Abstract
Sphingomyelin (SM) and ceramide-phosphoethanolamines (cer-PE) are related lipids present in mammals and insects, respectively. Owing to the critical roles that cer-PE play in eukaryotic cellular function, there is a need to develop methods that provide accurate quantitation of these compounds. Results obtained in this study demonstrate that Drosophila contains cer-PE’s with unsaturated sphingoid base cores as well as low levels of cer-PE’s that possess saturated sphingoid base cores. Specifically, the method developed in this study enabled the quantitation of picogram amounts of cer-PE containing both unsaturated d14:1Δ4 and d16:1Δ4 and saturated d14:0 sphingoid base cores. Using this method cer-PE compounds with both saturated and unsaturated sphingoid base core were initially identified by neutral loss scanning, followed by quantitation using single reaction monitor scans (SRM). The SRM scans measured a product ion originating from the sphingoid base backbone, rather than from the head group, increasing the specificity and the sensitivity of the quantation measurement.
Keywords: Drosophila, Liquid chromatography, Neutral loss-single ion monitoring scans, Selected reaction monitoring, Quantitation, Ceramide-phosphorylethanolamines, Sphingoid bases
Introduction
Sphingolipids are a group of highly conserved functional entities that regulate a number of cellular processes in eukaryotes [1–7]. Sphingolipids such as sphingomyelins (SM), ceramides, ceramides-1-phosphates, glucosylceramides, hexosylceramides and ceramide phosphorylethanolamines etc., are characterized by sphingoid base backbones (Fig. 1). These complex lipids provide structural framework for plasma membrane organization [8], formation and functioning of plasma membrane lipid rafts (9), cell signaling and transduction events [10–14], as well as regulation of diverse cellular processes such as growth, differentiation, apoptosis, and angiogenesis [15–22].
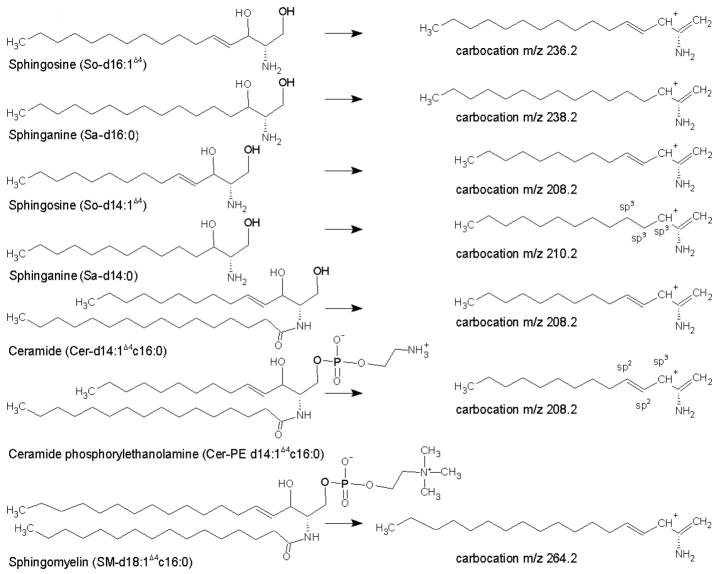
Figure 1.
Structures for some of the representative sphingoid base containing compounds and their associated double dehydrated carbocations. A core chain for a sphingoid base structure contains a 2-amino-1,3-dihydroxy moiety, and a notation for eg., d14:1Δ4 is a dihydroxy 14 carbon core structure with double bond on the fourth carbon atom starting from the hydroxyl terminus of the core chain. The notation c16:0 is for number of carbon atoms linked to the amino side chain, as in cers, SM, and cer-PE’s.
Sphingolipid abnormalities are associated with a number of maladies including inborn errors of metabolism, atherosclerosis, diabetes, Alzheimer’s disease, and photoreceptor degeneration [2, 3, 23, 24]. Sphingolipids from mammalian cells such as sphingoid bases, ceramides, monohexosylceramides, dihexosylceramides, and SM have been well characterized and quantitated using a variety of liquid chromatograhy-mass spectrometry (LC-MS) techniques [25–34]. In mammalian cells, the predominant forms that contain sphingoid base backbones are the c18 and c20 unsaturated sphingosines and their corresponding saturated dihydro-sphinganines, while in Drosophila the c14 and c16 species predominate [2, 16, 35–37]. Sphingomyelins and ceramide-phosphoethanolamines (cer-PE) are related lipids, however, the former contain a phosphoryl-choline moiety while the latter possesses a phosphoryl-ethanolamine moiety (Fig. 1). With mounting evidence showing relationships between aberrant cer-PE and ceramides levels and various diseases [8, 16, 17, 24, 38], the need to accurately quantitate these compounds has become increasingly more critical. Although several excellent methods for quantitating ceramides have been reported [16, 27–32, 39, 40], documented methods for quantitating ceramide phosphoryl ethanol amine compounds (cer-PE) are not without their biases and almost all the methods that are reported make use of precursor and neutral loss scans for cer-PE quantitation [37, 41–45]. Ultraviolet absorbance-based assays are not possible for many sphingolipids because of the lack of chromogenic functionalities on cers, cers-PE, and SMs, etc. Previously, Fryst et. al., [17, 38] have reported the presence of independent bases such as sphingosines, sphinganines (i.e. dihydrosphingosines, and sphingadienes in Drosophila) and Rietveld et. al., [37] described the presence of cer-PE d14:1Δ4 c20:0 and d14:1 Δ4 c22:0 species by precursor ion scans.
The direct detection and multiplexed quantitation capabilities of mass spectrometry (MS)-based approaches offer a number of advantages for the analysis of lipidomic compounds [15, 25]. In this study we present a reverse phase liquid chromatography-tandem MS (LC-MS/MS) methods that utilizes neutral loss scans for identification and single reaction monitoring for quantitation of various cer-PE’s molecular species containing unsaturated sphingoid bases. Using this method we were able to quantitate previously unidentified low abundant cer-PE’s containing saturated sphingoid base cores. We also report here for comparative sake a method for quantitating ceramides extracted from Drosophila based on non-polar HPLC-ESI-MS/MS.
Experimental section
Reagents
N-Lauroyl-D-erythro-sphingosylphosphoethanolamine (cer-PE-d17:1Δ4 c12:0) was purchased from Avanti Polar Lipids Inc Alabaster, AL). All HPLC grade reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of Drosophila samples
Control w1118 Drosophila were obtained from Bloomington Stock Center at Indiana University. Drosophila stocks were cultured on standard corn meal agar and maintained at 25 °C. Flies were anesthetized by delivering CO2 gas and euthanized on dry ice. For tissue isolation, whole flies were homogenized in 12.5 mM ammonium bicarbonate (NH4HCO3) containing protein inhibitors and centrifuged at 5000 g at 4 °C. The supernatant was collected and protein concentration was measured using the BIO-RAD DC protein assay kit (Bio-Rad, Hercules, CA).
