Abstract
The recent success of channelrhodopsin in optogenetics has also caused increasing interest in enzymes that are directly activated by light. We have identified in the genome of the bacterium Beggiatoa a DNA sequence encoding an adenylyl cyclase directly linked to a BLUF (blue light receptor using FAD) type light sensor domain. In Escherichia coli and Xenopus oocytes, this photoactivated adenylyl cyclase (bPAC) showed cyclase activity that is low in darkness but increased 300-fold in the light. This enzymatic activity decays thermally within 20 s in parallel with the red-shifted BLUF photointermediate. bPAC is well expressed in pyramidal neurons and, in combination with cyclic nucleotide gated channels, causes efficient light-induced depolarization. In the Drosophila central nervous system, bPAC mediates light-dependent cAMP increase and behavioral changes in freely moving animals. bPAC seems a perfect optogenetic tool for light modulation of cAMP in neuronal cells and tissues and for studying cAMP-dependent processes in live animals.
Keywords: Bacterial Metabolism, Biophysics, Cyclic AMP (cAMP), Cyclic Nucleotides, Enzyme Catalysis, Enzyme Inactivation, Neurobiology, Photoreceptors, Optogenetics
Introduction
Non-invasive manipulation of intercellular processes by light-activated proteins has recently developed as an emerging scientific field (1). The wide application of channelrhodopsin (ChR)2 in the neurosciences as an inheritable protein with retinal as a ubiquitous chromophore has strongly promoted the young field of optogenetics (2, 3). ChR is so well appreciated in this context because it modulates the membrane voltage as a universal parameter relevant for basically all neuronal cells. The protein is small and non-toxic, the cofactor retinal is available in all animal cells, and a once transformed organism inherits the light sensitivity to the next generation. This success stimulated the demand for novel genetically encoded light-activated proteins that modulate other general cellular parameters such as the second messengers Ca2+, cAMP, cGMP, or inositol triphosphate. To become useful tools for cell biology and neuroscience, such light-gated enzymes would have to work well in all classical experimental systems and use ubiquitous cofactors as light sensors.
Promising examples in this direction are the light-gated adenylate cyclases PACα and PACβ of the unicellular flagellate Euglena gracilis (euPACs), where they serve as an α2β2 photoreceptor complex that senses light for photophobic responses and phototaxis (4, 5). However, both euPACs are large proteins with two BLUF photoreceptor domains (F) (6) and two cyclase domains (C) in an FCFC arrangement (see Fig. 1a) functioning in the flagellate as a tetrameric complex. The purified protein complex shows some cyclase activity in the dark that is stimulated 80-fold in the light. In Xenopus oocytes and in HEK cells, the activity of euPACα was much higher than that of euPACβ (7), suggesting that euPACα would be an appropriate tool for manipulating cAMP levels in host cells and animals. In fact, ubiquitous expression of euPACα in Drosophila leads to a lethal cAMP increase, whereas pan-neuronal euPACα expression yielded strong effects on the grooming behavior of adult fruit flies in response to blue light (7). Despite these promising experiments, PAC proteins were not widely accepted for the study of neuronal or developmental cAMP-dependent processes. The main obstacles are the large molecular mass of above 100 kDa, low solubility, significant dark activity, and only moderate activation by light (7).
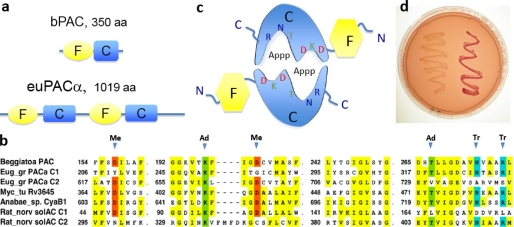
FIGURE 1.
Concept of light-activated cyclase. a, schematic arrangements of the photoreceptive BLUF domain (F) and the catalytic domain (C). b, part of the bPAC cyclase amino acid sequence aligned to the corresponding regions of other Type III cyclases; metal-binding Asps (Me) are shaded in red, essential adenine-binding Lys and Thr are in green, and transition state-stabilizing Asn and Arg are in blue. c, model of the dimeric bPAC with flavin-binding BLUF domain (F) in yellow and the catalytic domain (C) in blue. d, cyclase activity in an adenylate cyclase-deficient E. coli strain before (left) and after (right) transformation with bPAC on a MacConkey agar plate. Red color indicates rescue of maltose metabolism due to cAMP production.
Here we introduce a novel PAC from Beggiatoa, a sulfide-oxidizing bacterium that colonizes large areas of sea ground in the form of widely extended microbial mats (8). The chemolithoautotrophic metabolism allows Beggiatoa to utilize oxygen or nitrate as electron acceptors during sulfide oxidation (9, 10). A recently deposited genome sequence revealed the presence of a gene, putatively encoding a 350-amino acid protein, consisting of a blue light-sensing BLUF domain linked C-terminally to a Type III adenylyl cyclase (see Fig. 1a) (11). All amino acids considered as critical for the catalytic mechanism are conserved as highlighted in Fig. 1b. The amino acids are arranged in such a way that we expect the protein to function as a homodimer in accordance the structure of Type III cyclases (see Fig. 1c and Ref. 11). (see Fig. 1c).
