Abstract
Rapamycin was administered in food to genetically heterogeneous mice from the age of 9 months and produced significant increases in life span, including maximum life span, at each of three test sites. Median survival was extended by an average of 10% in males and 18% in females. Rapamycin attenuated age-associated decline in spontaneous activity in males but not in females. Causes of death were similar in control and rapamycin-treated mice. Resveratrol (at 300 and 1200 ppm food) and simvastatin (12 and 120 ppm) did not have significant effects on survival in male or female mice. Further evaluation of rapamycin’s effects on mice is likely to help delineate the role of the mammalian target of rapamycin complexes in the regulation of aging rate and age-dependent diseases and may help to guide a search for drugs that retard some or all of the diseases of aging.
Keywords: TOR, Rapamycin, Life span, Resveratrol
AGENTS that can extend the life span of mice are of interest for two reasons: they can provide new models of delayed aging to teach us more about what controls aging rate and how aging leads to disease; and in addition, they serve as a first step toward eventual development of pharmaceuticals to slow aging and retard diseases in humans. The National Institute on Aging Intervention Testing program (ITP) has previously reported significant increases in life span caused by aspirin and nordihydroguaiaretic acid in male mice (1) and by rapamycin in both male and female mice (2). The design of the ITP (3) emphasizes the use of genetically heterogeneous mice to mitigate against idiosyncrasies that can complicate interpretation of data from a single inbred or F1 hybrid stock and includes parallel replication of protocols at three sites, the University of Texas (UT), University of Michigan (UM), and The Jackson Laboratory (TJL), with standard operating protocols that attempt to reproduce key elements of the environmental conditions at each site. Sufficient numbers of mice are used in each yearly cohort to give more than 80% power to detect an increase or decrease of 10% in mean life span, with respect to controls of the same sex, even if only two of the three sites can contribute data to the pooled analysis.
Our previous report (2) showed significant increases in longevity in male and female mice exposed to rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR) protein kinase, from 20 months of age (Cohort 2005). The effects were seen at each site evaluated independently. Rapamycin led to an increase in the fraction of mice alive at the 90th percentile survival age, taken as an index of maximal life span. The report also presented preliminary results from a separate group of mice (Cohort 2006) that had received rapamycin from 9 months of age, and which had reached the median survival age at the time the data were analyzed. In the current report, we now present an analysis of the completed data set from the mice in Cohort 2006, showing extension of median and maximal life span in mice given rapamycin from 9 months of age, and comparing the effect of rapamycin with two other drugs of interest, resveratrol, a polyphenol with pleiotropic effects, and simvastatin, a representative statin that in humans modulates lipid profiles by lowering low-density lipoproteins (LDL cholesterol).
METHODS
Mice
The test populations were produced by a cross between two different F1 parents: (BALB/cByJ × C57BL/6J)F1 mothers (JAX stock 100009) and (C3H/HeJ × DBA/2J)F1 fathers (JAX stock 100004). These were referred to as UM-HET3, reflecting their original development at the UM as a genetically heterogeneous population for gene mapping. Each mouse in the test population was thus genetically unique, but shared half of its (nuclear) genome with every other mouse. Mice were bred over a period of 6–8 months at each site, and each cage of weanlings was assigned to one of the test groups (control or drug treatment) by use of a random number table. A written protocol was used by each site that included details of husbandry, including source and amount of bedding; cage ventilation; frequency of cage changing; insertion of electronic identification devices at age 42 days; and other details of husbandry. Each colony was tested for pathogen status as described (3) at 3-month intervals; all such tests were negative for Cohorts 2005 and 2006. Mice were housed at initial densities of three per cage (males) or four per cage (females). Mouse chow (Purina 5LG6) containing test agents, as well as control chow without drugs, was prepared at one site (TestDiet, Richmond, IN) and shipped to each of the three test sites at intervals of approximately 4 months. Mice were inspected daily. If a cage in which mice had incurred serious bite wounds was found, all mice in the cage were humanely euthanized and considered as censored at that age for survival statistics. Mice were also removed from the population if they died as a cause of an experimental accident (e.g. cage flooding), if their ID chip was lost or became dysfunctional, or if they were exposed to an experimental procedure (eg, blood drawing for determination of drug levels in serum) to which the remainder of the population had not been exposed. Of the 2173 mice initially entered into Cohort 2006, 125 (6%) were removed from the longevity group for one of these reasons, including 53 at TJL, 25 at UM, and 47 at UT. At the time of analysis (May 12, 2010), four mice (0.2%) were still alive, all at TJL: three mice in the rapamycin group, and one in the high dose simvastatin group, at ages 1277–1308 days.
As reported previously (2), the three test sites differed in the source of mouse chow provided to the mothers of the test mice and also in the source of mouse chow provided to the test mice before the introduction of drug-containing food. Although each of these diets conformed to the National Institutes of Health–31 standard, it is possible that these variations in early diet history may have contributed to the differences among sites in body weight trajectories, with both male and female mice at UM lighter in weight than mice at the other two sites (3), and perhaps contributed as well to intersite differences in survival rates.
Table 1 lists the agents used for Cohort 2006, together with the suppliers, age of initial exposure to the drug, and the doses used. As in our previous report (2), rapamycin was given in an encapsulated form that is water-soluble only in nonacidic conditions, to protect it from release and degradation in the stomach before its entry into the small intestine.
Table 1.
