Summary
Compared with neural crest-derived melanocytes, retinal pigment epithelium (RPE) cells in the back of the eye are pigment cells of a different kind. They are a part of the brain, form an epithelial monolayer, respond to distinct extracellular signals, and provide functions that far exceed those of a light-absorbing screen. For instance, they control nutrient and metabolite flow to and from the retina, replenish 11-cis-retinal by re-isomerizing all-trans-retinal generated during photoconversion, phagocytose daily a portion of the photoreceptors’ outer segments, and secrete cytokines that locally control the innate and adaptive immune systems. Not surprisingly, RPE cell damage is a major cause of human blindness worldwide, with age-related macular degeneration a prevalent example. RPE replacement therapies using RPE cells generated from embryonic or induced pluripotent stem cells provide a novel approach to a rational treatment of such forms of blindness. In fact, RPE-like cells can be obtained relatively easily when stem cells are subjected to a two-step induction protocol, a first step that leads to a neuroectodermal fate and a second to RPE differentiation. Here, we discuss the characteristics of such cells, propose criteria they should fulfill in order to be considered authentic RPE cells, and point out the challenges one faces when using such cells in attempts to restore vision.
Keywords: ES cells, induced pluripotent stem cells, age-related macular degeneration, retinitis pigmentosa, cell-based therapy, retinal pigment epithelium
The importance of the retinal pigment epithelium for vision
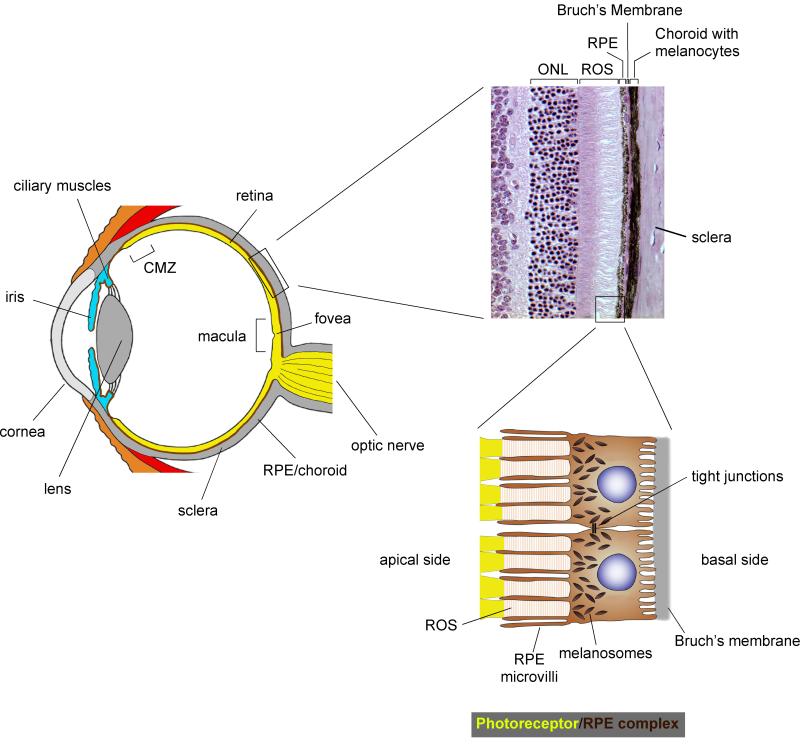
The vertebrate eye has three major components: (i) an optic apparatus, which consists of cornea, lens and iris; (ii) a photosensitive layer, the retina, which is composed of a neuronal network capable of processing electrical signals that originate primarily in the retina’s rods and, in animals with color vision, cones; and (iii) a light-absorbing, protective casing, which is provided by the pigmented choroid and the retinal pigment epithelium (RPE) (Fig. 1) (Land and Nilsson, 2002). Functionally, these elements resemble the parts of a modern photographic camera, but the analogy has limitations. The RPE, for instance, has multiple support functions for the retina and does much more than simply absorb light. It is part of the blood–retina barrier and together with the selectively permeable Bruch’s membrane provides control over ion, nutrient, and metabolite transport between the retina and the fenestrated capillaries of the choroid (Adijanto et al., 2009; Li et al., 2009; Quinn et al., 2001). This is no minor feat given that the retina has a very high cell density and is highly active metabolically. The RPE also has a specific function in the visual cycle. Vision begins with the photoconversion of opsin-bound 11-cis-retinal to all-trans-retinal, leading to a conformational change in opsin, release of the all-trans-retinal, and initiation of the phototransduction cascade that terminates in the conversion of light quanta into an electrical signal. In a series of steps, the all-trans retinal is then transported to the RPE, re-isomerized by the isomerase RPE65, and transported back to the photoreceptors (Cai et al., 2009; von Lintig et al., 2010). In this way, precious retinal is recycled and made available for renewed use by photoreceptors. Moreover, because light damages the photoreceptors’ outer segments through photooxidation of lipids, lipoproteins, and other molecules, there is a continuous need for outer segment turnover. In fact, each day, the photoreceptors add about 10% of the length of outer segments to the segments’ base, and RPE cells remove a similar amount from their apical tips. In this, RPE cells are quite active: for instance, in the human fovea, one RPE cell phagocytoses and digests the tips of more than 20 outer segments daily (the fovea, measuring about 1 mm across, is the area of the retina with the highest spatial resolution in the center of the macula which measures about 6 mm across and is responsible for the central visual field, see Fig. 1) (Curcio et al., 1990; Kevany and Palczewski, 2010; Mallavarapu and Finnemann, 2010; Snodderly et al., 2002; Strauss, 2005). Lastly, by secreting a host of factors, including copious amounts of tumor growth factor-β (TGF β) and cytotoxic T lymphocyte antigen-2a (CTLA-2a), the RPE locally regulates both the innate and the adaptive immune system (Horie et al., 2010; Kvanta, 1994; Sugita et al., 2008).
Figure 1.
Anatomy of the camera-style mammalian eye and photoreceptor/retinal pigment epithelium (RPE) complex. Left: schematic cross-section through the human eye showing its main structural components that allow focusing of the light onto the photoreceptor layer of the retina. Top: Histologic section of an adult mouse eye showing photoreceptor nuclei in the outer nuclear layer (ONL), rod outer segments (ROS), RPE, Bruch’s membrane, and the choroid with its neural crest-derived melanocytes. Bottom: Diagram showing two RPE cells with their microvilli contacting the rod outer segments. The intimate anatomic relationship of RPE cells with photoreceptors and Bruch’s membrane allow the cells to have multiple roles as support cells for the retina (see text). The cells also carry a dense array of apical melanosomes that serve as a light screen in the back of the eye.
Given the intimate anatomic and functional relationship of RPE cells and photoreceptors, it is not surprising that the first manifestations of primary RPE disorders are often problems with vision (Longbottom et al., 2009; Nussenblatt et al., 2009; Swaroop et al., 2009). Such is the case with age-related macular degeneration (AMD), a major cause of still untreatable blindness worldwide. AMD comes in two forms, a dry form which is characterized by regional loss of the RPE without neovascularization and a rarer wet form, in which new vessels emerge from the choroid and penetrate under or into the retina. A hallmark of AMD, and a portent of its full development, is the presence of large, coalescing ‘drusen’ which are excretions that accumulate between RPE cells and Bruch’s membrane and within Bruch’s membrane. Drusen might originate from a phototoxically and oxidatively damaged RPE and can build up particularly in the area of the macula. AMD is commonly associated with inflammation that is possibly stimulated by drusen, involves both the innate and adaptive immune system, and is influenced by genetic and environmental risk factors (Gupta et al., 2003; Kaarniranta and Salminen, 2009; Ma et al., 2009; Nussenblatt et al., 2009; Swaroop et al., 2009). Although the precise role of inflammation during the onset of disease remains to be determined (for further discussion, see below), alleles of genes regulating inflammatory processes have clearly been recognized as significant risk factors for AMD. Among them are, for instance, the tyrosine402-to-histidine substitution in complement factor H, and alleles of ApoE and complement factor C2/BF (Baird et al., 2006; Edwards et al., 2005; Gold et al., 2006; Haines et al., 2005; Klein et al., 2005).
