Abstract
Elevated levels of lysophosphatidylcholine (lysoPC), present in oxidatively damaged low-density lipoprotein (oxLDL), are implicated in cardiovascular complications. LysoPC is generated by free radical-catalyzed oxidation of polyunsaturated PCs to oxidatively-truncated phosphophatidylcholines (oxPCs). It is known that oxPCs are especially susceptible to hydrolysis by platelet-activating factor acetylhydrolase, a phospholipase (PL) A2 that exists in plasma largely in association with LDL. Drugs that aim to prevent the generation of lysoPC by inhibiting this PLA2-catalyzed hydrolysis are in advanced clinical trials. We now report that spontaneous deacylation oxPCs, such as 1-palmityl-2-(4-hydroxy-7-oxo-5-heptenoyl)-sn-glycero-3-phosphocholine, occurs readily under physiological conditions of temperature and pH (t1/2 = 30 min at 37 °C and pH 7.4). We also show that this reaction proceeds through an intramolecular transesterification mechanism. Because anti-phospholipase drugs cannot block this nonenzymatic pathway to lysoPC, additional therapeutic measures may be needed to avoid the pathological consequences of the newly discovered biomolecular chemistry of oxPCs.
Keywords: Oxidized phospholipid, 2-lysophosphatidylcholine, oxidized low-density lipoprotein, cardiovascular disease
Introduction
Phosphoipid oxidation is accompanied by spontaneous deacylation
Previously, we studied free radical-induced oxidative degradation of phospholipids using the biologically relevant myeloperoxidase (MPO)/H2O2/NO2− system, that employs glucose/glucose oxidase to continuously generate H2O2 (1), to initiate autoxidation under physiomimetic conditions (pH 7.4, 37 °C). We characterized a variety of oxidatively-truncated ether phospholipids that are generated through autoxidation of the arachidonic acid (AA) ester of 2-lyso platelet activating factor (lysoPAF, 1-alkyl-sn-glycero-3-phosphocholine) in small unilamellar vesicles (1). Now we find that this oxidative degradation of AA-lysoPAF also releases lysoPAF, while little or no lysoPAF is generated upon autoxidation of the corresponding linoleic acid (LA) ester of 2-lysoPAF (Fig 1). We then showed that 2-lyso-phosphatidylcholine (lysoPC, 1-acyl-sn-glycero-3-phosphocholine) is also generated upon autoxidation of the corresponding AA ester of 2-lysoPC (data not shown). In view of the biological activities of 2-lysoPC, these observations piqued our interest.
Figure 1.
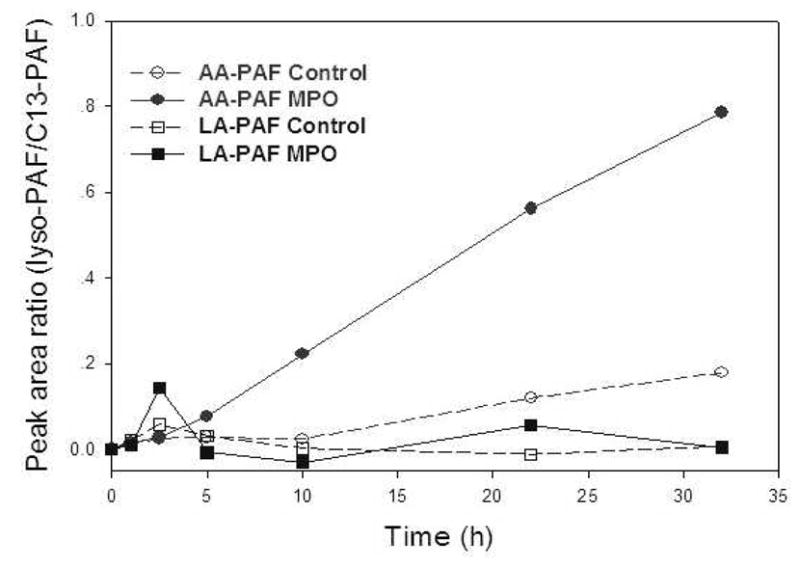
Generation of lysoPAF during the oxidation of AA-PAF but not LA-PAF. LysoPAF was determined by LC-ESI-MS/MS analysis of lipid extracts at various times using C13-PAF as internal standard.
The generation of lysoPC in vivo has biological significance
LysoPC is an important major lysophospholipid that is generated as a breakdown product of lipoprotein PCs. This production of lysoPC has been considered to be a result of endogenous phospholipase A2 (PLA2) activation (2). Present in the cell membranes or the polar surface of oxidized lipoproteins, lysoPC plays important physiological and pathophysiological roles including vascular development, reproduction, myelination, neuronal diseases and cancer in both humans and animals. Specifically, elevated levels of lysoPC have been linked to the cardiovascular complications associated with diabetes, atherosclerosis, ischemia (3, 4), renal failure during hemo-dialysis (5), rheumatoid arthritis (6), asthma (7), sepsis (8), hyperlipidemia (9), endometriosis (10) and psoriasis (11). Lyso-PC is generated from oxidation of low-density lipoprotein (LDL), accounting for nearly one-third (12) to one half (13) of the PC equivalents in oxidized (ox)LDL. Until now, it has been known that lysoPC can be produced under physiological conditions by PLA2-mediated hydrolysis of phosphatidylcholine (14), or from the hydrolysis of oxidized PC by PAF-acetylhydrolase (15). This occurs upon oxidation of LDL promoted by iron or copper ions (16), free radical initiators (17), lipoxygenase (18) or UV radiation (19).
