Abstract
Stem cells play an important role in restoring cardiac function in the damaged heart. In order to mediate repair, stem cells need to replace injured tissue by differentiating into specialized cardiac cell lineages and/or manipulating the cell and molecular mechanisms governing repair. Despite early reports describing engraftment and successful regeneration of cardiac tissue in animal models of heart failure, these events appear to be infrequent and yield too few new cardiomyocytes to account for the degree of improved cardiac function observed. Instead, mounting evidence suggests that stem cell mediated repair takes place via the release of paracrine factors into the surrounding tissue that subsequently direct a number of restorative processes including myocardial protection, neovascularization, cardiac remodeling, and differentiation. The potential for diverse stem cell populations to moderate many of the same processes as well as key paracrine factors and molecular pathways involved in stem cell-mediated cardiac repair will be discussed in this review.
Keywords: Paracrine Factors, Stem Cells, Cardiac Repair, Cardiac Regeneration
Introduction
Myocardial infarction (MI) is one of the major causes of cardiovascular mortality and morbidity, especially congestive heart failure [1]. Despite major advances in drug and interventional therapies, surgical procedures and organ transplantation, restoration and regeneration of the damaged myocardium remains a tremendous challenge. Stem cell therapy has generated significant interest and to date preclinical research has documented its therapeutic potential. Clinical studies, still in early stages, have reported that this therapeutic modality may lead to an overall improvement of cardiac function [2, 3]. The precise underlying mechanisms of stem cell action are still under debate. There is a growing body of evidence supporting the hypothesis that stem cells can enhance endogenous repair and regenerative processes through the release and actions of paracrine factors.
Numerous studies have demonstrated that stem cells contribute to tissue repair and regeneration by releasing important paracrine factors in a dynamic spatial-temporal manner that can lead to cell survival, angiogenesis, tissue repair and remodeling, as well as cellular regeneration [4-7]. Moreover it has been postulated that the cross-talk facilitated by stem cells in the cardiac microenvironment includes both direct autocrine communication as well as paracrine-mediated signaling with surrounding cells.
Various types of adult stem cells that have demonstrated therapeutic potential with potential paracrine activities can be broadly categorized as bone marrow-derived, circulating, and resident to the heart [7]. Studies that have claimed the use of bone marrow derived stem cells have included a broad range of cells from purified mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs), to bone marrow-derived mononuclear cells (BM-MNCs), and unfractionated bone marrow cells (BMCs). Evidence supports the contribution of paracrine mediators to the actions of these cells [6-10]. The category of circulating stem/ progenitor cells is represented principally by endothelial progenitor cells (EPCs) that can be isolated from circulation and have been reported to restore blood flow to ischemic myocardium via paracrine mechanisms[11]. Resident cardiac progenitor/stem cells (CPCs) are believed to represent self-renewing populations of cells confined to specific niches within the heart that may be stimulated to proliferate and differentiate as a result of paracrine effects[12]. While embryonic stem cells (ESCs) continue to hold promise for regenerative research, their role in paracrinemediated cell therapy has not been extensively studied. Recent data suggest that multiple factors secreted by these cells may be important for their therapeutic effect [9, 10]. The recent discovery of induced pluripotent stem cells by Takahashi and Yamanaka circumvents ESC-related concerns [13] and holds much promise for cardiac regenerative medicine [14, 15]. Still, their contribution to paracrine-mediated stem cell therapy remains unknown at present.
The majority of studies relating to paracrine-mediated cardiac repair have utilized adult stem cells and therefore this review will primarily focus on the mediation of these cells in regulating the healing process in the heart via secretion of key regulatory molecules (Figure 1).
Figure 1.
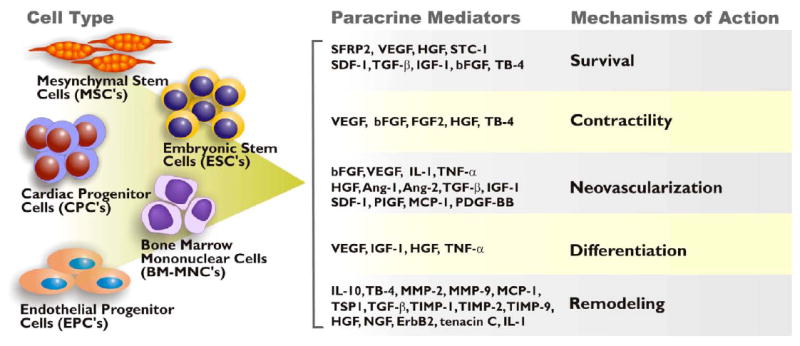
Summary of stem cell-secreted paracrine factors. The proposed cardiac repair mechanisms these factor modulate include cell survival, neovascularization, remodeling, differentiation, contractility, and regeneration.
Bone marrow mononuclear cells (BM-MNCs)
Bone marrow mononuclear cells (BM-MNCs) represent a crude mixture of self-renewing mononuclear cells isolated from gradient fractionation of bone marrow aspirates [16]. As such, they comprise a heterogeneous population of stem/progenitor cells (HSCs, MSCs and EPCs), stromal elements (bone specula, fat and fibroblasts), and mature blood cells [17]. BM-MNCs are the most utilized stem cell population for clinical trials concerning ischemic and chronic heart disease with currently over 100 open studies worldwide [18]. In culture, BM-MNC conditioned media has been shown to preserve the contractile potential of adult cardiomyocytes. Specifically, fractional shortening and Ca2+ ratio amplitude of myocytes incubated with BM-MNC supernatants were better preserved than myocytes cultured in control medium [19]. Additionally, microvessel density and fractional shortening was improved in animals administered with BM-MNC supernatant [19]. In the latter case, systemic injection in addition to intramyocardial delivery of BM-MNC conditioned media was found necessary for an improvement of cardiac function.
BM-MNC delivery to ischemic tissue have been shown to significantly increase tissue levels of angiogenic ligands such as bFGF and VEGF and cardiac levels of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) [20]. The authors concluded that the released factors likely contributed to angiogenic induction in BM-MNC treated animals. Interestingly, BM-MNCs secrete significant amounts of IL-10 and it has been recently reported that the protective effects of BM-MNCs are at least partially mediated by this protein. Specifically, IL-10 depleted BM-MNCs failed to reduce infarct size, neutrophil accumulation, and neovascularization relative to wild-type counterparts. Instead, these cells appear to impact remodeling by interfering with T-cell recruitment, collagen deposition, and reactive hypertrophy [21].