Preparation of ceramide-phosphoethanolamines extracts
Lipid extracts were prepared according to the method described by Merrill et al [26, 27]. In brief, to 100 μL of Drosophila cell extracts (total protein content 2.2 mg) 0.5 mL of methanol (CH3OH), 0.25 mL of chloroform (CHCl3), 50 μL of water (H2O) was added. In addition, 30 μL of each 25 μM solution of LM-6002 and N-Lauroyl-D-erythro-sphingosylphosphoethanolamine (2-ammonioethyl-(2R,3S, E)-2-dodecaanamide-3-hydroxyheptadec-4-enyl phosphate) prepared in 2/1 v/v CHCl3/CH3OH solution was added providing 750 pmoles of each internal standard. The lipid aggregates were dispersed by sonicating four times using a Branson tip sonicator set at an amplitude of 30% for 10 seconds. The samples were incubated overnight at 48 °C with shaking. After cooling the mixture to ambient temperature, 75 μL of 1 M methanolic potassium hydroxide (KOH) solution was added to the samples followed by incubation at 37 °C for 2 hrs with shaking. The sample solution was divided into two portions. One portion was neutralized with 7 μL of glacial acetic acid (CH3COOH) and extracted twice using a mixture of 2:1 (v/v) H2O:CHCl3. The lower organic portions were collected, combined, and evaporated to dryness using a Savant Speed Vac Concentrator (GMI, Ramsey, MN). The dried sample was re-suspended in ~300–400 μL of 1:3 (v/v) CHCL3 and non polar mobile phase A (please see next section). The other half of the sample was evaporated to a volume of approximately 25 μL and reconstituted by adding 300–400 μL of a 1:1 (v/v) mixture of reverse mobile phase A and reverse mobile phase B. After vortexing for 1 min and centrifugation using a desk-top centrifuge (Eppendorf Centrifuge 5415 D) for two min, the supernatant was collected.
Liquid chromatography tandem mass spectrometry
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was performed using a TSQ Discovery triple quadrupole tandem mass spectrometer (Thermo Electron Corp., San Jose, CA, USA) equipped with an electrospray ionization (ESI) source. The mass spectrometer was coupled to an Agilent 1100 series HPLC system.
Ceramides were separated using normal phase chromatography employing a binary system and a 7.5 cm × 3.0 mm × 3 μm Supelcosil LC-NH2 column operating at a flow rate of 250 μL/min. The mobile phase buffer A composed of 5 mM ammonium acetate (CH3COONH4) dissolved in 20 mL CH3OH, 15 mL CH3COOH, 270 mL acetonitrile (CH3CN), 300 mL ethyl acetate (CH3COOCH2CH3), and 400 mL hexanes and buffer B solution consisted of 99:1 (v/v) CH3OH: CH3COOH containing 5 mM CH3COONH4. The LC-MS/MS experiments were performed by injecting 10 μL of sample onto the column via an auto-sampler. After sample injection, the lipid compounds were eluted from the column by increasing the initial gradient from 0% to 2.75% mobile phase B over a 2.75 min period, and held there for 0.75 min. The gradient was increased to 18% B over the next 1.25 min and held there for 1.25 min. The gradient was then increased to 35% B over the next 1.5 min and held at this level for 1 min. The gradient was increased to 50% B over the next 1 min, and held there for 0.5 min, followed by an increase to 100% B over the next 0.5 min. After 1.5 min at 100% B, the gradient was brought back to its initial condition over 0.5 min and the column was equilibrated for 4.5 min prior to the next injection. The total run time for each ceramide sample was 17 min. Samples were analyzed in triplicate.
Ceramide-phosphoethanolamine compounds were separated using reverse phase HPLC and a 2.1 mm × 5 cm × 5 μm Supelco Discovery C18 column operating at a flow rate of 250 μl/min and maintained at 37 °C. Mobile phase A consisted of 74/25/1 (v/v/v) H2O:CH3OH:HCOOH (formic acid), containing a final concentration of 5 mM HCOONH4 (ammonium formate). Mobile phase B consisted of 99:1 (v/v) CH3OH:HCOOH containing a final concentration of 5 mM HCOONH4. For each LC-MS/MS experiment, 10 μL of sample was injected onto the column using an auto-sampler. After injection, the cer-PE were eluted by increasing the initial gradient from 10% to 95 %B buffer over 3.5 min. The gradient was then increased to 100% B over the next 12.5 min and held there for 1 min, followed by a drop to 10% B over 0.1 min. The column was then equilibrated for 3.9 min. The LC run time for each analysis was 21 min. Each sample was analyzed in triplicate.
The mass spectrometer was calibrated using a solution of polytyrosine-1,3,6 as per the manufacturer’s recommendation (Thermo Electron Corp.). The tune file for the present analytes was obtained by optimizing ESI source conditions at a LC flow rate of 250 μL/min and using a standard cer-PE d17:1Δ4 c12:0 compound. The optimized source parameters were as follows: ionization mode, positive; sheath gas pressure, 20 psi; auxiliary gas pressure, 3 (arbitrary units); ion spray needle voltage, 4,500 V; capillary temperature, 377 °C; skimmer offset, 7 V. No source fragmentation was observed when reference compounds were analyzed via direct infusion using this skimmer potential. Collision energy between 28 and 30 V provided optimal fragmentation of [M+H]+ ions to product ions at m/z 208.2, 210.3, 236.2, 238.3 and 250.2, corresponding to doubly dehydrated product ion fragments for cer-PE with So-d14:1Δ4, Sa d14:0, So-d16:1Δ4, Sa d16:0, and So-d17:1Δ4 sphingoid base backbones of the cer-PE’s, respectively.
Collision induce dissociation was performed using nitrogen gas within Q2, which was offset from Q1 by 10 V. For neutral loss scans Q3 was set to allow passage of m/z 141.2 corresponding to the loss of phosphorylethanolamine moiety while scanning Q1 for different precursor ions of m/z 591.6, 633.5, 661.5, 689.60, 715.7, 717.6 and 719.4. Single reaction monitoring (SRM) was accomplished by setting Q1 and Q3 to pass the precursor and product ions, respectively. For experiments conducted in SRM mode for cer-PE’s, two different MS/MS methods were constructed, and each method was partitioned into three segments. The first (5 min) and the third segments (8.5 min) of each method contain no analyte transitions. In the second segment of each method, which has a duration of 7.5 min, seven analyte precursor-to-product ion transitions belonging to either the cer-PE d14:1 or cer-PE d18:1 series were included along with the transitions related to the respective cer-PE internal standards. Likewise, to perform SRM experiments for ceramides, three different methods were constructed with each method demarcated into two segments with the analytes being incorporated in the first segment.
The acquisition parameters common to all analytes were: scan width (m/z) 0.1; scan time, 0.20 sec for each transition; peak width (FWHM) 0.70 for both Q1 and Q3; and collision pressure 1.5 mTorr. Other acquisition parameters and the chromatographic retention times of the compounds measured are listed in the Tables 1–3. Data acquisition and analysis were accomplished using Xcalibur software v.2.0.5 (Thermo Electron Corp.). Quantitation data was corrected for the carbon number difference between a given molecular species and the selected internal standard (i.e., to include difference in degrees of freedom due to varying carbon chain lengths which results in different ionization efficiencies) according to the following formula; z1 = (1+ 0.011n + 0.0112 n (n−1)/2)/(1 + 0.011s + 0.0112 s(s−1)/2) (31).
Table 1.
