Abstract
Background and Purpose
Abnormal vascular remodeling triggered by hemodynamic stresses and inflammation is believed to be a key process in the pathophysiology of intracranial aneurysms. Numerous studies have shown infiltration of inflammatory cells, especially macrophages, into intracranial aneurysmal walls in humans. Using a mouse model of intracranial aneurysms, we tested whether macrophages play critical roles in the formation of intracranial aneurysms.
Methods
Intracranial aneurysms were induced in adult male mice using a combination of a single injection of elastase into the cerebrospinal fluid and angiotensin-II-induced hypertension. Aneurysm formation was assessed three weeks later. Roles of macrophages were assessed utilizing clodronate liposome-induced macrophage depletion. In addition, the incidence of aneurysms was assessed in mice lacking monocyte chemotactic protein-1 (MCP-1, CCL2), and mice lacking matrix metalloproteinase-12 (MMP-12, macrophage elastase).
Results
Intracranial aneurysms in this model showed leukocyte infiltration into the aneurysmal wall, the majority of leukocytes being macrophages. Mice with macrophage depletion had a significantly reduced incidence of aneurysms compared to control mice (1/10 vs. 6/10; P < 0.05). Similarly, there was a reduced incidence of aneurysms in mice lacking MCP-1, compared to incidence of aneurysms in wild-type mice (2/10 vs. 14/20, P < 0.05). There was no difference in the incidence of aneurysms between mice lacking MMP-12 and wild-type mice.
Conclusions
These data suggest critical roles of macrophages and proper macrophage functions in the formation of intracranial aneurysms in this model.
Keywords: Intracranial aneurysm, stroke, inflammation, animal model, macrophage
Introduction
Potential roles of inflammation in the pathophysiology of intracranial aneurysms—both ruptured and unruptured— have been suggested by observational and genetic studies.1-6 Macrophage infiltration has been well-documented in both ruptured and unruptured intracranial aneurysms in humans.2, 3, 7 A higher degree of inflammation in aneurysms seems to be associated with aneurysmal wall destruction and rupture.3, 7
We have recently showed that macrophages and macrophage-derived cytokines are critical for hemodynamically-induced outward vascular remodeling.8, 9 Vascular remodeling coupled with inflammation is considered as a key part in the pathophysiology of intracranial aneurysms.4, 10 Sustained vascular remodeling may lead to aneurysmal growth and rupture.1, 11 By mediating inflammation and hemodynamically-induced vascular remodeling, macrophages may play critical roles in the development, growth, and rupture of intracranial aneurysms.
In this study, we examined whether macrophages are critical for the formation of intracranial aneurysms using a mouse model of intracranial aneurysms that replicates key features of human intracranial aneurysms. First, we assessed the effects of macrophage depletion by clodronate liposome on the formation of aneurysms. Second, aneurysm formation was assessed in mice lacking monocyte chemotactic protein-1 (MCP-1, CCL2). MCP-1 is a chemotactic factor that is critical for proper macrophage functions. MCP-1 knockout mice have reduced macrophage/monocyte counts and impaired macrophage functions. Therefore, they have been used as a genetic equivalent of mice with pharmacological depletion of macrophages and monocytes in various physiological and pathological settings.12, 13
Materials and Methods
Experiments were conducted in accordance with the guidelines approved by the University of California, San Francisco, Institutional Animal Care and Use Committee.
We used the elastase-induced intracranial aneurysms in 8 to 9 week old hypertensive mice as previously described.11 In this model, two well-known clinical factors associated with human intracranial aneurysms—hypertension and the disruption of elastic lamina— were combined to induce intracranial aneurysm formation in mice. We performed a single stereotaxic injection of elastase into the cerebrospinal fluid at the right basal cistern. A volume of 2.5 μL of elastase solution (17 milli-units) was injected at a rate of 0.2 μL/min (Ultramicropump, World Precision Instruments). Hypertension was induced by a continuous subcutaneous infusion of angiotensin-II at 1000 ng/kg/min for three weeks via an implanted osmotic pump (Alzet pump, Durect).11, 14
Systolic blood pressure was measured in mice before treatment, one week after elastase injection, and two weeks after elastase injection using the tail cuff method. After three weeks, we sacrificed the mice and perfused the animals with bromophenol blue dye. Two blinded observers assessed the formation of intracranial aneurysms by examining of Circle of Willis and its major branches under a dissecting microscope (10×). Intracranial aneurysms were operationally defined as a localized outward bulging of the vascular wall in the Circle of Willis or in its major primary branches, as previously described.10, 11 After inspecting the Circle of Willis, the whole brain samples were frozen in OCT for immunohistochemical staining.