Mesenchymal Stem cells
Perhaps one of the most extensively investigated stem cell types for their paracrine-mediated effects are the mesenchymal stem cells (MSCs). MSCs are multipotent adult stromal stem cells that differentiate into a variety of tissues including muscle, cartilage, bone, skin, and fat. While traditionally isolated from the bone marrow, cells with MSC-like properties have also been isolated from other tissues including skeletal muscle[22], peripheral blood[23], adipose tissue [24] and lung [25]. While a major utility of MSC is focused on direct transdifferentiation to regenerate of bone and cartilage for skeletal therapies [26], in the treatment of cardiovascular disease, MSCs have gained much recognition for their paracrine-mediated protective effects on stressed or injured myocardium [27-30]. Additionally, MSCs have been reported to secrete factors that protect the heart from ischemia, activate neovascularization, improve contractility and bioenergetics, and attenuate fibrosis following myocardial injury.
a) Myocardial Protection
Experimental studies recapitulating the prosurvival effects of stem cell therapy via the administration of cell-free conditioned medium in both in vitro and in vivo platforms have established that mesenchymal stem cells can lead to increased cardiomyocyte survival via a paracrine mechanism. Gnecchi et al. have demonstrated that conditioned media from MSCs exposed to hypoxia was cytoprotective of isolated adult rat ventricular cardiomyocytes and significantly reduced infarct size in a rodent infarct model after MSC transplantation [31]. In particular, it was observed that conditioned media from MSCs overexpressing the Akt gene (Akt-MSCs) inhibited apoptosis of isolated cardiomyocytes exposed to hypoxia as demonstrated by a reduction of morphologic evidence of necrotic or apoptotic cell death and an attenuation of Caspase 3 release [31]. Follow up functional genomics studies to identify the key Akt-MSCreleased paracrine factors responsible for mediating protection of the injured myocardium revealed that Sfrp2, a member of the Wnt signaling pathway, is significantly upregulated in Akt- MSCs compared to control MSCs and its attenuation by siRNA silencing abrogated Akt-MSCmediated cytoprotective effects [32]. More recent studies conducted by members of our group indicate that a novel secreted protein, Hypoxic induced Akt regulated Stem cell Factor (HASF), that is upregulated in Akt-MSCs subjected to normoxia or hypoxia, may mediate survival effects in isolated hypoxic cardiomyocytes via PKC-ε signaling which in turn, may provide cardioprotection by blocking activation of mitochondrial death channels [33].
Furthermore, Uemura and colleagues recently reported that preconditioning of MSCs enhanced their survival and ability to attenuate LV remodeling, which was attributable, in part, to paracrine effects [34]. Moreover, work conducted by Prockop et al. has shown that MSCs subjected to UV irradiation, secrete stanniocalcin-1 (STC-1), a peptide hormone that modulates mineral metabolism and is required for protection from UV- induced cell death. It would be of interest to test whether stanniocalcin-1 might play a similar role in cardioprotection by MSCs via paracrine mechanisms[35]. Interestingly in another study it was shown that ablation of the TNF receptor 1 (TNFR1) but not TNFR2 from mouse MSCs increased the MSC growth factor production and enhanced their cardioprotective effects after transplantation in the injured myocardium [36]. Based on this evidence it was further postulated that TNFR1 signaling may damage MSC paracrine effects and decrease MSC-mediated cardioprotection, whereas TNFR2 likely mediates beneficial effects in MSCs.
Importantly Nguyen et al. have recently shown using a swine model of acute MI that intracoronary injection of either concentrated MSC-derived growth factors or control medium significantly reduced cardiac troponin-T elevation and improved echocardiographic parameters [30]. Further analysis demonstrated reduced levels of fibrosis and cardiomyocyte apoptosis
b) Neovascularization
To date, accumulating evidence supports the hypothesis that the predominant mechanisms driving angiogenesis and arteriogenesis, post-MI, are orchestrated via the release of stem-cell derived paracrine factors. MSCs in particular, secrete high levels of proangiogenic and proarteriogenic factors such as the angiopoietins, VEGF, bFGF, and HGF all of which have been implicated in MSC-mediated neovascularisation [37, 38]. Moreover, Markel and colleagues have shown that VEGF is a crucial mediator of MSC-mediated effects in the injured rat myocardium by demonstrating that its ablation negatively impacts stem cell-mediated myocardial recovery following ischemia [39]. Additionally, MSC administration in rodent models of permanent occlusion leads to increased capillary density in several studies [38, 40]. Recently, Zhou et al. have shown that cell transplantation of autologous MSCs in the heart of a porcine model of chronic ischemia resulted in improved cardiac function associated with increased vascularity[41].
Multiple factors regulate the expression of pro-angiogenic factors in MSCs, of which Toll-like receptor 2 (TLR2) has been resent shown to control the increase in VEGF production following cytokine or ischemic treatment [42]. In brief, using MSC derived from mice in which the TLR2 was knocked out, Abarbanell et al. have shown that ablation of TLR2 resulted in reduced expression of VEFG from the MSCs and affected their repair capacity. Since it has been shown that that TLR2 mediates VEGF production via ERK- and activator protein-1-dependent pathways [43], it remains to be tested if the Erk signaling pathway is involved in regulating the TLR2 mediated effects in the production of VEGF from MSCs.
Interestingly, a recent study by Webber et al. utilized a novel heparin-presenting injectable nanofiber network in order to bind and deliver factors released from hypoxic conditioned MSC media to the heart following coronary ligation [44]. Conditioned media-loaded heparin-binding peptide amphiphile (HBPA) nanofibers injected into the left ventricle following ischemic injury improved contractility significantly compared to untreated controls. Interestingly these effects on contractility were primarily observed when HBPA was loaded with proteins <30kDa. Initial attempts to extract crucial paracrine factors showed that recombinant VEGF- and bFGF-loaded nanofiber networks were able to recapitulate the media effects. Similar experiments in a hind limb ischemia model showed that revascularization could partially account for the improved functionality [44].
c) Metabolism
The influence of stem cell therapy on the metabolic fate of infarcted hearts is an area of investigation that has received relatively little attention. In the healthy non-ischemic heart, almost all ATP produced is as a result of oxidative phosphorylation in the mitochondria. The acetyl-coA necessary to fuel the citric acid cycle is primarily generated via the β-oxidation of fatty acids with the remaining sources being from the oxidation of pyruvate (from both glycolysis and lactate oxidation)[6, 45]. Heart failure is characterized by a change in substrate preference from fatty acid oxidation to increased glucose uptake and a subsequent shift from net lactate consumption to production[45]. Additionally, the infarct border zone is characterized by abnormal bioenergetics including a high-energy phosphate content and phosphocreatine-to-ATP ratio and is believed to correspond to the severity of left ventricular contractile dysfunction [46].