In addition to AMD, primary RPE pathology is also associated with some forms of retinitis pigmentosa (RP), a disorder that first affects the peripheral field of vision before it encroaches on its more central parts (Busskamp et al., 2010; Cronin et al., 2010). Some forms of RP with RPE pathology are clearly heritable, such as the one caused by mutant alleles of the RPE-specific retinaldehyde-binding protein-1 (RLBP1, also called CRALBP, Maw et al., 1997). Furthermore, there are a number of other primary RPE pathologies that lead to vision loss, many also associated with known mutations. Among them is the subtype of Leber’s amaurosis in which RPE65 is mutated (Morimura et al., 1998), and BEST disease where the channel protein bestrophin-1 (BEST1, also called VMD2 or TU15B) is mutated (Kramer et al., 2000; Zhang et al., 2010). Therefore, one can conclude that the RPE is critically important for the normal functioning of the retina and hence the visual perception of the world around us.
Stemming vision loss with stem cells
As the overall human disease burden because of primary RPE defects is already staggering and expected to increase dramatically with increasing longevity, it is of paramount importance to speed exploration of potential therapies. Recently, the idea of cell replacement therapies has gained traction. The goal is to replace lost or abnormal cells — RPE alone or in conjunction with other key cell types such as photoreceptors — in the hope that the replacement cells would correctly integrate into the still existing albeit damaged cellular network, and then function for prolonged times to ameliorate the effects of cell loss on vision.
Replacement cells could potentially come from endogenous progenitors. For instance, in amphibians and fish, the ciliary margin zone (CMZ, see Fig. 1) contains stem cells that have the capacity to generate retinal neurons throughout the animal’s life (Craig et al., 2008; Karl and Reh, 2010; Sanchez Alvarado and Tsonis, 2006). In these animals and to a limited extent in birds and rodents, certain types of retinal neurons can also be generated from a retinal glial cell called Mueller glia (Bernardos et al., 2007; Das et al., 2006; Fischer and Reh, 2001). Another form of endogenous repair could come from the ‘transdifferentiation’ of RPE into retina that one observes in urodele amphibians such as newts and salamanders (Araki, 2007; Mitashov, 1996), a phenomenon likely related to the embryonic development of retina and RPE. Unlike the pigment cells of the integument and the choroid, which are developmentally derived from the neural crest, RPE cells are derived from the neuroepithelium as are retinal cells (Bharti et al., 2006) (for a definition of these cells, see Box 1). Following retinectomy in newts and salamanders, the RPE dedifferentiates and then redifferentiates to replace not only the lost retina with all its cellular constituents but also to rebuild a normal RPE (Araki, 2007; Mitashov, 1996). Compared with these exceptional amphibians, the regenerative capacities of the adult retina are somewhat reduced in other amphibians, such as frogs and toads, further reduced in fish and birds, and nearly absent in mammals (Karl and Reh, 2010). Nevertheless, it is conceivable that even humans retain evolutionary remnants of the repair capacities of other vertebrates and that one could harness such repair potentials therapeutically. There is a concern, however, that diseases with a genetic component operating cell autonomously in RPE or photoreceptor cells might not be countered by stimulating endogenous progenitors as these cells would carry the same genetic defects. In diseases of aging such as AMD, however, a newly differentiated cell, even when derived from an adult precursor, may have its clock reset or may at least not be as badly damaged as one born years earlier. Hence, endogenous repair may allow for a period of healthy function before disease processes overtake again.
Table Box 1. Definitions.
ES cells
Human embryonic stem cells are derived from blastocysts and share many features with cells from the epiblast of the embryo proper. They are characterized by the capacity to self-renew indefinitely and to generate differentiated cells of all three germ layers. By comparison, mouse embryonic stem cells, also derived from blastocysts, correspond to inner cell mass cells and have different biologic properties. For a discussion of these differences, see Hanna et al. (2010).
iPS cells
Induced pluripotent stem cells are derived by transdifferentiation from a differentiated somatic cell, following the forced expression of a combination of pluripotency factors, such as OCT4, SOX2, NANOG, KLF4, c-MYC or LIN28. They are also characterized by an indefinite capacity to self-renew and generate differentiated cells of all three germ layers. Because iPS cells are derived from differentiated somatic cells, they can be generated from the same patient who may benefit from cell replacement therapies. This offers the theoretical advantage that the cells carry the same histocompatibility antigens as the potential recipient, but has the disadvantage that they also carry the same genetic risk factors that may be the cause of the patient’s disorder. Patient-specific iPS cells are in fact an ideal cellular source to investigate pathogenetic mechanisms associated with patient-specific risk factors, and for drug screening.
Neuroectodermal cells
Neuroectodermal cells originate from the ectodermal germ layer and are the precursors to neurons and glial cells of the central nervous system. RPE cells originate from neuroectodermal cells.
Neural crest cells
Neural crest cells originate from cells at the border between the neuroectoderm and the surface ectoderm. They are a transient population of cells that give rise to neurons and glial cells of the peripheral nervous system. They also produce a variety of other cells such as smooth muscle cells, cartilage cells, and melanocytes, including choroidal melanocytes and a portion of the pigmented cells in ciliary body and iris.
An alternative to endogenous repair is to replace lost cells with cells derived from embryonic (ES) or adult stem cells or induced pluripotent stem (iPS) cells (see Box 1). Such cells can be grown in culture to large numbers and can be coaxed, ideally, into producing differentiated cells regardless of the time kept as stem cells (Lengner, 2010). In fact, major advances have recently been made in the derivation of photoreceptor cells and RPE cells from ES cells or iPS cells, and the hope is high that RPE cells may be among the first of the ES or iPS-derived cells to provide clinical benefits (Buchholz et al., 2009; Carr et al., 2009a,b; Idelson et al., 2009; Kawasaki et al., 2002; Klimanskaya et al., 2004; Lu et al., 2009; Meyer et al., 2009; Osakada et al., 2009a,b). Also, the eye may be ideal for such cell-based therapies as it is of necessity translucent, and so the transplanted cells can be visualized both during and after surgery. Moreover, the number of cells required for this purpose may be relatively small. For instance, it has been estimated that just 60 000 RPE cells would be required to cover the macular region and preserve central vision. Furthermore, potential improvements of visual function can be accurately and rapidly measured, and complications such as overgrowth can be effectively addressed by local rather than systemic treatment. Here, we review recent work showing the feasibility of generating RPE cells from human ES and iPS cells and discuss criteria these cells should fulfill for successful implantation and restoration of visual function.
RPE cells made from ES and iPS cells
One of the first reports on in vitro differentiation of RPE-like cells was published in 2002 by Kawasaki et al. (2002). By co-culturing a monkey ES cell line with the stromal cell line PA6, the authors showed that after 3 weeks of culture, about 8% of the ES cell colonies contained polygonal pigmented cells that were positive for Pax6, a paired homeodomain transcription factor found in the developing retina and RPE and not normally in neural crest-derived melanocytes. A follow-up study (Haruta et al., 2004) showed that these cells expressed typical RPE markers, were able to phagocytose latex beads, and transiently enhanced the survival of host photoreceptors after transplantation into so-called Royal College of Surgeons (RCS) rats. In these animals, photoreceptors degenerate because RPE cells cannot phagocytose photoreceptor outer segments because of a mutation in the receptor tyrosine kinase MERTK (MER stands for expressed in ‘monocytes and tissues of epithelial and reproductive origin’ and TK for tyrosine kinase) (D’Cruz et al., 2000; Dowling and Sidman, 1962; Edwards and Szamier, 1977). In a seminal study, Klimanskaya et al. (2004) then demonstrated that human ES cells can spontaneously differentiate into RPE-like cells without any stromal cells or other feeder layers when cultured in the absence of FGF for 4–6 weeks. By gene expression profiling, the cells were more closely related to human fetal RPE than to RPE cell lines, and they were capable of phagocytosis of fluorescently labeled rod outer segments (ROS). Intriguingly, however, they could spontaneously dedifferentiate to non-RPE-like cells and differentiate back again to RPE-like cells, indicating they were phenotypically unstable.