Intramolecular transesterification is a likely mechanism for the nonenzymatic generation of lysoPC
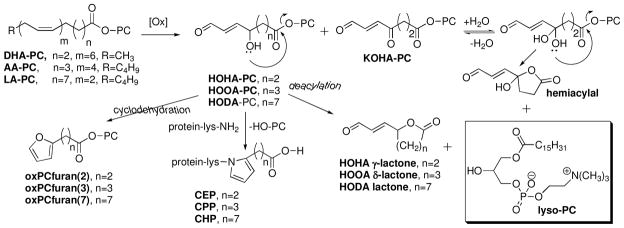
The contrasting behavior of AA- and LA-containing phospholipids led us to postulate a mechanism for the spontaneous generation of lysoPC involving intramolecular attack on the ester carbonyl carbon by a pendant hydroxyl. Such a process is somewhat reminiscent of the biologically relevant, spontaneous intramolecular transesterification involved in exon self-splicing, wherein an adenosyl hydroxyl group attacks a phosphodiester linkage displacing the 3′-hydroxyl terminus of the upstream exon. The putative mechanism is depicted in Fig 2 for the deacylation of oxPCs in which the sn-2 acyl group contains a γ-hydroxy-α,β-unsaturated aldehyde. We have shown that such oxidatively-truncated phospholipids, i.e., HOHA-PC, HOOA-PC, and HODA-PC, are generated in vivo by free radical-induced oxidation of polyunsaturated PCs, specifically, docosahexaenoic acid (DHA) ester of 2-lysoPC, AA-PC, and LA-PC respectively (20, 21). Such an intramolecular transesterification would be expected to occur readily if it involves the formation of a 6-membered lactone byproduct from AA-PC through 2-(5-hydroxy-8-oxooct-6-enoyl)-PC (HOOA-PC), and to be strongly disfavored if it requires formation of a 10-membered lactone from LA-PC through 2-(9-hydroxy-12-oxodec-10-enoyl)-PC (HODA-PC). Furthermore, it is expected to occur most readily for DHA-PC since it would involve the formation of a 5-membered lactone from 2-(4-hydroxy-7-oxohept-5-enoyl)-PC (HOHA-PC). An alternative mechanism involving acid-catalyzed hydrolysis would not readily explain the structural selectivity of these spontaneous deacylations. We now report that such deacylations can proceed rapidly (t1/2 ~ 30 min) at physiological pH = 7.4 and are inhibited by acid. The intramolecular transesterification mechanism is supported by the isolation and characterization of lactone byproducts (Fig 2).
Figure 2.
Postulated mechanism for the spontaneous deacylation of γ-hydroxy- (HOHA-PC and HOOA-PC) or γ-keto- (KOHA-PC) α,β-unsaturated aldehydic ester PCs that generates lysoPC and lactones or cyclic hemiacylals. The γ-hydroxy-α,β-unsaturated aldehydic ester PCs can also (i) form adducts with the ε-amino group of protein lysyl residues to generate ω-carboxyalkyl pyrroles (34), and (ii) undergo cyclization and dehydration to produce
Materials and Methods
Syntheses of γ-hydroxy-α,β-unsaturated aldehydic ester of PCs, oxidized PC-furans, and lactone by-products
Pure authentic standard samples of γ-hydroxy-α,β-unsaturated aldehydic esters of PCs (HOHA-PC, HOOA-PC, and HODA-PC), oxfuran-PCs were prepared as described previously (20, 22–24) with the following modified procedure for preparing the oxfurans. For oxPC-furan(3) as an example, dry furan (10 mL, 0.13 mol) was added to a suspension of AlCl3 (26 g, 0.19 mol) and methyl 4-chloro-4-oxobutyrate (8.4 mL, 0.06 mol) in methylene chloride (800 mL) under argon, and the mixture was stirred overnight. The reaction mixture was then poured onto ice. Aluminum hydroxide was dissolved by addition of concentrated HCl. After extraction with chloroform, the organic layer was dried over anhydrous sodium sulfate to give crude methyl 4-(furan-2-yl)-4-oxobutanoate, which was used without further purification. The crude ester (2.2 g, 0.01 mol), hydrazine monohydrate(1.2 mL, 0.02 mol), potassium hydroxide(1.6 g, 0.028 mol), and triethylene glycol (150 mL) were refluxed for 2 h. After cooling to room temperature, water (100 mL) was added. The solution was acidified with HCl to pH 3 and the organic layer dried over anhydrous sodium sulfate. The organic layer was evaporated and the product purified by flash column (ethyl acetate/hexanes = 1:1) to give oxPC-furan(3) (1.52 g, 15%). 1H NMR spectral data is in agreement with that reported previously (25). Pure authentic samples of the deacylation products, HOHA lactone and HOOA lactone, were prepared as described elsewhere (26).
High performance liquid chromatograpic separation and purification of γ-hydroxy-α,β-unsaturated aldehydic esters of 2-lysoPCs
High performance liquid choromatographic (HPLC) purification was performed using HPLC grade solvents (Fisher Scientific) with a model 600 solvent delivery system, 717plus autosampler, and 2996 photodiode array detector (PDA) from Waters (Milford, MA), and a SEDEX 75 evaporative light scattering (ELS) detector from Sedere (France). Generally, ELS is an excellent detection method for all phospholipids and adaptable to most gradient programs and solvents, including chloroform. The elution gradient is shown in Table S1. The flow was 1.0 mL/min for a column by Phenomenex (25 cm, 4.6 mm i.d. LUNA C18 ODS-5 μm). γ-Hydroxy-α,β-unsaturated aldehydic esters of 2-lyso-PCs were purified by HPLC to separate the different PC esters from lysoPC. HOHA-PC was detected by both ELS and UV, but lysoPC was only detected by ELS (Fig S1).
