Fig. 2A). In addition, PEPCK mRNA gene expression is shown to be increased by Wy14,643 and glucocorticoid in a synergistic manner (30), suggesting that the precise control of gene expression of G6Pase and PEPCK may be different.
Although mRNA levels of PPARα in the Wy-14,643- or fenofibrate-treated primary cultured hepatocyte were not changed (supplemental Fig. 1, A and C), G6Pase gene expression was increased in a dose-dependent and time-dependent manner (Fig. 2, A and G). In addition, the mRNA level of PPARα in the livers of fasted wild-type mice showed a marginal change, although the protein levels of PPARα and G6Pase were increased (Fig. 1B). These results suggest that increased endogenous PPARα ligands might be responsible for the increase in the stability of PPARα by reducing ubiquitination-mediated proteasomal degradation of PPARα (57).
Glucocorticoid and glucagon have been shown to activate gluconeogenic gene expression either by the glucocorticoid receptor, PGC-1α, or cAMP-responsive element-binding protein in the fasting liver (58). To understand the correlation between the PPARα agonist and dexamethasone or cAMP in primary cultured hepatocytes, we compared the effects of these agents on G6Pase and PEPCK mRNA levels. The degree of G6Pase mRNA induction was similar among these agents. In contrast, the dexamethasone-treated group showed the highest PEPCK mRNA level. Additionally, dexamethasone showed synergistic effects with Wy14,643 in both G6Pase and PEPCK gene transcription (Fig. 2, E and F). These data are consistent with PPARα and glucocorticoid effects on the G6Pase promoter (supplemental Fig. 2C). Dexamethasone is shown to up-regulate PPARα gene expression (59), and its effect was increased in time-dependent manner up to 24-h incubation time (supplemental Fig. 1A).
The current study provides evidence that up-regulation of the G6Pase gene can be mediated by direct action of PPARα and adds to our understanding of the transcriptional control of G6Pase gene expression. Despite an improvement in the lipid parameters and resulting amelioration in the overall metabolic profile by PPARα agonist treatment (27), it should be taken into account that PPARα agonists potentially activate gluconeogenic genes, specifically G6Pase. Studying the diverse effects of PPARα will provide insight for development of selective PPARα modulators that reduce hepatic gluconeogenesis while maintaining lipid-reducing effects. Understanding the diversity of PPARα may also establish a role for PPARα in the liver of diabetic animals. This study provides a novel avenue for therapeutic intervention in high fat diet-induced diabetes.
Supplementary Material
Acknowledgment
We thank Dr. Frank J. Gonzalez for providing PPARα-null mice.
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea, funded by the Ministry of Education, Science, and Technology Grant 2009-0080655 (Y.-H. A.) and by Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea, Grant A091014 (M.-Y. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- T2DM
- type 2 diabetes mellitus
- Gck
- glucokinase
- G6Pase
- glucose-6-phosphatase
- L-PK
- liver-type pyruvate kinase
- PEPCK
- phosphoenolpyruvate carboxykinase
- PPAR
- peroxisome proliferator-activated receptor
- PPRE
- PPAR response element
- qPCR
- quantitative real-time PCR
- RXR
- retinoid X receptor
- PGC-1α
- peroxisome proliferator-activated receptor gamma coactivator 1 alpha
- HNF-4α
- hepatic nuclear factor 4 alpha.
REFERENCES
- 1. Boden G., Chen X., Stein T. P. (2001) Am. J. Physiol. Endocrinol. Metab. 280, E23–E30 [DOI] [PubMed] [Google Scholar]
- 2. Bergman R. N., Ader M. (2000) Trends Endocrinol. Metab. 11, 351–356 [DOI] [PubMed] [Google Scholar]
- 3. Lam T. K., Carpentier A., Lewis G. F., van de Werve G., Fantus I. G., Giacca A. (2003) Am. J. Physiol. Endocrinol. Metab. 284, E863–E873 [DOI] [PubMed] [Google Scholar]
- 4. Goodridge A. G. (1987) Annu. Rev. Nutr. 7, 157–185 [DOI] [PubMed] [Google Scholar]
- 5. Massillon D., Barzilai N., Hawkins M., Prus-Wertheimer D., Rossetti L. (1997) Diabetes 46, 153–157 [DOI] [PubMed] [Google Scholar]