Retention times and compound specific selected reaction monitoring (SRM) parameters for some of the ceramides analyzed in this study. The methods comprised of two segments, and all the peaks are displayed in the first segment. In each method to attain maximum sensitivity only 5–7 ceramides were included, in which precursor ion to double dehydrated product ion arising from sphingoid base is monitored.
| Ceramide Analyte | Precursor [M+H]+ → Product ion | Retention Time (min) | Collision Enery (V) |
|---|---|---|---|
| Method 1 | |||
| d18:1Δ4 c12:0 (ISTD) | 482.48 → 264.24 | 1.76 | 25 |
| d14:1Δ4 c14:0 | 454.43 → 208.29 | 1.77 | 25 |
| d14:1Δ4 c16:0 | 482.48 → 208.29 | 1.76 | 25 |
| d14:1Δ4 c18:1 | 508.49 → 208.30 | 1.75 | 26 |
| d14:1Δ4 c18:0 | 510.49 → 208.30 | 1.74 | 26 |
| d14:1Δ4 c20:0 | 538.50 → 208.30 | 1.73 | 36 |
| d14:1Δ4 c22:0 | 566.52 → 208.30 | 1.72 | 36 |
| Method 2 | |||
| d18:1Δ4 c12:0 (ISTD) | 482.48 → 264.24 | 1.76 | 25 |
| d14:1Δ4 c24:1 | 592.57 → 208.30 | 1.71 | 27 |
| d14:1Δ4 c24:0 | 594.58 → 208.30 | 1.70 | 27 |
| d16:1Δ4 c14:0 | 482.46 → 236.30 | 1.75 | 25 |
| d16:1Δ4 c16:0 | 510.49 → 236.30 | 1.74 | 26 |
| d16:1Δ4 c18:0 | 538.52 → 236.30 | NI | 26 |
| Method 3 | |||
| d18:1Δ4 c12:0 (ISTD) | 482.48 → 264.24 | 1.76 | 25 |
| d16:1Δ4 c20:0 | 566.55 → 236.30 | 1.71 | 26 |
| d16:1Δ4 c22:0 | 594.58 → 236.30 | 1.68 | 27 |
| d16:1Δ4 c24:1 | 620.59 → 236.30 | 1.81 | 28 |
| d16:1Δ4 c24:0 | 622.60 → 236.30 | 1.77 | 28 |
10 μL of solution was injected by an auto-sampler for LC-ESI-MS/MS experiments. A universal tuned tube lens value from polytyrosine calibration was used.
Table 3.
Retention times and compound specific selected reaction monitoring (SRM) parameters for some of the ceramide phosphoryl ethanolamines containing saturated sphingoid base core. The methods comprised of one segment. In each method to attain maximum sensitivity only seven cer-PE were included, in which precursor ion to double dehydrated product ion arising from sphingoid base is monitored
| Cer-PE Analyte SRM Transitions: | Precursor [M+H]+ → Product ion | Retention Time (min) | Collision Enery (V) | Tube Lens (V) |
|---|---|---|---|---|
| Method 1 for d14:0 | ||||
| d17:1Δ4 c12:0 (ISTD) | 591.46 → 250.19 | 6.17 | 28 | 94 |
| d14:0 c16:0 | 607.49 → 210.28 | - | 28 | 96 |
| d14:0 c18:0 | 635.49 → 210.28 | 7.17 | 29 | 98 |
| d14:0 c20:0 | 663.53 → 210.28 | 7.96 | 30 | 100 |
| d14:0 c22:0 | 691.50 → 210.28 | 8.92 | 30 | 102 |
| d14:0 c24:0 | 719.58 → 210.28 | 9.96 | 30 | 102 |
| Method 2 for d16:0 | ||||
| d17:1Δ4 c12:0 (ISTD) | 591.46 → 250.19 | 6.19 | 28 | 94 |
| d16:0 c14:0 | 607.49 → 238.20 | NI | 28 | 96 |
| d16:0 c16:0 | 635.49 → 238.20 | NI | 29 | 98 |
| d16:0 c18:0 | 663.53 → 238.20 | NI | 30 | 100 |
| d16:0 c20:0 | 691.50 → 238.20 | NI | 30 | 102 |
| d16:0 c22:0 | 719.58 → 238.20 | NI | 30 | 106 |
10 μL of solution was injected by an auto-sampler for LC-ESI-MS/MS experiments. NI = Not Identified.
Results and discussion
Methods for the analysis of different sub-classes of sphingolipids from mammalian cells are well established (13, 26–34, 40). Masukawa et. al. developed a reversed-phase (RP) HPLC-APCI-MS method for comprehensively profiling isobaric and isomeric ceramides. However, their method is a qualitative technique for identifying ceramides from the profiles of multimass chromatograms. In addition, the sensitivity of their method requires greater than 5–10 ng of material to be injected on to a column in order to detect the ceramides (40). Likewise, Merril et al. (28) developed excellent methods for quantitating ceramides and sphingomyelins. Methods that are selective but not specific (45) for the quantitation of multi cer-PE species from non-mammalian cells, however, have not been reported.
Ceramide-phosphorylethanolamines can be analyzed by RP-HPLC-ESI-MS/MS as these analytes are soluble in aqueous and protic solvents and form good ion pairs with ammonium salts of formic and acetic acids resulting in greater chromatographic resolution. Therefore we have developed assays based on RP-HPLC that can qualitatively identify cer-PE’s using single ion monitoring-neutral loss (SIM-NL) scans, followed by quantitation by selected reaction monitoring (SRM) of the cer-PE analytes including the isobaric and the isomeric species. More reproducible chromatographic separation, a nominally slow solvent flow-rate of 250 μL/min, and the buffering conditions used in our method aid in attaining the selectivity and sensitivity for quantitating cer-PE.
Identification of cer-PE using RP-HPLC-ESI-MS/MS and neutral loss scanning
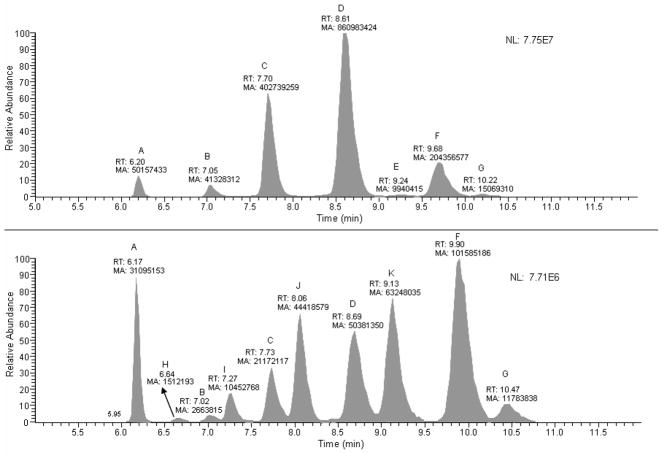
Ceramide-phosphoryethanolamines extracted from wild type Drosophila were identified by single ion monitoring neutral loss scanning (SIM-NL) using a neutral loss of 141.2 Da corresponding to the mass of the phosphorylethanolamine moiety. Parent ion masses, corresponding to the [M+H]+ of different cer-PE molecular ions, of m/z 591.6, 633.5, 661.5, 689.60, 715.7, 717.6 and 719.4 were used in one SIM-NL experiment. As depicted in the upper panel of Fig. 2, seven peaks were observed with retention times between 6.20 and 10.22 min. These peaks correspond to; (A) the internal standard cer-PE d17:1Δ4 c12:0, (B) cer-PE d14:1Δ4 c18:0 and/or cer-PE d16:1Δ4 c16:0, (C) cer-PE d14:1Δ4 c20:0 and/or cer-PE d16:1Δ4 c18:0, (D) cer-PE d14:1Δ4 c22:0 and/or cer-PE d16:1Δ4 c20:0, (E) cer-PE d14:1Δ4 c24:1 and/or cer-PE d16:1Δ4 c22:1 (F) cer-PE d14:1Δ4 c24:0 and/or cer-PE d16:1Δ4 c22:0, and (G) cer-PE d14:0 c24:0 and/or cer-PE d16:0 c22:0. These assigned analytes correspond to cer-PE species that are comprised of an unsaturated sphingoid base backbone (i.e., species containing a Δ4 double bond on the fourth carbon atom starting from the terminal carbon containing an hydroxyl functional group), except for peak G, which corresponds to the cer-PE containing a saturated sphingoid base skeleton. The molecular ion peaks could be unequivocally assigned to their correct molecular structures based on the SRM experiments. In these SRM experiments the highly specific double dehydrated product ion that arises from dissociation of the sphingoid base backbone cores of the cer-PE molecules are observed at the same retention times as the parent ion. In addition, these product ions are unique to the sphingoid base architectures seen within cer-PE.