Liquid Chromatography/Mass Spectrometry
LC/ESI/MS/MS analysis of product mixtures from PUFA-PAF MPO oxidation and decomposition of γ-hydroxy-α,β-unsaturated aldehydic esters of PCs were performed on a Quattro Ultima (Micromass, Wythenshawe, UK) connected to an Alliance 2690 Separations Module (Waters). The source temperature was maintained at 120 °C, the desolvation temperature was kept at 250 °C, the drying gas (N2) was maintained at ca. 450 L/h, the cone gas flow was kept at ca. 70 L/h, and the multiplier was set at an absolute value of 500. Optimized parameters for detecting AA-PAF, LA-PAF, LysoPC, LysoPAF, oxfuran-PCs, γ-hydroxy-α,β-unsaturated aldehydic esters of PCs, KOHA-PC, HOHA lactone-DNPH and HOOA lactone-DNPH were determined using authentic samples. MS scans at m/z 80–1000 were obtained for standard compounds (Table S2). Argon was used as collision gas at a pressure of 5 psi for MS/MS analysis, and the collision energy was optimized for each compound. For multiple reaction monitoring (MRM) experiments, the optimum collision energy (giving the strongest signal) was determined for each m/z ion pair (Table S3).
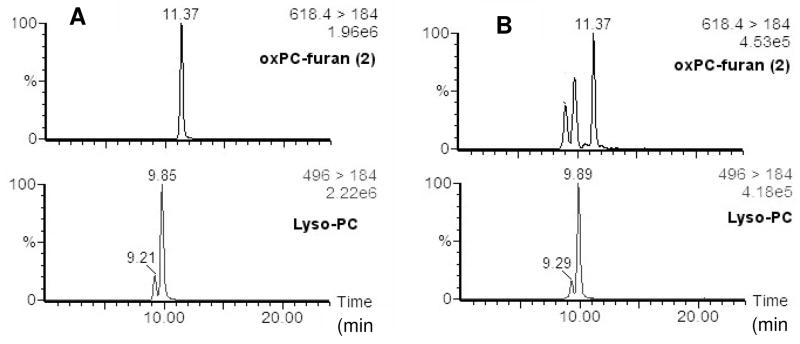
Online chromatographic separation was achieved using on 150 × 2.0 mm i.d. Prodigy ODS-5 μm column (Phenomenex), using binary solvent gradients (water and methanol) at 200 μL/min. For the PUFA-PAF MPO oxidation study, methanol and water were supplemented with 0.2% formic acid to increase the ion signal under positive mode. The gradient started with 85% methanol/water and maintained for 5 min, then increased to 100% methanol linearly over 5 min. The elution was held at 100% methanol for 20 min, then reversed to 85% methanol/water in 0.5 min. Finally, 85% methanol/water was maintained for 9.5 min at to re-equilibrate the column. For LC-ESI/MS/MS studies of the decomposition of γ-hydroxy-α,β-unsaturated aldehydic esters of PCs and KOHA-PC, methanol and water were supplemented with 2 mM ammonium acetate, and the reactant and product were detected in the positive ion mode. The gradient started with 85% methanol/water and increased to 100% methanol over 5 min. The elution was maintained at 100% methanol for 12 min, and then reversed to 85% methanol/water in 1 min. Finally, 85% methanol/water was maintained for 6 min to re-equilibrate the column. For HOHA lactone-DNPH and HOOA lactone-DNPH studies, both methanol and water were supplemented with 2 mM ammonium acetate under negative mode. The gradient started with 30% methanol/water and maintained for 3 min, then increased to 100% methanol linearly over 4 min. The elution was maintained at 100% for 10 min, and then reversed to 85% methanol/water in 0.1 min. Finally, 30% methanol/water was maintained for 5.9 min to re-equilibrate the column. Because of the acid sensitivity of this type of PC esters, 2 mM ammonium acetate was used as an additive, and multiple reaction monitoring (MRM) allowed for simultaneous quantification of specific isomers based upon unique transitions between the mass-to-charge ratio of the parent ion [M+H]+ in the positive mode and characteristic daughter ions for each species as well as their characteristic liquid chromatography retention times. In previous studies, phosphatidylcholine was detected in samples as a common daughter ion, m/z 184. Single peaks with the retention times of lysoPC and oxPC–furan(2) that are identical to the each standard (Fig 3A) were detected in the MRM chromatogram of the reaction product mixture (Fig 3B).
Figure 3.
MRM HPLC chromatograms of (A) authentic oxPC-furan (2) and lysoPC standard samples, (B) reaction product mixtures from incubation of HOHA-PC for 7 h in pH 5 sodium acetate buffer solution (2mM).
HOHA lactone-DNPH and HOOA lactone-DNPH derivatives
Authentic standards were prepared from the pure lactone (5 mg) in a 3.7 dram vial to which was added acetonitrile (2 mL) containing 2,4-dinitrophenylhydrazine (DNPH, 5 mg) and formic acid (40 μL). The mixture was sealed under nitrogen, kept at room temperature for 30 min, and the solvent evaporated under a stream of nitrogen. The residue was dissolved in water (1 mL), extracted with ethyl acetate (1 mL), the organic solvent evaporated, and the residue stored under argon and kept at − 80 °C until LC-MS/MS analysis.