Macrophage depletion
Macrophage depletion was achieved by an intravenous injection of liposome-encapsulated dichloromethylene diphosphonate (clodronate liposome).15 We used 8 to 9-week-old male C57BL/6J mice (n = 10 in each group). Clodronate was a gift from Roche Diagnostics GmbH (Mannheim, Germany). Animals received clodronate liposome intravenously two days before elastase and angiotensin-II treatment. This regimen was reported to cause a reduction of macrophages to less than 10% of the baseline count.9, 16 Animals in the control group received the same volume of phosphate-buffered saline-containing liposome (PBS liposome). We assessed the efficiency of macrophage depletion by examining macrophages in the spleen using immunohistochemistry, as previously described.9, 16
Incidence of aneurysms in MCP-1 knockout mice and MMP-12 knockout mice
In addition, the incidence of aneurysms was assessed in MCP-1 knockout mice and MMP-12 knockout mice (n = 10). Wild-type mice with the same background (both C57BL/6J) were used as control mice (n = 20).
Immunohistochemical analysis
Details of immunohistochemical analysis were described in Online Data Supplement.
Statistical analysis
All results were expressed as mean ± SD. Differences between multiple groups were analyzed by one-way ANOVA, followed by the Tukey-Kramer post hoc test. Fisher's exact test was used to analyze the incidence of aneurysms. Statistical significance was taken at P < 0.05.
Results
Presence of macrophages in experimental intracranial aneurysm
Figure 1 shows representative intracranial aneurysms in hypertensive mice that received a single injection of elastase into the cerebrospinal fluid. Large saccular aneurysm formation was found along the Circle of Willis or its major branches, which is consistent with our previous study (A-D).11 Most aneurysms were larger than 250 μm in diameter, approximately 2 to 5 times larger than their parent arteries, as we previously reported (A-D). Some mice had multiple aneurysms (D).
Figure 1. Representative intracranial aneurysms in hypertensive mice that received a single injection of elastase into the cerebrospinal fluid.
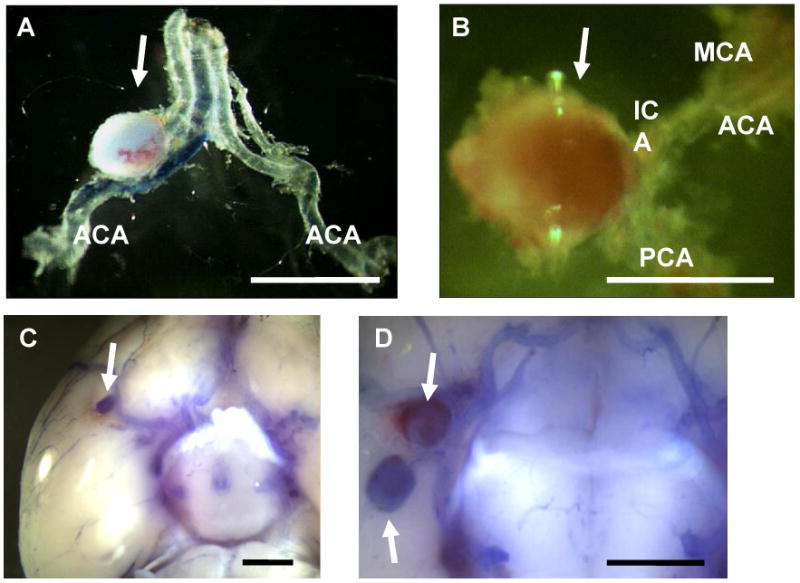
Arrows indicate aneurysms. Large aneurysm formation was found along the Circle of Willis or its major branches (A-D). Dissection of aneurysms revealed saccular shape of the aneurysms (A, B). Some of the mice had multiple aneurysms (D). Bar = 1 mm, ACA: anterior cerebral artery, MCA: middle cerebral artery, PCA: posterior cerebral artery, ICA: internal carotid artery.