We proved the bPAC activity first in Escherichia coli, analyzed the spectral properties on the purified protein, and tested the applicability and kinetics in Xenopus oocytes, rat hippocampal pyramidal cells, and the Drosophila CNS in light and darkness. Despite its small size, bPAC performed in most respects superior to euPAC. bPAC shows lower dark activity and a better stimulation of the activity in the light. We demonstrate that light-induced cAMP elevations in neurons are highly reproducible and proportional to the light dose, making non-invasive light control of cAMP possible in cell biology and the neurosciences.
EXPERIMENTAL PROCEDURES
Cyclase Activity in E. coli
E. coli-optimized synthetic DNA encoding the photoactivated cyclase bPAC of Beggiatoa sp. (accession number GU461307) was purchased from Mr. Gene (Regensburg, Germany) and was cloned in-frame behind the N-terminal His6 tag and SUMO epitope into a pET SUMO vector (Invitrogen). The protein was expressed in E. coli strain BTH101 at 30 °C in 200 μm isopropyl-1-thio-β-d-galactopyranoside for 2 h. Both transformed and non-transformed cells were plated on MacConkey agar (Difco), pH 7.5, containing 1% maltose and incubated at 30 °C overnight in darkness or in white light (average intensity: 8 W m−2 white light).
Protein Purification
For purification, the bPAC SUMO fusion construct was expressed in E. coli strain BL21 DE3 at 18 °C in LB induced with 60 μm isopropyl-1-thio-β-d-galactopyranoside for 48 h. bPAC was purified on cobalt-containing nitrilotriacetic acid resin (Clontech) in 50 mm NaH2PO4 (pH 7.5), 300 mm NaCl, 0.1 mm phenylmethylsulfonyl fluoride (PMSF) buffer according to the supplier's instructions. The eluate was dialyzed two times against 200 volumes of buffer and concentrated by ultrafiltration (Amicon Ultracel, molecular weight cut-off 10,000, Millipore).
Oocyte Electrophysiology
Oocytes from Xenopus laevis and human cystic fibrosis transmembrane conductance regulator (CFTR) cRNA were prepared as described before (12). We used bPAC DNA codon-optimized for expression in human cells (0810735_Beggiatoa_Mammal_pMK, GenbankTM accession number GU461307 or GU461306). The DNA (Mr. Gene) was inserted between the BamHI and BsiWI of the pGEMHE vector (13), a derivative of pGEM3z (Promega, Madison, WI) including a C-terminal myc tag. The Nhe-linearized plasmids were used for the in vitro generation of cRNA with the mMESSAGE mMACHINE T7 Ultra kit (Ambion, Austin, TX). For the cyclic nucleotide-gated cation channel (CNG channel) assay, 100 pg of bPAC mRNA and 20 ng of RNA encoding the olfactory CNG channel variant C460W/E583M (14) (kindly provided by J. W. Karpen, Oregon University) were injected into oocytes and incubated for 3–5 days at 16–18 °C in Ringer solution (96 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 5 mm MOPS or 5 mm HEPES (pH 7.5) with streptomycin (1 mg/ml) and penicillin (1 mg/ml)). For the CFTR assay, we injected 20 pg of bPAC RNA and 1 ng of CFTR RNA. Current recordings were performed with a GeneClamp 500 (Axon) at sampling rates between 500 Hz and 5 kHz. For recordings under pulsed light conditions, oocytes were excited with 450 nm light of a 75-W Xenon lamp (Osram). The oocyte in the two-electrode voltage clamp experiment was protected from intense ambient light so that exposure did not exceed 84 nW/cm2. Oocytes were monitored with a binocular under orange (>515 nm) light.
Immunological Quantification of cAMPi
cAMP was detected by a competitive immunoassay (Correlate-EIA, Assay Designs, Ann Arbor, MI) as described previously (7). Cyclase activity of purified recombinant protein was also assessed with this assay by incubation of 10 μg of bPAC in 24 μl of a solution of 300 mm KCl, 50 mm Hepes-Tris, pH 7.4, 1 mm MgCl2, 100 μm MgATP at room temperature (21 °C). The reaction was stopped with 220 μl of 0.1 m HCl.