A study conducted by our group demonstrated that the infusion of Akt-MSCs prevented cardiac metabolic remodeling following myocardial infarction. This was determined by measurement of phosphocreatine levels, rate of glucose uptake, and cytosolic pH all of which appeared better preserved in Akt-MSC-treated hearts when compared to unmodified MSC-treated and untreated controls as early as 72 hours following cardiac insult [47]. Since the results obtained from infusion with unmodified MSCs were similar to untreated controls, the overexpression of Akt appears to be regulating the normalized energetics observed in the surviving myocardium of Akt-MSC-treated animals. Additionally, these results corroborate earlier reports by Lim et al. and Feygin et al. who demonstrated normalized phosphocreatine levels in swine models of permanent coronary occlusion 4 weeks following myocardial infarction [48, 49].
d) Contractility
The impact of MSC administration on cell contractility due to paracrine mechanisms has also been observed by our group and others [31, 50]. This may due to the consequence of cardiomyocyte protection and/or direct release of inotropic factors. Mesenchymal transplantation of autologous bone marrow stem cells in a non-ischemic (doxorubicin-induced) model of heart failure by Dhein et al. led to an improvement in long-term cardiac hemodynamic function[50]. This was assessed by measurement of β—adrenoceptor density and contractility 28 days following cardiac insult. Rabbit hearts injected intramyocardially with MSCs exhibited an attenuation in the downregulation of adrenoceptor density and a significant increase in contractility. The β—adrenoceptor density downregulation was especially marked in the septum and left ventricle compared to sham-operated counterparts. The authors speculated that the improved hemodynamics lead to a decrease in catecholamine levels and a subsequent attenuation in β—adrenoceptor downregulation. While the mechanisms underlying these observations is yet unknown, the possibility that MSCs may be mediating these effects via the release of paracrine factors is supported by the work conducted in this study and by others. In fact, the possibility that MSCs transdifferentiate was excluded by the same group in a recent report showingthat transplanted MSCs fail to express cardiac markers one month following transplantation [51].
e) Remodeling
Paracrine factors released by transplanted stem cells may alter the extracellular matrix and favorably influence post-infarction remodeling of the heart chambers. It has been shown in animal models that MSC transplantation decreases fibrosis in the heart and other organs including lung [52], liver [53] and kidney [54]. Specifically, direct injection of hMSCs into ischemic rat myocardium decreased fibrosis, apoptosis and left ventricular dilatation while increasing myocardial thickness; this resulted in the prevention of systolic and diastolic cardiac dysfunction without evidence of myocardial regeneration [55]. Additionally, MSC transplantation significantly attenuated the increase in cardiac expression of collagen types I and III, tissue inhibitor of metalloproteinase-1 (TIMP1) [56], and tumor growth factor beta (TGF-â) in infarcted myocardium [57]. Since MSCs express a number of molecules involved in the biogenesis of extracellular matrix such as adrenomedullin, thymosin-β, thymocollagenase, metalloproteinases (MMPs), serine proteases and their inhibitors, it has been suggested that transplanted MSCs can inhibit fibrosis through paracrine actions [58]. Likewise, transplantation of MSCs led to decreased fibrosis in a rat model of dilated cardiomyopathy via the decrease in MMP-2 and MMP-9 protein expression [59]. Similar results by Ohnishi et al., have led to the postulation that MSCs exhibit paracrine-mediated antifibrotic effects[60]. Collectively, these studies suggest that MSCs may have a direct effect on extracellular matrix remodeling via secretion of extracellular matrix modulating proteins.
When injected into injured tissue, stem cells may also attenuate local inflammation by releasing signaling molecules within the immediate microenvironment. MSCs transplanted into ischemic tissue led to decreased expression of the pro-inflammatory cytokines TNF-α, IL-1α and IL-6, which are known to regulate left ventricular remodeling [56]. Likewise, MSC transplantation into a rat model of acute myocarditis attenuated the increase in CD68+ inflammatory cells and myocardial monocyte chemoattractant protein-1 (MCP-1) expression [61]. Furthermore, isolated adult rat cardiomyocytes (ARVCs) cultured in the presence of MSC conditioned media were more resistant to MCP-1-induced injury. T lymphocytes from post-infarcted mice cocultured with cardiac fibroblasts also led to an increase in pro-collagen expression [62], suggesting that the in vivo suppression of T lymphocyte accumulation and/or function may also inhibit fibrosis.
In addition Tang et al. have recently shown that engineering of MSCs to overexpress SDF1 affected their capabilities in regulating cardiac remodeling after injury [63]. Specifically, SDF-MSC-treated hearts showed higher levels of antifibrotic factor HGF expression and significant reduction of the expression of collagens I and III and matrix metalloproteinase 2 and 9.
f) Cardiac differentiation
Despite evidence suggesting that MSC capacity to undergo cardiac differentiation is limited, recent evidence suggests that MSCs might contribute to cardiac regeneration by indirectly affecting cardiac progenitor stem cell proliferation and differentiation. Of note, the Nagaya group has shown that MSC conditioned medium protected CPCs from hypoxia-induced apoptosis and enhanced their proliferative capacity [60]. Interestingly, they were also able to detect enhanced gene expression of cardiac myocyte markers in CPCs treated with MSCderived supernatants. In addition, it was recently shown that selection of MSCs based on STRO-1 expression yields a population with higher clonogenic, multipotent and proliferative capacity [64]. The conditioned medium from this selected cell population also showed enhanced capability in inducing cardiac cell proliferation and migration and endothelial cell migration and tube formation[64].
While no specific paracrine mediators for CPC activation have been reported as yet, it can be postulated that MSCs secrete molecules influencing cardiac differentiation. It has been reported that MSCs express BMPs, Wnt pathway modulators and FGF [65, 66], all of which represent key regulators of cardiac cells differentiation and commitment, suggesting that cardiac expansion may be directed by paracrine mechanisms. However, whether these molecules contribute to the paracrine regenerative capacity of MSCs by activation of resident cardiac progenitors remains to be investigated.
Endothelial progenitor cells (EPCs)
EPCs are thought to be primarily bone marrow-derived and following ischemia, home to sites of injury to restore the endothelial lining of damaged blood vessels. The balance between endothelial dysfunction and recovery may be predictive of certain cardiovascular risk factors [67]. Since mature endothelial cells (ECs) have limited regenerative potential, the possibility that circulating EPCs may mediate endothelial regeneration has generated much interest in terms of their therapeutic potential. Recent evidence reveals a role for EPC derived paracrine mechanisms in the prevention of oxidative stress-induced apoptosis of EPCs and mature differentiated ECs [68]. Specifically, EPC conditioned media significantly reduced levels of intracellular oxidative stress and reduced apoptosis in hydrogen peroxide-treated human umbilical vein endothelial cells (HUVECs). Interestingly, the neutralization of VEGF, HGF, IL-8 and MMP-9 did not attenuate the cytoprotective effects of EPC conditioned media suggesting that these cells may exert their influence via alternative mechanisms likely relating to intracellular antioxidant pathways [68]. Paracrine mechanisms have also been shown to account for EPC-mediated angiogenic effects. In particular, VEGF and stromal derived factor 1 (SDF-1) released by EPC into conditioned media promote the migration of mature ECs and the formation of capillaries via differentiation-independent mechanisms [69]. This was further validated by a recent report by Di Santo et al. demonstrating that delivery of EPC conditioned media to ischemic tissue could confer similar angiogenic effects [70]. The release of cardiotrophic factors by EPCs has also been implicated in the regulation of cardiac remodelling post-MI [71]. Intracoronary injection of conditioned media from EPCs in a porcine model of MI resulted in increased cardiomyocyte size. In vitro experiments demonstrated that the conditioned media from EPCs increased cell mass of cultured cardiomyocytes and that these effects were partly mediated by TGF-β1 [71]. Furthermore, EPCs also release a variety of proinflammatory cytokines such as tissue factor 1 and MCP-1. A better understanding of the paracrine profile of EPCs should provide insights into the full biologic and therapeutic potentials [72, 73].