In the last few years, many studies have independently confirmed and significantly extended the above-mentioned early investigations of in vitro RPE generation. Vugler et al. (2008), for instance, showed that upon transplantation in RCS rats, ES-derived RPE cells can phagocytose photoreceptor outer segments in vivo. Buchholz et al. (2009) generated RPE-like cells from human iPS cells and showed them to share gene expression and functional characteristics with RPE-like cells derived from ES cells. Carr et al. (2009a) studied the interaction of explanted human retina with ES-derived RPE-like cells in vitro and found that expression of MERTK on the RPE-like cells was essential for outer segment phagocytosis. The same group (Carr et al., 2009b) showed visual function improvements in RCS rats transplanted with human iPS-derived RPE-like cells. In most of the above studies, however, survival of transplanted cells in vivo did not exceed 13–15 weeks, and only recently has survival beyond 30 weeks been reported (Lu et al., 2009).
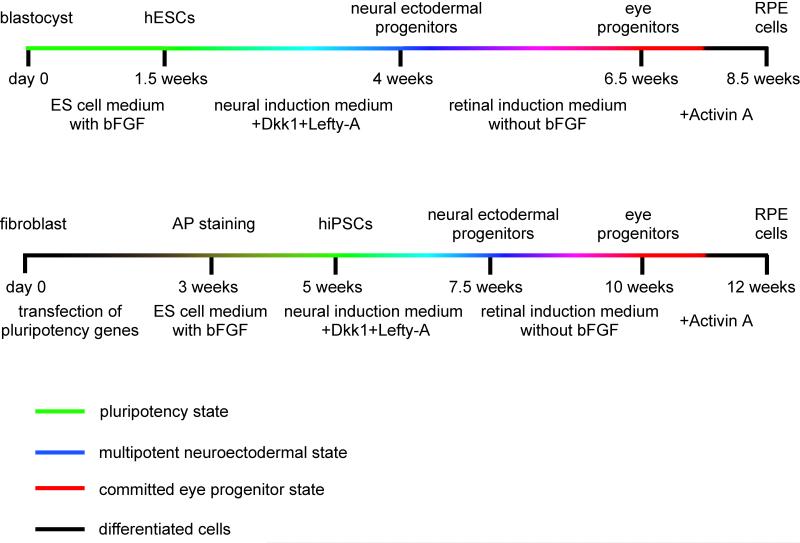
A number of additional studies also explored variations in culture protocols, described in more detail below, to improve on the efficiency of deriving RPE-like cells in vitro from stem cells (Idelson et al., 2009; Meyer et al., 2009; Osakada et al., 2009a,b). Despite such variations, however, three common threads run through all of these studies. First, all protocols are two-step protocols, with a first step designed to convert ES or iPS cells into cells with neuroectodermal characteristics, and a second step in which the neuroectodermal cells are then differentiated into RPE-like cells (Fig. 2). Second, using such two-step protocols, RPE-like cells can be reliably obtained, though only after weeks in culture. Third, it is never the whole ES or iPS cell culture that turns into RPE-like cells. This last point is particularly important as it may reflect the fact that ES or iPS cultures are likely heterogeneous to begin with. Also, the generation of RPE-like cells might depend on the presence of neighboring non-RPE-like cells. Nevertheless, once RPE-like cells are generated, they can be isolated, for instance by manual picking, and propagated. Much as observed in the above-mentioned earlier studies (Klimanskaya et al., 2004), however, the cultures may not remain phenotypically pure beyond 5–8 passages, either because the RPE-like cells themselves are not phenotypically stable, or because minor populations of contaminant cells may proliferate more efficiently than RPE cells. In fact, the production and propagation of cultures comprised of a single differentiated cell type derived from ES or iPS cells is still a significant challenge in today’s stem cell biology.
Figure 2.
Schematic diagrams of the differentiation protocols used to obtain retinal pigment epithelium (RPE) cells from ES or induced pluripotent stem cells. While the time periods required to obtain human ES cells and iPS cells differ by several weeks, the time to obtain RPE cells from them is similar. In a first step, neuroectodermal progenitors are generated from ES/iPS cells by exposing the cells to inhibitors of the WNT signaling pathway such as Dickkopf-1 (DKK1) or Nodal, and Lefty-A. In a second step, RPE cells are generated from neuroectodermal cells upon plating onto a laminin coated dish and culturing in a medium lacking FGF but containing activin A. Note that both neuroectodermal and RPE cells can be obtained from ES/iPS cells without the addition of Dkk1/Nodal or Activin A, but the efficiency of obtaining RPE cells under such conditions is much lower compared with conditions containing the respective factors.
Cell lines and culture protocols influence the efficiency of RPE generation
The choice of cell lines from which to derive RPE-like cells is a critical parameter for success because different ES and iPS cell lines vary in their tendency to differentiate into given cell lineages (Feng et al., 2010; Kim et al., 2010). Nevertheless, RPE-like cells have been obtained from a number of distinct cell lines, including the ES lines H9, Shef1 and Shef7 or Hes1 and Hes4, and the iPS lines IMR90-3 and IMR90-4 (Buchholz et al., 2009; Idelson et al., 2009; Meyer et al., 2009; Vugler et al., 2008; Yu et al., 2007). Recent studies suggest, however, that iPS cells may retain epigenetic marks of the cell type of origin and so may not be fully equivalent to ES cells (Kim et al., 2010). It has been proposed therefore that the best source of iPS cells for deriving RPE cells might be the RPE itself, but this idea still needs rigorous experimental verification.
The fact that ES and iPS lines are not all interchangeable has led to great variations among the published protocols used for the conversion of ES/iPS cells into neuroectodermal cells. For instance, Osakada et al. (2009a) used LEFTY-1, an inhibitor of the NODAL pathway (Shen, 2007), and DKK1, an inhibitor of the WNT pathway (Niehrs, 2006). Meyer et al. (2009) used endogenous inhibitors of the WNT, FGF, and BMP pathways. The choice of these inhibitors is based on the fact that during embryonic development, inhibition of the NODAL, WNT, and BMP pathway is associated with induction of anterior and neuronal fates (Niehrs, 2006; Shen, 2007; Stern, 2005). Indeed, in ES cell cultures, these factors have been used to consistently generate neural progenitors (Ikeda et al., 2005; Osakada et al., 2008, 2009a,b; Watanabe et al., 2005). A somewhat surprising and seemingly serendipitous observation was made by Idelson et al. (2009) who have used nicotinamide in conjunction with activin A and obtained an impressive augmentation in the number of neural progenitors and RPE-like cells. Activin A is a known inducer of RPE cell differentiation in the chick (Fuhrmann et al., 2000), but nicotinamide has so far been used mostly for pancreatic stem cell differentiation protocols where it is known to stimulate cell proliferation (Shi, 2010). Idelson et al. (2009) suggested that the augmentation in ES cell differentiation seen with nicotinamide is likely because of its anti-apoptotic properties, rather than to a direct action on the differentiation of neuroectodermal or, ultimately, RPE cells. The mechanism of nicotinamide action, however, still needs to be determined.
In contrast to the variations in the protocols used for the first step of RPE induction, a consensus has emerged that removal of FGF from the media can help convert ES and iPS cells into RPE cells. Indeed, mouse and avian models have shown that FGF, an inducer of neurogenesis, is an inhibitor of RPE differentiation (Nguyen and Arnheiter, 2000; Pittack et al., 1997). In developing chicken and mouse embryos, FGFs are secreted by the surface ectoderm overlying the optic neuroepithelium and induce the formation of a neural retina from the seemingly homogeneous population of neuroepithelial cells that make up the optic cup. The cells that are directly exposed to the FGF source will form the retina, whereas the more distant cells will form the RPE (Nguyen and Arnheiter, 2000; Pittack et al., 1997). In fact, in mouse embryos, removal of the surface ectoderm or genetic interference with the normal downregulation of the microphthalmia-associated transcription factor MITF (Hodgkinson et al., 1993) in the future retina lead to formation of an RPE monolayer from the cells that normally develop into retina. Conversely, addition of FGFs to the developing RPE or mutations in Mitf turns this tissue into a second retina (Bharti et al., 2006; Horsford et al., 2005; Nguyen and Arnheiter, 2000). Once the initial fate determination has been achieved, however, RPE cells seem to be resistant to the action of FGFs, some of which are expressed at high levels by the developing adjacent retina (Kurose et al., 2004; McWhirter et al., 1997).