Preparation and oxidation of small unilamellar vesicles (SUV) in vitro
SUV were prepared from hydrated lipids as described elsewhere.(24, 27) Briefly, 950 μg AA-PAF or LA-PAF in freshly distilled chloroform, with 1,2-dinonadecanoyl-sn-glycero-3-phosphocholine as a lipid carrier, was evaporated under nitrogen gas flow. SUV were prepared in argon-sparged sodium phosphate buffer (50 mM, pH 7.0), supplemented with 100 μM of DTPA by extrusion (20 times) through a 0.1 μm polycarbonate filter using an Avanti Mini-Extruder Set (Avanti Polar Lipids, Inc., Alabaster, AL). The vesicles are split into two parts, one for control and the other for MPO oxidation. The following reagents are added in order under cooling with ice and the oxidations were started with the addition of D-glucose. The vesicles (1 mg total lipids/mL) were incubated in the presence of MPO (30 nM), glucose oxidase (100 ng/mL), glucose (100 μg/mL) and NaNO2 (500 μM) in sodium phosphate buffer (50 mM) with DTPA (200 μM) at 37 °C. The reaction was stopped by adding butylated hydroxytoluene (BHT, 40 μM) and catalase (150 μM). 13C-PAF (30 ng) was added before extraction as internal standard. The lipids were extracted by the modified method of Bligh and Dyer,(28) the extracts were dried and stored in vials under argon at −80 °C. For the γ-hydroxy-α,β-unsaturated aldehydic esters of PCs decomposition studies, incubations were performed in a total volume of 1 mL containing various buffers (50 mM sodium acetate for pH 4 and 5, MES/KOH for pH 6, HEPES for pH 7.4 and 8 values) supplemented with 100 μM of DTPA and 1 μg substrate. After incubation, DT-PC (50 ng) was added before extraction as an internal standard, the lipids extracted by the method of Bligh and Dyer,(28) and the extracts dried and stored in vials under argon at −80 °C.
Results
Spontaneous deacylation of pure phospholipids demonstrates nonenzymatic generation of lyso PC
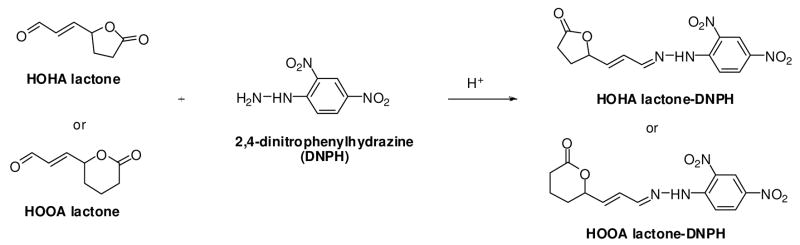
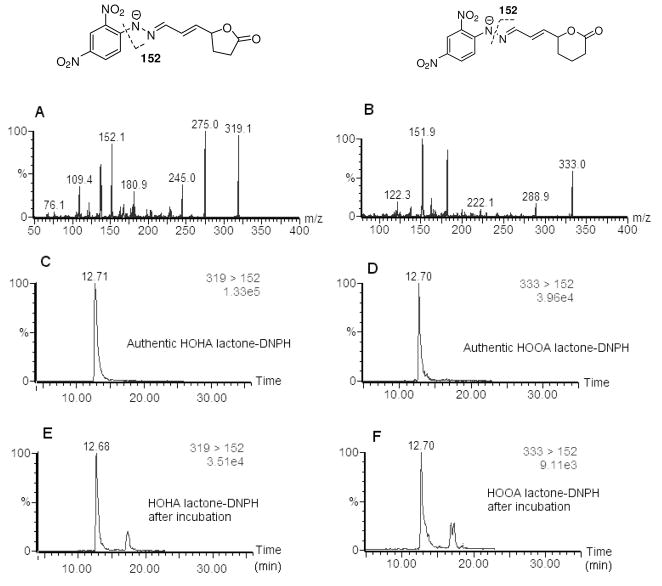
Monitoring the generation of oxidatively-truncated phospholipids in oxLDL is complicated by the proclivity of those that incorporate γ-hydroxy-α,β-unsaturated aldehyde functionality to form covalent ω-carboxyalkyl pyrrole adducts with proteins (Fig 2). Furthermore, hydrolysis catalyzed by LDL-associated PAF acetylhydrolase would compete with the spontaneous process. Therefore, to test the predictions summarized in Fig 2, and to determine the effects of pH on the putative spontaneous lactonization, pure individual oxPCs were vortexed for 10 sec in various aqueous pH buffers (pH 4 ~ 8) supplemented with a metal chelator, diethylenetriaminepentaacetic acid (DTPA), to block transition metal-induced oxidation. The resulting PC vesicles were then incubated at 37 °C, and sampled at various times. Lipids were extracted using a slightly modified Bligh and Dyer method,(28) dried under an argon stream at room temperature, and stored at −80 °C until analysis. Deacylated PCs from the γ-hydroxy-α,β-unsaturated aldehydic esters of lysoPC were quantified by LC-ESI/MS/MS. Besides the consumption of the oxidized lipid and the production of lysoPC, furans generated by cyclodehydration were also monitored. However, monitoring the production of the lactone byproducts, HOHA lactone or HOOA lactone, was not readily achieved by direct ESI-MS/MS analysis owing both to their neutral functionality and low molecular weight. Therefore, these lactone products were derivatized under mild conditions with 2,4-dinitrophenylhydrazine (DNPH) in the presence of a catalytic amount of formic acid in acetonitrile (Fig 4). A major advantage of the DNPH derivatization is that it reduces the volatility of low molecular weight compounds. HOHA lactone-DNPH and HOOA lactone-DNPH have parent ions ([M - H]−, m/z 319) and ([M - H]−, m/z 333), respectively. Also, both produce a dominant daughter ion m/z 152. Initially, the HPLC chromatograms of pure samples of HOHA lactone-DNPH and HOOA lactone-DNPH derivatives were monitored by ESI/MS/MS. LC-MS/MS analysis in the negative mode gave characteristic daughter fragments (Fig 5). Analytical methods for the simultaneous quantification of γ-hydroxy-α,β-unsaturated aldehydic esters of PCs, lysoPC, carboxyalkylfuran esters of 2-lysoPC (oxPC-furans), HOHA lactone-DNPH and HOOA lactone-DNPH were then developed using HPLC with on-line tandem mass spectrometry. LC-ESI/MS/MS standard curves were used to quantify these species with 1,2-ditridecanoyl-sn-glycero-3-phosphatidylcholine (DT-PC) and cinnamaldehyde-DNPH as internal standards (Fig S3).