A cerebral artery from the sham operation group revealed an endothelial cell lining with a thin layer of smooth muscle cells, as previously described (Figure 2A).11 In contrast, intracranial aneurysms had a partially thickened vascular wall with inflammatory-like cell infiltration (Figure 2B). Endothelial cell layers seemed to be generally intact, but smooth muscle cell layers had thickened in the area with intense inflammatory cell infiltration.
Figure 2. H&E, pan-leukocyte, and macrophage staining.
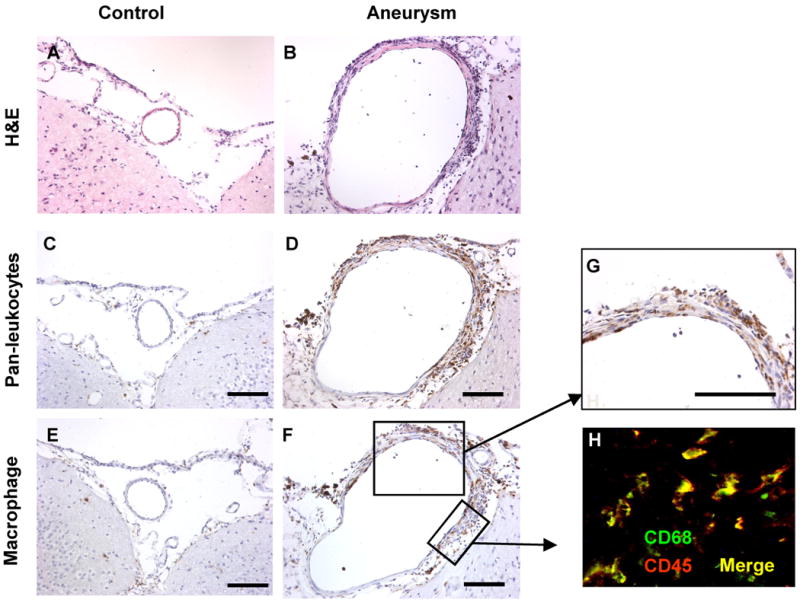
A. A cerebral artery from a mouse in the sham operation group showed an endothelial cell lining with a thin layer of smooth muscle cells. B. Intracranial aneurysms had a partially thickened vascular wall with inflammatory-like cell infiltration. Endothelial cell layers seemed to be generally intact, but smooth muscle cell layers had thickened in the area with intense inflammatory cell infiltration. C, E. Cerebral artery from a mouse in the sham operation group showed a lack of inflammatory cells. D, G. Intracranial aneurysms had numerous leukocytes. Distribution of macrophages was similar to that of leukocytes. F. Double staining with anti-CD68 and anti-CD45 revealed that a majority of leukocytes in intracranial aneurysms in this model were macrophages.
Pan-leukocyte staining using anti-CD45 antibody and macrophage staining using anti-CD68 antibody in the cerebral artery from the sham operation group showed a lack of inflammatory cells and macrophage infiltration (Figure 2C and 2E). In intracranial aneurysms, numerous leukocytes (CD45 positive cells) were detected in the adventitia and media of the aneurysmal wall (Figure 2D), especially in the thickened part of aneurysmal wall, which is generally consistent with observations in human intracranial aneurysms.2, 3, 7 Macrophage staining showed macrophage infiltration into the aneurysmal wall with a distribution similar to that of leukocytes (Figure 2F-G). Double staining with anti-CD68 and anti-CD45 revealed that a majority of leukocytes in intracranial aneurysms in this model were macrophages (Figure 2H).