Sources and Doses of Test Agents
| Dose |
|||||
| Test agent | Source | Age at initiation (mo) | mg/kg food | mg/kg mouse weight per day | mg per mouse per day |
| Rapamycin | LC Labs; Southwest Research Institute | 9 | 14 | 2.24 | 0.067 |
| Simvastatin high | Merck | 10 | 120 | 20 | 0.60 |
| Simvastatin low | Merck | 10 | 12 | 2 | 0.06 |
| Resveratrol | Orchid/Lalilabs | 12 | 300 | 50 | 1.5 |
| Resveratrol | Orchid/Lalilabs | 12 | 1200 | 200 | 6 |
Note: Calculation of dose per mouse and dose per kg mouse body weight per day assume that an average mouse weighs 30 g and consumes 5 g of mouse diet per day. The rapamycin was given in encapsulated form as described in (2).
Activity Testing
Approximately 100 control mice (half males) at each site, plus approximately 50 mice in each drug-exposed group, were evaluated at 7 months and then (for survivors) again at 18 months for several indices of spontaneous in-cage activity, using a computer-controlled Micromax apparatus (Accuscan, Columbus, OH). Mice were removed from their home cage at approximately 8:00 AM and placed individually in fresh cages in the testing room. Laser light (at a low intensity, which does not harm the mice) was detected by sensors outside the cage to count the number of times in which a mouse interrupts the laser beam. The mice were left in the chamber for 50 hours, with free access to food and water and the same light–dark cycle to which they were accustomed in their home cages. The analysis system provided measures of total number of beam breaks over arbitrarily small time windows; we typically tabulated the data using 1-hour windows. The data were also analyzed to reveal the proportion of activity that took place with lights on or off, and the proportion of activity attributable to stereotypic movement (ie, movement in place) versus ambulatory movement. The data at age 7 months provided a baseline for each mouse to permit calculation of “change scores” at the later age, which are taken as the best estimate of age effect for each individual mouse. The tested mice were included in the populations evaluated for longevity effects.
Necropsies and Histopathology
Each mouse in the study was examined at least once each day. The date of death was recorded for each mouse found dead. In other cases, mice that were deemed to be so sick that survival for more than another 48 hours is considered unlikely were euthanized; the date of euthanasia is taken as the best estimate of the date of natural death. This decision is made based on a symptom checklist that is used at each site. Specifically, a mouse was considered severely moribund if it exhibited more than one of the following clinical signs: (a) inability to eat or to drink; (b) severe lethargy, as indicated by reluctance to move when gently prodded with a forceps; (c) severe balance or gait disturbance; (d) rapid weight loss over a period of 1 week or more; or (e) an ulcerated or bleeding tumor. This symptom checklist complied with the rules of each of the three institutions related to humane treatment of mice and was also consistent with the experimental goals. Each mouse found dead, or euthanized, was retained for possible inclusion in a necropsy study. Incisions were made in the cranium, thorax, and abdomen, and the entire body was immersed in 10% neutral buffered formalin and kept in a sealed container before transfer to the pathology unit after an interval of up to 4 years.
Among the mice in Cohort 2006, 110 were evaluated for end-of-life pathology: approximately 9 males and 10 females, from each of the three sites, from the control and rapamycin-treated populations, selected from each site by use of a random number table. Cause of death was inferred, where possible, based on gross evaluation, followed by histopathologic examination of a standard set of tissues from each mouse by an experienced veterinary pathologist. Tumors were deemed the cause of death based on tumor type, size, number, and distribution. Cause of death for mice with inflammatory or degenerative lesions was based on the location and severity of the lesions and the likelihood that such lesions were severe enough to cause morbidity and mortality. Many animals had small localized tumors and various degenerative lesions, which were deemed unlikely to have contributed to their death.
Statistical Methods
For each question of interest, the initial analysis evaluated the null hypothesis that each of the six treatment groups (rapamycin, two doses of simvastatin, two doses of resveratrol, and controls) had the same level of the test variable. These initial analyses pooled data from all three sites to provide maximal statistical power. Males and females were evaluated separately. For survival data, the initial comparison used the log-rank test; for weight and activity data, the initial test was by analysis of variance (ANOVA). When the initial test refuted the null hypothesis at p = .05, we then followed up with tests comparing each drug-treated group, individually, to the control population, again pooling across sites and for each gender separately. Log-rank tests for effects on survival included mice removed from the study (described above), as lost to follow-up at the age of removal; these mice were not included in estimates of median life span nor in calculations of 90th percentile survival.
RESULTS
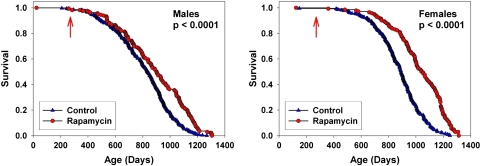
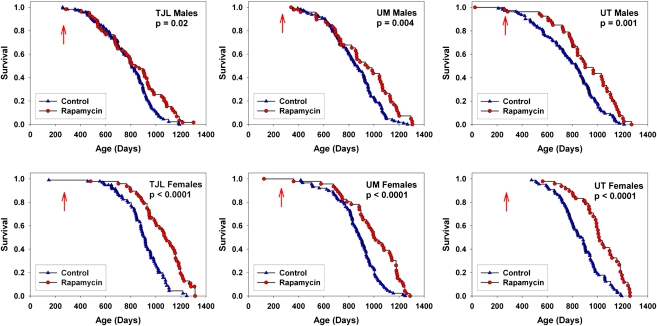
We have previously reported (2) that male and female UM-HET3 mice given rapamycin from 20 months of age had longer life spans than controls, and that rapamycin administered from 9 months of age led to lower mortality when evaluated at the median age for control survival. At the current analysis point, the groups of mice exposed to rapamycin from 9 months (Cohort 2006) had reached the point where 99.8% of the animals died, and no animal remained alive at an age younger than the 99th percentile for control group survival. Rapamycin was found to lead to improved survival in both males and females when pooling across test sites (Figure 1), and to significant effects at each test site considered separately (Figure 2). Table 2 presents calculated values for median age and 90th percentile age for each group. For males, rapamycin led to an increase of 10% in median age, averaged across the three sites, and an increase of 16% in the 90th percentile age. For females, the corresponding values were 18% for median, and 13% for 90th percentile ages.