Spectroscopy
Single wavelength kinetics were recorded in a Cary300bio (Varian, Palo Alto) UV-visible spectrometer at 489 nm. The protein was excited with a 455 nm LED (1-W Royal Blue, Luxeon Star, effective power 0.9 milliwatt mm−2). For recordings of transient absorption spectra, a faster setup was used comprising a Shamrock 303i imaging spectrograph with an Andor iStar ICCD (Andor Technology, Belfast, Ireland). The spectrum of the late photoproduct was recorded 2 s after the application of a 500-ms flash of a 455 nm LED (0.9 milliwatt mm−2). The probe light from a short arc xenon lamp (XBO 75 W) was applied by an optical fiber, transferred to a spectrograph, and then mapped on the ICCD chip of the camera. To minimize actinic effects, the probe light was interrupted with a VS-25 shutter (Uniblitz, San Francisco) to 90-ms dark/10-ms light. Furthermore, the intensity was reduced until 10 subsequent dark spectra appeared constantly. The spectra shown in Fig. 3a are averages of 10 discrete spectra, further smoothed by low pass-filtering the amplitude representation of the Fourier series expansion.
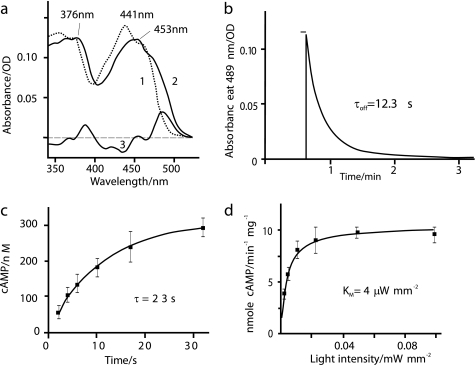
FIGURE 3.
Spectral properties. a, absorption spectra of purified bPAC in its dark-adapted (trace 1) and light adapted (trace 2) state. The difference between the two is shown as a line (trace 3). OD, optical density. b, decay of the red-shifted intermediate that is considered as the signaling state. The fit is seen as a white line. The protein was excited for 3 s with a 455 nm LED, and the absorbance change was recorded at 489 nm. c, cAMP concentration at different time delays in the dark after a 4-s 475 nm light pulse; 300 mm KCl, 50 mm Tris-Hepes, pH 7.4, 21 °C, n = 3 with double determinations for each cAMP value. d, light (475 nm) intensity dependence of cAMP production by purified bPAC, conditions as in C, illumination for 60 s, and immediate quenching with 9-fold volume of 0.1 m HCl. n = 2 with double determinations for each cAMP value. Plotted are mean values with S.D. and a Michaelis-Menten fit curve, yielding a Km of 3.7 ± 0.4 μW mm−2.
Electrophysiology of Hippocampal Neurons
Hippocampal slice cultures from Wistar rats were prepared at postnatal days 4–5 as described previously (15), according to Swiss veterinary regulations. Neurons were co-transfected with DNA encoding bPAC, CNG-A2 (C460W/E583M), and RFP (tdimer2, a gift from R. Y. Tsien, University of California, San Diego, CA) under control of the neuron-specific synapsin 1 promoter using a Helios Gene-Gun (Bio-Rad). Whole-cell patch clamp recordings were conducted at 30 °C in artificial cerebrospinal fluid (16) containing 1 μm tetrodotoxin, using potassium gluconate intracellular solution. For photostimulation of bPAC, a 100-W mercury arc lamp was controlled by a mechanical shutter (Uniblitz), attenuated by a series of neutral density filters and an eGFP excitation filter (BP470/40). Light intensity was measured at the back aperture of the objective (LUMPlan 60 × 0.9NA) and divided by the field of view (0.1 mm2).
Generation and Analysis of Transgenic Drosophila
The bPAC cDNA was subcloned into the pUASt fly transformation vector via EcoRI and KpnI restriction sited, and transgenic flies were generated by standard procedures (BestGenes Inc., Chino Hills, CA). Flies containing elav-Gal4 and appropriate PAC transgenes were F1 progeny of homozygous parental lines. cAMP was quantified from groups of 10 brains dissected in Drosophila Ringer solution under red light and 10 min with blue light (455 nm, 20 milliwatts mm−2) when appropriate. Immediately afterward, brains were processed following the manufacturer's instructions (catalog number 900-066, Assay Designs).
Grooming Assay
For behavioral experiments, we used female Drosophila aged 3–5 days after eclosion in an assay modified after Ref. 7. Illumination regimes contained either dim red light (>650 nm, 10 milliwatts mm−2) or intense blue light (455 nm, 40 milliwatts mm−2) to provoke “freezing behavior” in a light-dependent manner. Delay times for behavioral changes were determined from videotapes of individual animals.
Statistical Analysis
Numerical data are presented as mean ± S.E. Statistical differences were analyzed using Student's t test.