Effects of macrophage depletion on intracranial aneurysm formation
Ten mice underwent macrophage depletion treatment with clodronate liposome two days before the induction of intracranial aneurysms, and another ten mice received PBS liposome. All twenty mice received a single stereotaxic injection of elastase into the cerebrospinal fluid to disrupt the elastic lamina, and a continuous infusion of angiotensin-II to induce hypertension.
When we examined the mice three weeks after aneurysm induction, mice that received macrophage depletion treatment using clodronate liposome had a reduced incidence of intracranial aneurysms compared to mice that received PBS liposome (mice with intact macrophages) (1/10 vs. 6/10; 10% vs. 60%, P < 0.05) (Figure 3A), indicating a critical role of macrophages in the formation of intracranial aneurysms in this model.
Figure 3. Macrophage depletion and intracranial aneurysm formation.
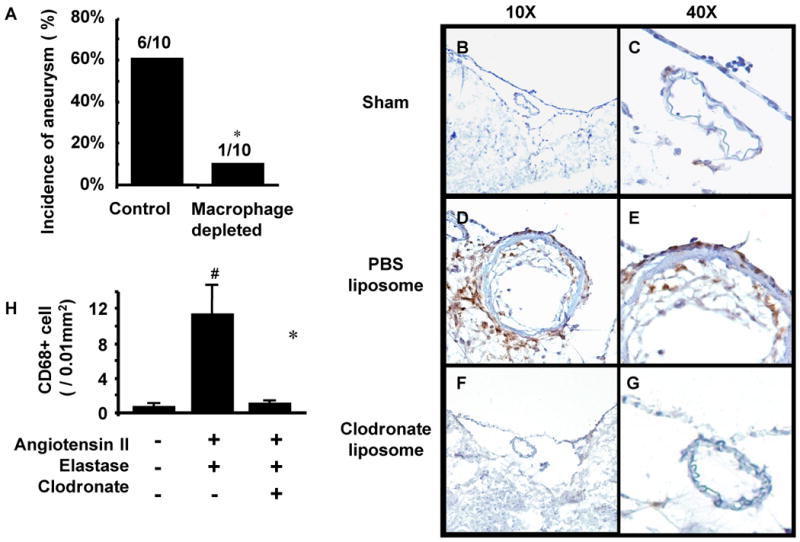
A. Mice with macrophage depletion treatment using clodronate liposome had a reduced incidence of intracranial aneurysms compared to mice that received PBS liposome (P < 0.05). B-G. While there were an abundant number of macrophages in the intracranial aneurysms from PBS liposome treated mice, middle cerebral arteries from mice in either the sham operation group or the macrophage depletion group did not show macrophage infiltration into the vascular wall. H. The number of macrophages was higher in mice treated with elastase, angiotensin-II, and PBS liposome compared to mice in the sham group or macrophage depletion group (n = 5 in each group, P < 0.05).
While there were abundant macrophages in the intracranial aneurysm from a PBS liposome-treated mouse (Figure 3D, 3E), middle cerebral arteries from mice in either the sham operation group (Figure 3B, 3C) or the macrophage depletion group (F, G) did not show macrophage infiltration into the vascular wall.
Quantification of macrophages (n = 5 in each group) showed that the number of macrophages was higher in the mice treated with elastase, angiotensin-II, and PBS liposome treated mice compared to mice in the sham group or macrophage depletion group (0.5 ± 0.3 vs. 11.1 ± 3.3, P < 0.05; 0.5 ± 0.3 vs. 1.0 ± 0.3, P < 0.05) (Figure 3H).
Immunohistochemical staining for CD68 positive cells (monocyte / macrophage) in the spleen (n = 5 at each time point) was used to assess the efficiency and time-course of clodronate liposome treatment as previously described (Figure 4A-B).9, 16 Treatment with clodronate liposome decreased the CD68 positive area in the spleen by 88% from the baseline (28.8 ± 4.1 vs. 3.2 ± 1.0%, P < 0.05), showing an effective macrophage / monocyte reduction by the clodronate treatment. At two weeks, CD68 positive area returned to the baseline (28.8 ± 4.1 vs. 23.1 ± 2.6%).