Figure 2.
A neutral loss-single ion monitoring (NL_SIM) scan profiles. The upper panel displays profiles for the cer-PE’s containing an unsaturated core in their structures, and the lower panel displays the profile for cer-PE’s containing saturated core structure, as well as [M+2+H]+ contributed peaks from the cer-PE’s containing an unsaturated core.
(A) cer-PE d17:1Δ4 c12:0 (B) cer-PE d14:1Δ4 c18:0, (C) cer-PE d14:1Δ4 c20:0 (D) cer-PE d14:1Δ4 c22:0 (E) cer-PE d14:1Δ4 c24:1 (F) cer-PE d14:1Δ4 c24:0 (G) cer-PE d14:0 c24:0 (H) cer-PE d14:0 c16:0 (I) cer-PE d14:0 (J) cer-PE d14:0 c20:0 and (K) cer-PE d14:0 c22:0 respectively.
The results show a direct correlation between the number of methylene units and retention times of the analytes. Unfortunately, monitoring the neutral loss of m/z 141.2 for the phosphorylethanolamine moiety, does not allow molecular species that contain the same number and type of atoms, but differ in the number of CH2 groups, to be distinguished. For example, it cannot be determined whether peak D represents cer-PE d14:1Δ4 c22:0 or cer-PE d16:1Δ4 c20:0 as these molecules have identical retention times under the chromatographic conditions used in this study. In both cases the number and type of atoms in each molecule are the same, with their only difference being the number of carbon and hydrogen atoms within their carbon chains.
To identify cer-PEs containing saturated sphingoid base cores, another SIM-NL experiment was performed in which parent ion m/z’s of 591.5, 607.5, 635.5, 663.5, 691.6, 717.7, 719.7, and 721.4 corresponding to the cer-PE d17:1Δ4 c12:0 (internal standard), cer-PE d14:0 c18:0 and/or cer-PE d16:0 c16:0, cer-PE d14:0 c20:0 and/or cer-PE d16:0 c18:0, cer-PE d14:0 c22:0 and/or cer-PE d16:0 c20:0, cer-PE d14:0 c24:1 and/or cer-PE d16:0 c22:0, cer-PE d14:0 c24:0 and/or cer-PE d16:0 c22:0, and cer-PE d14:0 c24:0 and/or cer-PE d16:0 c22:0, respectively, were monitored. Ten peaks (labeled A-J) were observed with retention times between 6.17 and 10.47 min (Fig. 2, lower panel). Peak A corresponds to the cer-PE d17:1Δ4 c12:0 internal standard, while the rest of the peaks belong to the saturated series of cer-PE’s. Peaks B, C, D, and F in this lower panel of Fig 2 were observed to elute with similar retention times as peaks labeled B, C, D, and F in the upper panel. The peaks in the lower panel are two m/z units higher and have peak areas <5% of their counterparts in the upper panel. Therefore, peaks B, C, D, and F in the lower panel correspond to the [M+H+2]+ species of these molecules, resulting from the C-13 contribution of the unsaturated cer-PE’s, rather than arising from d-saturated sphingoid base cer-PE’s. The remaining peaks in the lower panel (labeled H through K) with m/z values of 607.5, 635.5, 663.5, and 691.6 likely originate from d-saturated cer-PE’s and may be assigned to cer-PE d14:0 c16:0 and/or d16:0 c14:0, cer-PE d14:0 c18:0 and/or d16:0 c16:0, cer-PE d14:0 c20:0 and/or d16:0 c18:0, and d14:0 c22:0 and/or cer-PE d16:0 c20:0, respectively. It is also evident that the d-saturated [M+H]+ and 13C contributed d-unsaturated [M+H+2]+ analyte peaks that have the same m/z values are displayed in pairs, and that d saturated cer-PE’s have higher retention times compared to the corresponding d-unsaturated cer-PE’s. The area of peak F in lower panel is approximately 50% of the same labeled peak in upper panel, suggesting this peak may be a combination of both a saturated sphingoid base cer-PE d14:0 c24:1 and/or d16:0 c22:1 as well as [M+H+2]+ contribution of ca. 5 % from the corresponding unsaturated analyte cer-PE d14:1 c24:0 and/or d16:1 c22:0. Peaks G and A (the internal standard), are used as reference peaks to check the retention times for cross analysis.
Quantitation is truly representative if similar reaction pathway is followed for the analytes and the internal standard
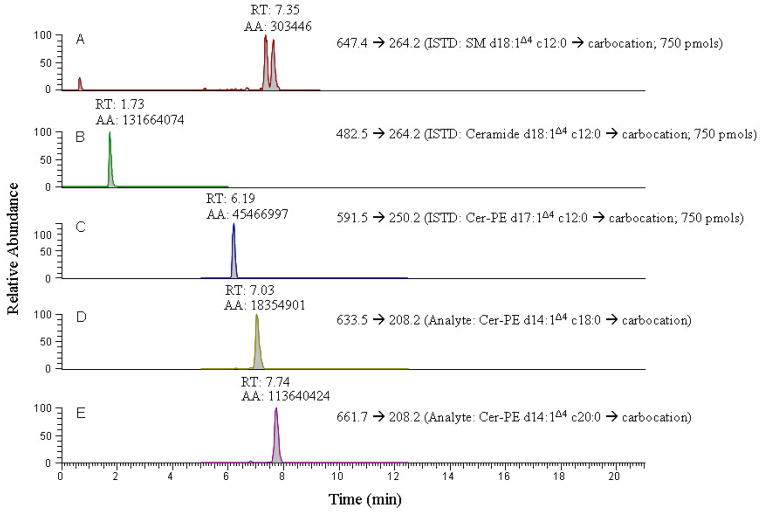
Quantitation of cer-PE’s has previously been accomplished using neutral loss and precursor ion scanning (37, 41–45), and Rao et al., (16) have employed multiple reaction monitoring (MRM) scanning to acquire sphingolipid data within Drosophila. Unfortunately the experimental details provided within this study were limited to the m/z values of the product ion species that were monitored and if they could identify and quantify the presence of either saturated cer-PE’s or unsaturated cer-PE d16:1Δ4 species. Both saturated cer-PE’s and unsaturated cer-PE d16:1Δ4 species are usually present in very low abundance. Furthermore, this study used ceramide d18:1Δ4 c12:0 and SM-d18:1Δ4 c12:0 as the internal standards for analysis of ceramides and cer-PE’s. It is well established that only stable isotope enriched analogs of the analytes or, in the event of their non-availabilty, compounds having minimal physico-chemical differences from potential analytes of interest are to be used as internal standards. It should be noted that even if closely related internal standards of the analytes are used, the quantitative data can be erroneous if different product ion transitions are monitored for the internal standard and the analytes of interest since the magnitude and ionization efficiencies of different product ions formed may vary (see Fig. 3). Optimal quantitative results are obtained if molecular ion intensities are compared for both analytes and their internal standards. However, factors such as the complexity of the sample matrix and the concentration of the analyte of interest require quantitative measurements be based on MS/MS techniques such as SRM, MRM, and NL scanning.
Figure 3.
A comparative extracted ion chromatogram from SRM scans of precursor → double dehydrated carbocation product fragment for the three different internal standards each at a 750 pmol concentration and two cer-PE analytes from drosophila, providing evidence that only cer-PE d17:1Δ4 c12:0 ISTD provides correct quantitation.