It is not clear why the simple omission of FGFs from ES or iPS cells is sufficient to induce the RPE fate. One can speculate that RPE is a primary fate, perhaps because under normal developmental conditions, RPE cells are among the first to differentiate from the neuroepithelium. That RPE may be a primary fate is also suggested by the observation that ES or iPS-derived neuroectodermal cells express MITF which they then actively downregulate in order to become retinal progenitors but maintain in order to become RPE-like cells (Meyer et al., 2009). Alternatively, ES cells subjected to the reported induction protocols may generate a number of different cell types more or less randomly, and the ready appearance of RPE-like cells might simply reflect the fact that their pigmentation is noticed right away. In any event, the above information suggests three future goals: (i) to increase the efficiency with which RPE-like cells can be obtained, (ii) to characterize the generated cells thoroughly at the molecular, biochemical, and functional level, and (iii) to investigate the mechanisms that determine the phenotypic stability of the RPE-like cells.
Molecular characteristics of ES/iPS cell-derived RPE cells
Until now we have referred to the ES/iPS cell-derived RPE cells as ‘RPE-like’ cells. But what, in fact, is an authentic RPE cell? Is there more to it than the ‘four p’s’, polygonal, polarized, pigmented, and phagocytic? It is important to provide an operational definition of an RPE cell for at least two reasons. First, it would make comparisons between ES/iPS cell-derived RPE cells generated in different laboratories more meaningful and second, it might maximize the likelihood of finding fully functional RPE cells suitable for therapeutic applications.
A good starting point toward this goal is to compare ES/iPS cell-derived RPE cells with RPE cells obtained from an in vivo source. Such analyses were performed extensively by almost all recent studies, using many different assays, including gene expression profiling. Nevertheless, in most cases, the comparison was done with fetal and not adult human RPE. Where adult human RPE was included in the comparison, the ES cell-derived RPE cells were found to resemble more closely the fetal RPE (Buchholz et al., 2009; Carr et al., 2009a; Klimanskaya et al., 2004; Liao et al., 2010; Lu et al., 2009). This is not surprising as the cultured RPE cells still divide and express developmental markers including MITF and PAX6 (Bharti et al., 2008; Vugler et al., 2008). Although upon transplantation, the levels of these developmental markers and of markers for dividing cells such as Ki67 are reduced (Vugler et al., 2008), a concern remains that for therapeutic purposes, differentiated cells might be transplanted into the adult that are, in fact, embryonic and dividing. This may be of particular importance when thinking about transplanting such cells into AMD patients where the eye is flooded with cytokines which could potentially maintain the cells in a proliferative state (Nussenblatt et al., 2009). Hence, in order to reduce the risk of tumor or teratoma formation, it may be wise to explore possibilities to render the cells post-mitotic before transplantation. This might be achieved by modulating the activities of factors that affect in vitro RPE proliferation (Li et al., 2007) or whose mutations are known to lead to RPE hyperproliferation, such as the cyclin-dependent kinase inhibitor p27 or the modulator of canonical WNT signaling, encoded by the gene mutated in familial adenomatous polyposis coli (Defoe et al., 2007; Marcus et al., 1997). Indeed, before cultured RPE cells are used in human patients, the activity of their growth-regulating genes should be checked even when the cells seem stable, mature and post-mitotic.
Despite the fact that the ES/iPS cell-derived RPE cells have a general gene expression profile more typical of fetal RPE, they also express markers shared with postnatal RPE. These include differentiation markers associated with pigmentation, such as tyrosinase (TYR) and the premelanosomal protein-17 (PMEL17), channel proteins, such as Bestrophin-1 (BEST1), markers associated with phagocytosis, such as MERTK and the focal adhesion kinase (FAK), growth factors, such as pigment epithelium-derived factor (PEDF), and the visual cycle genes RPE65 and RLBP1. But how many markers should be shared between the ES/iPS cell-derived cells and RPE cells in vivo, fetal or adult, to be confident the cells in question will retain their identity and function? The initial study by Klimanskaya et al. (2004) used comparisons based on present/absent calls and showed that ES cell-derived RPE cells and fetal human RPE share expression of a large number of genes. A recent study using hierarchical clustering of gene expression profiles found that human ES cell-derived RPE, but not human iPS cell-derived RPE, clustered together with fetal human RPE. As might be expected, none of the ES/iPS-derived RPE clustered with human melanocytes that are neural crest derived (Liao et al., 2010). Pair-wise comparisons also showed more similarities between human fetal RPE and ES cell-derived RPE than iPS cell-derived RPE. Further it appears that many of the genes differentially upregulated at least 1.5 fold in ES cell-derived RPE compared to fetal RPE are involved in cell proliferation and neural differentiation, and down-regulated genes in visual perception. Genes upregulated in iPS cell-derived RPE versus fetal RPE were found to be predominantly involved in epithelial development and inflammatory responses, while some of the downregulated genes are, as in the case of ES cell-RPE versus fetal RPE, involved in visual perception (Liao et al., 2010). These results highlight the need for a thorough functional characterization of these in vitro generated cells.
It will also become important to determine whether genes with dissimilar expression levels are involved in any of the subtle features that characterize the native adult RPE. For instance, from birth to adulthood, the size of human or monkey RPE cells generally increases (Robb, 1985; Robinson and Hendrickson, 1995; Snodderly et al., 2002; Streeten, 1969). Furthermore, their size in the retina’s periphery, which is rod-dominated, is bigger than their size in the center of the human fovea, which is cone-dominated (Harman et al., 1997; Robinson and Hendrickson, 1995; Snodderly et al., 2002; Strauss, 2005; Streeten, 1969). Also, the length of RPE microvilli varies with the length of the photoreceptors’ outer segments they contact (Kivela et al., 2000; Streeten, 1969). It is possible, of course, that such subtle cell-to-cell differences are purely adaptive, induced by the microenvironment in which an individual RPE cell finds itself, but the capacity for such adaptations may not be fully developed in the ES/iPS cell-derived cells.
Using an extensive global expression profiling of native fetal, native adult and cultured fetal RPE, Strunnikova et al. have recently identified a set of 154 ‘RPE signature’ genes whose expression levels were similar in the three sample sets and at least 10-fold over the median of the corresponding genes’ expression levels in 78 tissues from throughout the body (Maminishkis et al., 2006; Strunnikova et al., 2010). Several of these genes encode proteins with critical RPE functions, for example in melanogenesis, cell adhesion, or the visual cycle. Others encode epithelial proteins such as epithelial channels and transporters, or matrix remodeling proteins, and still others proteins known to be involved in ophthalmic diseases, or the genes mapped to genomic regions associated with such diseases. One might argue therefore that expression of this set of genes in an ES/iPS cell-derived cell would mark it as an authentic RPE cell.
A particular role in the expression of mRNAs and proteins is also played by microRNAs (miRs). Interestingly, like many other epithelia, both adult and fetal RPE cells express microRNAs such as miR-200a, miR-204, miR-205, and miR-211 (Wang et al., 2010). Most of these microRNAs are well-known for their effects on mRNAs encoding the stem cell factors SOX2 and KLF4 (Wellner et al., 2009). They are also involved in inducing and maintaining stable epithelial monolayers through the action on mRNAs encoding proteins associated with epithelial-to-mesenchymal transition (EMT) and cell migration (Park et al., 2008; Wellner et al., 2009). For example, in fetal human RPE cultures, miR-204 directly targets the mRNA for TGFβ receptor 2, a classical EMT-inducing protein. In fact, a reduction in miR-204 leads to increased TGFβ receptor expression, indirectly resulting in a reduction of the tight junction proteins CLAUDIN 10, 16 and 19 (Wang et al., 2010). Conversely, disruption of cell–cell contacts in cultured adult mouse RPE leads to upregulation of EMT-inducing factors including the transcription factor ZEB1, which in turn leads to downregulation of tight junction proteins and particular miRs (Liu et al., 2010; Park et al., 2008; Tamiya et al., 2010; Wellner et al., 2009). Therefore, it is important to determine at what level these miRs are expressed in ES/iPS cell-derived RPE cells, and whether their expression levels correlate with the epithelial state of such cells.