Resident cardiac progenitor cells
The recent discovery of endogenous or resident cardiac progenitor cells (CPCs) has generated much interest regarding their identity(s) and proliferative/differentiation potentials. Emerging evidence suggests that resident CPCs can function in a paracrine manner [74, 75]. Conditioned media from human cardiosphere and cardiosphere derived cells (CDCs) have been shown to enhance the survival of cardiomyocyte to hypoxia as well as induce Matrigel tube formation of ECs [76]. VEGF and HGF were found to be two secreted factors responsible for these effects. In addition, injection of CDCs into infarcted myocardium increased expression of Akt, decreased Caspase 3 activity and apoptosis, and increased capillary density. While direct differentiation of CDCs accounted for a small portion of the new capillaries and cardiomyocytes, human CDCs enhanced the number of cKit+ and Nkx2.5+ cells in the infarct border zone, suggesting activation of endogenous cardiac progenitors [76]. Likewise, intracoronary administration of cKit+ CPCs into rat hearts following acute ischemia not only reduced infarct size and fibrosis through differentiation into cardiomyocytes and vascular cells, but also induced proliferation of resident cKit+ CPCs in the infarct zone presumably through a paracrine mechanism [77]. These initial studies warrant further investigation to determine how paracrine or autocrine signals from resident CPCs affect the myocardial repair post-MI.
Embryonic stem cells
Of all stem cells populations, embryonic stem cells (ESCs) possess the most regenerative potential and as such remain an attractive prospect for cardiac cell therapy. ESCs have the propensity to spontaneously differentiate in vitro into cardiomyocytes. Presumably this ability is controlled by spatial and temporal coordination of surface and secreted differentiation factors produced by adjacent cells or through autocrine mechanisms. A number of these secreted factors have been identified and utilized to induce cardiogenesis of ESCs [78]. In addition, proteomic analysis of hESC conditioned media yielded cytokines and growth factors involved in cardiac remodeling and proliferation of neonatal cardiomyocytes, including thrombospondin, TGF-β, MMP-2/-9, TIMP-1/-2/-9, HGF, NGF, and ErbB2 [10]. In an ischemic-reperfusion model of cardiac injury, Crisostomo et al. observed that pre-ischemic infusion of ESCs conferred significantly greater improvement of cardiac function post-MI compared with saline or MSC controls. Interestingly, ESC-conditioned media alone while being cytoprotective did not provide significant improvement of myocardial function in the same injury model [9]. The authors of this study surmise that in the case of ESC-mediated effects on injured cardiac tissue, other stem cell protective mechanisms may be responsible for cardioprotection in addition to paracrine mechanisms. In addition to ESCs, embryonic-derived endothelial progenitor cells (eEPCs) have been shown to exhibit cytoprotective effects on both cardiomyocytes and endothelial cells exposed to hypoxia and reoxygenation by the secretion of thymosin-β4 [79], an activator of the PI3K/Akt pathway [80].
Autocrine mechanisms in stem cell maintenance
It has been postulated that the cross-talk facilitated by stem cells in the cardiac microenvironment includes both direct autocrine communication as well as paracrine-mediated signaling with surrounding cells [6]. In other words, the biology of stem cells within their niche is dynamic, and likely governed by the spatial and temporal release of factors from themselves at any given time. Autocrine/paracrine feedback is believed to trigger CPC activation in response to stress. Secreted growth factors such as IGF-1, HGF, and SDF-1 generated by stress-induced cardiomyocytes have been shown to bind to receptors on CPCs consequently activating production of these ligands on CPCs themselves[81]. Activation of resident CPCs in response to environmental stimuli promotes the proliferation and differentiation of these cells and is sustained even after its initial catalyst has dissipated[81]. Survival and self-renewal in a variety of stem cell lineages appear to be mediated by autocrine mechanisms. For example, the maintenance, differentiation and expansion of hematopoietic stem cells has been attributed in part to VEGF[82] and likewise, FGF is speculated to maintain MSC self-renewal[83]. We have recently demonstrated that silencing Sfrp2 in a pluripotent mouse cell line leads to activation of Wnt signaling in an autocrine manner [84]. Similarly, MSCs overexpressing Sfrp2 exhibit an enhanced regenerative capacity and appear to modulate their own propagation via downregulation of key Wnt targets [85]. Additionally, cardiac differentiation in embryonic stem cells has been reported to be modulated by intracrine signaling such as the effects of certain growth regulatory peptides on transcriptional responses of the same cells [86].
FUTURE DIRECTIONS AND CHALLENGES
Identification of Paracrine Factors
The evidence for stem cell-derived paracrine factors mediating cardiac repair represents an important step forward in our understanding of stem cell biology. Identifying which factors mediate these effects, the molecular pathways involved and the relevant temporal and spatial expression patterns will strengthen our knowledge of how adult stem cells affect the complex processes of cardiac protection, neovascularization, remodeling, metabolism and regeneration (Figure 2).
Figure 2.
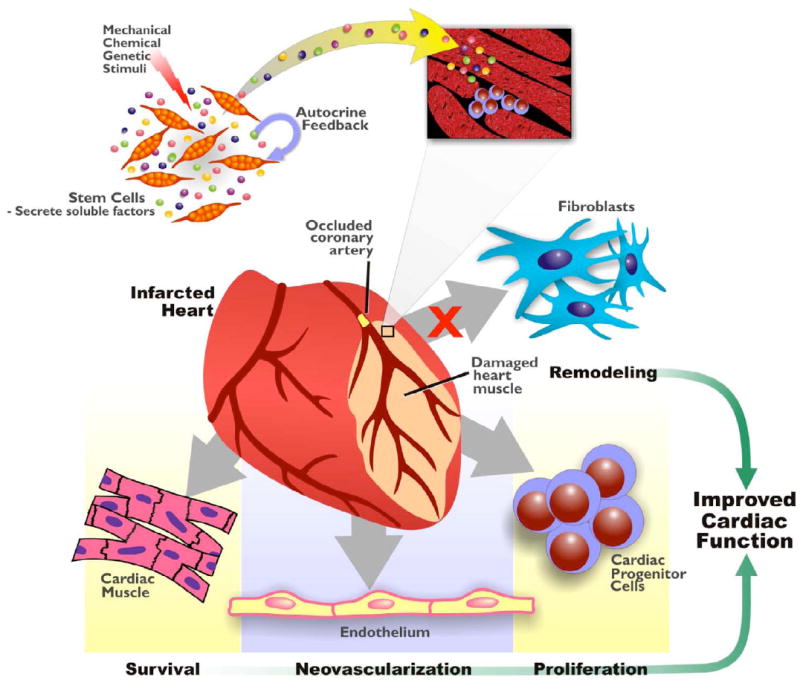
Autocrine/Paracrine mechanisms in cardiac repair. In response to environmental stimuli, stem cells release soluble factors that dynamically alter the myocardial microenvironment. These biologically active molecules exert effects on a variety of different cardiac cell types including the originating stem cells themselves. Paracrine actions of these factors lead to tissue protection, repair and regeneration while stem cell self-renewal and expansion is also regulated via autocrine feedback.