The size, composition, and architecture of linear side chains of cer and cer-PE must be considered when developing a quantitative method to measure these molecules. Since differences in their conformations, orientations, geometries and degrees of freedom affect their ionization efficiencies, accurate quantitation of cer and cer-PE can only be obtained if multiple internal standards possessing a broad range of aliphatic chain composition and degrees of unsaturation are used (28). Unfortunately, because of the limited availibility of stable isotope-labeled cer-PE analogues, we used the recently available internal standard cer-PE d17:1Δ4 c12:0 and partly corrected the data for number of carbon atoms variation in the analytes with respect to the internal standard by applying mathematical correction (31) to the quantitative data. If viable internal standards are not available, accurate and precise quantitation can still be performed if the relative response factor (RRF) for analytes can be measured with respect to the internal standard. This requires analyte reference standards, however, the diversity and complexity involved in the biological samples makes obtaining reference standards and multiplex internal standards for every compound either very expensive or impossible.
A specific method for analysis of ceramides using non-polar HPLC-ESI-MS/MS
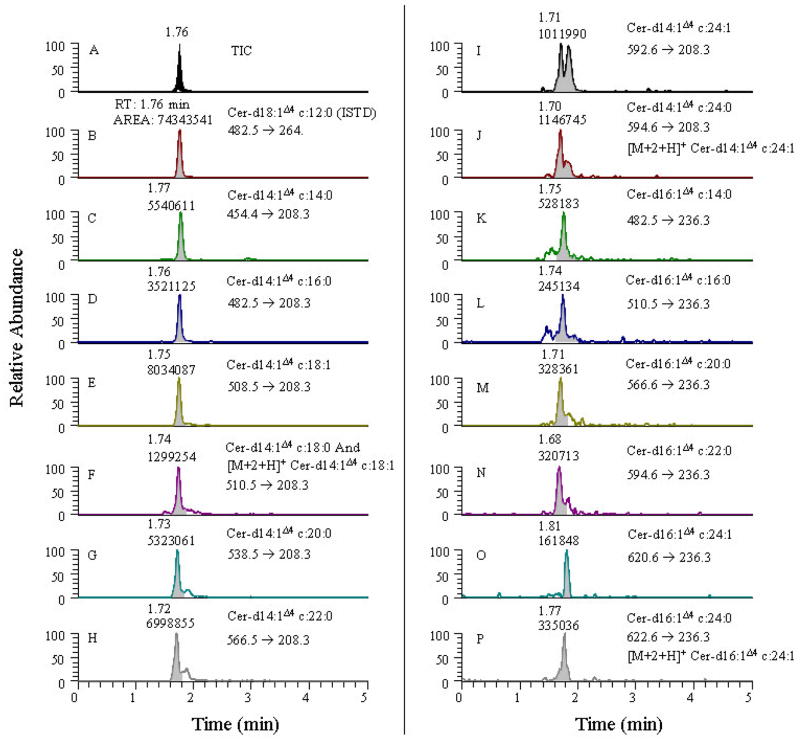
A specific method is able to quantitatively measure the compounds of interest unequivocally without separation in the presence of other impurities and degradation products. As shown in Fig. 4, quantitation of ceramides using non-polar HPLC belongs in this category since all types of ceramide species migrate together due to their strong lipophilic nature. Under the conditions of the non-polar HPLC method developed in this study, each head-group class could not be resolved into individual ceramides. Although reverse phase LC can resolve some individual constituents, the sensitivity is markedly decreased (39, 40, 47). Thus, mass filters are required to identify and quantitate individual ceramide species when using non-polar HPLC, as the use of MS/MS reduces the need for complete chromatographic resolution of each component.
Figure 4.
An extracted ion specific chromatogram from SRM scans of precursor → double dehydrated carbocation product fragment for the ceramides d14:1Δ4 and d16:1Δ4 containing analytes from drosophila. Panel A displays TIC for the ceramide head group. Panels B-P displays extracted ion chromatograms for the ceramide internal standard various other ceramide analytes.
Using mass filters to resolve specific compounds has limitations. Isobaric species that produce similar fragment ions and analytes having the same precursor and product ion transitions (due to similarities in their chemical composition and fragmentation chemistries) cannot be resolved using mass filters. For example, it is difficult to distinguish between d16:1Δ4 c14:1Δ4 and d16:1Δ4 c14:1Δ6 ceramides. In this case, the [M+H]+ of the precursor ion and the product ion transition that is being monitored is a carbocation arising from the sphingoid base core are identical. The only difference between these molecules is in the position of the double bonds present on the fourth and sixth carbon atom of the amino side chain of the two analytes, respectively, which is not distinguishable in the SRM analysis. In addition, these analytes are difficult to resolve using HPLC due to either poor selectivity and/or retentivity (selectivity factor, α and capacity factor, k parameters, etc.) or because of the similarity in their chemical structure and/or physico-chemical properties. In such cases, mass filters have limited utility in measuring individual cer-PE’s. If authentic standard reference analytes are not available and the analytes of interest moves as a single head group then the contribution from isobaric species has to be taken into consideration. However, if good separation can be achieved, interference from isobaric impurities and other close structurally and chemically relating species can be eliminated.
Another problem that arises when measuring closely related species with overlapping m/z values is the [M+1+H]+ and [M+2+H]+ carbon isotopic contributions from one molecular ion species of lower molecular weight to a higher molecular weight ion species. An example of this situation is illustrated in the results from a SRM experiment designed to quantitate various ceramide species having only mono-unsaturated sphingoid base cores in their structures, shown in Fig. 4. The product ion monitored in these cases is the double dehydrated carbocation fragment (see Fig. 1 for the chemical structure). In this example, the parent ions differ by only two mass units between the pair of analytes that contain a saturated or an unsaturated carbon side chain linked to the amino group respectively, as in the case of cer d14:1Δ4 c18:1 (panel E, Fig. 4) and cer d14:1Δ4 c18:0 (panel F, Fig. 4) ions. Here, the area for the peak in panel F is roughly 15% of the corresponding peak in panel E. Although it is possible to correct for the 13C isotopic contribution by calculating the number of carbon atoms from the contributing species if the contributing and receiving species are present in identical amounts, the problem will be manifested if the contributing species is present in a significantly greater abundance compared to the overlapping species receiving the 13C isotopic [M+2+H]+ contribution. The result is vastly erroneous quantitative results for the latter compound. Likewise panel pairs I and J, and O and P in Fig. 4 are similarly related. If the main sphingoid base chain itself is either saturated and/or unsaturated (e.g., cer d14:1Δ4 c18:0 and cer d14:0 c18:0 and in cases where the amido-linked carbon side chains are identical), the monitored product ion will differ by two mass units, and such pairs tend to be resolvable using HPLC and can be readily identified. In our experiments we were not successful in isolating such ceramide species, perhaps owing to their low abundances. Further, if the main sphingoid base core itself differs by the number of –CH2-groups then the m/z values of double dehydrated sphingoid base product ion differs (Fig. 1) significantly. This difference makes the quantitation for such molecular ions simpler as in the case of cer d14:1Δ4 c16:0 (Panel D of Fig. 4) and cer d16:1Δ4 c14:0 (panel K) in which the precursor ions have the same m/z values (i.e., m/z 482.5), while the monitored product ions differ (m/z 208.3 and 236.3, respectively).