In addition to simply assessing gene expression levels, there is also a need to characterize mRNA isoforms, be they generated by alternative promoter choice or alternative splicing, and ultimately the protein isoforms and their activities as they represent the business end of gene expression. In addition, it is important to define a set encompassing genes that are not normally expressed in adult native RPE and whose aberrant expression might interfere with the normal function of adult RPE cells (for a summary, see Table 1).
Table 1.
Molecular and functional criteria of authentic human retinal pigment epithelium (RPE) cells
| Feature | Testable criteria | References |
|---|---|---|
| RPE signature gene set (fetal and adult) |
Maintenance of expression of the RPE signature set of genes, including genes for melanogenesis, channel proteins, tight junction proteins, visual cycle, response to sensory perception, oxidoreductase activity, phagocytic activity, and transporter activity |
Strunnikova et al., 2010 |
| Micro-RNAs (fetal or adult) |
miR-200 genes, including miR-200a, miR-204*, miR-205 and miR-211*; miR-184, miR-187, and miR-302b/d** confirmed in adult human RPE |
Wang et al., 2010 |
| Pluripotent, fetal, and non-epithelial genes |
Absence of expression of genes associated with early development, including genes marking ES or induced pluripotent stem cells or neuroectodermal cells of the optic vesicle/optic cup: OCT4, SOX2, NANOG, KLF4, MYC, LIN28, high levels of PAX6, MITF Absence of expression of oncogenes but presence of expression of tumor suppressor genes Absence of expression of genes associated with epithelial-to-mesenchymal transition: ZEB1, TGFβ receptor-2 |
Wellner et al., 2009; Liu et al., 2010; Wang et al., 2010; and see text |
| Modulators of gene expression, mRNA isoforms, and protein activity |
RPE derived from iPS cells should not have an epigenetic memory of their tissue origin except when derived from RPE or neuroectodermal progenitors. Characteristics of promoter choice, splicing patterns, and post-translational protein modifications such as phosphorylation, sumoylation, or ubiquitination (dependent on E3 ligases and de-ubiquitinases). |
Kim et al., 2010; Bharti et al., 2008; and see text |
| Cell proliferation | Ideally, cells should be post-mitotic, i.e. not express Ki67 or incorporate BrdU |
See text |
| Polarization – a critical determinant of epithelial function |
Polarized distribution of channels, receptors, transporters, and associated proteins, located on the apical and the basal sides of the RPE Polarized constitutive secretion of macromolecules: Predominantly apical: MCP-1, IL-6, IL-8, PEDF, TGFβ1/2 Predominantly basal: VEGF Increase in secretion of both angiogenic (IL-6, IL-8, MCP-1) and angiostatic (IP-10, MCP-3, ITAC, RANTES) cytokines upon stimulation with pro-inflammatory cytokines |
Hughes et al., 1998; Maminishkis et al., 2006; Strauss, 2005; Shi et al., 2008; Li et al., 2009; Crane et al., 2000; Bryant and Mostov, 2008; Nejsum and Nelson, 2009; Marmorstein, 2001; and unpublished observations |
| Physiology of the cell – Transepithelial resistance (TER) Resting membrane potentials Fluid transport Subretinal space |
The presence of tight junctions should allow for a TER of several hundred Ω·cm−2. Maintenance of cell polarity by the continued presence of occludins and claudins. Apical and basolateral membrane resting potentials of approximately −50 to −60 mV, resulting in a transepithelial resting potential (TEP) of 2–10 mV (apical side, positive relative to basal side) Approximately 5–10 μl × cm−2 × h−1 Ability to regulate volume and chemical composition of subretinal space |
Maminishkis et al., 2006; Hughes et al., 1998; Strauss, 2005; Yang et al., 2003; Rizzolo, 2007; Li et al., 2009,Adijanto et al., 2009; Li et al., 2009 |
| Phagocytosis | Capable of phagocytosis of rod/cone outer segments | Kevany and Palczewski, 2010; Mallavarapu and Finnemann, 2010 |
| Cell geometry | Confluent monolayer of polygonal epithelium | |
| Immunologic requirements |
Should express normal levels of cytokines and complement- associated proteins. Ideally, when patient-derived, should not express patient-specific risk factor genes. For transplantations, careful consideration should be given to the question of whether MHC-matched or allogeneic cells should be used because the distinct types of cells may have differential sensitivities to different types of ongoing immune reactions. |
Sugita et al., 2009; Liao et al., 2010; and detailed comments in text |
Functional characteristics of ES/iPS cell-derived RPE cells
An assay frequently used for functional assessment of the in vitro generated RPE cells is phagocytosis of photoreceptor outer segments. For this assay, Buchholz et al. (2009) and Idelson et al. (2009) used in vitro generated, fluorescently marked outer segments and Carr et al. (2009a) used intact human retina and immunostaining for the photoreceptor protein opsin. These studies showed that the ES/iPS cell-derived RPE cells are capable of phagocytosis in vitro as well as in vivo after transplantation and that in vitro the phagocytic activity is comparable to that of cultured fetal human RPE. Fetal RPE, however, may not be entirely appropriate for comparison as its phagocytic activity may be lower than that of adult RPE. In addition to phagocytosis, the RPE is fundamentally important for regulating the chemical composition and volume of the photoreceptor/RPE interface. A number of tests have been developed to assess such additional functions in vitro. For instance, like other epithelial monolayers, RPE monolayers also express the tight junction proteins JAM-C, CLAUDIN 10, 16 and, at highest level, CLAUDIN 19 which allow the cells to become mechanically and electrically stable (Economopoulou et al., 2009; Maminishkis et al., 2006; Rizzolo, 2007). In fact, human RPE cells in culture develop a transepithelial electrical resistance of several hundred Ω·cm−2 which compares favorably with that of native mammalian RPE (approximately 200–300 Ω·cm−2) (Maminishkis et al., 2006; Wang et al., 2010). They also exhibit a resting apical membrane potential of approximately −50 to −60 mV, generated primarily by the apical presence of Kir7.1 potassium channels, and a resting basolateral membrane potential of similar magnitude, generated in part by a basolaterally located chloride channel, the cystic fibrosis transmembrane conductance regulator CFTR (Li et al., 2009; Rizzolo, 2007; Yang et al., 2003). CFTR is activated by a protein kinase A-mediated increase in cAMP concentrations. Experimentally, CFTR activation can be achieved by exposing the cells to forskolin, which activates cAMP; IBMX, which inhibits phosphodiesterase; or dibutyl cAMP, a membrane-permeable cAMP analog. CFTR channels can also be activated by interferon-γ that stimulates the JAK-STAT pathway and, ultimately, PKA (Blaug et al., 2003; Li et al., 2009). Moreover, CFTR channels indirectly modulate fluid transport across the epithelium, and so fluid absorption measurements can also serve to assess the functionality of the cells (Hughes et al., 1998; Li et al., 2009). Lastly, cytokines such as MCP-1, IL-6, IL-8, PEDF, and TGF-β1/2 are normally secreted constitutively predominantly from the apical side, while others such as VEGF are predominantly secreted from the basal side. The expression and polarized secretion of several of these cytokines significantly increases both on the apical and basal sides upon stimulation of RPE cells with pro-inflammatory cytokines (Shi et al., 2008). Hence, polarized secretion of specific cytokines and increased secretion upon stimulation may serve as additional functional criteria for ES/iPS cell-derived RPE cells in culture (Crane et al., 2000; Marmorstein, 2001; Bryant and Mostov, 2008; Shi et al., 2008; Li et al., 2009; Nejsum and Nelson, 2009; Li and Miller, unpublished). A summary of functional tests one might consider to assess RPE authenticity is given in Table 1.
Toward in vivo replacement of damaged RPE cells
Although the ultimate goal is to use ES/iPS cell-derived RPE cells in human patients, and although the results from animal studies are sometimes misleading when it comes to transfer them to humans, animal models of primary RPE degeneration are nevertheless invaluable for the initial optimization of cell culture and surgical techniques. The model of choice is the above-mentioned RCS rat whose RPE is unable to phagocytose photoreceptor outer segments and whose photoreceptors degenerate over a period of 3 months after birth (Dowling and Sidman, 1962; Edwards and Szamier, 1977). Indeed, these rats have been used widely to assess the performance of ES/iPS cell-derived RPE cells after injection into the subretinal space. Alternatively, the RPE can be damaged specifically by sodium iodate before cell transplantation (Franco et al., 2009).