Figure 1.
Survival curves comparing control to rapamycin-treated mice, pooled across sites. The p values reflect outcome of stratified log-rank test. The arrows at 270 days indicate the age at which rapamycin treatment was initiated.
Figure 2.
Survival curves for control and rapamycin-treated mice, each site plotted individually. The p values reflect outcome of log-rank tests. The arrows at 270 days indicate the age at which rapamycin treatment was initiated.
Table 2.
Statistical Estimates of Survival Differences Between Control and Rapamycin-Treated Mice
| TJL male | UM male | UT male | Mean male | TJL female | UM female | UT female | Mean female | |
| Control, median | 800 | 851 | 780 | 889 | 891 | 843 | ||
| Rapamycin, median | 841 | 932 | 888 | 1077 | 1008 | 1006 | ||
| % Change | 5 | 10 | 14 | 10 | 21 | 13 | 19 | 18 |
| Control, P90 | 1,002 | 1,061 | 985 | 1,064 | 1,061 | 1,101 | ||
| Rapamycin, P90 | 1,155 | 1,211 | 1,167 | 1,220 | 1,211 | 1,204 | ||
| % Change | 15 | 14 | 18 | 16 | 15 | 14 | 9 | 13 |
Note: Estimates of median survival age or (P90) age at which 90% of the mice had died excluding mice removed from the study as defined in the Methods section. Percent change calculated as (rapamycin − control)/(control). Mean values are averages of the values calculated for each of the three test sites. UT = University of Texas; UM = University of Michigan; TJL = The Jackson Laboratory.
Inferences about maximal survival, that is, survival to exceptional ages were evaluated by the test of Wang and colleagues (4) and found to be significant both for the pooled data sets and for each combination of gender and site considered separately. For males, 3% of the controls and 24% of the rapamycin-treated mice were alive at the age of 90% mortality. For females, 3% of controls and 25% of rapamycin-treated mice were alive at the 90th percentile point of the joint survival distribution. By Fisher’s exact test, these proportions differ at p < .001 for the pooled distributions and for males and females at each site considered individually, except that p < .002 for the males and females at TJL. We conclude that rapamycin increases life span in male and female mice of a genetically heterogeneous stock and does so reproducibly at each of the three test sites.
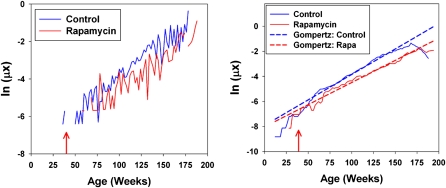
Figure 3 shows calculated mortality risks as a function of age for control and rapamycin groups, combining across sites and gender; the left panel shows the raw mortality plots for deaths pooled into 2-week intervals, and the right panel shows the smoothed plots with fitted Gompertz lines superimposed. Visual inspection suggests a right-shift in the mortality trajectory caused by rapamycin treatment such that treated animals experience a roughly 20-week delay in the onset of senescent mortality. Rapamycin treated mice also have significantly lower intercept values (2.3 × 10−5 vs 2.6 × 10−5 for controls). These observations would be consistent with the idea that rapamycin acts rapidly to reduce age-specific mortality, but the small number of observed deaths immediately following treatment make it impossible to test the hypothesis directly. Although there is evidence that rapamycin treatment also alters the rate of change in mortality with age (0.0050 vs 0.0058 in rapamycin and control cohorts, respectively), this result should be interpreted with caution because significant differences in the sizes of the experimental and control cohorts may differentially bias early-life mortality estimates and skew measures of mortality slopes (5).
Figure 3.
Raw (left) and smoothed (right) plots of the logarithm of the mortality risk for control and rapamycin-treated mice as a function of age. Data have been pooled across gender and test site. Dashed diagonal lines on the right panel indicate fits to the Gompertz model. Arrows at 39 weeks indicate start of rapamycin exposure.
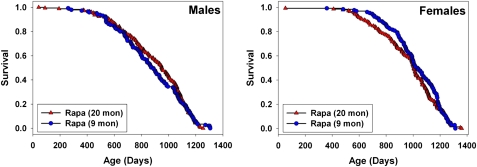
Figure 4 presents a graphical comparison between the results of the current study, and the results of the study comparing mice exposed to rapamycin from 20 months of age. The two data sets were not produced simultaneously, but they do represent work done using the same conditions of drug preparation, diet, water source, housing, and genetic stocks at the same three sites, with only a 1 year lag between start dates. For male mice, starting rapamycin at 9 months rather than at 20 months did not lead to any improvement in survival; the two cohorts were not significantly different by log-rank test, at p = .74. For female mice, there is a suggestion that earlier exposure to rapamycin may have led to some slight decline in mortality risk before ∼1000 days of age, but the log-rank test is not significant (p = 0.31). The Wilcoxon–Breslow test, which does not assume that the difference in risk between the test groups is constant at all ages and which gives more weight to earlier deaths, was also nonsignificant for females, at p = 0.09 (two tailed). It will be of interest to determine if gender differences in rapamycin effect are seen using higher or lower doses of this drug, and to see if males and females differ in the biochemical or physiological responses to rapamycin treatment.
