Abstract
A theoretical model is proposed to describe the heat capacity function and the phase behavior of binary mixtures of phospholipids and cholesterol. The central idea is that the liquid-ordered state (Lo) is a thermodynamic state or an ensemble of conformations of the phospholipid, characterized by enthalpy and entropy functions that are intermediate between those of the solid and the liquid-disordered (Ld) states. The values of those thermodynamic functions are such that the Lo state is not appreciably populated in the pure phospholipid, at any temperature, because either the solid or the Ld state have much lower free energies. Cholesterol stabilizes the Lo state by nearest-neighbor interactions, giving rise to the appearance of the Lo phase. The model is studied by Monte Carlo simulations on a lattice with nearest-neighbor interactions, which are derived from experiment as much as possible. The calculated heat capacity function closely resembles that obtained by calorimetry. The phase behavior produced by the model is also in agreement with experimental data. The simulations indicate that separation between solid and Lo phases occurs below the melting temperature of the phospholipid (Tm). Above Tm, small Ld and Lo domains do exist, but there is no phase separation.
Introduction
Our understanding of the interactions between phospholipids and cholesterol (Chol) is still incomplete, as demonstrated by the continuous work on the problem in recent years (1–5). The effect of cholesterol on the excess heat capacity (ΔCp) associated with the main phase transition of phosphatidylcholines (PC) has intrigued researchers for a long time (6–10). Fig. 1 shows typical changes in ΔCp of dipalmitoylphosphatidylcholine (DPPC) caused by incorporation of cholesterol in the bilayer, determined by differential scanning calorimetry (DSC) (8). When cholesterol is added to PC multilamellar vesicles (MLVs), ΔCp decreases substantially and the main phase transition temperature (Tm) decreases slightly; at still higher cholesterol concentrations, a significant broadening of ΔCp is observed, and the maximum moves to higher temperatures. Eventually, the phase transition becomes extremely broad and hard to detect.
Figure 1.
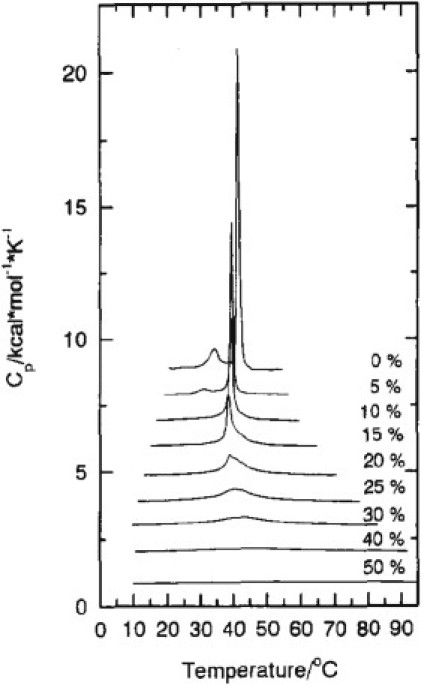
Effect of increasing amounts of cholesterol on the excess heat capacity function of DPPC MLVs. (Reprinted with permission from Huang et al. (8). Copyright 1993 American Chemical Society.)
Ipsen et al. (11) proposed a model that became the standard description of the phase behavior in PC/Chol mixtures. In pure DPPC, the main phase transition occurs at Tm = 41.5°C, between a solid and a liquid-disordered (Ld) phase. At high cholesterol content, however, the phospholipid exists in a state of high chain order, akin to the solid, but with fast long-range mobility, akin to the liquid, which was called a liquid-ordered phase (Lo). The model yielded ΔCp functions for DPPC/Chol mixtures (12) that closely resembled those obtained experimentally by DSC. The broad, high-temperature melting at Chol ≥ 20 mol % was reproduced, which was associated with an Lo→Ld phase transition. Furthermore, based on this model Ipsen et al. (11,12) calculated a phase diagram that subsequently received significant experimental corroboration (9,13,14). The canonical phase diagram for DPPC/Chol is shown in Fig. 2 A (4).