Multiple groups have identified a plethora of potential cytoprotective molecules by differential expression microarray analysis [32, 69]. Likewise, proteome and secretome profiling has emerged as a viable option for analysis of paracrine factors. However, the analysis of secreted proteins using proteomic techniques is currently hampered by sample preparation. Proteins are usually secreted at low concentrations in culture media subsequently requiring selective precipitation or ultrafiltration for analysis. In addition, culture media components, such as salts and serum proteins, interfere with most proteomics techniques. Despite these limitations, several groups have successfully used proteomics to identify important paracrine factors [74, 87, 88]. Comparative proteomics of rat neonatal cardiomyocytes versus CPCs identified 33 secreted proteins, including known cardioprotective factors adrenomedulin, connective tissue growth factor, and IL-1 receptor-like 1 (ST2)[74]. Likewise, identifying biologically active protein fragments and nonpolypeptide metabolites, including phospholipids [89], fatty acid chains, inotropic compounds, antioxidants and hormones, released by adult progenitor cells in response to hypoxia may aid in cardiac repair and remodeling. Genetic and metabolic profiling approaches have been successful in identifying coronary artery disease signatures at a submicromolar level using serum samples[90]. Methods such as these can be utilized for further identification of paracrine mediators secreted at lower concentrations. A recent report by Chen et al. claims that hESC-derived MSCs are capable of secreting microparticles enriched in pre-microRNAs [91]. Interestingly, these vesicles were readily taken up by H9C2 cardiomyoblasts implying that MSCs may utilize pre-microRNAs to facilitate signaling with neighboring cells. MicroRNA profiling using microarray analyses is rapidly yielding distinct microRNA expression signatures for different experimental models of cardiac disease [92]. It would be tempting to speculate that stem cells could potentially be used to deliver microRNAs in addition to protein factors for cardiac cell repair therapy.
Ex vivo Enhancement of Stem Cell Paracrine Effect
Knowledge of how paracrine mediators of adult stem cells mediate their cardioprotective and regenerative effects can provide insight into ways of modifying the cells ex vivo to enhance their function, viability and retention in target tissue [93]. Multiple genetic engineering strategies have been applied to stem cells to increase their cell viability and enhance the production of secreted factors capable of cardioprotection both in vitro and in vivo, including Akt [8], SFRP2 [85], Bcl-2 [94], Hsp-20 [95] and hypoxia regulated heme oxygenase-1 [96]. In each case, engineered MSCs were shown to survive better in ischemic tissues and to exert more powerful cytoprotection and/or pro-neovascularization effects compared with control MSCs. Moreover, MSCs have been used as a vehicle for gene therapy to deliver secreted gene products such as VEGF [97], HGF [98], bFGF [99], adrenomedullin [100], and SDF-1 [63, 101], allowing the cells to exert more powerful paracrine or autocrine actions leading to improved viability in the hypoxic environment [37];[102]. Since the expression of some receptors specific for chemokines upregulated in infarcted myocardium is poor in stem cells such as MSCs, our group hypothesized that genetic overexpression of such receptors in these cells would improve viability and engraftment. Recently, our group demonstrated that indeed, ectopic expression of the chemokine receptor CCR1 increased MSC survival, engraftment and migration in the infarcted territory [103]. Similarly, increasing expression of CXCR4 on MSCs had been shown to augment their attachment and migration into the infarcted zone following intravenous administration [104]. Both studies signify the power of chemokine receptor expression modification for enhanced mobility of adult stem cells to the site of injury. Additionally, combinatorial approaches by transducing MSCs with both Akt and Angiopoietin-1 together enhanced long-term engraftment and maintenance of these cells beyond the acute phase of MI [105]. Furthermore, cardiac overexpression of the serine/threonine kinase Pim-1 led to increased proliferative activity of both cardiomyocytes and CPCs. Specifically, following infarction, Pim-1 activated CPC cycling via asymmetric cell division suggesting that it may represent a valuable target for generating differentiated cardiac cells in response to injury, while maintaining CPC reserves in the heart [106]. In addition to modifications to MSC and CPCs, Pitx2c overexpressing ESCs have been shown to not only have enhanced capacity towards cardiomyocyte differentiation, but also secrete heparin-binding paracrine factors that restore cardiac function after MI [107].
An alternative approach to improve paracrine effects is represented by cell preconditioning. Preconditioning of MSCs in hypoxia prior to their transplantation has been shown to improve cell survival and stimulate paracrine mechanisms for heart repair [34]. More recently it was also shown that hypoxic preconditioning of bone marrow-derived mesenchymal stem cells (MSCs) with two cycles of 30-min hypoxia/reoxygenation significantly enhanced their survival in the ischemic environment of the infarcted heart. Interestingly it was concluded that these effects were mediated by regulation of mir-210 and inhibition of Caspase 8 expression [108]. In addition, growth factors or other molecules have been used for preconditioning with successful results demonstrating that these strategies enhance cell survival and differentiation and stimulate the release of soluble factors [109]. For instance, hyaluronan-mixed esters of butyric and retinoic acid (HBR) elicit a remarkable increase in the transcription of cardiac lineage-promoting genes and cardiac differentiation in ESCs and MSCs [110]. Transplantation of MSCs preconditioned ex vivo with HBR into infarcted rat myocardium led to complete normalization of myocardial performance and to a dramatic reduction in scar formation via regeneration and paracrine mechanisms. Likewise, preconditioning MSCs with SDF-1 [111], TGF-α [112] or TGF- β [113] led to decreased fibrosis after MI. Together these lines of data suggest that ex vivo modification is a promising way to enhance the therapeutic potential of stem cells.
Potential Challenges to Paracrine Factor Therapy
The demonstration that stem cells secrete therapeutic factors represents a potential breakthrough. Characterization of those paracrine mediators may lead to the possibility of replacing stem cell-based therapy with soluble factor-based therapy; an easier approach clinically. However, limitations for the clinical translation of the current findings still exist (Table 1).
Table 1.