Figure 4.
Comparison of survival for rapamycin administered from 9 or from 20 months of age in separate yearly cohorts, pooled across sites. See text for discussion of statistical significance tests. Data for the mice exposed to rapamycin from 20 months of age are from Harrison and colleagues (2).
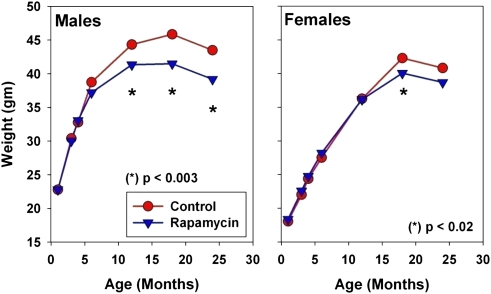
Figure 5 shows mean body weight values for control and drug-treated mice (Cohort 2006) for each sex. Mice given rapamycin from 9 months of age are lighter in weight than controls; the effect is significant for males at 12, 18, and 24 months of age, but in females only at 18 months. The effect in males is less than 10% of the control weight at all ages, and less than 6% in females.
Figure 5.
Body weight values for control mice and for mice exposed to rapamycin from 9 months of age. The p values reflect t tests, pooling across sites, for the hypothesis that rapamycin has no effect on body weight.
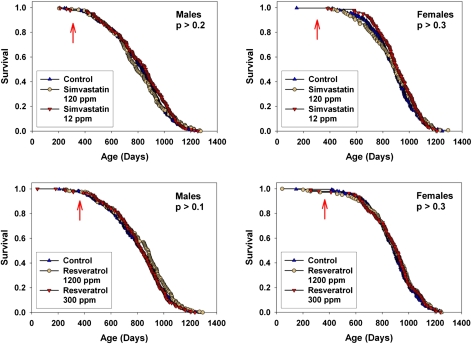
Other mice in Cohort 2006 were exposed either to simvastatin (at 12 or 120 ppm) or to resveratrol (at 300 or 1200 ppm) from the age of 10 or 12 months, respectively. Figure 6 shows the survival curves, for data pooled across sites, at each combination of dose and sex. Neither of these drugs affected survival at either high or low doses in either sex. Nor did either of the drugs lead to a significant improvement in survival at any one of the test sites considered separately (not shown). The doses of resveratrol used were twofold and eightfold higher than those used for the study showing a beneficial effect of resveratrol on median survival in C57BL/6 mice on a high-fat diet (6). Supplementary Figure 1 shows levels of resveratrol found in the blood of UM-HET3 mice consuming 300 or 1200 ppm resveratrol, as tested between 9 AM and 11 AM; resveratrol was detected in three of the five mice on the low dose, and in all five of the mice consuming the higher dose of resveratrol. Supplementary Figure 2 shows that our preparation of simvastatin, at 120 ppm, was effective in mitigating the severity of bacterial infection in 18 month old BALB/c mice. Neither resveratrol nor simvastatin had a significant effect on weight in males or females at any dose tested, pooling across test sites, when evaluated at 18 or 24 months (data not shown.).
Figure 6.
Survival curves for mice exposed to simvastatin from 10 months of age (top), or to resveratrol from 12 months (bottom). Data are pooled across sites. Males are shown in the left panels, and females in the right panels. Each curve shows results for both high and low doses. The p values reflect log-rank tests, stratified by test site. None of the agents had a significant effect on life span, compared with control mice, at any dose tested. The arrows indicate the age at which the drug treatment was initiated.
Fifty-two males and 58 females (8–10 controls and 8–12 rapamycin-treated mice, of each sex, from each site) were used for a study of terminal pathology. The mice submitted for pathology were selected by a random number procedure, excluding only those animals that died at an age of less than 12 months. Of these 110 mice, 17 (9 controls, 8 rapamycin treated) were found to have undergone severe autolysis and were not processed for histopathology. The pathologist (Wilkinson) was able to infer a most likely cause of death for 90 cases (46 controls and 44 rapamycin) and was unable to assign a cause of death in the 3 remaining cases. Table 3 summarizes the cause of death diagnoses. The most common causes of death among controls were lymphoma, hemangiosarcoma, and lung carcinoma, which together accounted for 70% of the cases. These three diagnoses were also the most common in the rapamycin-treated mice, accounting for 66% of the diagnoses. Lung carcinoma was somewhat more common among the rapamycin mice (34% vs 15% for controls, p = .04), and hemangiosarcoma and lymphoma were slightly more common among controls (not significant: p = 0.33 and p = .1, respectively). There were no significant differences between controls and rapamycin-treated mice in the incidence of adrenal degeneration (19% of rapamycin mice, vs 7% of controls, p = .07), adrenal hyperplasia, myocardial degeneration, myocardial fibrosis, skeletal atrophy, pulmonary adenoma, pulmonary adenocarcinoma (fatal combined with nonfatal cases), endometrial hyperplasia (in females), or testicular atrophy (in males). Eleven percent of the rapamycin mice, and 7% of the controls had a non-neoplastic illness as the likely cause of death. Table 3 also shows the mean age at death for each of the diseases found in at least two mice of each kind. Although the amount of data is limited, there is no evidence to suggest that rapamycin accelerates any specific form of neoplasia. In summary, rapamycin, though it delayed death, did not lead to a dramatic change in the range of lethal or nonlethal illnesses found among these mice once they did die.