Figure 2.
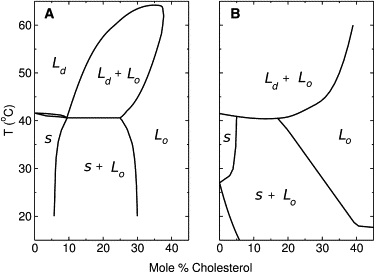
(A) Canonical phase diagram for DPPC/Chol, based on theory and several lines of experimental data. (B) Variation based also on the 13C-NMR signal of the lipid carbonyl group (8). In these diagrams, S represents a solid phase.
In recent years, interest in PC/Chol mixtures has resurged, motivated mainly by two developments. First, an alternative view, the idea that interactions between phospholipids and cholesterol can be described by formation of condensed complexes with defined stoichiometry was proposed by McConnell et al. (15,16). The broad, high-temperature shoulder in ΔCp (Fig. 1) was interpreted as thermal dissociation of the complexes, and was qualitatively reproduced by calculations (17). Although formation of complexes between PC and Chol is an old and criticized idea, the new concept does not require highly specific and well-defined chemical interactions, but rather transient associations between PC and cholesterol (18).
Second, developments in fluorescence microscopy, combined with the ability to produce giant unilamellar vesicles with diameters ≥10 μm, allowed direct visualization of liquid-liquid phase separation in phospholipid/cholesterol mixtures (19–24). Ld/Lo phase separation was observed in ternary mixtures of cholesterol with two phospholipids, one of which is typically saturated, with a high Tm (ordered), and the other is unsaturated (or has short acyl chains), with a low Tm (disordered). However, in binary mixtures of PC/Chol, no micron-size phase separation was observed (19), and it now appears that no Ld/Lo phase separation occurs in DPPC/Chol (25). This is contrary to a strict interpretation of the immiscibility in PC/Chol systems in terms of the phase diagram of Fig. 2 A, which includes a large region of Ld/Lo phase coexistence. The condensed complex model, on the other hand, predicted a closed loop in ternary mixtures of a high-melting and a low-melting PC with cholesterol in the liquid state (18,26), which means that no Ld/Lo phase separation would exist in the PC/Chol binary mixtures.
Recently, we investigated domain formation in ternary mixtures of a high- and a low-melting PC with cholesterol by Monte Carlo simulations (27). A simple lattice model of the membrane was used, with phospholipids and cholesterol interacting only with nearest neighbors. The unlike nearest-neighbor interaction Gibbs free energies were represented by a parameter designated by ωAB, which is equivalent to the Flory parameter in polymer physics (28,29),
| (1) |
where gAA and gBB are the Gibbs energies of interaction between two A or two B molecules, and gAB is the Gibbs energy of interaction between one A and one B molecule. The simulations showed that phase separation occurs in the ternary mixture, but not in any of the three binary mixtures (27). This result requires that a favorable interaction exist between the ordered lipid and cholesterol (ωAB < 0) combined with unfavorable interactions (ωAB > 0) of the disordered lipid with both the ordered lipid and cholesterol. This is all that is necessary for a closed loop to exist in the ternary phase diagram, with no need to invoke formation of complexes (1,27).
However, the model we used does not account for phase transitions, which is a serious limitation. That model cannot describe the fundamental behavior of mixtures containing cholesterol over the phospholipid phase transition, which may be biologically relevant because sphingomyelins have Tm close to 37°C. A model that includes the phase transition is necessary and useful, but it must be simple to be extended to ternary mixtures, which are of greater biological interest. The model used by Ipsen et al. (12), which combines the 10-state Pink and q-state Potts models, is too complicated for this purpose. The condensed complex model, on the other hand, results in a ΔCp that exhibits a large spurious transition at high cholesterol content, below the lipid main phase transition (17). In addition, its energetic parameters are much too large (1).