Potential challeges and resolutions to paracrine factor identification, production, and therapy.
| Challenges to Paracrine Factor Therapy | Solutions | |
|---|---|---|
| Identification | -Difficult to isolate low concentrations | -New proteomic/secretomic standard libraries for mass spectometry |
| -Difficult to analyze non-protein components | ||
| Administration | -Large scale production | -Bioreactor / high throughput column isolation |
| -Route | -Bioengineered delivery platforms for continuous delivery (dermal patch, stents) | |
| -Maintenance of theraputic levels | ||
| Pharmacokinetics / Pharmacodynamics | -Protein stability | -polymer / nanofibers |
| -Potential adverse effects of theraputic doses | ||
| -Immunological concerns | ||
One of the biggest challenges is designing a therapeutic regimen based on the intricate interaction of paracrine factors during the acute and chronic phases of cardiac disease. This is complicated by the likely scenario that there is no single paracrine mediator but rather a complex of proteins involved in mediating stem cells effects. Indeed, recent proteomic data suggests that only the fraction of conditioned media from human MSCs containing products >1000 kDa (100-220 nm) provided cardioprotection in a mouse model of ischemia and reperfusion injury. This observation suggests that the critical paracrine factors mediating these effects are likely part of a complex of molecules [114]. The complex interaction between multiple factors may work synergistically to enhance the reparative potential of conditioned media versus single factor therapy.
Many of the proteins induce distinct actions at different concentrations and at specific phases of repair post MI. In particular, members of the TGF superfamily members have pleiotrophic effects; activating opposing and diverse cell responses in a concentration-dependent manner [115]. Likewise, the timing of MMP and TIMP expression plays a significant role in the outcomes of cardiac fibrosis and remodeling [116]. Substantial differences between animal models and humans further complicate the scenario. For example, it has been shown that a single dose of specific growth factors is effective in enhancing neovascularization in animals but not in humans [117]. A more thorough understanding of temporal and spatial interaction between paracrine factors and their roles in distinct phases of the disease is needed.
Before any therapeutic biologic can be applied for widespread clinical use, commercial pharmacokinetics/pharmacodynamic consideration must be taken into account. Large scale production and isolation of these factors for therapy is hampered by commercial costs associated with animal component-free cell systems. Protein stability, specificity, route of delivery, and immunogenicity in vivo need to be addressed. For example, oral administration is constrained by limitations on absorption, stability and transit time within the GI track. Likewise, intravenous injection of proteins is encumbered by serum proteases which rapidly degrade factors. Currently, a variety of modification have been developed to increase administered proteins uptake, bioavailability, and solubility [118]. Biopolymers [119] and nanofibers [120] are promising tools for delivery platforms based on subcutaneous implantable devices or endovascular stents. Together, protein modification and delivery tools, while still in the development phase, represent great promise for protein therapy in the future.
The use of a single or a cocktail of proteins remains a more attractive clinical option than delivery of isolated adult stem cells. Nevertheless, biologic/protein therapy is still hindered by our lack of knowledge of this tightly controlled, complex mechanism in cardiac repair and regeneration. Continual advancements in protein and peptide therapeutics such as improved methods for large scale production, chemical modifications that enhance stability and more efficient delivery methods will facilitate the development of protein therapy for cardiac repair and regeneration.
Conclusion
It is evident that the improvement in cardiac function following stem cell therapy can be attributed mainly to the release of key paracrine factors by stem cells in the injured myocardial microenvironment. A growing body of evidence strongly suggests that these secreted molecules mediate a number of protective mechanisms including cell survival, neovascularization, remodeling, and proliferation. The regulatory machinery governing paracrine factor release appears to be complex and dependent on spatiotemporal parameters. Advances in profiling technologies continue to identify significant secreted factors that mediate cardiac repair mechanisms. The potential for magnifying stem cell-mediated paracrine effects using “engineered”, “conditioned” or other ex vivo manipulated stem cells will significantly propel this type of therapy forward and provide invaluable information regarding stem cell biology.
Acknowledgments
We would like to express our appreciation to Steven Conlon, Department of Pathology, Duke University for his artistic contribution to the illustrations in this review. Research conducted by our group mentioned in this review was was supported by National Heart, Lung, and Blood Institute grants RO1 HL35610, HL81744, HL72010, and HL73219 (to V.J.D.); the Edna Mandel Foundation (to V.J.D. and M.M.); and the Foundation Leducq (to V.J.D.). M.M. is also supported by an American Heart Association National Scientist Development Award (10SDG4280011). M.G. is supported by the Fondazione IRCCS Policlinico San Matteo Pavia, Italy; the Ministero Italiano dell’Universitàe della Ricerca (MIUR); the Ministero della Salute; the Fondazione Cariplo; and the Fondazione Banca del Monte di Lombardia.
Footnotes
Disclosures The authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005 Jul;23(7):845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 3.Melo LG, Pachori AS, Kong D, Gnecchi M, Wang K, Pratt RE, et al. Molecular and cell-based therapies for protection, rescue, and repair of ischemic myocardium: reasons for cautious optimism. Circulation. 2004 May 25;109(20):2386–93. doi: 10.1161/01.CIR.0000128597.37025.00. [DOI] [PubMed] [Google Scholar]
- 4.Zhou P, Wirthlin L, McGee J, Annett G, Nolta J. Contribution of human hematopoietic stem cells to liver repair. Semin Immunopathol. 2009 Sep;31(3):411–9. doi: 10.1007/s00281-009-0166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008 Nov 21;103(11):1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1(1):4. doi: 10.1186/1755-1536-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005 Apr;11(4):367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 9.Crisostomo PR, Abarbanell AM, Wang M, Lahm T, Wang Y, Meldrum DR. Embryonic stem cells attenuate myocardial dysfunction and inflammation after surgical global ischemia via paracrine actions. Am J Physiol Heart Circ Physiol. 2008 Oct;295(4):H1726–35. doi: 10.1152/ajpheart.00236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaFramboise WA, Petrosko P, Krill-Burger JM, Morris DR, McCoy AR, Scalise D, et al. Proteins secreted by embryonic stem cells activate cardiomyocytes through ligand binding pathways. J Proteomics. Mar 10;73(5):992–1003. doi: 10.1016/j.jprot.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003 Jan 28;107(3):461–8. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 12.Maxeiner H, Krehbiehl N, Muller A, Woitasky N, Akinturk H, Muller M, et al. New insights into paracrine mechanisms of human cardiac progenitor cells. Eur J Heart Fail. Jul;12(7):730–7. doi: 10.1093/eurjhf/hfq063. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009 Aug 4;120(5):408–16. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009 Feb 27;104(4):e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004 Apr 6;109(13):1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004 Oct 1;95(7):742–8. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 18.Allan R, Kass M, Glover C, Haddad H. Cellular transplantation: future therapeutic options. Curr Opin Cardiol. 2007 Mar;22(2):104–10. doi: 10.1097/HCO.0b013e3280210657. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006 Aug;291(2):H886–93. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 20.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001 Aug 28;104(9):1046–52. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 21.Burchfield JS, Iwasaki M, Koyanagi M, Urbich C, Rosenthal N, Zeiher AM, et al. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ Res. 2008 Jul 18;103(2):203–11. doi: 10.1161/CIRCRESAHA.108.178475. [DOI] [PubMed] [Google Scholar]