Figure 4.
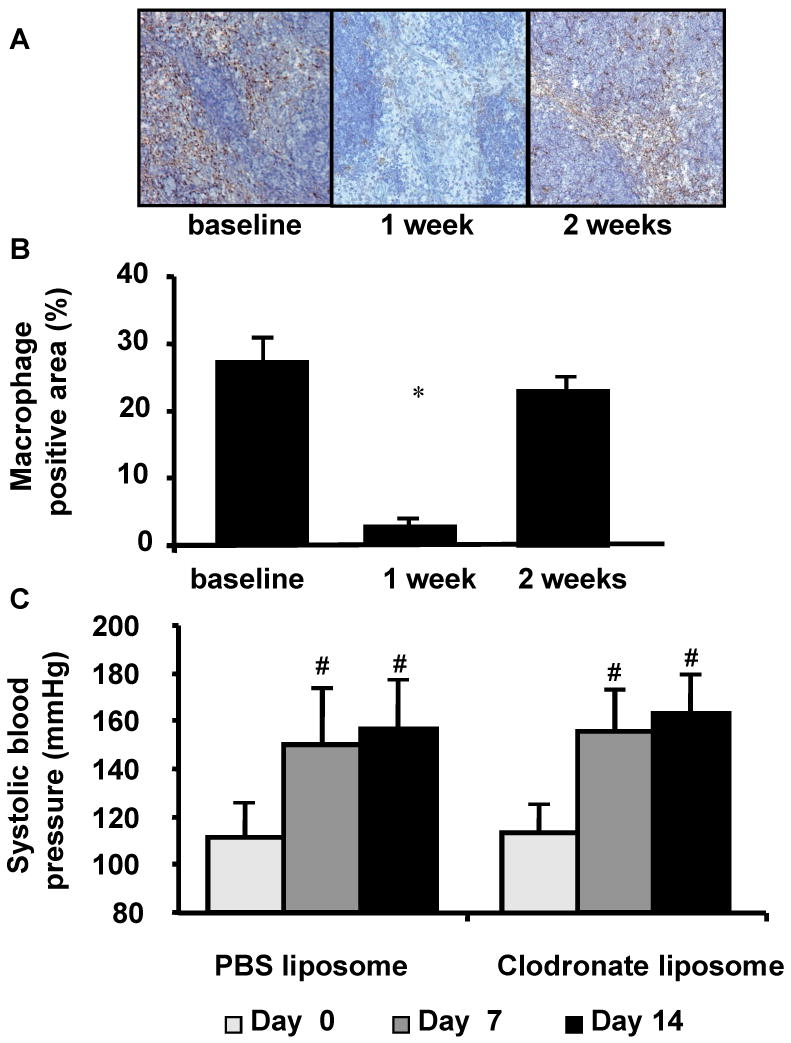
A. Macrophage staining of the spleen from mice that received clodronate liposome. Brown area indicates macrophage positive area. B. Clodronate liposome treatment decreased CD68 positive area in the spleen by 88% from the baseline (P < 0.05), showing an effective macrophage / monocyte reduction by the clodronate treatment. At two weeks, CD68 positive area returned to the baseline. C. Successful induction of hypertension by angiotensin-II.
Angiotensin-II treatment caused hypertension in both groups. After one week and two weeks, systolic blood pressure was higher than the baseline. Macrophage depletion treatment did not affect systolic blood pressure (Figure 4C).
Reduced incidence of intracranial aneurysm in MCP-1 knockout mice
Since MCP-1 knockout mice have reduced monocyte/macrophage counts and impaired macrophage functions,17 we used MCP-1 knockout mice to further test the critical role of macrophages in the formation of intracranial aneurysms. Twenty wild-type mice and ten MCP-1 knockout mice underwent intracranial aneurysm induction.