This report describes an attempt to fill this gap, using a simple thermodynamic model that is approximate but tractable. This model is applied to DPPC/Chol binary mixtures and is investigated using Monte Carlo simulations on a triangular lattice. The lipid interactions are represented by parameters that are consistent with experiment. The calculated ΔCp(T) is in fairly good agreement with that obtained experimentally by DSC. The model correctly produces phase separation in DPPC/Chol, or not, in agreement with the experimental observations.
The Model
The central idea of the model is that the Lo state is a thermodynamic state of the phospholipid characterized by an enthalpy and an entropy that are intermediate between the Ld and solid states (Fig. 3 A). The values of those thermodynamic functions are such that the Lo state is not appreciably populated in pure DPPC. Below Tm, the free energy of the solid lies much below those of the Ld and Lo states (Fig. 3 B); above Tm, the free energy of Ld is much lower than those of Lo and solid states. Therefore, the transition of pure DPPC occurs from the solid to the Ld phase (Fig. 3 C). The Lo state is scarcely populated, except in the region of the main phase transition, where it stabilizes solid/Ld interfaces (but even at Tm, Lo < 7 mol %). In the presence of cholesterol, however, the free energy of the Lo state is lowered by preferential nearest-neighbor interactions with cholesterol, and its population increases. This is qualitatively illustrated in Fig. 3 C where the addition of ≈20 mol % Chol brings the Lo line (dashed) close to the solid, which is the reference state (horizontal axis).
Figure 3.
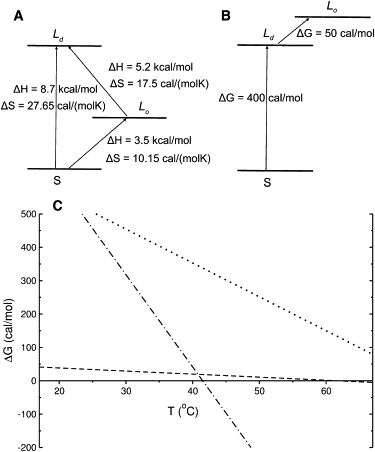
Thermodynamic model used in the simulations. (A) Enthalpy of the three states of DPPC, solid (S), Lo, and Ld.. (B) Gibbs free energy of the three states close to room temperature (300 K). (C) Qualitative illustration of the temperature dependence of the free energy for the solid, which is the reference state (solid line at ΔG = 0), the Ld state (dash-dotted line), and the Lo state of the phospholipid in the absence (dotted line) and in the presence of ∼20% Chol (dashed line).
The concept proposed is fundamentally similar to the classical model of Monod et al. (30) for oxygen binding to hemoglobin. Hemoglobin was assumed to exist in two states, T and R, which interconvert through a conformational change. The T state dominates in the absence of oxygen and has low affinity for it. The R state is not appreciably populated in the absence of oxygen, but has high affinity for it. Thus, binding of oxygen stabilizes the R state, increasing its population. In the DPPC/Chol model, cholesterol plays a role similar to that of oxygen: it stabilizes the Lo state. The classical alternative for binding of oxygen to hemoglobin is the model of Koshland et al. (31), in which the R state does not exist in the absence of oxygen, but is formed concomitant with ligand binding to the protein (induced fit). The analogous model in DPPC/Chol would not allow for Lo to exist in the absence of cholesterol. But in this case, the implementation of the model is much more complicated because numerous restrictions must be imposed in the various moves and transitions in the Monte Carlo simulations. In the case of hemoglobin, binding of oxygen is described similarly well by both models. I expect that both models would work well in DPPC/Chol, but the model chosen is much simpler to implement. It also seems to me that the PC conformations that prefer to interact with cholesterol should be possible in its absence.
The DPPC/Chol binary system was studied with this model. A leaflet of the lipid bilayer is represented by a triangular lattice with periodic boundary conditions. Each site represents a phospholipid (in the solid, Lo, or Ld states) or a cholesterol molecule. The free energy of each lattice site is determined by its interactions with nearest neighbors, represented by the ωAB parameters, and, in the case of DPPC, by the enthalpy of its state. Monte Carlo simulations were performed to obtain the equilibrium properties of the lattice, such as the excess heat capacity (ΔCp) and the phase behavior. A complete description of simulation methods is given in the Supporting Material.