RESULTS
To test the function of the protein encoded by the published bPAC sequence, we cloned bPAC into a cAMP-deficient E. coli strain in which lactose and maltose cannot be fermented (17). On MacConkey agar plates, cAMP-deficient bacteria produce ammonia and form slow growing white colonies (Fig. 1d). Expression of bPAC rescued their maltose metabolism, as seen from the improved growth and red color of transformed colonies in Fig. 1d. Differences between light- and dark-grown plates were not detected. We concluded that bPAC is a functional adenylyl cyclase.
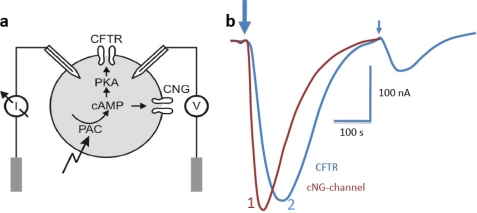
To test the light dependence of the new cyclase in a eukaryotic cell, we have expressed bPAC in Xenopus oocytes in conjunction with two cAMP-dependent ion channel systems (Fig. 2a). First, 100 pg of bPAC-RNA were injected into oocytes in combination with RNA encoding an olfactory CNG channel (14). After 3–4 days of expression, we measured large light-induced inward currents that peaked ∼50 s after the light pulse (Fig. 2b). Current decay was slow, and the resting conductance was reached after ∼5 min in the dark. In a second approach, we employed the CFTR, an anion channel with indirect cAMP dependence and higher sensitivity. The cAMP-dependent protein kinase (PKA) phosphorylates CFTR, thus triggering transition to the open state (Fig. 2a). CFTR currents had slower rise times, peaking ∼100 s after the light pulse, but decayed faster when compared with the CNG currents (Fig. 2b). To estimate absolute cAMP levels under light and dark conditions, oocytes expressing bPAC only (2 ng of bPAC-RNA) were tested for cAMP using an ELISA assay. After 4 days of expression in the dark, cAMP levels were 3.5 μm, slightly but significantly above the level of control oocytes (2.0 μm). Following a 1-min light pulse, cAMP levels reached values up to 140 μm. Assuming that bPAC dark activity was responsible for the 1.5 μm increase above baseline, these values correspond to an ∼100-fold increase in cyclase activity after illumination.
FIGURE 2.
bPAC activity in Xenopus oocytes. a, principle of the electrical assay. CFTR is activated by phosphorylation via an oocyte-endogenous PKA, whereas the CNG channel is directly activated by cAMP binding. I, current; V, voltage. b, photocurrents evoked by a 500-ms light pulse (450 nm) after coexpression of bPAC and CNG channel (dark red trace (trace 1)) and currents evoked by an 8-s (large arrow) or a 100-ms (small arrow) light pulse after co-injection of bPAC and CFTR (blue trace (trace 2)). Currents were measured at −40 mV for CFTR and CNG channels. In both test systems, the current reached values up to about −0.3 μA.
To investigate the relation between the bPAC photoreceptor states and enzymatic activity, we expressed bPAC in E. coli and purified it via affinity chromatography. The absorption spectrum of the purified bPAC showed the typical BLUF fine structure with a maximum at 441 nm (18) (Fig. 3a). Upon irradiation, the absorption band became less structured and was shifted by 12 nm to longer wavelengths, in accordance to the photochemical properties of other BLUF photoreceptors. The recovery of the dark state (Fig. 3b) was relatively fast with a τoff = 12 s at pH 7.5 in phosphate buffer at room temperature. Next, we measured the timing of cyclase activity in vitro. After a light flash of 4 s, cAMP continued to rise in the dark with a time constant τ = 23 ± 2 s at pH 7.4 (Fig. 3c), which is in fair agreement with the decay of the BLUF signaling state.
A parameter of great practical importance is the light intensity dependence of the enzymatic activity of bPAC. We measured cAMP concentrations in test tubes with purified bPAC protein after illumination for 1 min with blue light of variable intensity and obtained a Michaelis-Menten-type saturation curve (475 nm, Fig. 3d) with a half-saturation constant of 3.7 ± 0.4 μW mm−2. This low value is consistent with a photocycle in the range of 10 s and demonstrates the very high sensitivity of bPAC to light. Dark activity of bPAC was 33 ± 5 pmol of cAMP min−1 mg−1 of protein. For the maximal activity in the light, we obtained a value of 10 ± 2 nmol of cAMP/min/mg of protein (Fig. 3d), corresponding to a 300-fold increase in enzymatic activity. This large dynamic range combined with its high sensitivity to light made bPAC a promising tool to modulate brain function.