MCP-1 knockout mice had a lower incidence of aneurysms compared to wild-type mice (2/10 vs. 14/20; 20 vs. 70%, P < 0.05) (Figure 5A). Immunohistochemical staining of cerebral arteries for macrophages showed a lack of macrophage infiltration in MCP-1 knockout mice (Figure 5B). Quantification of macrophages in the middle cerebral artery (n = 5 in each group) showed reduced macrophage infiltration to the middle cerebral artery in MCP-1 knockout mice compared to macrophage infiltration in wild-type mice (2.8 ± 1.4 vs. 10.9 ± 0.8, P < 0.05, Figure 5E).
Figure 5.
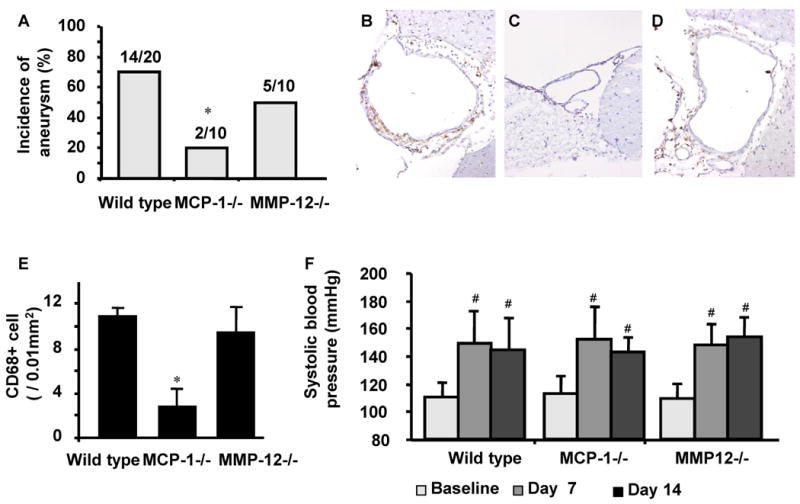
A. MCP-1 knockout mice had a lower incidence of aneurysms compared to wild-type mice (P < 0.05). There was no difference in the incidence of intracranial aneurysms between MMP-12 knockout mice and wild-type mice. B-D. While macrophage infiltration was observed in the cerebral arteries of wild-type mice (B) and MMP-12 knockout mice (D), cerebral arteries in MCP-1 knockout mice showed a lack of macrophage infiltration (C). F. Successful induction of hypertension by angiotensin-II.
Macrophages produce matrix metalloproteinases (MMP) that are critical for vascular remodeling.8, 9 Previously, we have shown that high activity of MMPs in intracranial aneurysms of this model.11 MMP inhibitor, doxycycline, significantly reduced the incidence of intracranial aneurysms.11 MMP-9 knockout mice, but not MMP-2 knockout mice, have a reduced incidence of intracranial aneurysms.11 While MMP-9 is the main gelatinase produced by macrophages, MMP-12 represents the major elastase from macrophages. MMP-12 could be the proteinase responsible for facilitating structural changes of elastic lamina, resulting in physiological and pathological vascular remodeling.18 Therefore, we investigated the roles of MMP-12 in the formation of intracranial aneurysms. However, there was no difference in the incidence of intracranial aneurysms between MMP-12 knockout mice and wild-type mice (50% vs. 70%). Moreover, immunohistochemical staining for macrophages (CD68 positive cells) showed a similar number of macrophages that accumulated in MMP-12 knockout mice compared to the number of macrophages in wild-type mice (9.4 ± 2.3 vs. 10.9 ± 0.8, Figure 5E).
Continuous infusion of angiotensin-II increased systolic blood pressure from the baseline after one week and two weeks in MCP-1 knockout mice and MMP-12 knockout mice. There was no significant difference between wild-type mice and MCP-1 or MMP-12 knockout mice at one week and two weeks (Figure 5F).