For the main transition of DPPC, Tm = 41.5°C (32,33) and ΔH = 8.7 kcal/mol (34). The solid→Lo transition enthalpy was set to ΔH1 = 3.5 kcal/mol, from which follows that ΔH = 5.2 kcal/mol for Lo→Ld transition (ΔH1 + ΔH2 = ΔH). The corresponding entropies of the transitions are then determined by the imposed conditions that ΔG1 = 450 cal/mol (solid→Lo) at 300 K and ΔS1 + ΔS2 = ΔS, which equals 27.65 cal/mol/K for the solid→Ld transition of pure DPPC (Fig. 3, A and B).
The work of Regen et al. (35,36) using the nearest-neighbor recognition method constitutes one of the major sources of experimental data for lipid-lipid interaction parameters. Values of ωAB derived from those data were recently compiled and vary between −350 and +400 cal/mol (1). Several other experimental approaches yield ωAB of similar magnitudes (27,37–46). In DPPC/Chol, the van 't Hoff enthalpy was determined from the temperature dependence of ωAB (47), providing an estimate of the interaction enthalpy, ΔHAB. In the Ld phase, both ωAB and ΔHAB ≈ 0 for DPPC/Chol (47). In the Lo phase, the interaction is favorable, with a significant temperature dependence, reflected in ΔHAB = −2.1 kcal/mol (47), which was included in the simulations. For example, at 60°C, ωAB = −100 cal/mol, but at 20°C, ωAB = −340 cal/mol. In the solid, the PC/Chol interaction is unfavorable, judging from distearoylphosphatidylcholine (DSPC)/Chol mixtures (48), with ωAB ≈ +370 cal/mol (1).
In pure DPPC, ωAB for the Ld/solid interaction was assigned by matching the excess heat capacity maximum (ΔCp,max) obtained in the simulations to the value known from experiment. In DPPC MLVs, ΔCp,max = 10–70 kcal/mol/K have been reported using DSC (6,7,40,49–51). In choosing ωAB = 360 cal/mol for Ld/solid in the simulations, a value of ΔCp,max = 33 kcal/mol/K, in the midrange of the experimental values, is obtained. A simplifying assumption was to use the same value of ωAB = 330 cal/mol for both the Lo/solid and Lo/Ld interactions, because otherwise Tm would depend on those interaction parameters, with either the solid or the Ld state being stabilized relative to the other. Table 1 lists the complete set of parameters used in the calculations.
Table 1.
Lipid-lipid interaction parameters, transition enthalpies, and transition entropies used in the Monte Carlo simulations
| Lipid pair (A/B) | ωAB (cal/mol) | ΔH (kcal/mol) | ΔS (cal/mol/K) |
|---|---|---|---|
| Solid/Ld | +360 | 8.7 | 27.65 |
| Solid/Lo | +330 | 3.5 | 10.15 |
| Lo/Ld | +330 | 5.2 | 17.5 |
| Chol/Ld | +20 | — | — |
| Chol/Lo | −340∗ | — | — |
| Chol/solid | +350 | — | — |
Value at 20°C. This parameter is temperature-dependent, with ωAB = −2120 + 6.07 T cal/mol (T in K).
Results
Approximately 180 Monte Carlo simulations were performed, each with 5 × 105 to 5 × 106 Monte Carlo cycles, in pure DPPC and in DPPC/Chol mixtures containing 10, 15, 20, 25, and 30 mol % Chol, as a function of temperature. Those simulations were used to obtain the equilibrium configuration of the membrane, represented by a 100 × 100 triangular lattice, from which observable, average properties were calculated. Of particular interest were the heat capacity and the phase behavior of the mixtures.