Figure 4.
Derivatization of HOHA lactone and HOOA lactone with 2,4-dinitrophenylhydrazine (DNPH).
Figure 5.
Negative ESI-MS/MS spectrum of HOHA lactone-DNPH (A), HOOA lactone-DNPH (B), and HPLC chromatogram of authentic HOHA lactone-DNPH (C), HOOA lactone-DNPH (D), found HOHA lactone-DNPH (E) and HOOA lactone-DNPH (F) after incubation.
The rate of lysoPC generation decreases as chain length between hydroxyl and carboxyl increases
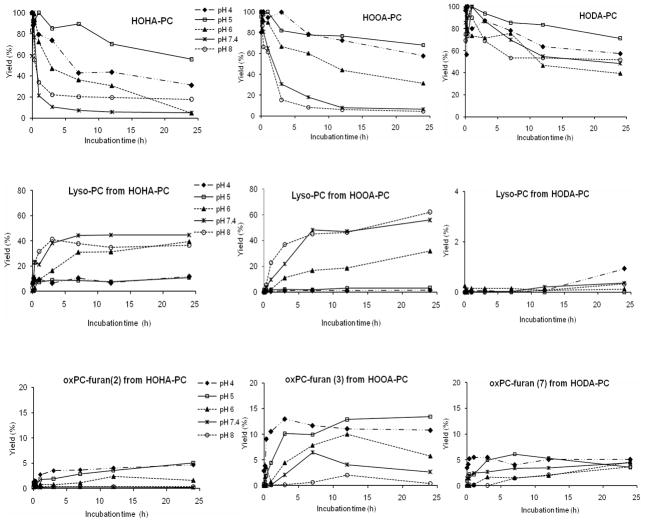
Our observation that free radical-induced oxidation of AA-PAF, but not LA-PAF, results in the generation of lyso-PAF (Fig 1) led us to postulate an intramolecular transesterification mechanism for the deacylation. We first tested the postulated effect on the ease of lyso-PC generation of the carbon chain length between the ester and hydroxyl groups at the sn-2 position in the oxPCs. As shown in Fig 6, the decomposition of HOHA-PC (t1/2 = 30 min) was more rapid than deacylation of the homologous HOOA-PC (t1/2 = 2 h) at 37 °C under neutral conditions (pH 7.4). After 24 h, HOHA-PC and HOOA-PC both decomposed more than 90%. In contrast, after 24 h, decomposition of HODA-PC was only 50% complete. As predicted, lysoPC generation from HOHA-PC was more rapid than that from HOOA-PC (40–50% yield from both), and less than 2% yield was produced from HODA-PC (Fig 6B) under the same reaction conditions.
Figure 6.
Time and pH dependence at 37 °C for (A) decomposition of γ-hydroxy-α,β-unsaturated aldehydic esters of 2-lysoPC, (B) generation of lysoPC and (C) generation of ω-carboxyalkylfuran esters of 2-lysoPC.
The spontaneous, nonenzymatic, reactions of γ-hydroxy-α,β-unsaturated aldehydic esters of 2-lysoPC were examined under various pH conditions. The decomposition of HOHA-PC to generate, inter alia, lysoPC occurs more readily (t1/2 = 30 min) under neutral conditions (pH 7.4) as compared to weakly acidic (t1/2 = 3 h at pH 6) or more acidic conditions (t1/2 = 6 h at pH 4). Thus acid inhibits the decomposition. Furthermore, deacylation of HOHA-PC is more rapid than deacylation of the homologous HOOA-PC at all pHs examined. Under acidic conditions (pH 4) a major product from HOOA-PC is the 2-(ω-carboxypropyl)furan ester of 2-lysoPC (oxPC-furan(3), 13%) (Fig 6C) and lysoPC is generated in less than 2% yield whereas decomposition of HOHA-PC under these conditions generates only a minor amount of the homologous 2-(ω-carboxyethyl)furan ester of 2-lysoPC (oxPC-furan(2), 2%) and much more lysoPC (10%). This contrasting behavior is again consistent with the expectation that intramolecular transesterification is much more favorable for HOHA-PC than for HOOA-PC.
Initially yields of lactone byproducts equal those of lysoPC
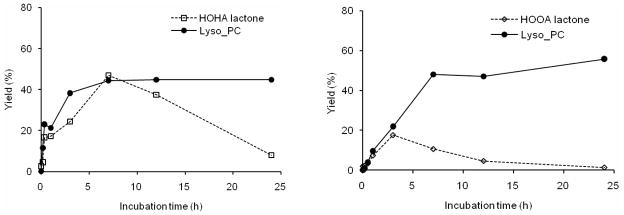
To confirm that the postulated byproducts, HOHA γ-lactone and HOOA δ-lactone, are generated by the putative intramolecular transesterification leading to deacylation of γ-hydroxy-α,β-unsaturated aldehydic esters of 2-lysoPC, the reaction product mixture was derivatized with DNPH in acetonitrile. The DNPH adducts generated in the reaction product mixture were identified by LC-ESI/MS/MS comparison with pure samples obtained by unambiguous total syntheses. The amounts of HOHA γ-lactone-DNPH and HOOA δ-lactone-DNPH products were monitored by LC-ESI/MS/MS (Fig 7). Yields of HOHA γ-lactone-DNPH and HOOA δ-lactone-DNPH increased with time, initially equaled those of lysoPC, and reached their maxima in less than 7 h or 3 h, respectively. However, the yields of HOHA γ-lactone-DNPH and HOOA δ-lactone-DNPH decreased during further incubation likely owing, inter alia, to lactone hydrolysis. Furthermore, the maximum yield of HOOA δ-lactone-DNPH is lower than that of HOHA γ-lactone-DNPH. This is the anticipated consequence of both the slower expected rate of formation and faster rate of hydrolysis of the six-membered ring HOOA δ-lactone-DNPH than the more stable five-membered ring HOHA γ-lactone-DNPH.