Discussion
In this study, we have shown the critical roles of macrophages in the formation of intracranial aneurysms in mice. We used a recently developed intracranial aneurysm model in which intracranial aneurysms were induced by a combination of single stereotaxic injection of elastase into the cerebrospinal fluid and pharmacologically-induced hypertension in mice. Intracranial aneurysms in this mouse model closely resemble histological changes that are observed in human intracranial aneurysms.11 Using this model, we first showed infiltration of inflammatory cells, mostly macrophages, into the aneurysmal wall. Second, mice with pharmacological depletion of macrophages had a significantly reduced incidence of intracranial aneurysms compared to mice with intact macrophages. In addition, MCP-1 knockout mice, mice with reduced monocyte/macrophage counts and impaired macrophage function, had a significantly reduced incidence of intracranial aneurysms compared to wild-type mice. These findings strongly indicate that macrophages play critical roles in the formation of intracranial aneurysms in this model, especially during the early stages of aneurysmal formation and growth.
Intracranial aneurysms are commonly found in locations where abnormal hemodynamic stresses are exerted on the vascular wall.19 Abnormal hemodynamic stresses trigger an inflammatory process by activating endothelial cells and monocytes/macrophages. These cells secrete proteinases, including MMPs and elastases.1 MMPs can destabilize the vascular wall directly by facilitating vascular remodeling by digestion of the vascular matrix, and indirectly by activating and releasing of other proteinases and angiogenic factors.20 We have previously shown critical roles of macrophages and MMPs in adaptive vascular remodeling of large arteries.9 Intracranial aneurysms may represent a result of maladaptive vascular remodeling in which inflammatory cells maintain active and abnormal remodeling processes that lead to aneurysm growth and rupture.1
Similar to our study, Aoki et al. used MCP-1 knockout mice in a different mouse intracranial aneurysm model in which intracranial aneurysms were induced by a combination of four manipulations over five months: treatment with beta aminopropio-nitrile (irreversible lysyl oxidase inhibitor), unilateral carotid artery ligation, bilateral posterior renal artery ligation, and high-salt drinking water.21 In their study, aneurysmal changes, defined as disruption of elastic lamina with or without the formation of aneurysms, were less frequent in MCP-1 knockout mice compared to wild-type mice, which is generally consistent with our data.21 In their study, there was a weak trend for MCP-1 knockout mice to have a reduced incidence of aneurysm compared to wild-type mice (10% vs. 20%), while our study showed a statistically significant reduction of the incidence of aneurysms in MCP-1 knockout mice compared to wild-type mice (20% vs. 70%). Such difference between these two studies might be due to a difference in severity of the phenotype between these two models. Aneurysms induced by a single injection of elastase into the cerebrospinal fluid in hypertensive mice tend to be larger and macroscopically apparent.11 In contrast, the mouse aneurysm model used by Aoki et al. yielded smaller aneurysms with more subtle histological changes.21, 22
Previously, potential roles of MMPs were shown in the formation of intracranial aneurysms.6, 11, 22 We have shown that a broad-spectrum inhibitor of MMPs can suppress the formation of intracranial aneurysms. Although MMP-2 was not critical for the formation of intracranial aneurysms, mice lacking MMP-9 had a reduced incidence of aneurysms.11 Macrophage-derived MMP-9 may be playing critical roles in the formation of intracranial aneurysms.22 In this study, we assessed roles of another key MMP that is produced by macrophages—MMP-12, a macrophage elastase. However, a lack of MMP-12 did not affect the incidence of aneurysms. Unlike MMP-9, MMP-12 may not play a significant role in the formation of intracranial aneurysm. Since exogenous elastase was used to induce aneurysms in our model, it may be the case that the early processes that require endogenous elastases such as MMP-12 may have been bypassed in this model. Alternatively, roles of macrophages and MMPs may play different roles between different stages—early and late stages— of aneurysm formation and growth.
Clodronate liposome, the treatment we used to deplete macrophages, may have unknown side effects. However, in our experiments, the animals that received clodronate liposome did not show any apparent signs of adverse effects. In our previous study, we have shown that clodronate liposome treatment did not have effects on other leukocyte subpopulation, platelets, or red blood cells.9 Our methods to deplete macrophages did not completely deplete the target cell population. This may have resulted in an incomplete suppression of aneurysm formation. Alternatively, other cell types may have been compensated due to a relative lack of macrophages.