Selective method for the analysis of ceramide phosphorylethanolamines using RP-HPLC-ESI-MS/MS in SRM mode
A selective method is the one in which the analytes are well separated and can be measured quantitatively and unequivocally without any bias (46). Although some methods for the identification and quantitation of cer-PE’s are reported in literature almost all of the methods employ either precursor ion scans and/or neutral loss scans (37, 41–45). Precursor ion and NL scanning produce spectra with each spectrum revealing an analyte species containing a common fragment. The use of NL and precursor scanning techniques for analyte quantitation, however, has limitations. Firstly, both the precursor scans and NL scans more often are used for compound screening and identifying groups of individual parent ions belonging to a class containing a common fragment ion, although many laborotories have used these techniques for quantitation. Secondly, as Sullards and Merrill (27), and Han and Gross (30, 31) have noted, sphingolipids cannot be quantitated with an acceptable degree of accuracy using precursor ion scans. They have pointed out that their dissociation rates vary greatly and depend upon the collision energies applied. Lower molecular weight species predominate at lower collision energies while higher molecular weight species predominate at higher collision energies (27). In precursor ion scans and neutral loss scans one cannot tailor the collision energy and other compound specific instrumental parameters such as tube lens voltage for each compound. Hence optimum results for each individual compound cannot be obtained. Thirdly, precursor ion and neutral loss scans have limited dwell time on ions of interest and yield poor sampling efficiency, resulting in, lower sensitivity for the analytes of interest. Finally, precursor scans more often produces broad, tailing peaks. This tailing is due to the inter-scan voltage delay in shuttling between the Q1 and Q3 quadrupoles, which results in a detection time-lag. However, it should also be emphasized that with appropriate gas pressure applied and appropriately adjusted collision energies and tube lenses, and if enough sampling data is acquired for each of all the innumerable analyte peaks which in biological samples more often might not be possible, than some tailing can be reduced in precursor ion scans. All these factors contribute to inaccuracy in the quantitative measurements.
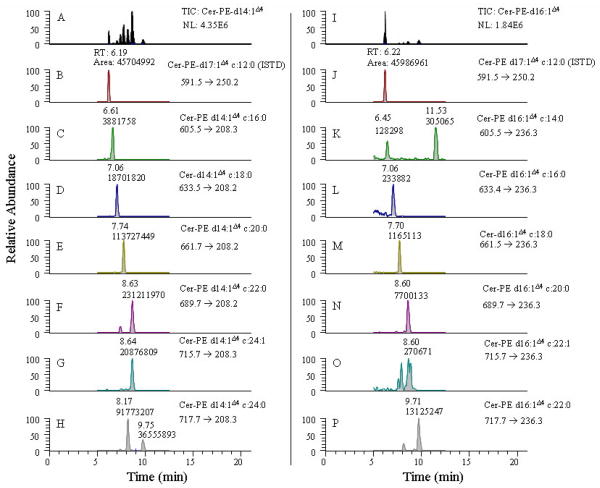
The cers-PE analytes extracted from Drosophila were analyzed using SRM as shown in Fig. 5. After identification of the cers-PE containing saturated and unsaturated sphingoid base cores, the analytes were quantitated using SRM. The SRM conditions were optimized by analysis of a cer-PE d17:1Δ4 c12:0 standard at an HPLC flow rate of 250 μL/min. For all other cer-PE analytes, compound optimization was performed by measuring the m/z value of the cer-PE analytes via neutral loss scans of m/z 141.2 corresponding to loss of the phosphoryl ethanolamine fragment through either direct infusion of cell solution or by reverse phase LC. The optimized parameters were obtained through the combined use of MS, MS/MS, and SRM modes. For the cer-PE’s that could not be measured using NL scanning but were included in the SRM method, the m/z value for the specific analyte transition was calculated based on the exact molecular weights of the precursor and product ions of the most dominating isotopic atoms. Where individual optimized collision energies could not be obtained for an analyte, a universal value is applied that is found to give the most appropriate response. Furthermore, the discrepancies in the actual fractional values of the m/z values of these analytes were adjusted to match the instrument calibration response as observed with other analytes whose MS response had been optimized. Although the product transitions that were monitored in the present experiment are not the top-most product component and this double dehydrated carbo-cationic product fragment ion is supposedly believed to be less sensitive as shown for d18:1Δ4 sphingomyelin species in Fig. 3, nevertheless, its observation indicates good sensitivity in the present assay. The double dehydrated carbo-cationic product ion was monitored in the SRM experiment, because it specifically represents the core chain of the cer-PE species. Less than 10 pmols (ca. 6 ng) of cer-PE internal standard was injected on-column for quantitation. The observation of a peak for cer-PE d16:1Δ4 c16:0 (panel L of Fig. 5) corresponding to approximately 32 pg of lipid analyte from a biological matrix, illustrates the high sensitivity of this method for measuring endogenous lipids.
Figure 5.
An extracted ion selective chromatogram from the SRM scans of precursor → double dehydrated carbocation product fragment for the ceramide phosphorylethanol amine d14:1Δ4 and d16:1Δ4 containing analytes from drosophila. Panel A and I displays TIC for the cer-PE d14:1Δ4 and cer-PE d16:1Δ4 core. Panel B and J displays extracted ion chromatogram for the internal standard. Panels C-H displays extracted ion chromatograms for the core group d14:1Δ4 cer-PE analytes. Panels K-P displays extracted ion chromatograms for the core group d16:1Δ4 cer-PE analytes.
Several SRM peaks corresponding to cer-PE d14:1Δ4 and d16:1Δ4 species extracted from Drosophila were resolved and quantitated using the described method. The results show that cer-PE d14:1Δ4 species are at least ten-fold higher in concentration than the corresponding cer-PE d16:1Δ4 species in the samples measured in this study. While the cer-PE d16:1Δ4 species have similar retention times as cer-PE d14:1Δ4, they are distinguished from each other by monitoring product ions at m/z 208.2 and 236.2 for the cer-PE d16:1Δ4 and cer-PE d14:1Δ4 species, respectively.
Some of the components, such as cer-PE d14:1Δ4c22:0 (panel F) and cer-PE d14:1Δ4c24 (panel H), display multiple peaks within the general retention time range observed for cer-PE’s. (Fig 5). These observations suggest these peaks represent isomers of these compounds. The two peaks observed for cer-PE d16:1Δ4c14:0 (panel K), however, are not isomeric. The peak eluting at 11.53 min is an isobaric peak, as its retention time falls outside of the retention range observed for cer-PE’s, while the peak at 7.45 min is due to cer-PE d16:1Δ4c14:0. A full scan spectrum did not exhibit any observable signals using NL and precursor ion scanning experiments either via direct infusion or HPLC.
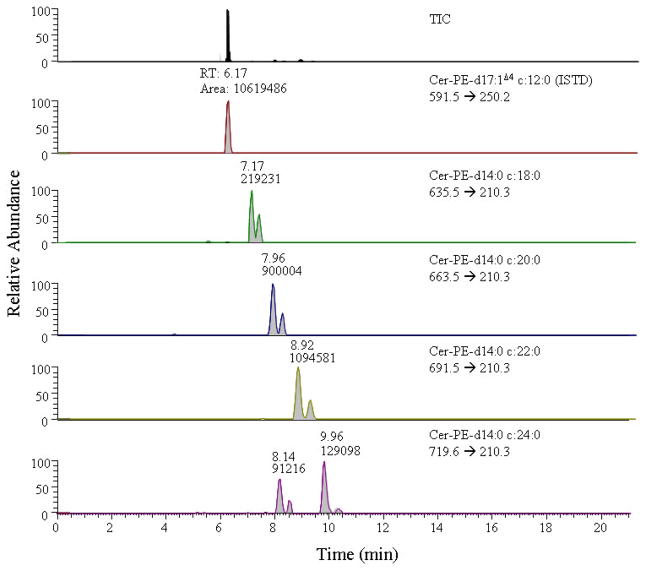
Neutral loss scanning could not determine whether the peaks labeled H to K in Fig. 2 correspond to cer-PE d14:0 and/or cer-PE d16:0 saturated species. The identity of these analytes can only be conclusively established in concordance with NL scanning by performing SRM. Selected reaction monitoring was performed and the precursor ions corresponding to the respective product ion transitions m/z 210 or 238 for the cer-PE d14:0 and/or cer-PE d16:0 saturated core series of analytes, respectively, were monitored. As shown in Fig. 6, only transitions corresponding to cer-PE d14:0 species were observed as no signals corresponding to cer-PE d16:0 analytes were detected. The peaks at retention times of 7.17, 7.96, 8.92, and 9.96 min arise from the transitions m/z 635.5 → 210.3, m/z 663.5 → 210.3, m/z 691.5 → 210.3, and m/z 719.6 → 210.3. These peaks (and peaks I to K and G in Fig. 2, which have the similar retention times) correspond to cer-PE d14:0 c18:0, cer-PE d14:0 c20:0, cer-PE d14:0 c22:0, and cer-PE d14:0 c24:0, respectively. Cer-PE d14:0 c24:0 (panel F, Fig. 6) exhibited two peaks at 8.14 min, which may be due to another isomeric form rather than an isobaric species as the retention times falls within the general elution range of cer-PE analytes. In addition, the doublet observed for these peaks is indicative of the signals that originate from the saturated sphingoid core base series. The sensitivity of the signals for the carbocation obtained from the unsaturated sphingoid base core is consistently higher than that from the product fragment arising from the saturated sphingoid base core, because in the former the carbocation is stabilized due to the allylic nature of the group. Nevertheless, the observance of these transitions, which can measure less than 100 pg of the saturated core series, indicates the potential to assay sub picomolar levels of analytes from biological samples.