Figure 7.
Coproduction of 2-lysoPC and lactone byproducts at 37 °C and pH 7.4 from γ-hydroxy-α,β-unsaturated aldehydic esters of 2-lysoPC. Left panel: time courses for the production of HOHA-lactone and lysoPC from HOHA-PC. Right panel: time course for the production of HOOA-lactone and lysoPC from HOOA-PC. Lactone yields were determined after conversion to DNPH derivatives.
A diverse functional and structural array of oxPCs are susceptible to spontaneous deacylation
Spontaneous deacylation is not limited to oxPCs that incorporate a hydroxyl group on the γ- or δ-carbon adjacent to the ester functionality in their oxidized acyl moiety. Oxidatively truncated PCs that incorporate an aldehyde or ketone carbonyl on the γ- or δ-carbon adjacent to the ester functionality should also spontaneously deacylate through hydration of the carbonyl group. As expected, 2-(4-oxo-7-oxohept-5-enonyl)-PC, (KOHA-PC), which has a ketone carbonyl on the γ-carbon adjacent to the ester functionality, spontaneously deacylates with a similar t1/2 ~ 40 minutes (Fig 8) as the hydroxy analogue HOHA-PC. Presumably, the enedione functional array in KOHA-PC is in rapid equilibrium with a hydrate that cyclizes to generate a hemiacylal with concomitant release of lysoPC (see Fig 2).
Figure 8.
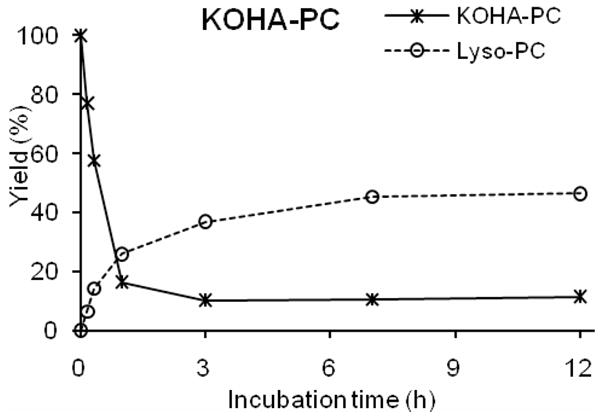
Time course for decomposition of KOHA-PC and generation of 2-lysoPC.
Discussion
All of the processes shown in Fig 2, including the free radical-catlayzed generation of γ-hydroxy-α,β-unsaturated aldehydic esters of PCs, can occur nonenzymatically. Our previous studies have shown that this biomolecular chemistry has important biomedical consequences. Thus, besides the newly discovered role as a precursor of 2-lysoPC, HOOA-PC is a proinflammatory molecule that regulates leukocyte-endothelial interactions. It dose-dependently activates human aortic endothelial cells to bind monocytes and causes a dose-dependent increase in levels of monocyte chemotactic protein-1 and interleukin-8 – chemokines that are important in monocyte entry into chronic lesions (29). HOOA-PC also inhibits LPS-induced expression of E-Selectin, a major adhesion molecule that mediates neutrophil endothelial interactions (29). We subsequently found that HOHA-, HOOA-, HODA-PC, and more oxidized derivatives are ligands for the scavenger receptor CD36. On macrophages, signaling through CD36 promotes endocytosis of oxLDL and foam cell formation (24). In the eye, these oxPCs promote physiologically important endocytosis of oxidatively damaged photoreceptor rod cell outer segment tips by retinal pigmented endothelial cells (21). On platelets they promote aggregation, accounting for the prothrombotic phenotype that is linked with hyperlipidemia and oxidant stress (30). They also inhibit scavenger receptor B1-mediated selective uptake of cholesteryl esters in hepatocytes, and thus, may have an inhibitory effect on reverse cholesterol transport. 2-(ω-Carboxyethyl)pyrrole (CEP)-protein modifications (Fig 2), that are generated from HOHA-PC, promote an immune-mediated retinal atrophy associated with “dry” age-related macular degeneration, and angiogenesis associated with the choroidal neovascularization of “wet” age-related macular degeneration (31, 32). CEP- and CPP-protein modifications (Fig 2) also accumulate in melanoma where they promote angiogenesis and consequent tumor growth (33). Besides these pathological roles, they also exhibit physiological activity, promoting the angiogenesis required for wound healing (33).
A diverse functional and structural array of oxPCs is expected to be susceptible to nonenzymatic deacylation
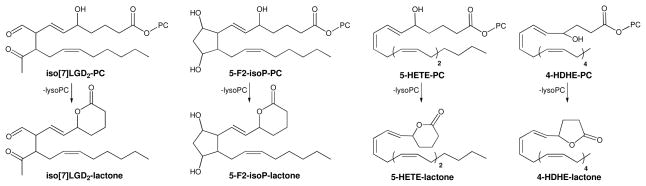
A wide variety of oxPCs – besides HOHA-, HOOA-, and HODA-PC – that incorporate hydroxyl or KOHA-PC that incorporates ketone functionality in their oxidized acyl moiety on the γ- or δ-carbon adjacent to the ester group are expected to undergo spontaneous deacylation. For example, isolevuglandins (34), isoprostanes (35), and hydroxypolyenoates such as iso[7]LGD2-PC and 5-F2-isoP-PC, and 5-HETE-PC or 4-HDHE-PC respectively (Fig 9) are expected to produce lysoPC through spontaneous deacylation. Thus, any oxPC that possesses a hydroxyl group (including that of a ketone hydrate) that is positioned to attack the ester linkage intramolecularly to form a 5-membered ring γ-lactone or 6-membered ring δ-lactone has an inherent proclivity towards spontaneous deacylation.