Most studies show that the transplanted cells die within 10–15 weeks, and survival of a high percentage of cells for up to 20–30 weeks seems the exception (Carr et al., 2009b; Idelson et al., 2009; Lu et al., 2009). Nevertheless, no monolayers of honeycomb-shaped cells resembling authentic RPE are formed. Rather, the cells aggregate in clumps, occasionally become dislodged in the retina proper, and do not form a smooth transition to the existing RPE. It is also unclear whether they assume the right polarity, with the apical side facing the outer segments and the basal side Bruch’s membrane, and whether a damaged Bruch’s membrane is regenerated. Nevertheless, they are capable of slowing down, though not stopping, photoreceptor loss. Some studies report an improvement of visual function as measured by improvements in electroretinograms (ERGs) and even behavioral assays such as those that measure eye or body movements in response to light (optokinetic responses) (Carr et al., 2009b; Idelson et al., 2009). The RPE also generates slow potentials in response to light that can be seen in the ERG (Samuels et al., 2010; Wu et al., 2004) but such RPE-mediated light-evoked responses have not so far been tested after transplantation. Furthermore, it remains unknown whether improved retinal function would translate into improved cortical representation of images, an improvement that can be seen in humans after transplantation of native RPE (da Cruz et al., 2007). In addition, some of the positive effects seen in the eyes injected with cultured cells might at least in part be because of attraction of immune cells, especially of macrophages that might help to phagocytose photoreceptor outer segments or even whole RPE cells that have phagocytosed but not digested outer segments (Carr et al., 2009b). It is also conceivable that all these retinal and visual improvements are because of the ability of the injected cells to supply extra amounts of growth and survival factors and not to their ability to phagocytose or provide other RPE-specific functions (Shi et al., 2008). In fact, ARPE19 cells, a spontaneously immortalized human RPE line (Dunn et al., 1996) that shares fewer characteristics with authentic RPE cells than ES/iPS cell-derived RPE cells, have also been able to partially rescue photoreceptor degeneration in RCS rats (Lund et al., 2001; McGill et al., 2004).
It would seem therefore that a fully functional replacement RPE should display an authentic RPE’s normal anatomy. In fact, the replacement RPE should be a monolayer precisely wedged between photoreceptors and Bruch’s membrane. In patients potentially benefitting from RPE replacements, however, Bruch’s membrane is likely injured by inflammatory processes, and replacement cells could be damaged by the ongoing inflammation even before they are potentially able to regenerate Bruch’s membrane. A solution to this problem might come from using RPE cells that are grown on artificial membranes and transplanted as intact monolayers along with their membranes. Several materials are being considered for this purpose, including poly(lactic-co-glycolic acid) (PLGA), poly(para-xylene) (Parylene), and polymers of carbamic acid-derived esters (polyurethans). Ideally, such polymers should be chemically inert, biocompatible, and allow for nutrient transport. Because of their hydrophobic nature, however, they do not support cell growth very well. Hence, they need to be chemically modified, and it remains to be seen whether such modified polymers will become clinically useful (Booij et al., 2010; Sheridan et al., 2004; Williams et al., 2005).
Immunologic considerations
Even if culture-derived RPE cells can be transplanted as correctly oriented and functional monolayers or can be made to organize in such monolayers after transplantation, concerns remain about whether they will be accepted immunologically and have the capacity to regulate the local immunity in a way similar to healthy RPE cells. As mentioned, at least for AMD, inflammation is an important if not critical component in the pathogenesis of the disease, and so any therapeutic regimen using cell transplantation has to take into consideration the mechanisms that underlie the inflammatory processes. Conceptually, we can think of several distinct immunologic scenarios that may lead to inflammation and that may impair the acceptance of grafted cells.
In a first scenario, retinal microglial cells may become activated either because they themselves are abnormal or because they may be stimulated by intracellular material from dying or dead cells. As a result of their activation, they may secrete large amounts of cytokines to which RPE cells may be particularly susceptible as recently shown in a mouse model (Ma et al., 2009). Under such conditions, grafted RPE cells might not survive regardless of their genotype, unless one first somehow interferes with microglial activation and cytokine accumulation.
In a second scenario, the primary source for elevated cytokine levels may not be microglial cells but T cells. Such T cells may have been stimulated initially by an infection in the periphery but by chance might cross-react against a molecule expressed in the vicinity of the endogenous RPE. Here, too, transplanted RPE cells, regardless of genotype, might be damaged as innocent bystanders.
In a third scenario, a peripheral infection might stimulate T cells that cross-react with an antigen X on the patient’s resident RPE cells. Here, either of two conditions would protect the transplanted RPE cells from attack: they could either lack antigen X, or they could possess antigen X but express totally different major histocompatibility complex (MHC) molecules from those expressed by the patient. Under both conditions, RPE cells would be protected because they would not be recognized by the patient’s T cells which have been stimulated to see antigen X in conjunction with self-MHC. Because it is unlikely that antigen X and its cross-reactive, immune-stimulatory peripheral antigen are known, the only choice for transplantation may be allogeneic RPE cells. Normally, however, each individual has a high frequency of T cells able to react with allogeneic cells. Nevertheless, if allogeneic donor cells are completely free of antigen-presenting cells (APCs) and do not themselves work as APCs capable of providing co-stimulatory molecules, they are likely to be tolerated in a host’s eye because they would not be able to activate the resident alloreactive T cells.
In a fourth scenario, the immune reaction against the patient’s RPE could be driven by the RPE cells directly. This would require that resident RPE cells release RPE antigens along with alarm signals and stimulate local APCs, or that they themselves act as APCs and stimulate ongoing auto-immune reactions. Under such conditions, only allogeneic transplanted RPE cells with totally mismatched MHC molecules would have a chance of survival, provided they cannot themselves become APCs. In fact, rather than acting as APCs, RPE cells have been reported to inhibit T-cell responses, for instance by expressing programmed cell death ligand-1 (PD-L1, also called B7H1) along with the appropriate MHC molecules (Sugita et al., 2009). Hence, one might consider grafting MHC-matched RPE cells expressing such inhibitory molecules as they might indeed help to subdue anti-RPE responses. Nevertheless, although patient iPS cell-derived RPE cells would best fulfill the criterion of an MHC match, such cells might still express genetic risk factors that operate in RPE cells themselves, for instance by conferring increased susceptibility to cytokine stress. Moreover, the costs associated with the derivation and quality control of such patient-specific cells are likely prohibitive.
Thus it seems that for several of the above theoretical considerations, allogeneic RPE cells may actually be a better choice for transplantation compared to syngeneic cells. Nevertheless, it will be important to first experimentally verify their long-term acceptance.
Conclusions
The recent progress made in generating RPE cells from ES or iPS cells in vitro provides great hopes for a curative treatment of RPE-mediated ocular diseases, in particular AMD. Nevertheless, as outlined in this review, we are still faced with major obstacles in the quest to restore vision with such cell-based therapies. These obstacles include a still experimental stage to efficiently generate RPE cells in vitro; a limited knowledge of the mechanisms that might render the cells molecularly and functionally stable; a lack of a precise list of functional properties absolutely required prior to transplantation and properties that the cells may acquire adaptively once they are correctly integrated into the host tissue; a paucity of information concerning the immunologic properties that guide long-term acceptance of cell grafts; and the sheer costs associated with any individualized cell-based therapy. Never before in medical history, however, has an in vitro approach offered such an opportunity to study the pathogenesis of a disease as has the availability of patient-specific cells that can be differentiated deliberately into a variety of cell types. It is possible therefore that the biggest gain from the novel approach might not come from using in vitro generated cells for cell replacement therapies but from an ever deeper understanding of disease mechanisms that may eventually lead to non-cell-based therapies, or better still, disease prevention. Nevertheless, we believe that the potential of replacing damaged RPE with in vitro generated healthy RPE should be explored to the fullest extent possible, though without losing sight of the right balance between enthusiasm and caution.