Table 3.
Apparent Cause of Death in Control and Rapamycin-Treated Mice
| Number of cases |
Percent of cases |
Mean age at death |
||||
| Diagnosis | Control | Rapamycin | Control | Rapamycin | Control | Rapamycin |
| Adrenal carcinoma | 2 | 4 | ||||
| Cardiac fibrosis | 1 | 2 | ||||
| Cardiomyopathy | 1 | 2 | ||||
| Fibrosarcoma | 3 | 1 | 7 | 2 | ||
| Hemangiosarcoma | 10 | 6 | 22 | 14 | 884 | 890 |
| Hydronephrosis | 1 | 2 | ||||
| Liposarcoma | 1 | 2 | ||||
| Liver carcinoma | 2 | 4 | 4 | 9 | 841 | 1006 |
| Lung carcinoma | 7 | 15 | 15 | 34 | 755 | 1004 |
| Lymphoma | 15 | 8 | 33 | 18 | 920 | 951 |
| Mammary carcinoma | 4 | 2 | 9 | 5 | 826 | 1142 |
| Melanoma | 1 | 2 | ||||
| Mouse urinary syndrome | 1 | 2 | ||||
| Nephritis | 1 | 2 | ||||
| Septicemia | 1 | 2 | ||||
| Skin carcinoma | 1 | 2 | ||||
| Thymoma | 1 | 2 | ||||
| Thyroid carcinoma | 1 | 2 | ||||
| Total diagnosable cases | 46 | 44 | ||||
Note: Mean age at death is calculated for those diagnostic categories with at least two cases in each of the two groups of mice.
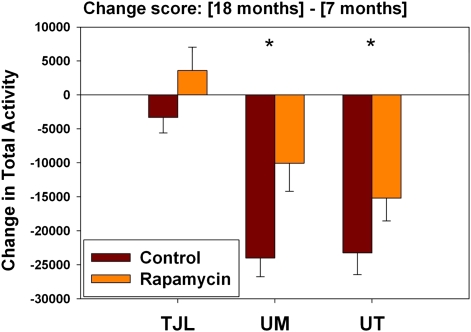
A subset of mice in each test group was tested at 7 months of age for spontaneous activity in a standard mouse cage over a 50-hour period. Most of these individuals were then retested at 18 months to provide a change score, corresponding to the difference in activity at the two ages for each mouse. This age effect was significant in both males and females, but the effect differed greatly in magnitude from site to site. We therefore used a two-factor ANOVA (site and treatment as predictors of activity change score), taking the significance of the treatment effect as our indicator of whether intervention had an effect on activity levels. This treatment effect was significant for males (p = .007) but not for females (p = .42). Including an interaction term (Site × Treatment) in the ANOVA did not alter the significance of the treatment effect in either sex. We therefore evaluated effects of each agent, compared with controls, in males only. Only rapamycin had a significant effect on total activity change scores (p = .003). Because the change scores were not normally distributed, we also used the nonparametric Wilcoxon rank sum test, and again found a significant effect for rapamycin compared with controls (p = .003). Figure 7 shows age effects on the total activity change score for the control and rapamycin males at each site. Age led to a decline in activity levels at each site, but these effects at UM and UT were much greater than at TJL (24,000 vs. 23,000 vs. 3,000 beam breaks, respectively), for reasons we do not understand. Rapamycin reduced these change scores by 58% at UM and by 35% at UT, and completely reversed the weaker trend seen at TJL, leading to a small increase in the number of beam breaks at the later age. When males at each site were evaluated independently, the effects were significant at UM and UT (p = .004 and p = .04, respectively), but not at TJL (p = 0.15). Rapamycin did not lead to a significant decline in activity change scores in female mice, either in the pooled data set or at any site considered separately, nor did simvastatin or resveratrol alter activity change scores at any dose in either sex (not shown). These data suggest that rapamycin may retard age effects on activity in males but are inconsistent and far from conclusive.
Figure 7.
Age effects on total activity in male mice, comparing control to rapamycin-treated mice. The y-axis shows change score, calculated as (activity at 18 months) minus (activity at 7 months in the same mouse). A negative score indicates that the mouse was less active at 18 than at 7 months. (For comparison, the number of beam breaks at 7 months of age for males was approximately 45,000 at The Jackson Laboratory [TJL], 75,000 at University of Michigan [UM], and 71,000 at University of Texas [UT].) The bars show mean and SEM for the change scores. Number of mice tested: 35, 43, and 43 controls and 19, 19, 24 rapamycin-treated mice at TJL, UM, UT, respectively. Asterisks indicate a significant difference between control and rapamycin males at p = .004 at UM and p = .04 at UT.
We used the same data set to evaluate the ratio of daytime (ie, “lights on”) to nighttime activity. Young control mice are more active at night than during the day: the day:total ratio was 0.47 in males and 0.45 in females. In male mice, aging from 7 to 18 months diminishes this ratio to 0.42 (p = .0005 by paired t test); the amount of daytime activity diminishes more rapidly than nighttime activity over this 11-month period. The age effect, for males, is seen at all three sites, with values of 0.03, 0.06, and 0.05 for TJL, UM, and UT, respectively. Rapamycin did not have a significant effect on this day:total ratio in pooled males or at any one of the test sites, but the age effect was significantly diminished by resveratrol at both the high dose (p = .003 for pooled males, and p = .02 and .01 for TJL and UM, respectively) and at the low dose for this agent (p = .03 for pooled males). Neither dose of simvastatin had a significant effect on the day:total ratio. Age also diminished the day:total ratio in females, but not to a significant extent in the pooled data set. Of the drugs tested, only high-dose resveratrol had a significant effect on the day:total ratio in females (p = .04 in pooled females).