- 22.Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001 Sep 1;264(1):51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- 23.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2(6):477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001 Apr;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 25.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest. 2005 Aug;85(8):962–71. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 26.Chanda D, Kumar S, Ponnazhagan S. Therapeutic potential of adult bone marrowderived mesenchymal stem cells in diseases of the skeleton. J Cell Biochem. May 19; doi: 10.1002/jcb.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999 Nov 9;100(19 Suppl):II247–56. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 28.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001 Apr 5;410(6829):701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 29.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004 Jul 9;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen BK, Maltais S, Perrault LP, Tanguay JF, Tardif JC, Stevens LM, et al. Improved Function and Myocardial Repair of Infarcted Heart by Intracoronary Injection of Mesenchymal Stem Cell-Derived Growth Factors. J Cardiovasc Transl Res. Feb 26; doi: 10.1007/s12265-010-9171-0. [DOI] [PubMed] [Google Scholar]
- 31.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006 Apr;20(6):661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 32.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007 Jan 30;104(5):1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Guo J, Mirotsou M, Mu H, Zhang L, Zhang Z, et al. Novel Stem Cell Paracrine Factor Protects Cardiomyocytes Through Protein Kinase C Epsilon Selective Mechanism. Circulation. 2009;120:S846. [Google Scholar]
- 34.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006 Jun 9;98(11):1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 35.Block GJ, DiMattia GD, Prockop DJ. Stanniocalcin-1 regulates extracellular ATP-induced calcium waves in human epithelial cancer cells by stimulating ATP release from bystander cells. PLoS One. 2010;5(4):e10237. doi: 10.1371/journal.pone.0010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly ML, Wang M, Crisostomo PR, Abarbanell AM, Herrmann JL, Weil BR, et al. TNF receptor 2, NOT tnf receptor 1, enhances mesenchymal stem cell-mediated cardiac protection following acute ischemia. Shock. Jun;33(6):602–7. doi: 10.1097/SHK.0b013e3181cc0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005 Jul;46(1):7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 38.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004 Mar 30;109(12):1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 39.Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, et al. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2308–14. doi: 10.1152/ajpheart.00565.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004 Dec;287(6):H2670–6. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Wang S, Yu Z, Hoyt RF, Jr, Sachdev V, Vincent P, et al. Direct injection of autologous mesenchymal stromal cells improves myocardial function. Biochem Biophys Res Commun. 2009 Dec 18;390(3):902–7. doi: 10.1016/j.bbrc.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abarbanell AM, Wang Y, Herrmann JL, Weil BR, Poynter JA, Manukyan MC, et al. Toll-like receptor 2 mediates mesenchymal stem cell-associated myocardial recovery and VEGF production following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. May;298(5):H1529–36. doi: 10.1152/ajpheart.01087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varoga D, Paulsen F, Mentlein R, Fay J, Kurz B, Schutz R, et al. TLR-2-mediated induction of vascular endothelial growth factor (VEGF) in cartilage in septic joint disease. J Pathol. 2006 Nov;210(3):315–24. doi: 10.1002/path.2059. [DOI] [PubMed] [Google Scholar]
- 44.Webber MJ, Han X, Prasanna Murthy SN, Rajangam K, Stupp SI, Lomasney JW. Capturing the stem cell paracrine effect using heparin-presenting nanofibres to treat cardiovascular diseases. J Tissue Eng Regen Med. 2010 Mar 10; doi: 10.1002/term.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005 Jul;85(3):1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 46.Hu Q, Wang X, Lee J, Mansoor A, Liu J, Zeng L, et al. Profound bioenergetic abnormalities in peri-infarct myocardial regions. Am J Physiol Heart Circ Physiol. 2006 Aug;291(2):H648–57. doi: 10.1152/ajpheart.01387.2005. [DOI] [PubMed] [Google Scholar]
- 47.Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, et al. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009 Apr;27(4):971–9. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, et al. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006 Jun 1;70(3):530–42. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Feygin J, Mansoor A, Eckman P, Swingen C, Zhang J. Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1772–80. doi: 10.1152/ajpheart.00242.2007. [DOI] [PubMed] [Google Scholar]
- 50.Dhein S, Garbade J, Rouabah D, Abraham G, Ungemach FR, Schneider K, et al. Effects of autologous bone marrow stem cell transplantation on beta-adrenoceptor density and electrical activation pattern in a rabbit model of non-ischemic heart failure. J Cardiothorac Surg. 2006;1:17. doi: 10.1186/1749-8090-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garbade J, Dhein S, Lipinski C, Aupperle H, Arsalan M, Borger MA, et al. Bone marrow-derived stem cells attenuate impaired contractility and enhance capillary density in a rabbit model of Doxorubicin-induced failing hearts. J Card Surg. 2009 Sep-Oct;24(5):591–9. doi: 10.1111/j.1540-8191.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 52.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005 Aug;33(2):145–52. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdel Aziz MT, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, Ahmed HH, et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem. 2007 Aug;40(12):893–9. doi: 10.1016/j.clinbiochem.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 54.Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009 Dec;27(12):3063–73. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 55.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006 Jun;290(6):H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 56.Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007 Aug;30(3-4):97–104. doi: 10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 57.Xu X, Xu Z, Xu Y, Cui G. Effects of mesenchymal stem cell transplantation on extracellular matrix after myocardial infarction in rats. Coron Artery Dis. 2005 Jun;16(4):245–55. doi: 10.1097/00019501-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007 May;25(5):1166–77. doi: 10.1634/stemcells.2006-0347. [DOI] [PubMed] [Google Scholar]
- 59.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005 Aug 23;112(8):1128–35. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 60.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007 Aug 21;581(21):3961–6. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 61.Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007 Jan;42(1):88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Yu Q, Watson RR, Marchalonis JJ, Larson DF. A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol. 2005 Aug;289(2):H643–51. doi: 10.1152/ajpheart.00073.2005. [DOI] [PubMed] [Google Scholar]
- 63.Tang J, Wang J, Guo L, Kong X, Yang J, Zheng F, et al. Mesenchymal stem cells modified with stromal cell-derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol Cells. Jan;29(1):9–19. doi: 10.1007/s10059-010-0001-7. [DOI] [PubMed] [Google Scholar]
- 64.Psaltis PJ, Paton S, See F, Arthur A, Martin S, Itescu S, et al. Enrichment for STRO-1 expression enhances the cardiovascular paracrine activity of human bone marrow-derived mesenchymal cell populations. J Cell Physiol. May;223(2):530–40. doi: 10.1002/jcp.22081. [DOI] [PubMed] [Google Scholar]