Heat capacity
The excess heat capacity functions of DPPC/Chol mixtures were calculated from the Monte Carlo simulations through the fluctuation-dissipation theorem (28),
| (2) |
where 〈H2〉 − 〈H〉2 denotes the fluctuations in enthalpy. The results are shown in Fig. 4. As the cholesterol content in DPPC increases, ΔCp,max initially moves to lower temperatures, consistent with the freezing point depression observed experimentally. When cholesterol reaches 20 mol %, the curve also broadens significantly, and on further increase in concentration the broad maximum moves to higher temperatures. This is consistent with the DSC data (Fig. 1). In the simulations, ΔCp(T) depends on the parameters chosen, both in the terms of position of the maximum and the width of the transition, but there are no dramatic qualitative changes when the parameters change slightly.
Figure 4.
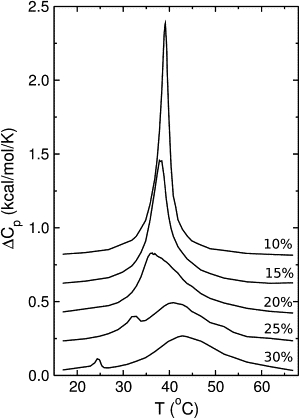
Excess heat capacity function calculated from the Monte Carlo simulations for different concentrations of Chol in DPPC, indicated next to each curve in mol % Chol. The parameters used are listed in Table 1.
A small, local maximum in ΔCp is observed in DPPC/Chol 75:25 at 32°C, and in DPPC/Chol 70:30 at 25°C. This has not been observed by DSC at 30 mol % Chol, but some studies do show a slight local maximum at 25 mol % Chol in DSC scans (5). The occurrence of this local maximum in the simulations depends on the choice of ΔH1 (solid→Lo). If ΔH1 is reduced, the small maximum decreases and shifts to lower temperatures, and when ΔH1 < 2 kcal/mol, this transition becomes essentially indiscernible in the heat capacity.
Phase behavior
The phospholipid transition is conveniently followed by a plot of the fractions of DPPC in each of the three states as a function of temperature (Fig. 5). In DPPC/Chol 80:20 (Fig. 5 A), as in pure DPPC, the main transition occurs from the solid (dotted line) to the Ld state (solid line). The population of the Lo state (dashed line) reaches a maximum at the transition midpoint. (This is also true in pure DPPC, in this model, but the Lo state does not even reach 7 mol %.) In DPPC/Chol 70:30 (Fig. 5 B) there is an initial solid→Lo transition, which is completed at ≈25°C, followed by a broad Lo→Ld transition.
Figure 5.
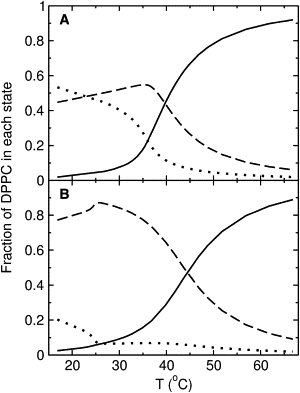
Fractions of DPPC molecules in each state in mixtures of DPPC/Chol 80:20 (A) and 70:30 (B): Ld (solid line), Lo (dashed), and solid states (dotted line).
Fig. 6 shows snapshots of the lattice along the course of the transition in DPPC/Chol 80:20. Below Tm, clear solid/Lo phase separation is observed (Fig. 6 A, 29°C), but as the temperature increases, the large domains disappear (Fig. 6, B and C, 36 and 39°C). Above Tm of DPPC, where the phase diagram indicates Ld/Lo phase coexistence, only small domains are actually observed (Fig. 6 D, 43°C).
Figure 6.