In summary, data from this study strongly indicated critical roles of macrophages in the formation of intracranial aneurysms in mice. Macrophages and macrophage-derived cytokines may be maintaining abnormal and active aneurysmal wall remodeling that lead to aneurysmal growth and rupture. Pharmacological therapy that modifies inflammation mediated by macrophages may be studied for the prevention of progression, growth, and rupture of intracranial aneurysms.
Supplementary Material
Acknowledgments
None
Funding
This study was funded by NIH R01NS055876 (TH), NIH R01NS027713 (WLY) and NIH P01NS04415 (TH, WLY)
Footnotes
Conflict of interest / Disclosures
None
References
- 1.Hashimoto T, Meng H, Young WL. Intracranial aneurysms: Links between inflammation, hemodynamics and vascular remodeling. Neurol Res. 2006;28:372–380. doi: 10.1179/016164106X14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45:1137–1146. doi: 10.1097/00006123-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999;30:1396–1401. doi: 10.1161/01.str.30.7.1396. [DOI] [PubMed] [Google Scholar]
- 4.Shi C, Awad IA, Jafari N, Lin S, Du P, Hage ZA, Shenkar R, Getch CC, Bredel M, Batjer HH, Bendok BR. Genomics of human intracranial aneurysm wall. Stroke. 2009;40:1252–1261. doi: 10.1161/STROKEAHA.108.532036. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Mineharu Y, Inoue S, Yamada S, Matsuda F, Nozaki K, Takenaka K, Hashimoto N, Koizumi A. Search on chromosome 17 centromere reveals tnfrsf13b as a susceptibility gene for intracranial aneurysm: A preliminary study. Circulation. 2006;113:2002–2010. doi: 10.1161/CIRCULATIONAHA.105.579326. [DOI] [PubMed] [Google Scholar]
- 6.Kim SC, Singh M, Huang J, Prestigiacomo CJ, Winfree CJ, Solomon RA, Connolly ES., Jr Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery. 1997;41:642–666. doi: 10.1097/00006123-199709000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Frosen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, Jaaskelainen J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 8.Ota R, Kurihara C, Tsou TL, Young WL, Yeghiazarians Y, Chang M, Mobashery S, Sakamoto A, Hashimoto T. Roles of matrix metalloproteinases in flow-induced outward vascular remodeling. J Cereb Blood Flow Metab. 2009;29:1547–1558. doi: 10.1038/jcbfm.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuki Y, Matsumoto MM, Tsang E, Young WL, van Rooijen N, Kurihara C, Hashimoto T. Roles of macrophages in flow-induced outward vascular remodeling. J Cereb Blood Flow Metab. 2009;29:495–503. doi: 10.1038/jcbfm.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats. Surg Neurol. 1978;10:3–8. [PubMed] [Google Scholar]
- 11.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, Ishibashi T, Kuwano M, Ono M. Infiltration of cox-2-expressing macrophages is a prerequisite for il-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 14.Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, Makino H, Hashimoto T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 2010;55:1267–1274. doi: 10.1161/HYPERTENSIONAHA.109.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 16.Danenberg HD, Fishbein I, Gao J, Monkkonen J, Reich R, Gati I, Moerman E, Golomb G. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106:599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 17.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown PM, Zelt DT, Sobolev B. The risk of rupture in untreated aneurysms: The impact of size, gender, and expansion rate. J Vasc Surg. 2003;37:280–284. doi: 10.1067/mva.2003.119. [DOI] [PubMed] [Google Scholar]
- 19.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 20.Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: Interaction with no. Arterioscler Thromb Vasc Biol. 2000;20:E120–126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- 21.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke. 2009;40:942–951. doi: 10.1161/STROKEAHA.108.532556. [DOI] [PubMed] [Google Scholar]
- 22.Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke. 2007;38:162–169. doi: 10.1161/01.STR.0000252129.18605.c8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