DISCUSSION
The mTOR enzyme, a protein kinase, helps to integrate cellular activities in responses to nutrients, stress, and extracellular signals, including hormones and locally produced growth factors (7,8). Involvement of mTOR in regulation of aging was suggested by evidence that impairment of mTOR activity in flies (9), worms (10,11), and yeast (12) could lead to life span extension, and by observations that mTOR function was lower in several mouse models of slow aging, including data on dwarf mice (13) and in mice subjected to caloric restriction (14). Our previous report (2) was consistent with these earlier studies showing that rapamycin treatment initiated at 20 months of age led to significant increase in life span in both male and female mice at each of three test sites. Similarities between the effects of rapamycin treatment and those of a calorie-restriction diet have supported speculation (7,15) that the ability of calorie-restriction to extend rodent life span may be mediated at least partly by downregulation of mTOR in one or more tissues. Because ribosomal S6 protein kinase (S6K) is among the major targets of mTOR action, a report that deletion of the gene for S6K1 increases life span in female mice (16) and retards age effects on bone, immunity, and motor function provides strong justification for additional work on mTOR pathways as modulators of aging and late-life illnesses.
Our new data extend our previous observations in several ways. They provide a fully independent replication to show that rapamycin can extend mouse life span in males and females, and they show that the effect does not depend on the age at which drug exposure is begun, at least between 9 and 20 months of age. Our surrogate for maximum life span, that is, the proportion of mice in each population alive at the 90th percentile age, was significantly affected by rapamycin in the Cohort 2006 population at each of the three sites. The data on spontaneous activity levels suggest that rapamycin may have retarded the effects of aging on activity, although the effect seems restricted to males, and interpretation is complicated by site-to-site variations in the size of the age effect. When rapamycin is initiated at 9 months of age, it leads to a decline in the rate of weight gain, which is apparent in males by 12 months of age and in females by 18 months. This is consistent with the known effects of rapamycin on protein translation and cell size. The size of this effect is smaller than that typically seen in mice subjected to calorie-restriction diets sufficient to extend life span, and further studies will be needed to determine whether rapamycin effects body mass via changes in lean mass, level of adiposity, or both. Similarly, it will be informative to see if rapamycin has beneficial effects on mice subjected to a calorie-restriction diet.
A comparison of survival results between mice exposed to rapamycin from 9 months of age (this report), and those in our previous study using rapamycin from 20 months onward, showed no significant advantage of starting the agent at the earlier age. Interpretation is complicated, however, by the trend (two-tailed p = .09 by Wilcoxon–Breslow test) for improved survival in female mice and by differences in midlife diet formulations at UT and UM in the original study (2). Inferences based on comparison of nonsimultaneous data sets have well-known disadvantages. In principle, however, an agent that slowed the overall process of aging would be expected to have a stronger effect if started at a relatively early age. Thus, if later work confirms that rapamycin treatment starting in later life is fully as effective as treatment started early in adult life, this could be taken as evidence that the agent is not modulating aging so much as limiting the pace of, or vulnerability to, late life illnesses, such as the neoplastic diseases that lead to most of the deaths in UM-HET3 mice. Additional data on age-sensitive traits unrelated to major lethal diseases will help to shed light on this important issue.
A recent report (17) has also shown increased life span in aged mice exposed to rapamycin. C57BL/6Nia mice were treated with rapamycin at 4 mg/kg body weight by intraperitoneal injection every other day for 6 weeks starting at age 22 months and were found to have significantly increased survival (p < .01), compared with vehicle-injected controls when followed over the ensuing 30 weeks. This treatment protocol was also shown to improve the function of hematopoietic stem cells in the aged mice and to improve responses to influenza vaccine and thus protect the aged mice from a potentially lethal viral challenge. Although rapamycin and other mTOR inhibitors are used clinically as immunosuppressive agents, more work will be needed to determine under what circumstances this drug leads to stronger or weaker protective immunity.
The principal cause of death of UM-HET3 is cancer, with the largest proportion of deaths attributable to lymphoma (2). Therefore, it is possible that rapamycin may extend life span at least in part through delay or prevention of cancer. In fact, inhibitors of mTOR are under both preclinical and clinical investigation for treatment of cancer (18–23). A rapidly expanding list of rapamycin analogs (rapalogs) are being used for treatment of cancer (19,20). Used alone, rapalogs have had mixed results. Overall, the responses of patients to treatment have been modest. However, in some cancers such as lymphoma, for example, rapalogs used as a single agent have shown encouraging response rates. In others, however, the response rates are less impressive, with the clinical outcome being to stabilize the disease rather than producing tumor regression (19). Planned studies in the ITP will determine the extent to which the life-extending effects of rapamycin are related to the delay or prevention of cancer.