Figure 9.
Isolevuglandin, isoprostane, and hydroxyl polyunsaturated fatty acyl phospholipids. Isolevuglandins and isoprostanes such as iso[7]LGD2-PC and 5-F2-isoP-PC, respectively, as well as 5- or 6-hydroxy fatty acyl-PCs are expected to produce lysoPC and 5-membered ring γ-lactones or 6-membered ring δ-lactones through spontaneous deacylation.
Nonenzymatic lysoPC production will blunt the efficacy of antiphospholipase drugs
Inhibition of PLA2 is an emerging field of lysoPC-based targeted drug development in atherosclerosis. Drugs, such as Darapladib (SB 480848), targeted at inhibiting this enzymatic pathway (36) are currently in phase III clinical trials (37). However, the oxidative generation of γ-hydroxy- and γ-keto-α,β-unsaturated aldehydic ester oxPCs occurs nonenzymatically in vivo (Fig 2), and the phospholipid deacylations reported herein are not the result of enzyme-catalyzed lipolysis. Rather they occur because of an inherent chemical reactivity of certain oxidized phospholipids themselves that occurs readily and spontaneously, i.e., nonenzymatically, under conditions of temperature and pH found in biological systems. The nonenzymatic deacylation of γ-hydroxy- and γ-keto-α,β-unsaturated aldehydic ester oxPCs, uncovered in the present study, is especially noteworthy in the context of therapeutic measures to prevent pathology associated with the accumulation of lysoPC. It remains to be determined to what extent this previously unrecognized biomolecular chemistry, that produces lysoPC from oxPCs, will blunt the efficacy of anti-phospholipase drugs that block the release of lysoPC from oxidized phospholipids, and whether it will necessitate additional therapeutic measures to prevent the accumulation of lysoPC in vivo.
Supplementary Material
Acknowledgments
This work was supported by NIH grants EY016813, GM21249, and HL053315. We thank Dr. Mingjiang Sun for helpful discussions.
Footnotes
Supporting Information Available: Materials and General Methods, Tables S1–3, Figures S1–3
References
- 1.Chen X, Zhang W, Laird J, Hazen SL, Salomon RG. Polyunsaturated phospholipids promote the oxidation and fragmentation of gamma-hydroxyalkenals: formation and reactions of oxidatively truncated ether phospholipids. J Lipid Res. 2008;49:832–846. doi: 10.1194/jlr.M700598-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang R, Rodrigues B, MacLeod KM. Lysophosphatidylcholine potentiates phenylephrine responses in rat mesenteric arterial bed through modulation of thromboxane A2. J Pharmacol Exp Ther. 2006;317:355–361. doi: 10.1124/jpet.105.097964. [DOI] [PubMed] [Google Scholar]
- 3.Shi A, Yoshinari M, Iino K, Wakisaka M, Iwase M, Fujishima M. Lysophosphatidylcholine molecular species in low density lipoprotein and high density lipoprotein in alloxan-induced diabetic rats: effect of probucol. Exp Clin Endocrinol Diabetes. 1999;107:337–342. doi: 10.1055/s-0029-1212123. [DOI] [PubMed] [Google Scholar]
- 4.Shi AH, Yoshinari M, Wakisaka M, Iwase M, Fujishima M. Lysophosphatidylcholine molecular species in low density lipoprotein of type 2 diabetes. Horm Metab Res. 1999;31:283–286. doi: 10.1055/s-2007-978734. [DOI] [PubMed] [Google Scholar]
- 5.Sasagawa T, Suzuki K, Shiota T, Kondo T, Okita M. The significance of plasma lysophospholipids in patients with renal failure on hemodialysis. J Nutr Sci Vitaminol (Tokyo) 1998;44:809–818. doi: 10.3177/jnsv.44.809. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs B, Schiller J, Wagner U, Hantzschel H, Arnold K. The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: investigations by 31P NMR and MALDI-TOF MS. Clin Biochem. 2005;38:925–933. doi: 10.1016/j.clinbiochem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Mehta D, Gupta S, Gaur SN, Gangal SV, Agrawal KP. Increased leukocyte phospholipase A2 activity and plasma lysophosphatidylcholine levels in asthma and rhinitis and their relationship to airway sensitivity to histamine. Am Rev Respir Dis. 1990;142:157–161. doi: 10.1164/ajrccm/142.1.157. [DOI] [PubMed] [Google Scholar]
- 8.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Fan P, Shimoji E, Itabe H, Miura S, Uehara Y, Matsunaga A, Saku K. Modulating effects of cholesterol feeding and simvastatin treatment on platelet-activating factor acetylhydrolase activity and lysophosphatidylcholine concentration. Atherosclerosis. 2006;186:291–301. doi: 10.1016/j.atherosclerosis.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Murphy AA, Santanam N, Morales AJ, Parthasarathy S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T-lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab. 1998;83:2110–2113. doi: 10.1210/jcem.83.6.4823. [DOI] [PubMed] [Google Scholar]