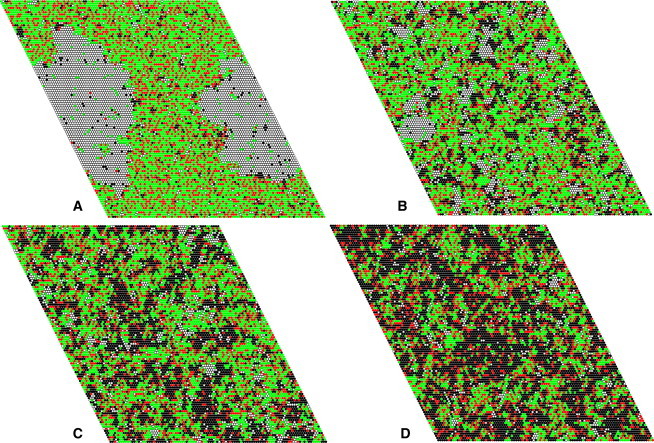
Snapshots of the Monte Carlo simulations of DPPC/Chol 80:20 at four different temperatures along the transition: (A) 29, (B) 36, (C) 39, and (D) 43°C. The different colors represent Chol (red) and DPPC in Ld (black), Lo (green), and solid states (white).
Distribution functions of the domain sizes in DPPC/Chol 80:20 are shown in Fig. 7 A for the solid state at 29°C (gray) and 36°C (black), and in Fig. 7 B for the Ld state at 39°C (black) and 43°C (gray). Thus, well-defined solid domain sizes are observed in the solid/Lo coexistence region, indicative of phase separation (Figs. 6 A and 7 A). But in the Ld/Lo coexistence region, the domain distribution is very broad, indicating that no true phase separation occurs (Figs. 6 D and 7 B).
Figure 7.
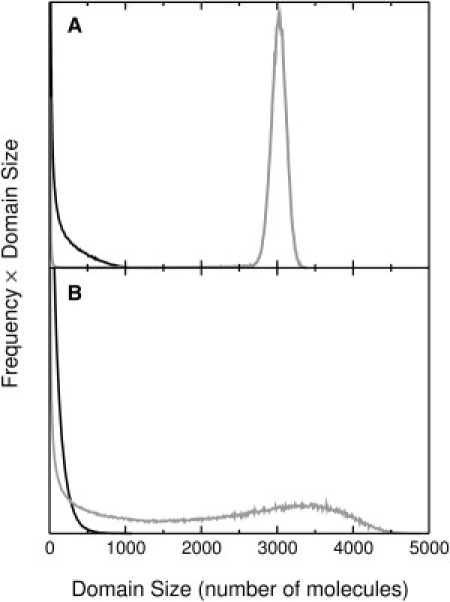
Distributions of domain sizes in DPPC/Chol 80:20 (107 mcc). (A) Solid domains at 29°C (gray) and 36°C (black). At 29°C there are ≈3400 DPPC solid molecules, which corresponds to ∼42% of the DPPC. (B) Liquid-disordered (Ld) domains at 39°C (black) and 43°C (gray). At 43°C there are ≈4800 DPPC Ld molecules, which corresponds to ∼60% of the DPPC. The ordinate scale is the same in both panels.
It is also interesting to consider the mixture DPPC/Chol 70:30. Here, the main transition occurs from Lo→Ld (Fig. 5 B). The snapshots of the lattice show what is occurring. At low temperatures, solid/Lo phase separation exists (Fig. 8, A and B), but it disappears abruptly above 25°C, when the system becomes entirely Lo phase (Fig. 8 C). This solid→Lo transition corresponds to the weak local maximum in ΔCp (Fig. 4). At high temperatures, in the Ld/Lo coexistence region, no phase separation exists but only small clusters (Fig. 8 D, 44°C).
Figure 8.
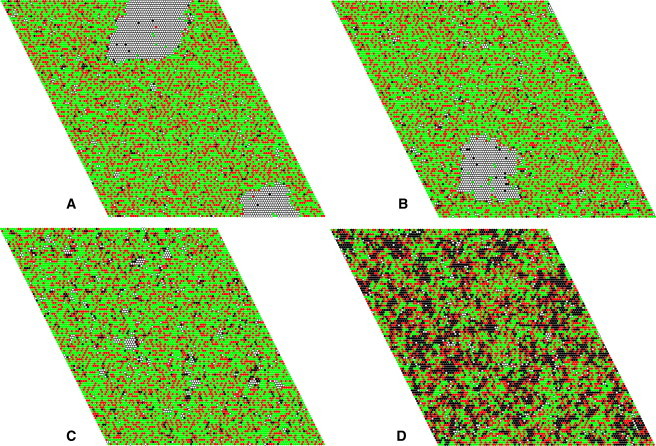
Snapshots of the Monte Carlo simulations of DPPC/Chol 70:30 at four different temperatures along the transition: (A) 17, (B) 22, (C) 27, and (D) 44°C. The colors represent Chol (red) and DPPC in Ld (black), Lo (green), and solid states (white).