One of the major goals of the ITP is to evaluate possible anti-aging effects of drugs that have shown promising effects in invertebrate models, in cell culture assays thought to be relevant to the aging process, or in rodent studies (24). Resveratrol was chosen for study because of promising data (25) from animal models of cancer, heart disease, stroke, and immunity and because of data suggesting it could activate sirtuins, a class of protein deacetylases implicated in control of life span in yeast (26), flies (27), and worms (28). Resveratrol at 22 mg/kg body weight per day had also been shown to improve the median survival of male C57BL/6Nia mice subjected from 1 year of age to a high-fat diet (with 60% of caloric content as fat) (6). A subsequent report (29), based on the complete survival curve with terminal necropsy data, showed that improved survival of mice given resveratrol on the high-fat diet could be attributed to reduction of deaths due to lipid accumulation in the liver and subsequent pulmonary congestion and edema. Mice given resveratrol on a standard diet (AIN-93G) were found to be relatively resistant to age effects on aortic elasticity, cataract formation, bone mineralization, and gene expression patterns, but did not show any survival advantage over controls (29). Our resveratrol data thus serve to confirm the absence of any effect of this agent on mouse life span, using doses two- to eightfold higher than the dose studied by Pearson and colleagues and using genetically heterogeneous mice of both sexes rather than male C57BL/6Nia mice alone. Several groups have reported that resveratrol is able to increase life span in Drosophila (30,31) and Caenorhabditis elegans (30,32,33), and a short-lived species of fish (34), though others have reported that this agent did not increase life span in either of the first two species (35). In addition, the idea that the pharmacological effects of resveratrol are mediated by direct activation of SIRT1 has been challenged by data suggesting that the agent does not activate the deacetylase activity of SIRT1 in vitro when enzyme function is evaluated using natural substrates rather than the fluorochrome-conjugated substrates used in earlier experiments (36–38). Clearly more work is called for to identify the pathway(s) by which resveratrol modulates age-sensitive traits in mice and imparts beneficial effects in several rodent models of age-dependent diseases. It is possible that mice given resveratrol from an earlier age might show an increase in life span, and ITP Cohort 2007 includes a test of resveratrol, at 50 mg/kg body weight, begun at 4 months of age in UM-HET3 mice.
Simvastatin was tested, in part, because studies have indicated that statins can directly modify the aging process. For example, Assmus and colleagues (39) reported that atorvastatin delays replicative senescence in human endothelial cells. A subsequent study by the same laboratory concluded that the statin-induced delay in senescence was mediated through attenuated reactive oxygen species production (40). Narumiya and colleagues (41) reported that statins increase the mRNA expression of the age-suppressor gene, klotho, in mouse epithelial cells. All of these aging effects appear to be secondary to changes in geranylgeranyl diphosphate, which lies downstream of 3-hydroxy-3-methyl-glutaryl-CoA reductase (41). Statins have also been shown to be cardioprotective. Increased endothelial nitric oxide synthase (NOS) appears to play a significant role in statin-induced cardioprotection because at least three studies have reported that the protective effect is prevented by NOS inhibitors (42–44). As reviewed by Wolfrum and colleagues (43), statins are known to have both acute and long-term effects on NOS. Other studies have shown that statins may prevent infections (45). Moreover, epidemiological studies have shown that statin use is associated with reduction in certain cancers (46,47). It is unclear why statins did not increase life span in the present study, given the spectrum of effects it has on diseases of aging.
Follow-up studies are now underway to address several of the issues and opportunities raised by these findings. One such study will evaluate doses of rapamycin both higher and lower than the dose used for the initial longevity studies: It is unlikely that the initial dose selected will turn out to have been optimal for life span extension, but it is difficult to guess whether the optimal dose will prove to be higher or lower. Cross-sectional histopathology will help to identify earlier stages of common age-dependent illnesses, including those that do not typically lead to death, to provide a more comprehensive picture of which forms of pathophysiology are retarded (or, perhaps, accelerated) by the drug. Studies of multiple age-dependent physiological outcomes will also be included. These physiological endpoints, along with the cross-sectional histopathology, will help to show whether the life span improvement represents merely a global inhibition of neoplastic disease, or rather an authentic antiaging effect that includes anticancer protection as just one among many consequences. The follow-up studies will also evaluate biochemical changes in multiple tissues, to see which tissues show long-term and short-term inhibition of mTOR and modulation of mTOR-dependent cellular feedback circuitry. Although it is plausible to assume that the effects of rapamycin on health and longevity reflect simply downregulation of mTOR and mTOR-dependent downstream pathways, it is also possible that some of the physiological effects are caused by compensatory upregulation of enzymes involved in mTOR-dependent feedback mechanisms. It is equally plausible that the health benefits of rapamycin principally reflect a change in one or a few cell types, perhaps located in hypothalamus, vascular endothelium, a hormone-producing cell type, or a stem cell compartment, whose beneficial effects on other cell types are independent of the level of mTOR function in the downstream target tissues. Evaluation of rapamycin effects on rodent models of specific diseases is likely to be very informative in this regard. For example, two recent studies have evaluated rapamycin effects on different mouse models of Alzheimer’s disease using the same rapamycin preparation used in the ITP studies. Each study found that rapamycin rescued memory deficits and slowed pathological manifestations in these neurodegenerative models (48,49). Analysis of effects on other rodent (and perhaps canine) models of late-life diseases should prove equally informative. It will also be helpful to learn if rapamycin can further improve the longevity benefits produced by specific mutations (50) or diets (51) or other drugs (1). Researchers have learned much about the biology of aging by studies of caloric restriction and of antiaging mutations, and the advent of effective antiaging drugs should provide additional leverage for new work on the factors that time the coordinated appearance of age-related decline. In addition, work on antiaging interventions holds more promise for the eventual development of protective medicines than approaches that entail modification of germline genes or lifelong compliance with extreme dietary restrictions.