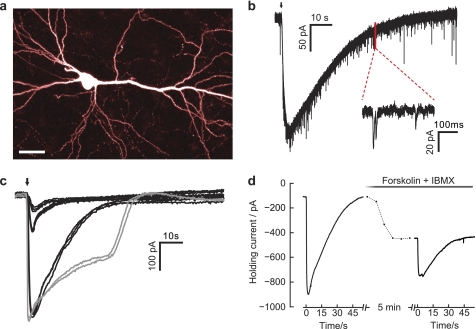
To test its applicability to neurons, we have expressed bPAC in CA1 pyramidal cells in conjunction with CNG channels and dimeric red fluorescent protein (tdimer2). Red fluorescent labeling of PAC-transfected cells is advantageous because the wavelength for RFP excitation (∼540 nm) is well beyond the BLUF absorption, which makes it easy to select cells for electrophysiological recordings without activating bPAC. 10 days after transfection, neurons were viable and had a normal appearance (Fig. 4a). Dim light pulses of 100-ms duration (470 nm, 0.12 milliwatt mm−2) evoked large inward currents (Fig. 4b), indicating rapid cAMP production in the transfected neurons. As expected, extension of pulse duration induced stronger and longer lasting CNG currents (Fig. 4c). At high light intensities (100 milliwatts mm−2), CNG currents rapidly saturated but were still fully reversible (Fig. 4c, gray curves). In control experiments with non-transfected neurons, identical illumination did not evoke any currents. At all tested light doses, CNG currents peaked rapidly (<3 s) and were highly reproducible, indicating precise control of intracellular cAMP concentration (cAMPi) by light. Pharmacological stimulation of endogenous AC with 100 μm forskolin and simultaneous inhibition of phosphodiesterases with 100 μm IBMX also activated CNG currents but with a much slower time course (3–4-min rise time, Fig. 4d). Interestingly, although combined forskolin/IBMX application is considered a very strong stimulation leading to “chemical Long-Term Potentiation” (19), forskolin/IBMX application did not fully occlude light-induced CNG currents, suggesting that bPAC outperformed the pharmacological mixture.
FIGURE 4.
Assessing bPAC function in hippocampal neurons. a, CA1 pyramidal cell expressing bPAC, CNG-A2, and RFP (two-photon imaging at 980 nm; scale bar, 30 μm). b, light-evoked cAMP-gated current at 0.14 milliwatt/mm (2). Arrow, 100-ms light pulse. The enlarged inset shows miniature excitatory postsynaptic currents. c, light-evoked cAMP-gated currents in one pyramidal cell at four different light doses (black traces, 0.14 milliwatt/mm2 for 50, 100, and 1000 ms; gray traces, 109 milliwatts/mm2 for 1 s). Traces were low pass-filtered at 10 Hz to remove miniature excitatory postsynaptic currents. At all stimulation intensities, currents were fully reversible and highly reproducible. CNG currents saturated at 0.14 milliwatt × s mm−2. d, light-evoked cAMP-gated currents before and after forskolin (100 μm) + IBMX (100 μm) wash-in. During forskolin/IBMX wash-in (dashed line, 5 min), holding current increased from −108 pA to −446 pA. Forskolin/IBMX application only partially occluded light-induced currents.
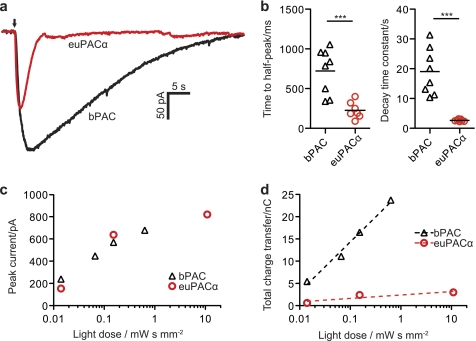
To directly compare the activities of bPAC and euPACα, we performed a second set of experiments on hippocampal neurons by co-transfection with euPACα, CNG, and RFP, using the same amounts of DNA as in bPAC experiments. In response to light pulses of saturating intensity, both PACs were able to induce large photocurrents (up to 1 nA), sufficient to induce action potential firing in many neurons under current clamp conditions. bPAC-induced currents peaked later than those of euPAC (bPAC, one-half peak after 723 ms ± 101 ms; euPACα, one-half peak after 227 ms ± 40 ms) and were much longer lasting (bPAC, τdecay 19.0 s ± 2.8 s; Fig. 5, a and b, euPACα, τdecay = 2.7 s ± 0.1 s), confirming the slow inactivation of bPAC. As a consequence, at light intensities that kept cAMP below CNG current saturation, peak currents were similar for both PACs (Fig. 5c), but in bPAC-transfected cells, the integrated current (total charge transfer) was significantly larger (Fig. 5d). Vice versa, to produce comparable amounts of cAMP in euPACα-expressing neurons, light dose had to be increased by at least 3 orders of magnitude. Because bPAC is able to produce large cAMP elevations in response to dim blue light pulses, it will be eligible for studies cAMP-mediated processes in cells deep below the surface of the brain. The previously described euPACα, on the other hand, might be advantageous for experiments in which subsecond temporal control is necessary and light dose is not a limiting factor.
FIGURE 5.