- 65.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10(5):223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009 Mar 15;433(1-2):1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004 Aug;15(8):1983–92. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 68.Yang Z, von Ballmoos MW, Faessler D, Voelzmann J, Ortmann J, Diehm N, et al. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010 Feb 24; doi: 10.1016/j.atherosclerosis.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 69.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005 Nov;39(5):733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, et al. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4(5):e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doyle B, Sorajja P, Hynes B, Kumar AH, Araoz PA, Stalboerger PG, et al. Progenitor cell therapy in a porcine acute myocardial infarction model induces cardiac hypertrophy, mediated by paracrine secretion of cardiotrophic factors including TGFbeta1. Stem Cells Dev. 2008 Oct;17(5):941–51. doi: 10.1089/scd.2007.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Ingram DA, Murphy MP, Saadatzadeh MR, Mead LE, Prater DN, et al. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1675–82. doi: 10.1152/ajpheart.00665.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Stefano R, Barsotti MC, Armani C, Santoni T, Lorenzet R, Balbarini A, et al. Human peripheral blood endothelial progenitor cells synthesize and express functionally active tissue factor. Thromb Res. 2009 Apr;123(6):925–30. doi: 10.1016/j.thromres.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 74.Stastna M, Abraham MR, Van Eyk JE. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 2009 Jun 5;583(11):1800–7. doi: 10.1016/j.febslet.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stastna M, Chimenti I, Marban E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. Jan;10(2):245–53. doi: 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. Mar 19;106(5):971–80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. Jan 19;121(2):293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007 Feb 19;204(2):405–20. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kupatt C, Bock-Marquette I, Boekstegers P. Embryonic endothelial progenitor cell-mediated cardioprotection requires Thymosin beta4. Trends Cardiovasc Med. 2008 Aug;18(6):205–10. doi: 10.1016/j.tcm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Muller S, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008 Apr 29;117(17):2232–40. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torella D, Ellison GM, Karakikes I, Nadal-Ginard B. Resident cardiac stem cells. Cell Mol Life Sci. 2007 Mar;64(6):661–73. doi: 10.1007/s00018-007-6519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002 Jun 27;417(6892):954–8. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 83.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deb A, Davis BH, Guo J, Ni A, Huang J, Zhang Z, et al. SFRP2 regulates cardiomyogenic differentiation by inhibiting a positive transcriptional autofeedback loop of Wnt3a. Stem Cells. 2008 Jan;26(1):35–44. doi: 10.1634/stemcells.2007-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18366–71. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ventura C, Branzi A. Autocrine and intracrine signaling for cardiogenesis in embryonic stem cells: a clue for the development of novel differentiating agents. Handb Exp Pharmacol. 2006(174):123–46. [PubMed] [Google Scholar]
- 87.Estrada R, Li N, Sarojini H, An J, Lee MJ, Wang E. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J Cell Physiol. 2009 Jun;219(3):563–71. doi: 10.1002/jcp.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pula G, Mayr U, Evans C, Prokopi M, Vara DS, Yin X, et al. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circ Res. 2009 Jan 2;104(1):32–40. doi: 10.1161/CIRCRESAHA.108.182261. [DOI] [PubMed] [Google Scholar]
- 89.Gellings Lowe N, Swaney JS, Moreno KM, Sabbadini RA. Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-beta-stimulated collagen production by cardiac fibroblasts. Cardiovasc Res. 2009 May 1;82(2):303–12. doi: 10.1093/cvr/cvp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah SH, Hauser ER, Bain JR, Muehlbauer MJ, Haynes C, Stevens RD, et al. High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol. 2009;5:258. doi: 10.1038/msb.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010 Jan;38(1):215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Condorelli G, Latronico MV, Dorn GW., 2nd microRNAs in heart disease: putative novel therapeutic targets? Eur Heart J. 2010 Mar;31(6):649–58. doi: 10.1093/eurheartj/ehp573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dzau VJ, Gnecchi M, Pachori AS. Enhancing stem cell therapy through genetic modification. J Am Coll Cardiol. 2005 Oct 4;46(7):1351–3. doi: 10.1016/j.jacc.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 94.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007 Aug;25(8):2118–27. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Zhao T, Huang W, Wang T, Qian J, Xu M, et al. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009 Dec;27(12):3021–31. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005 Oct 4;46(7):1339–50. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 97.Matsumoto R, Omura T, Yoshiyama M, Hayashi T, Inamoto S, Koh KR, et al. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005 Jun;25(6):1168–73. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 98.Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, et al. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003 Sep;8(3):467–74. doi: 10.1016/s1525-0016(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 99.Song H, Kwon K, Lim S, Kang SM, Ko YG, Xu Z, et al. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol Cells. 2005 Jun 30;19(3):402–7. [PubMed] [Google Scholar]
- 100.Jo J, Nagaya N, Miyahara Y, Kataoka M, Harada-Shiba M, Kangawa K, et al. Transplantation of genetically engineered mesenchymal stem cells improves cardiac function in rats with myocardial infarction: benefit of a novel nonviral vector, cationized dextran. Tissue Eng. 2007 Feb;13(2):313–22. doi: 10.1089/ten.2006.0133. [DOI] [PubMed] [Google Scholar]
- 101.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. Faseb J. 2007 Oct;21(12):3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 102.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003 Sep;9(9):1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 103.Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, et al. Genetic Modification of Mesenchymal Stem Cells Overexpressing CCR1 Increases Cell Viability, Migration, Engraftment, and Capillary Density in the Injured Myocardium. Circ Res. Apr 8; doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008 Mar;16(3):571–9. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 105.Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008 Feb 1;77(3):525–33. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- 106.Cottage CT, Bailey B, Fischer KM, Avitable D, Collins B, Tuck S, et al. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. Mar 19;106(5):891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guddati AK, Otero JJ, Kessler E, Aistrup G, Wasserstrom JA, Han X, et al. Embryonic stem cells overexpressing Pitx2c engraft in infarcted myocardium and improve cardiac function. Int Heart J. 2009 Nov;50(6):783–99. doi: 10.1536/ihj.50.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009 Nov 27;284(48):33161–8. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008 Oct;45(4):554–66. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ventura C, Cantoni S, Bianchi F, Lionetti V, Cavallini C, Scarlata I, et al. Hyaluronan mixed esters of butyric and retinoic Acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J Biol Chem. 2007 May 11;282(19):14243–52. doi: 10.1074/jbc.M609350200. [DOI] [PubMed] [Google Scholar]
- 111.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008 Jan;77(1):134–42. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 112.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. Jan;33(1):24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 113.Li TS, Hayashi M, Ito H, Furutani A, Murata T, Matsuzaki M, et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 2005 May 17;111(19):2438–45. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 114.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007 Nov;1(2):129–37. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 115.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007 May 1;74(2):184–95. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006 Feb 15;69(3):604–13. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 117.Post MJ, Laham R, Sellke FW, Simons M. Therapeutic angiogenesis in cardiology using protein formulations. Cardiovasc Res. 2001 Feb 16;49(3):522–31. doi: 10.1016/s0008-6363(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 118.Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Recent advances in protein and peptide drug delivery systems. Curr Drug Deliv. 2007 Apr;4(2):141–51. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 119.Balasubramanian V, Onaca O, Enea R, Hughes DW, Palivan CG. Protein delivery: from conventional drug delivery carriers to polymeric nanoreactors. Expert Opin Drug Deliv. Jan;7(1):63–78. doi: 10.1517/17425240903394520. [DOI] [PubMed] [Google Scholar]
- 120.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006 May 23;103(21):8155–60. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]