FUNDING
This work was supported by the National Institute on Aging at the National Institutes of Health (U01-AG022303 to R.A.M., U01-AG025707 and U01-AG022308 to D.E.H., R21-AG029313 to C.J.O., and U01-AG022307 and R01-AG13319 to R.S.); and the Department of Veterans Affairs (R.A.M. and R.S.). R.d.C. is supported by the Intramural Research Program of the National Institute on Aging.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://biomed.gerontologyjournals.org.
Acknowledgments
We thank Elizabeth Adler, Lisa Burmeister, Vivian Diaz, Sabrina Friedline, Melissa Han, Patricia J. Harrison, Bill Kohler, Pamela J. Krason, and Bee Stork for reliable technical assistance. D.S. is a Senior Scholar of the Ellison Medical Foundation and a consultant to GSK, a company developing medicines that target SIRT1.
References
- 1.Strong R, Miller RA, Astle CM, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RA, Harrison DE, Astle CM, et al. An aging interventions testing program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Promislow DE, Tatar M, Pletcher SD, Carey J. Below-threshold mortality and its impact on studies in evolutionary ecology. J Evol Biol. 1999;12:314–328. [Google Scholar]
- 6.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 11.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 12.Powers RW, III, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2005;60:293–300. doi: 10.1093/gerona/60.3.293. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci. 2009;64:711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaeberlein M, Kennedy BK. Ageing: A midlife longevity drug? Nature. 2009;460:331–332. doi: 10.1038/460331a. [DOI] [PubMed] [Google Scholar]
- 16.Selman C, Tullet JM, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane HA, Breuleux M. Optimal targeting of the mTORC1 kinase in human cancer. Curr Opin Cell Biol. 2009;21:219–229. doi: 10.1016/j.ceb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 21.Mahalingam D, Sankhala K, Mita A, Giles FJ, Mita MM. Targeting the mTOR pathway using deforolimus in cancer therapy. Future Oncol. 2009;5:291–303. doi: 10.2217/fon.09.9. [DOI] [PubMed] [Google Scholar]
- 22.Mita M, Sankhala K. bdel-Karim I, Mita A, Giles F. Deforolimus (AP23573) a novel mTOR inhibitor in clinical development. Expert Opin Investig Drugs. 2008;17:1947–1954. doi: 10.1517/13543780802556485. [DOI] [PubMed] [Google Scholar]
- 23.Mita MM, Mita A, Rowinsky EK. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther. 2003;2:S169–S177. [PubMed] [Google Scholar]
- 24.Warner HR, Ingram D, Miller RA, Nadon NL, Richardson AG. Program for testing biological interventions to promote healthy aging. Mech Ageing Dev. 2000;115:199–207. doi: 10.1016/s0047-6374(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 25.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 26.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 29.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans [erratum appears in Nature. 2004 Sep 2;431(7004):107] Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 31.Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 35.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 37.Kaeberlein M, McDonagh T, Heltweg B, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 38.Pacholec M, Chrunyk BA, Cunningham D, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assmus B, Urbich C, Aicher A, et al. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res. 2003;92:1049–1055. doi: 10.1161/01.RES.0000070067.64040.7C. [DOI] [PubMed] [Google Scholar]
- 40.Haendeler J, Hoffmann J, Diehl JF, et al. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- 41.Narumiya H, Sasaki S, Kuwahara N, et al. HMG-CoA reductase inhibitors up-regulate anti-aging klotho mRNA via RhoA inactivation in IMCD3 cells. Cardiovasc Res. 2004;64:331–336. doi: 10.1016/j.cardiores.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Kawabata H, Ryomoto T, Ishikawa K. Role of cardiac ATP-sensitive K+ channels induced by HMG CoA reductase inhibitor in ischemic rabbit hearts. Hypertens Res. 2001;24:573–577. doi: 10.1291/hypres.24.573. [DOI] [PubMed] [Google Scholar]
- 43.Wolfrum S, Grimm M, Heidbreder M, et al. Acute reduction of myocardial infarct size by a hydroxymethyl glutaryl coenzyme A reductase inhibitor is mediated by endothelial nitric oxide synthase. J Cardiovasc Pharmacol. 2003;41:474–480. doi: 10.1097/00005344-200303000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Verma S, Rao V, Weisel RD, et al. Novel cardioprotective effects of pravastatin in human ventricular cardiomyocytes subjected to hypoxia and reoxygenation: beneficial effects of statins independent of endothelial cells. J Surg Res. 2004;119:66–71. doi: 10.1016/j.jss.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Tleyjeh IM, Kashour T, Hakim FA, et al. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169:1658–1667. doi: 10.1001/archinternmed.2009.286. [DOI] [PubMed] [Google Scholar]
- 46.Murtola TJ, Tammela TL, Maattanen L, et al. Prostate cancer and PSA among statin users in the finnish prostate cancer screening trial. Int J Cancer. 2010;127:1650–1659. doi: 10.1002/ijc.25165. [DOI] [PubMed] [Google Scholar]
- 47.Mondul AM, Selvin E, De Marzo AM, Freedland SJ, Platz EA. Statin drugs, serum cholesterol, and prostate-specific antigen in the National Health and Nutrition Examination Survey 2001-2004. Cancer Causes Control. 2010 doi: 10.1007/s10552-009-9494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 51.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C Thomas; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.