Comparing bPAC- and euPACα-induced currents in neurons. a, following a 100-ms light pulse (140 μW mm−2, blue arrow), cAMP elevation was much longer lasting in CA1 pyramidal cells expressing bPAC, CNG-A2, and RFP (black trace) when compared with cells expressing euPACα, CNG-A2, and RFP (red trace). Traces were low pass-filtered to remove miniature excitatory postsynaptic currents. b, time to half-peak current and current decay time constant were significantly longer in bPAC-expressing neurons when compared with euPACα-expressing neurons (bPAC, n = 8 conditions (three light doses, five cells); euPAC: n = 7 conditions (four light doses, three cells); ***, p < 0.001). c, under subsaturating conditions, light dose dependence of peak currents was similar for bPAC- and euPACα-expressing neurons. d, total charge transfer (integrated current) was ∼8 times higher in bPAC-expressing neurons when compared with euPACα-expressing neurons. nC, nano Coulomb.
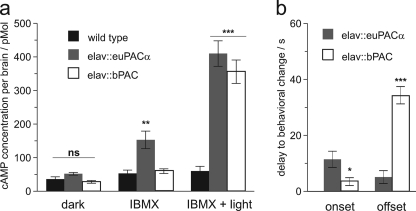
Applicability of bPAC was also tested in Drosophila. We targeted expression of bPAC to the Drosophila central nervous system (CNS) using euPAC as control. We used the neuron-specific elav-Gal4 driver and quantified cAMP levels in dissected brains (Fig. 6a). Basal cAMP dark levels did not significantly differ between genotypes (p > 0.05). The addition of 100 mm IBMX increased dark levels of cAMP in euPAC-expressing Drosophila but had no effect on bPAC transgenes, indicating significant dark activity of euPAC, but not bPAC (p < 0.01, see further details under “Experimental Procedures” and the figure legends). After photoactivation, both transgenes showed strongly increased levels of brain cAMP (p < 0.001).
FIGURE 6.
Transgenic bPAC and euPACα exhibit different levels of dark activity and affect grooming behavior in freely moving Drosophila. a, expression of euPACα transgenes (elav::euPACα) resulted in distinctive dark activity, which was revealed by the phosphodiesterase blocker IBMX. Dark activity was not observed upon bPAC expression (elav::bPAC) or in wild type Canton-S control animals. Photoactivation of either PAC transgene resulted in a 10-fold increase in cAMP with no statistical difference between final concentrations of cAMP derived from either euPACα or bPAC (n = 11/group). 100 μm IBMX was used to block phosphodiesterase activity; light activation of cyclase transgenes was performed in 100 μm IBMX and irradiation (5 min, 455 nm, 40 milliwatts mm−2). b, photoactivation of pan-neuronally expressed PAC transgenes affects grooming activity, resulting in stereotypic freezing behavior (7). bPAC-expressing flies freeze significantly faster in blue light than euPACα flies (n = 11/group). They also take significantly longer to resume grooming behavior in the dark. Data represent means ± S.E.; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.
In a small animal like Drosophila, photoactivation of PAC transgenes can be achieved through the cuticle of live animals. As described previously, grooming activity stops when cAMP is elevated throughout the CNS (7). Here we used this effect to compare in vivo applicability of bPAC and euPACα, focusing on the delay of behavioral changes after short illumination. When compared with euPAC transgenes, bPAC-expressing flies exhibited a significantly faster response onset (p < 0.05). After light-off, it took an average of 34 s until bPAC flies resumed grooming, confirming the more powerful and prolonged cyclase activity of bPAC (p < 0.01, Fig. 6b).
DISCUSSION
With the experiments described above, we introduce an adenylyl cyclase with properties that are superior for application in most respects over the previously described PACα of E. gracilis. We demonstrate that bPAC is functional in bacteria, fruit flies, frog oocytes, and rodent neurons and can be purified from E. coli for biophysical studies. The advantages of bPAC over euPACα are the following. (i) bPAC DNA is only about one-third the size of euPACα, and it is more conveniently handled in host vector systems, especially if viral vectors with limited maximal packing volume are used. (ii) Because of the fact that only one photoreceptor domain is present, modification of the photoreceptor kinetics (20) and the enzymatic active state lifetime will be more straightforward. (iii) It is likely that bPAC is active as a homodimer, like most prokaryotic Type III cyclases, resulting in a complex three or six times smaller than the tetrameric euPAC (4, 33). (iv) Light stimulation of the purified euPAC complex resulted in an 80-fold cyclase activity (4), whereas we determined a 300-fold increase in activity for bPAC. (v) Due to the long life time of the active state, the half-saturating light intensity is low, and bPAC needs ∼1000 times less light than euPACα to generate comparable steady state levels of cAMP in neurons.