Acknowledgements
We would like to express our gratitude to Drs Sally Temple, Jeffrey Stern, Ronald McKay, Christine Curcio, Monique Dubois-Dalcq and Polly Matzinger for invaluable comments on the manuscript, and Vinish Saini for help with the artwork in Fig. 1. This work was supported by the intramural program of the NIH, NINDS and NEI.
References
- Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS. CO2-induced ion and fluid transport in human retinal pigment epithelium. J. Gen. Physiol. 2009;133:603–622. doi: 10.1085/jgp.200810169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki M. Regeneration of the amphibian retina: role of tissue interaction and related signaling molecules on RPE transdifferentiation. Dev. Growth Differ. 2007;49:109–120. doi: 10.1111/j.1440-169X.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- Baird PN, Richardson AJ, Robman LD, Dimitrov PN, Tikellis G, McCarty CA, Guymer RH. Apolipoprotein (APOE) gene is associated with progression of age-related macular degeneration (AMD) Hum. Mutat. 2006;27:337–342. doi: 10.1002/humu.20288. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Nguyen MT, Skuntz S, Bertuzzi S, Arnheiter H. The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res. 2006;19:380–394. doi: 10.1111/j.1600-0749.2006.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Liu W, Csermely T, Bertuzzi S, Arnheiter H. Alternative promoter use in eye development: the complex role and regulation of the transcription factor MITF. Development. 2008;135:1169–1178. doi: 10.1242/dev.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaug S, Quinn R, Quong J, Jalickee S, Miller SS. Retinal pigment epithelial function: a role for CFTR? Doc. Ophthalmol. 2003;106:43–50. doi: 10.1023/a:1022514031645. [DOI] [PubMed] [Google Scholar]
- Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 2010;29:1–18. doi: 10.1016/j.preteyeres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, Clegg DO. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–2434. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- Cai X, Conley SM, Naash MI. RPE65: role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 2009;30:57–62. doi: 10.1080/13816810802626399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, Vugler A, Lawrence J, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol. Vis. 2009a;15:283–295. [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS ONE. 2009b;4:e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig SE, Calinescu AA, Hitchcock PF. Identification of the molecular signatures integral to regenerating photoreceptors in the retina of the zebra fish. J Ocul Biol Dis Infor. 2008;1:73–84. doi: 10.1007/s12177-008-9011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane IJ, Wallace CA, McKillop-Smith S, Forrester JV. Control of chemokine production at the blood-retina barrier. Immunology. 2000;101:426–433. doi: 10.1046/j.1365-2567.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin T, Raffelsberger W, Lee-Rivera I, et al. The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Differ. 2010;17:1199–1210. doi: 10.1038/cdd.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26:598–635. doi: 10.1016/j.preteyeres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J. Comp. Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev. Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Defoe DM, Adams LB, Sun J, Wisecarver SN, Levine EM. Defects in retinal pigment epithelium cell proliferation and retinal attachment in mutant mice with p27(Kip1) gene ablation. Mol. Vis. 2007;13:273–286. [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J. Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Economopoulou M, Hammer J, Wang F, Fariss R, Maminishkis A, Miller SS. Expression, localization, and function of junctional adhesion molecule-C (JAM-C) in human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2009;50:1454–1463. doi: 10.1167/iovs.08-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RB, Szamier RB. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science. 1977;197:1001–1003. doi: 10.1126/science.560718. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Feng Q, Lu SJ, Klimanskaya I, Gomes I, Kim D, Chung Y, Honig GR, Kim KS, Lanza R. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Franco LM, Zulliger R, Wolf-Schnurrbusch UE, Katagiri Y, Kaplan HJ, Wolf S, Enzmann V. Decreased visual function after patchy loss of retinal pigment epithelium induced by low-dose sodium iodate. Invest. Ophthalmol. Vis. Sci. 2009;50:4004–4010. doi: 10.1167/iovs.08-2898. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 2003;76:463–471. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. U S A. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman AM, Fleming PA, Hoskins RV, Moore SR. Development and aging of cell topography in the human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1997;38:2016–2026. [PubMed] [Google Scholar]
- Haruta M, Sasai Y, Kawasaki H, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest. Ophthalmol. Vis. Sci. 2004;45:1020–1025. doi: 10.1167/iovs.03-1034. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson E, Copeland NG, Jenkins NA, Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Horie S, Sugita S, Futagami Y, Yamada Y, Mochizuki M. Human retinal pigment epithelium-induced CD4(+)CD25(+) regulatory T cells suppress activation of intraocular effector T cells. Clin. Immunol. 2010;136:83–95. doi: 10.1016/j.clim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Horsford DJ, Nguyen MT, Sellar GC, Kothary R, Arnheiter H, McInnes RR. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development. 2005;132:177–187. doi: 10.1242/dev.01571. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Gallemore R, Miller SS. Transport Mechanisms in the retinal pigment epithelium. In: Marmor MF, editor. The Retinal Pigment Epithelium. Oxford University Press; New York: 1998. pp. 103–134. [Google Scholar]
- Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Osakada F, Watanabe K, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 2005;102:11331–11336. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaarniranta K, Salminen A. Age-related macular degeneration: activation of innate immunity system via pattern recognition receptors. J. Mol. Med. 2009;87:117–123. doi: 10.1007/s00109-008-0418-z. [DOI] [PubMed] [Google Scholar]
- Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010;16:193–202. doi: 10.1016/j.molmed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Suemori H, Mizuseki K, et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc. Natl. Acad. Sci. U S A. 2002;99:1580–1585. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivela T, Jaaskelainen J, Vaheri A, Carpen O. Ezrin, a membrane-organizing protein, as a polarization marker of the retinal pigment epithelium in vertebrates. Cell Tissue Res. 2000;301:217–223. doi: 10.1007/s004410000225. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–245. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- Kramer F, White K, Pauleikhoff D, et al. Mutations in the VMD2 gene are associated with juvenile-onset vitelliform macular dystrophy (Best disease) and adult vitelliform macular dystrophy but not age-related macular degeneration. Eur. J. Hum. Genet. 2000;8:286–292. doi: 10.1038/sj.ejhg.5200447. [DOI] [PubMed] [Google Scholar]
- Kurose H, Bito T, Adachi T, Shimizu M, Noji S, Ohuchi H. Expression of fibroblast growth factor 19 (Fgf19) during chicken embryogenesis and eye development, compared with Fgf15 expression in the mouse. Gene Expr. Patterns. 2004;4:687–693. doi: 10.1016/j.modgep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Kvanta A. Expression and secretion of transforming growth factor-beta in transformed and nontransformed retinal pigment epithelial cells. Ophthalmic Res. 1994;26:361–367. doi: 10.1159/000267502. [DOI] [PubMed] [Google Scholar]
- Land M, Nilsson D-E. Animal Eyes. Oxford University Press; Oxford, NY: 2002. [Google Scholar]
- Lengner CJ. iPS cell technology in regenerative medicine. Ann. N Y Acad. Sci. 2010;1192:38–44. doi: 10.1111/j.1749-6632.2009.05213.x. [DOI] [PubMed] [Google Scholar]
- Li R, Maminishkis A, Wang FE, Miller SS. PDGF-C and -D induced proliferation/migration of human RPE is abolished by inflammatory cytokines. Invest. Ophthalmol. Vis. Sci. 2007;48:5722–5732. doi: 10.1167/iovs.07-0327. [DOI] [PubMed] [Google Scholar]