- 6. Chu R., Lim H., Brumfield L., Liu H., Herring C., Ulintz P., Reddy J. K., Davison M. (2004) Mol. Cell. Biol. 24, 6288–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mithieux G. (1997) Eur. J. Endocrinol. 136, 137–145 [DOI] [PubMed] [Google Scholar]
- 8. Argaud D., Kirby T. L., Newgard C. B., Lange A. J. (1997) J. Biol. Chem. 272, 12854–12861 [DOI] [PubMed] [Google Scholar]
- 9. Xu C., Chakravarty K., Kong X., Tuy T. T., Arinze I. J., Bone F., Massillon D. (2007) J. Nutr. 137, 554–559 [DOI] [PubMed] [Google Scholar]
- 10. Lange A. J., Argaud D., el-Maghrabi M. R., Pan W., Maitra S. R., Pilkis S. J. (1994) Biochem. Biophys. Res. Commun. 201, 302–309 [DOI] [PubMed] [Google Scholar]
- 11. Grempler R., Kienitz A., Werner T., Meyer M., Barthel A., Ailett F., Sutherland C., Walther R., Schmoll D. (2004) Biochem. J. 382, 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Metzger S., Goldschmidt N., Barash V., Peretz T., Drize O., Shilyansky J., Shiloni E., Chajek-Shaul T. (1997) Am. J. Physiol. Endocrinol. Metab. 273, E262–E267 [DOI] [PubMed] [Google Scholar]
- 13. Argaud D., Zhang Q., Pan W., Maitra S., Pilkis S. J., Lange A. J. (1996) Diabetes 45, 1563–1571 [DOI] [PubMed] [Google Scholar]
- 14. Haber B. A., Chin S., Chuang E., Buikhuisen W., Naji A., Taub R. (1995) J. Clin. Invest. 95, 832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massillon D., Barzilai N., Chen W., Hu M., Rossetti L. (1996) J. Biol. Chem. 271, 9871–9874 [DOI] [PubMed] [Google Scholar]
- 16. Trinh K. Y., O'Doherty R. M., Anderson P., Lange A. J., Newgard C. B. (1998) J. Biol. Chem. 273, 31615–31620 [DOI] [PubMed] [Google Scholar]
- 17. Evans R. M., Barish G. D., Wang Y. X. (2004) Nat. Med. 10, 355–361 [DOI] [PubMed] [Google Scholar]
- 18. Kersten S., Desvergne B., Wahli W. (2000) Nature 405, 421–424 [DOI] [PubMed] [Google Scholar]
- 19. Rosen E. D., Hsu C. H., Wang X., Sakai S., Freeman M. W., Gonzalez F. J., Spiegelman B. M. (2002) Genes Dev. 16, 22–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michalik L., Auwerx J., Berger J. P., Chatterjee V. K., Glass C. K., Gonzalez F. J., Grimaldi P. A., Kadowaki T., Lazar M. A., O'Rahilly S., Palmer C. N., Plutzky J., Reddy J. K., Spiegelman B. M., Staels B., Wahli W. (2006) Pharmacol. Rev. 58, 726–741 [DOI] [PubMed] [Google Scholar]
- 21. Hsu M. H., Savas U., Griffin K. J., Johnson E. F. (2001) J. Biol. Chem. 276, 27950–27958 [DOI] [PubMed] [Google Scholar]
- 22. Schoonjans K., Staels B., Auwerx J. (1996) J. Lipid Res. 37, 907–925 [PubMed] [Google Scholar]
- 23. Patsouris D., Mandard S., Voshol P. J., Escher P., Tan N. S., Havekes L. M., Koenig W., März W., Tafuri S., Wahli W., Müller M., Kersten S. (2004) J. Clin. Invest. 114, 94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aoyama T., Peters J. M., Iritani N., Nakajima T., Furihata K., Hashimoto T., Gonzalez F. J. (1998) J. Biol. Chem. 273, 5678–5684 [DOI] [PubMed] [Google Scholar]
- 25. Motojima K., Passilly P., Peters J. M., Gonzalez F. J., Latruffe N. (1998) J. Biol. Chem. 273, 16710–16714 [DOI] [PubMed] [Google Scholar]
- 26. Guerre-Millo M., Gervois P., Raspé E., Madsen L., Poulain P., Derudas B., Herbert J. M., Winegar D. A., Willson T. M., Fruchart J. C., Berge R. K., Staels B. (2000) J. Biol. Chem. 275, 16638–16642 [DOI] [PubMed] [Google Scholar]
- 27. Kim H., Haluzik M., Asghar Z., Yau D., Joseph J. W., Fernandez A. M., Reitman M. L., Yakar S., Stannard B., Heron-Milhavet L., Wheeler M. B., LeRoith D. (2003) Diabetes 52, 1770–1778 [DOI] [PubMed] [Google Scholar]
- 28. Chou C. J., Haluzik M., Gregory C., Dietz K. R., Vinson C., Gavrilova O., Reitman M. L. (2002) J. Biol. Chem. 277, 24484–24489 [DOI] [PubMed] [Google Scholar]
- 29. Kersten S., Seydoux J., Peters J. M., Gonzalez F. J., Desvergne B., Wahli W. (1999) J. Clin. Invest. 103, 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cassuto H., Kochan K., Chakravarty K., Cohen H., Blum B., Olswang Y., Hakimi P., Xu C., Massillon D., Hanson R. W., Reshef L. (2005) J. Biol. Chem. 280, 33873–33884 [DOI] [PubMed] [Google Scholar]