The temporal precision of cAMP control by light is limited by the inactivation time of PAC but also by the activity of endogenous cyclic nucleotide phosphodiesterases. The correlation between the life time of the BLUF signaling state and activity of the cyclase tells us that the cyclase is not only activated by the BLUF domain but also inactivated by its transition back to the dark state. Moreover, the high phosphodiesterase activity in hippocampal neurons achieves a tight correlation between the BLUF signaling state and the cAMP level (19 s, Fig. 5b). In oocytes, this correlation is lost due to low phosphodiesterase activity and long diffusion pathways (Fig. 2b). Thus, neurons are ideal candidates for light control of cAMP signaling with high temporal accuracy. These kinetic comparisons have never been done for any euPAC because no recombinant full-length euPAC could be purified in an active and soluble state.
An important issue for application of light-activated enzymes is the difference in activity in light and darkness (dynamic range). Dark activity may pose an experimental problem because it changes the properties of transfected cells or tissues even during PAC expression before the actual experiment is started. The absence of any measurable dark conductance has been a great advantage of the channelrhodopsins. In contrast, most light-activated enzymes with a flavin-based BLUF- or (light, oxygen, voltage sensor)-type photoreceptor domain show significant dark activity, and their light activation is less than 10-fold (21, 22). This is also true for proteins with engineered photoreceptor function such as the light-activated Rac (10-fold) (23) or the light-modulated DNA-binding protein the Moffat group has recently designed (5.6 ± 2.5-fold) (24). An exceptional case is the designed light-activated kinase YF1, the activity of which is high in the dark and suppressed 1000-fold in the light (25). However, due to the microbial target, this kinase can hardly be employed in animals and, second, in most experiments, activation of the enzyme and not inactivation is preferred. This large dynamic range of bPAC with 300-fold light activation suggests that any potential problem associated with dark activity could be remedied simply by using a weaker promoter to drive bPAC expression. Thus, careful analysis of intracellular cAMP levels is mandatory in any experimental application of PAC transgenes, and various analytical techniques are available, e.g. immunodetection by ELISA, electrophysiological quantification, or use of FRET-based imaging techniques (7, 26, 27).
Across many species, the cAMP system in neurons has been shown to be crucial for learning and memory (28). Classical examples are the Drosophila learning mutants dunce and rutabaga that affect cAMP metabolism in opposite directions. From presynaptic short term plasticity to cAMP-inducible gene expression and long term plasticity, synapses transiently or persistently change their transmission characteristics in response to elevated cAMPi (29, 30). To manipulate cAMPi in neurons, most studies have used mutant animals or pharmacological agents (e.g. forskolin), methods that lack the precise temporal resolution, single-cell specificity, and quantitative control that can be readily achieved with light-activated PAC (7, 31). As an application example, optical control of cAMPi will allow researchers to dissect which forms of synaptic plasticity are triggered by pre- or postsynaptic signals, a distinction that has been notoriously difficult to make in the past. Furthermore, the sharp drop of the bPAC absorption spectrum at 500 nm might allow researchers to combine it with red-shifted variants of ChR2 (32) for independent optical control of cAMPi and the membrane voltage Em. This combination could be useful to probe second messenger systems within individual cells or to activate two populations of neurons by blue and green light, respectively. In summary, we show that the PAC from Beggiatoa is well tolerated by neurons and allows for rapid and reproducible control of cAMPi using very moderate levels of blue light.
Supplementary Material
Acknowledgments
We thank Tilo Mathes (Humboldt Universität zu Berlin, Germany) for fruitful discussion and Maila Reh, Christina Mrosek ((Humboldt Universität zu Berlin, Germany) Berlin, Germany), Doris Waffler, Elfriede Reisberg (Universität Würzburg, Germany), and Daniela Gerosa (Friedrich Miescher Institut, Basel, Switzerland) for excellent technical assistance. Wolfram Schleich (Friedrich Miescher Institut, Basel, Switzerland) performed pilot experiments on hippocampal neurons.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1-MH-086415-01 (to M. S.). This work was also supported by Deutsche Forschungsgemeinschaft Grants FOR526/1279 and the Cluster of Excellence Unifying Concepts in Catalysis (to P. H.), Grant SFB 567 (to G. N.), and Grant SCHW1410/1-1 (to M. S.).
This article was selected as a Paper of the Week.
- ChR
- channelrhodopsin
- BLUF
- blue light receptor using FAD
- PAC
- photoactivated cyclase
- bPAC
- photoactivated cyclase from Beggiatoa sp.
- euPAC
- photoactivated cyclase from E. gracilis
- CFTR
- cystic fibrosis transmembrane conductance regulator
- CNG
- cyclic-nucleotide gated
- IBMX
- 3-isobutyl-1-methylxanthine
- SUMO
- small ubiquitin-like modifier
- W
- watt.