- Li R, Maminishkis A, Banzon T, Wan Q, Jalickee S, Chen S, Miller SS. IFN-gamma regulates retinal pigment epithelial fluid transport. Am. J. Physiol. Cell Physiol. 2009;297:C1452–C1465. doi: 10.1152/ajpcell.00255.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JL, Yu J, Huang K, et al. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum. Mol. Genet. 2010 doi: 10.1093/hmg/ddq341. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lintig J, Kiser PD, Golczak M, Palczewski K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 2010;35:400–410. doi: 10.1016/j.tibs.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xin Y, Ye F, Wang W, Lu Q, Kaplan HJ, Dean DC. Taz-Tead1 links cell-cell contact to Zeb1 expression, proliferation and dedifferentiation in retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2010;51:3372–3380. doi: 10.1167/iovs.09-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longbottom R, Fruttiger M, Douglas RH, Martinez-Barbera JP, Greenwood J, Moss SE. Genetic ablation of retinal pigment epithelial cells reveals the adaptive response of the epithelium and impact on photoreceptors. Proc. Natl. Acad. Sci. U S A. 2009;106:18728–18733. doi: 10.1073/pnas.0902593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- Lund RD, Adamson P, Sauve Y, et al. Subretinal transplantation of genetically modified human cell lines attenuates loss of visual function in dystrophic rats. Proc. Natl. Acad. Sci. U S A. 2001;98:9942–9947. doi: 10.1073/pnas.171266298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS ONE. 2009;4:e7945. doi: 10.1371/journal.pone.0007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallavarapu M, Finnemann SC. Neural retina and MerTK-independent apical polarity of alphavbeta5 integrin receptors in the retinal pigment epithelium. Adv. Exp. Med. Biol. 2010;664:123–131. doi: 10.1007/978-1-4419-1399-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest. Ophthalmol. Vis. Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DM, Rustgi AK, Defoe D, Brooks SE, McCormick RS, Thompson TP, Edelmann W, Kucherlapati R, Smith S. Retinal pigment epithelium abnormalities in mice with adenomatous polyposis coli gene disruption. Arch. Ophthalmol. 1997;115:645–650. doi: 10.1001/archopht.1997.01100150647013. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic. 2001;2:867–872. doi: 10.1034/j.1600-0854.2001.21202.x. [DOI] [PubMed] [Google Scholar]
- Maw MA, Kennedy B, Knight A, Bridges R, Roth KE, Mani EJ, Mukkadan JK, Nancarrow D, Crabb JW, Denton MJ. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat. Genet. 1997;17:198–200. doi: 10.1038/ng1097-198. [DOI] [PubMed] [Google Scholar]
- McGill TJ, Lund RD, Douglas RM, Wang S, Lu B, Prusky GT. Preservation of vision following cell-based therapies in a model of retinal degenerative disease. Vision Res. 2004;44:2559–2566. doi: 10.1016/j.visres.2004.05.025. [DOI] [PubMed] [Google Scholar]
- McWhirter JR, Goulding M, Weiner JA, Chun J, Murre C. A novel fibroblast growth factor gene expressed in the developing nervous system is a downstream target of the chimeric homeodomain oncoprotein E2A-Pbx1. Development. 1997;124:3221–3232. doi: 10.1242/dev.124.17.3221. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc. Natl. Acad. Sci. U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitashov VI. Mechanisms of retina regeneration in urodeles. Int. J. Dev. Biol. 1996;40:833–844. [PubMed] [Google Scholar]
- Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc. Natl. Acad. Sci. U S A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejsum LN, Nelson WJ. Epithelial cell surface polarity: the early steps. Front. Biosci. 2009;14:1088–1098. doi: 10.2741/3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Nussenblatt RB, Liu B, Li Z. Age-related macular degeneration: an immunologically driven disease. Curr Opin Investig Drugs. 2009;10:434–442. [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009a;4:811–824. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J. Cell Sci. 2009b;122:3169–3179. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]
- Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development. 1997;124:805–816. doi: 10.1242/dev.124.4.805. [DOI] [PubMed] [Google Scholar]
- Quinn RH, Quong JN, Miller SS. Adrenergic receptor activated ion transport in human fetal retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2001;42:255–264. [PubMed] [Google Scholar]
- Rizzolo LJ. Development and role of tight junctions in the retinal pigment epithelium. Int. Rev. Cytol. 2007;258:195–234. doi: 10.1016/S0074-7696(07)58004-6. [DOI] [PubMed] [Google Scholar]
- Robb RM. Regional changes in retinal pigment epithelial cell density during ocular development. Invest. Ophthalmol. Vis. Sci. 1985;26:614–620. [PubMed] [Google Scholar]
- Robinson SR, Hendrickson A. Shifting relationships between photoreceptors and pigment epithelial cells in monkey retina: implications for the development of retinal topography. Vis. Neurosci. 1995;12:767–778. doi: 10.1017/s0952523800009020. [DOI] [PubMed] [Google Scholar]
- Samuels IS, Sturgill GM, Grossman GH, Rayborn ME, Hollyfield JG, Peachey NS. Light-evoked responses of the retinal pigment epithelium: changes accompanying photoreceptor loss in the mouse. J. Neurophysiol. 2010;104:391–402. doi: 10.1152/jn.00088.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A, Tsonis PA. Bridging the regeneration gap: genetic insights from diverse animal models. Nat. Rev. Genet. 2006;7:873–884. doi: 10.1038/nrg1923. [DOI] [PubMed] [Google Scholar]
- Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- Sheridan C, Williams R, Grierson I. Basement membranes and artificial substrates in cell transplantation. Graefes Arch. Clin. Exp. Ophthalmol. 2004;242:68–75. doi: 10.1007/s00417-003-0800-z. [DOI] [PubMed] [Google Scholar]
- Shi Y. Generation of functional insulin-producing cells from human embryonic stem cells in vitro. Methods Mol. Biol. 2010;636:79–85. doi: 10.1007/978-1-60761-691-7_5. [DOI] [PubMed] [Google Scholar]
- Shi G, Maminishkis A, Banzon T, Jalickee S, Li R, Hammer J, Miller SS. Control of chemokine gradients by the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2008;49:4620–4630. doi: 10.1167/iovs.08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM, Sandstrom MM, Leung IY, Zucker CL, Neuringer M. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2002;43:2815–2818. [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Streeten B. Development of the human retinal pigment epithelium and the posterior segment. Arch. Ophthalmol. 1969;81:383–394. doi: 10.1001/archopht.1969.00990010385017. [DOI] [PubMed] [Google Scholar]
- Strunnikova NV, Maminishkis A, Barb JJ, et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum. Mol. Genet. 2010;19:2468–2486. doi: 10.1093/hmg/ddq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Horie S, Nakamura O, et al. Retinal pigment epithelium-derived CTLA-2alpha induces TGFbeta-producing T regulatory cells. J. Immunol. 2008;181:7525–7536. doi: 10.4049/jimmunol.181.11.7525. [DOI] [PubMed] [Google Scholar]
- Sugita S, Usui Y, Horie S, Futagami Y, Aburatani H, Okazaki T, Honjo T, Takeuchi M, Mochizuki M. T-cell suppression by programmed cell death 1 ligand 1 on retinal pigment epithelium during inflammatory conditions. Invest. Ophthalmol. Vis. Sci. 2009;50:2862–2870. doi: 10.1167/iovs.08-2846. [DOI] [PubMed] [Google Scholar]
- Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet. 2009;10:19–43. doi: 10.1146/annurev.genom.9.081307.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya S, Liu L, Kaplan HJ. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Invest. Ophthalmol. Vis. Sci. 2010;51:2755–2763. doi: 10.1167/iovs.09-4725. [DOI] [PubMed] [Google Scholar]
- Vugler A, Carr AJ, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp. Neurol. 2008;214:347–361. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Wang FE, Zhang C, Maminishkis A, et al. MicroRNA-204/211 alters epithelial physiology. FASEB J. 2010;24:1552–1571. doi: 10.1096/fj.08-125856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- Williams RL, Krishna Y, Dixon S, Haridas A, Grierson I, Sheridan C. Polyurethanes as potential substrates for sub-retinal retinal pigment epithelial cell transplantation. J. Mater. Sci. Mater. Med. 2005;16:1087–1092. doi: 10.1007/s10856-005-4710-y. [DOI] [PubMed] [Google Scholar]
- Wu J, Peachey NS, Marmorstein AD. Light-evoked responses of the mouse retinal pigment epithelium. J. Neurophysiol. 2004;91:1134–1142. doi: 10.1152/jn.00958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Pan A, Swaminathan A, Kumar G, Hughes BA. Expression and localization of the inwardly rectifying potassium channel Kir7.1 in native bovine retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2003;44:3178–3185. doi: 10.1167/iovs.02-1189. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Stanton JB, Wu J, Yu K, Hartzell HC, Peachey NS, Marmorstein LY, Marmorstein AD. Suppression of Ca2+ signaling in a mouse model of Best disease. Hum. Mol. Genet. 2010;19:1108–1118. doi: 10.1093/hmg/ddp583. [DOI] [PMC free article] [PubMed] [Google Scholar]