- 11.Ryborg AK, Gron B, Kragballe K. Increased lysophosphatidylcholine content in lesional psoriatic skin. Br J Dermatol. 1995;133:398–402. doi: 10.1111/j.1365-2133.1995.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrecher UP, Zhang HF, Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9:155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- 13.Sakai M, Miyazaki A, Hakamata H, Sasaki T, Yui S, Yamazaki M, Shichiri M, Horiuchi S. Lysophosphatidylcholine plays an essential role in the mitogenic effect of oxidized low density lipoprotein on murine macrophages. J Biol Chem. 1994;269:31430–31435. [PubMed] [Google Scholar]
- 14.Parthasarathy S, Barnett J. Phospholipase A2 activity of low density lipoprotein: evidence for an intrinsic phospholipase A2 activity of apoprotein B-100. Proc Natl Acad Sci U S A. 1990;87:9741–9745. doi: 10.1073/pnas.87.24.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tew DG, Southan C, Rice SQ, Lawrence MP, Li H, Boyd HF, Moores K, Gloger IS, Macphee CH. Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low-density lipoproteins. Arterioscler Thromb Vasc Biol. 1996;16:591–599. doi: 10.1161/01.atv.16.4.591. [DOI] [PubMed] [Google Scholar]
- 16.Lynch SM, Frei B. Mechanisms of copper- and iron-dependent oxidative modification of human low density lipoprotein. J Lipid Res. 1993;34:1745–1753. [PubMed] [Google Scholar]
- 17.Ali S, Davis MG, Becker MW, Dorn GW., 2nd Thromboxane A2 stimulates vascular smooth muscle hypertrophy by up-regulating the synthesis and release of endogenous basic fibroblast growth factor. J Biol Chem. 1993;268:17397–17403. [PubMed] [Google Scholar]
- 18.Cathcart MK, McNally AK, Chisolm GM. Lipoxygenase-mediated transformation of human low density lipoprotein to an oxidized and cytotoxic complex. J Lipid Res. 1991;32:63–70. [PubMed] [Google Scholar]
- 19.Negre-Salvayre A, Lopez M, Levade T, Pieraggi MT, Dousset N, Douste-Blazy L, Salvayre R. Ultraviolet-treated lipoproteins as a model system for the study of the biological effects of lipid peroxides on cultured cells. II. Uptake and cytotoxicity of ultraviolet-treated LDL on lymphoid cell lines. Biochim Biophys Acta. 1990;1045:224–232. doi: 10.1016/0005-2760(90)90124-g. [DOI] [PubMed] [Google Scholar]
- 20.Gu X, Sun M, Gugiu B, Hazen S, Crabb JW, Salomon RG. Oxidatively truncated docosahexaenoate phospholipids: total synthesis, generation, and peptide adduction chemistry. J Org Chem. 2003;68:3749–3761. doi: 10.1021/jo026721t. [DOI] [PubMed] [Google Scholar]
- 21.Sun M, Finnemann SC, Febbraio M, Shan L, Annangudi SP, Podrez EA, Hoppe G, Darrow R, Organisciak DT, Salomon RG, Silverstein RL, Hazen SL. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J Biol Chem. 2006;281:4222–4230. doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng Y, Salomon RG. Total synthesis of ©-hydroxy-α,β-unsaturated aldehydic esters of cholesterol and 2-lysophosphatidylcholine. J Org Chem. 1998;63:7789–7794. [Google Scholar]
- 23.Gallasch BA, Spiteller G. Synthesis of 9,12-dioxo-10(Z)-dodecenoic acid, a new fatty acid metabolite derived from 9-hydroperoxy-10,12-octadecadienoic acid in lentil seed (Lens culinaris Medik.) Lipids. 2000;35:953–960. doi: 10.1007/s11745-000-0605-z. [DOI] [PubMed] [Google Scholar]
- 24.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 25.Sun M, Deng Y, Batyreva E, Sha W, Salomon RG. Novel bioactive phospholipids: practical total syntheses of products from the oxidation of arachidonic and linoleic esters of 2-lysophosphatidylcholine(1) J Org Chem. 2002;67:3575–3584. doi: 10.1021/jo0105383. [DOI] [PubMed] [Google Scholar]
- 26.Gu X, Zhang W, Salomon RG. Fe2+ catalyzes vitamin E-induced fragmentation of hydroperoxy and hydroxy endoperoxides that generates gamma-hydroxy alkenals. J Am Chem Soc. 2007;129:6088–6089. doi: 10.1021/ja0689785. [DOI] [PubMed] [Google Scholar]
- 27.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 28.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 29.Subbanagounder G, Deng Y, Borromeo C, Dooley AN, Berliner JA, Salomon RG. Hydroxy alkenal phospholipids regulate inflammatory functions of endothelial cells. Vascul Pharmacol. 2002;38:201–209. doi: 10.1016/s1537-1891(02)00170-2. [DOI] [PubMed] [Google Scholar]
- 30.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollyfield JG, Perez VL, Salomon RG. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol. 2010;41:290–298. doi: 10.1007/s12035-010-8110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur K, Salomon RG, O’Neil J, Hoff HF. (Carboxyalkyl)pyrroles in human plasma and oxidized low-density lipoproteins. Chem Res Toxicol. 1997;10:1387–1396. doi: 10.1021/tx970112c. [DOI] [PubMed] [Google Scholar]
- 35.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohler ER, 3rd, Ballantyne CM, Davidson MH, Hanefeld M, Ruilope LM, Johnson JL, Zalewski A. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2008;51:1632–1641. doi: 10.1016/j.jacc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 37.Rosenson RS. Future role for selective phospholipase A2 inhibitors in the prevention of atherosclerotic cardiovascular disease. Cardiovasc Drugs Ther. 2009;23:93–101. doi: 10.1007/s10557-008-6148-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.