REFERENCES
- 1. Gorostiza P., Isacoff E. Y. (2008) Science 322, 395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller G. (2006) Science 314, 1674–1676 [DOI] [PubMed] [Google Scholar]
- 3. Schoenenberger P., Zhang Schärer Y. P., Oertner T. G. (2010) Exp. Physiol. doi: 10.1113/expphysiol.2009.051219 [DOI] [PubMed] [Google Scholar]
- 4. Iseki M., Matsunaga S., Murakami A., Ohno K., Shiga K., Yoshida K., Sugai M., Takahashi T., Hori T., Watanabe M. (2002) Nature 415, 1047–1051 [DOI] [PubMed] [Google Scholar]
- 5. Ntefidou M., Iseki M., Watanabe M., Lebert M., Häder D. P. (2003) Plant Physiol. 133, 1517–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gomelsky M., Klug G. (2002) Trends Biochem. Sci. 27, 497–500 [DOI] [PubMed] [Google Scholar]
- 7. Schröder-Lang S., Schwärzel M., Seifert R., Strünker T., Kateriya S., Looser J., Watanabe M., Kaupp U. B., Hegemann P., Nagel G. (2007) Nat. Methods 4, 39–42 [DOI] [PubMed] [Google Scholar]
- 8. Hinck S., Neu T. R., Lavik G., Mussmann M., de Beer D., Jonkers H. M. (2007) Appl. Environ. Microbiol. 73, 7013–7022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mills H. J., Martinez R. J., Story S., Sobecky P. A. (2004) Appl. Environ. Microbiol. 70, 5447–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Møller M. M., Nielsen L. P., Jørgensen B. B. (1985) Appl. Environ. Microbiol. 50, 373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linder J. U., Schultz J. E. (2003) Cell. Signal. 15, 1081–1089 [DOI] [PubMed] [Google Scholar]
- 12. Weinreich F., Wood P. G., Riordan J. R., Nagel G. (1997) Pflugers Arch. 434, 484–491 [DOI] [PubMed] [Google Scholar]
- 13. Liman E. R., Tytgat J., Hess P. (1992) Neuron 9, 861–871 [DOI] [PubMed] [Google Scholar]
- 14. Rich T. C., Tse T. E., Rohan J. G., Schaack J., Karpen J. W. (2001) J. Gen. Physiol. 118, 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stoppini L., Buchs P. A., Muller D. (1991) J. Neurosci. Methods 37, 173–182 [DOI] [PubMed] [Google Scholar]
- 16. Schoenenberger P., Gerosa D., Oertner T. G. (2009) PLoS One 4, e8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karimova G., Pidoux J., Ullmann A., Ladant D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masuda S., Bauer C. E. (2002) Cell 110, 613–623 [DOI] [PubMed] [Google Scholar]
- 19. Otmakhov N., Khibnik L., Otmakhova N., Carpenter S., Riahi S., Asrican B., Lisman J. (2004) J. Neurophysiol. 91, 1955–1962 [DOI] [PubMed] [Google Scholar]
- 20. Bonetti C., Stierl M., Mathes T., van Stokkum I. H., Mullen K. M., Cohen-Stuart T. A., van Grondelle R., Hegemann P., Kennis J. T. (2009) Biochemistry 48, 11458–11469 [DOI] [PubMed] [Google Scholar]
- 21. Christie J. M., Swartz T. E., Bogomolni R. A., Briggs W. R. (2002) Plant J. 32, 205–219 [DOI] [PubMed] [Google Scholar]
- 22. Matsuoka D., Tokutomi S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13337–13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Y. I., Frey D., Lungu O. I., Jaehrig A., Schlichting I., Kuhlman B., Hahn K. M. (2009) Nature 461, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strickland D., Moffat K., Sosnick T. R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 10709–10714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Möglich A., Ayers R. A., Moffat K. (2009) J. Mol. Biol. 385, 1433–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dunn T. A., Feller M. B. (2008) Dev. Neurobiol. 68, 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaccolo M., Pozzan T. (2002) Science 295, 1711–1715 [DOI] [PubMed] [Google Scholar]
- 28. Bailey C. H., Bartsch D., Kandel E. R. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 13445–13452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen C., Regehr W. G. (1997) J. Neurosci. 17, 8687–8694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makhinson M., Chotiner J. K., Watson J. B., O'Dell T. J. (1999) J. Neurosci. 19, 2500–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schroll C., Riemensperger T., Bucher D., Ehmer J., Völler T., Erbguth K., Gerber B., Hendel T., Nagel G., Buchner E., Fiala A. (2006) Curr. Biol. 16, 1741–1747 [DOI] [PubMed] [Google Scholar]
- 32. Zhang F., Prigge M., Beyrière F., Tsunoda S. P., Mattis J., Yizhar O., Hegemann P., Deisseroth K. (2008) Nat. Neurosci. 11, 631–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Looser J., Schröder-Lang S., Hegemann P., Nagel G. (2009) Biol. Chem. 390, 1105–1111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.