Figure 6.
A TIC and an extracted ion selective chromatogram from SRM scans of precursor → double dehydrated carbocation product fragment for the cer-PE d14:0 containing analytes from drosophila.
Conclusion
Most previous methods have utilized NL scans and precursor scans and have primarily used head group losses to identify and quantitate the data as presumably the loss of head group fragment was the only product ion of sufficient abundance that was formed under their MS conditions. A reliable, efficient, and highly sensitive LC-MS2 method using SRM was developed to quantitate cer-PE containing both saturated and unsaturated sphingoid base core from Drosophila, after initially identifying them by NL scanning. For those cer-PE species that could not be observed in NL scanning experiments either due to their low abundances, their molecular formulas were calculated based on their structures, and their decimal weights were fractionally adjusted based on the m/z values observed for the other moderate to high abundance cer-PE species in our study. These low abundant cer-PE species were then included in the SRM method and the instrumental and other compounds specific parameters were adjusted in relation to the observed cer-PE species. The [M+H+2]+ 13C isotopic contributions from unsaturated cer-PE’s to the saturated cer-PE’s were also subsequently corrected. Because the product ion from the sphingoid base backbone is monitored, the resultant MS signal was of sufficient abundance to quantitate many of the cer-PE analytes that are present in amounts as low as few picograms. The efficacy of our method is also demonstrated in the ability to specifically quantitate very low abundance cer-PE’s with saturated sphingoid base cores extracted from Drosophila. To the best of our knowledge, these compounds have not previously been quantitated from Drosophila. Using the analogy as described above the method can easily be translated to identify and quantitate ceramides, sphingomyelins, and other cer-PE’s etc from both insects and mammalian samples.
Table 2.
Retention times and compound specific selected reaction monitoring (SRM) parameters for some of the ceramide phosphoryl ethanolamines analyzed in this study. The methods comprised of three segments, and all the peaks are displayed in the second segment. In each method to attain maximum sensitivity only seven cer-PE were included, in which precursor ion to double dehydrated product ion arising from sphingoid base is monitored.
| Cer-PE Analyte SRM Transitions: | Precursor [M+H]+ → Product ion | Retention Time (min) | Collision Enery (V) | Tube Lens (V) |
|---|---|---|---|---|
| Method 1 for d14:1Δ4 | ||||
| d17:1Δ4 c12:0 (ISTD) | 591.46 → 250.19 | 6.19 | 30 | 94 |
| d14:1Δ4 c16:0 | 605.54 → 208.30 | 6.61 | 29 | 96 |
| d14:1Δ4 c18:0 | 633.48 → 208.20 | 7.03 | 28 | 98 |
| d14:1Δ4 c20:0 | 661.71 → 208.19 | 7.74 | 28 | 98 |
| d14:1Δ4 c22:0 | 689.65 → 208.20 | 8.63 | 29 | 99 |
| d14:1Δ4 c24:1 | 715.67 → 208.29 | 8.64 | 30 | 100 |
| d14:1Δ4 c24:0 | 717.67 → 208.28 | 9.75 | 30 | 94 |
| Method 2 for d16:1Δ4 | ||||
| d17:1Δ4 c12:0 (ISTD) | 591.46 → 250.19 | 6.22 | 30 | 94 |
| d16:1Δ4 c14:0 | 605.54 → 236.30 | 6.45 | 30 | 94 |
| d16:1Δ4 c16:0 | 633.40 → 236.30 | 7.06 | 30 | 94 |
| d16:1Δ4 c18:0 | 661.52 → 236.25 | 7.70 | 30 | 106 |
| d16:1Δ4 c20:0 | 689.68 → 236.28 | 8.60 | 30 | 99 |
| d16:1Δ4 c22:0 | 715.67 → 236.30 | 8.60 | 30 | 94 |
| d16:1Δ4 c22:1 | 717.67 → 236.30 | 9.71 | 30 | 109 |
10 μL of solution was injected by an auto-sampler for LC-ESI-MS/MS experiments.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oskouian B, Saba JD. Death and taxis: what non-mammalian models tell us about sphingosine-1-phosphate. Semin Cell Dev Biol. 2004;15:529–540. doi: 10.1016/j.semcdb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Acharya U, Acharya JK. Enzymes of sphingolipid metabolism in Drosophila melanogaster. Cell Mol Life Sci. 2005;62:128–142. doi: 10.1007/s00018-004-4254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holthius JC, Pomorski T, Raggers RJ, Sprong H, Meer GV. The organizing potential of sphingolipids in intracellular membrane transport. Physiol Rev. 2001;81:1689–1723. doi: 10.1152/physrev.2001.81.4.1689. [DOI] [PubMed] [Google Scholar]
- 4.Dickson RC. Sphingolipid functions in Saccharomyces cerevisiae: comparison to mammals. Annu Rev Biochem. 1998;67:27–48. doi: 10.1146/annurev.biochem.67.1.27. [DOI] [PubMed] [Google Scholar]
- 5.Futerman AH, Hannun YA. The complex life of simple sphingolipids, EMBO. Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hla T. Physiological and pathological actions of sphingosine-1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel S, Milstein S. Exogenous and intracellularly generated sphingosine-1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003;31:1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- 8.Acharya U, Patel S, Koundakjian E, Nagashima K, Han X, Acharya JK. Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science. 2003;299:1740–1743. doi: 10.1126/science.1080549. [DOI] [PubMed] [Google Scholar]
- 9.Barenholz Y, Thompson TE. Sphingomyelin: biophysical aspects. Chem Phys Lipids. 1999;102:29–34. doi: 10.1016/s0009-3084(99)00072-9. [DOI] [PubMed] [Google Scholar]
- 10.Helms J, Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004;5:247–254. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- 11.Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non lipid biochemist. Advan Physiol Edu. 2007;31:5–16. doi: 10.1152/advan.00088.2006. [DOI] [PubMed] [Google Scholar]
- 12.Pyne S, Pyne NJ. Sphingosine-1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merrill AH, Jr, Sullards MC, Wang E, Voss KA, Riley RT. Sphingolipid metabolism: role in signal transduction and disruption by fumonisins. Environ Health Perspect. 2001;109(Suppl 2):283–289. doi: 10.1289/ehp.01109s2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prieschl EE, Baumruker T. Sphingolipids: second messengers, mediators and raft constituents in signaling. Immunol Today. 2000;21:555–560. doi: 10.1016/s0167-5699(00)01725-4. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Rao RP, Cholady TK, Masood MA, Southon E, Zhang H, Berthet C, Nagashim K, Veenstra TK, Tessarolla L, Acharya U, Acharya JK. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J Cell Biol. 2009;184:143–158. doi: 10.1083/jcb.200807176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao RP, Yuan C, Allegood JC, Rawat SS, Edwards MB, Wang X, Merrill AH, Jr, Acharya U, Acharya JK. Ceramide transfer protein function is essential for normal oxidative stress response and lifespan, Proceed. Nat Acad Sci (USA) 2007;104:11364–11369. doi: 10.1073/pnas.0705049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fyrst H, Zhang X, Herr DR, Byun HS, Bittman R, Phan VH, Harris GL, Saba JDJ. Identification and characterization by electrospray mass spectrometry of endogenous Drosophila sphingadienes. J Lipid Res. 2008;49:597–606. doi: 10.1194/jlr.M700414-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Dennis RD, Wiegandt H. Glycosphingolipids of the invertebrata as exemplified by a cestode platyhelminth, Taenia crassiceps, and a dipteran insect, Calliphora vicina. Adv Lipid Res. 1993;26:321–351. [PubMed] [Google Scholar]