- 31. Lee S. S., Pineau T., Drago J., Lee E. J., Owens J. W., Kroetz D. L., Fernandez-Salguero P. M., Westphal H., Gonzalez F. J. (1995) Mol. Cell. Biol. 15, 3012–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim T. H., Kim H., Park J. M., Im S. S., Bae J. S., Kim M. Y., Yoon H. G., Cha J. Y., Kim K. S., Ahn Y. H. (2009) J. Biol. Chem. 284, 15071–15083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leone T. C., Weinheimer C. J., Kelly D. P. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 7473–7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bihan H., Rouault C., Reach G., Poitout V., Staels B., Guerre-Millo M. (2005) FEBS Lett. 579, 2284–2288 [DOI] [PubMed] [Google Scholar]
- 35. Yoon J. C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C. R., Granner D. K., Newgard C. B., Spiegelman B. M. (2001) Nature 413, 131–138 [DOI] [PubMed] [Google Scholar]
- 36. Barthel A., Schmoll D. (2003) Am. J. Physiol. Endocrinol. Metab. 285, E685–E692 [DOI] [PubMed] [Google Scholar]
- 37. Bernal-Mizrachi C., Weng S., Feng C., Finck B. N., Knutsen R. H., Leone T. C., Coleman T., Mecham R. P., Kelly D. P., Semenkovich C. F. (2003) Nat. Med. 9, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 38. Memon R. A., Tecott L. H., Nonogaki K., Beigneux A., Moser A. H., Grunfeld C., Feingold K. R. (2000) Endocrinology 141, 4021–4031 [DOI] [PubMed] [Google Scholar]
- 39. Asayama K., Sandhir R., Sheikh F. G., Hayashibe H., Nakane T., Singh I. (1999) Mol. Cell. Biochem. 194, 227–234 [DOI] [PubMed] [Google Scholar]
- 40. Cherrington A. D. (1999) Diabetes 48, 1198–1214 [DOI] [PubMed] [Google Scholar]
- 41. Shulman G. I. (2000) J. Clin. Invest. 106, 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berger J., Moller D. E. (2002) Annu. Rev. Med. 53, 409–435 [DOI] [PubMed] [Google Scholar]
- 43. Lefebvre P., Chinetti G., Fruchart J. C., Staels B. (2006) J. Clin. Invest. 116, 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roe C. R., Coates P. M. (1995) in The Metabolic and Molecular Bases of Inherited Disease (Scriver C. R., Beaudet A. I., Sly W. S., Valle D.), pp. 1501–1533, McGraw-Hill, New York [Google Scholar]
- 45. Kelly D. P., Strauss A. W. (1994) N. Engl. J. Med. 330, 913–919 [DOI] [PubMed] [Google Scholar]
- 46. Chen X., Iqbal N., Boden G. (1999) J. Clin. Invest. 103, 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bandsma R. H., Van Dijk T. H., Harmsel At A., Kok T., Reijngoud D. J., Staels B., Kuipers F. (2004) J. Biol. Chem. 279, 8930–8937 [DOI] [PubMed] [Google Scholar]
- 48. Sugden M. C., Holness M. J. (2004) Diabetes 53, S71–S81 [DOI] [PubMed] [Google Scholar]
- 49. Oakes N. D., Cooney G. J., Camilleri S., Chisholm D. J., Kraegen E. W. (1997) Diabetes 46, 1768–1774 [DOI] [PubMed] [Google Scholar]
- 50. Suh H. N., Huong H. T., Song C. H., Lee J. H., Han H. J. (2008) Am. J. Physiol., Cell Physiol. 295, C1518–C1527 [DOI] [PubMed] [Google Scholar]
- 51. Rhee J., Inoue Y., Yoon J. C., Puigserver P., Fan M., Gonzalez F. J., Spiegelman B. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4012–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Massillon D., Arinze I. J., Xu C., Bone F. (2003) J. Biol. Chem. 278, 40694–40701 [DOI] [PubMed] [Google Scholar]
- 53. Boustead J. N., Stadelmaier B. T., Eeds A. M., Wiebe P. O., Svitek C. A., Oeser J. K., O'Brien R. M. (2003) Biochem. J. 369, 17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Juge-Aubry C., Pernin A., Favez T., Burger A. G., Wahli W., Meier C. A., Desvergne B. (1997) J. Biol. Chem. 272, 25252–25259 [DOI] [PubMed] [Google Scholar]
- 55. Yanuka-Kashles O., Cohen H., Trus M., Aran A., Benvenisty N., Reshef L. (1994) Mol. Cell. Biol. 14, 7124–7133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sugiyama T., Scott D. K., Wang J. C., Granner D. K. (1998) Mol. Endocrinol. 12, 1487–1498 [DOI] [PubMed] [Google Scholar]
- 57. Blanquart C., Barbier O., Fruchart J. C., Staels B., Glineur C. (2002) J. Biol. Chem. 277, 37254–37259 [DOI] [PubMed] [Google Scholar]
- 58. Puigserver P., Spiegelman B. M. (2003) Endocr. Rev. 24, 78–90 [DOI] [PubMed] [Google Scholar]
- 59. Lemberger T., Staels B., Saladin R., Desvergne B., Auwerx J., Wahli W. (1994) J. Biol. Chem. 269, 24527–24530 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.