- 19.Hildebrandt H, Jonas U, Ohashi M, Klaiber I, Rahmann H. Direct electrospray-ionization mass spectrometric analysis of the major ganglioside from crucian carp liver after thin layer chromatography, Comp. Biochem Physiol B. 1999;122:83–88. doi: 10.1016/s0305-0491(98)10152-9. [DOI] [PubMed] [Google Scholar]
- 20.Kolesnick R, Fuks Z. Ceramide: a signalfor apoptosis or mitogenesis? J Exp Med. 1995;181:1949–1952. doi: 10.1084/jem.181.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huwiler AT, Kolter J, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000;1485:63–99. doi: 10.1016/s1388-1981(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 22.Hannun YA, Obeid LM. The ceramide–centric universe of lipid mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 23.Ma D. Lipid mediators in membrane rafts are important determinants of human health and disease. Appl Physiol Nutr Metab. 2007;32:341–350. doi: 10.1139/H07-036. [DOI] [PubMed] [Google Scholar]
- 24.Acharya JK, Dasgupta U, Rawat SS, Yuan C, Sanxaridis PD, Yonamine I, Karim P, Nagashima K, Brodsky MH, Tsunoda S, Acharya U. Cell non-autonomous function of ceramidase in photoreceptor homeostasis. Neuron. 2008;57:69–79. doi: 10.1016/j.neuron.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masood MA, Xu X, Acharya JK, Veenstra TD, Blonder J. Enhanced detection of sphingoid bases via divalent ruthenium bipyridine complex derivatization and electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:495–502. doi: 10.1021/ac8019043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullards MC, Allegood JC, Kelly S, Wang E, Haynes CA, Park H, Chen Y, Merrill AH., Jr Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography-tandem mass spectrometry: “Inside-Out” sphingolipidomics. Methods Enzymol. 2007;432:83–115. doi: 10.1016/S0076-6879(07)32004-1. Chapt. 4. [DOI] [PubMed] [Google Scholar]
- 27.Sullards MC, Merrill AH., Jr Analysis of sphingosine1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Sci STKE. 2001;67:1–11. doi: 10.1126/stke.2001.67.pl1. [DOI] [PubMed] [Google Scholar]
- 28.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–224. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Sullards C. Analysis of sphingomyelin, glucosylceramide, ceramide, sphingosine, and sphingosine1-phosphate by tandem mass spectrometry. Methods Enzymol. 2000;312:32–45. doi: 10.1016/s0076-6879(00)12898-8. [DOI] [PubMed] [Google Scholar]
- 30.Han X. Characterization and direct quantitation of ceramide molecular species from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem. 2002;302:199–212. doi: 10.1006/abio.2001.5536. [DOI] [PubMed] [Google Scholar]
- 31.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 32.Liebisch GW, Reil M, Trümbach B, Arnecke R, Olgemöller B, Roscher A, Schmitz GJ. Quantitative measurement of different ceramide species from crude cellular extracts by electrospray ionization tandem mass spectrometry (ESI-MS/MS) J Lipid Res. 1999;40:1539–1546. [PubMed] [Google Scholar]
- 33.Adams J, Ann Q. Structure determination of sphingolipids by mass spectrometry. Mass Spectrom Rev. 1993;12:51–85. [Google Scholar]
- 34.Watson AWJ. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006;47:2101–2111. doi: 10.1194/jlr.R600022-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Eroglu C, Brugger B, Wieland F, Sinning I. Glutamate-binding affinity of Drosophila metabotropic glutamate receptor is modulated by association with lipid rafts. Proc Natl Acad Sci. 2003;100:10219–10224. doi: 10.1073/pnas.1737042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakowicz JR. Principles of Fluorescence Spectroscopy. Kluwer Academic/Plenum; New York: 1999. [Google Scholar]
- 37.Rietveld A, Neutz S, Simons K, Eaton S. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J Biol Chem. 1999;274:12049–12054. doi: 10.1074/jbc.274.17.12049. [DOI] [PubMed] [Google Scholar]
- 38.Fyrst H, Herr DR, Harris GL, Saba JD. Characterization of free endogenous C14 and C16 sphingoid bases from drosophila melanogaster. J Lipid Res. 2004;45:54–62. doi: 10.1194/jlr.M300005-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Mano M, Oda Y, Yamada K, Asakawa N, Katayana K. Simultaneous quantitative determination method for sphingolipid metabolites by liquid chromatography/ionspray ionization tandem mass spectrometry. Anal Biochem. 1997;244:291–300. doi: 10.1006/abio.1996.9891. [DOI] [PubMed] [Google Scholar]
- 40.Masukawa Y, Tsujimura H, Narita H. Liquid chromatography-mass spectrometry for comprehensive profiling of ceramide molecules in human hair. J Lipid Res. 2006;47:1559–1571. doi: 10.1194/jlr.D600007-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Moreau RA, Young DH, Danis PO, Powell MJ, Quinn CJ, Slawecki K, Beshah RA, Dilliplane RL. Identification of ceramide-phosphorylethanolamine in Oomycete plant pathogens: Pythium ultimum, Phytophthora infestans, and Phytophthora capsici. Lipids. 1998;33:307–317. doi: 10.1007/s11745-998-0210-1. [DOI] [PubMed] [Google Scholar]
- 42.Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X. Profiling membrane lipids in plant stress responses. J Biol Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 43.Welti R, Mui E, Sparks A, Wernimont S, Isaac G, Kirisits M, Roth M, Roberts CW, Botté C, Maréchal E, Mcleod R. Lipidomic analysis of Toxoplasma gondii reveals unusual polar lipids. Biochem. 2007;46:13882–13890. doi: 10.1021/bi7011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brugger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proceed Nat Acad Sci (USA) 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu F-Fu, Turk J. Structural determination of sphingomyelin by tandem mass spectrometry with electrospray ionization. Am Soc Mass Spectrom. 2000;11:437–449. doi: 10.1016/S1044-0305(99)00150-6. [DOI] [PubMed] [Google Scholar]
- 46.http://www.fda.gov/cder/guidance/
- 47.Cremesti AE, Fischl AS. Current methods for the identification and quantitation of ceramides: an overview. Lipids. 2000;35:937–945. doi: 10.1007/s11745-000-0603-1. [DOI] [PubMed] [Google Scholar]