In summary, the solid→Lo transition is sharp but the Lo→Ld transition is continuous.
Discussion
The Ld–Lo phase separation model
The canonical DPPC/Chol phase diagram shown in Fig. 2 A results from a combination of the original proposal of Ipsen et al. (11,12) with DSC, 2H-NMR, and electron paramagnetic resonance (EPR) data (4,9,13,52). Ipsen et al. (12) provided a compelling interpretation of ΔCp of PC/Chol mixtures using a microscopic interaction model. The PC conformations were represented by the 10-state Pink model, where one state corresponds to the liquid and the other nine, to the solid. One of the solid states is the all-trans, ground-state, and the other eight are excited conformations containing gauche isomers (53–55). In addition, the q-state Potts model was used to represent the crystallinity of the lipid states. The variable q takes a value between 1 and ≈30 (55), which accounts for matching domain boundaries in the solid (crystalline) state; but q is lost in the liquid state. In the absence of cholesterol, the Pink/Potts states are coupled, and the phase transition occurs from an ordered solid to a disordered liquid (Ld). In the presence of cholesterol, however, the transitions are decoupled, as cholesterol prefers to interact with ordered acyl chains without crystalline order. Above Tm of the phospholipid, “this leads to massive phase separation” (12) between two liquids, Ld and Lo (Fig. 2 A).
The shape of ΔCp calculated by Ipsen et al. (12) was in good agreement with DSC measurements. Namely, the broad high-temperature melting region at Chol ≈ 20 mol % is very nicely reproduced, and this broadening increases with cholesterol content, until the transition becomes virtually undetectable at Chol ≈ 40 mol %. A problem of the model, however, is its very large number of parameters, which render it unwieldy to extension to ternary mixtures. Also, it is not clear whether the agreement with DSC is quantitative because ΔCp was reported in arbitrary units (12). Finally, the parameters of the microscopic interaction model correspond to ωAB ≈ 1 kcal/mol in absolute value (11), which are ≈3 × larger than typical experimental values (1).
Does phase separation occur?
Several features of the phase diagram of Fig. 2 A are not consensual.
First, as pointed out by Marsh (4), the phase boundary between the solid/Lo and the all-Lo regions, which is drawn almost vertical in the canonical PC/Chol phase diagram, has been proposed to be much more slanted, even by Ipsen et al. (11,12), and to extend to higher cholesterol content (Fig. 2 B), both for DPPC/Chol and DSPC/Chol, based on the 13C-NMR signal of the lipid carbonyl group (8).
Second, fluorescence microscopy of giant unilamellar vesicles has shown that Ld/Lo phase separation occurs in ternary mixtures of cholesterol with a high- and a low-melting phospholipid, but not in binary mixtures of PC/Chol (19–24).
In the end, however, the criterion for phase separation needs to be thermodynamic, not a limit of resolution, which could be the case when light is used to visualize domains.
How large must domains be to be considered a phase and not just compositional fluctuations?
For the energy of a phase to be well defined, the contribution of the interface to the energy of a domain must be small (1,56). In very rough terms, a two-dimensional system with N molecules is large if the ratio of the boundary, of order N1/2, to the interior, of order N, is small (57). If by small we mean <1%, then for a system to be large in two dimensions, N1/2/N < 0.01, or N > 104. In fluorescence microscopy the detectable domain size is ∼1 μm, so that each domain contains ≈106 lipids, which is clearly large and corresponds to phase separation by this criterion. 2H-NMR is particularly well suited to assess phase separation because, if two-component spectra are observed (not just line broadening), then the domains must be larger than ≈100 nm (4). Domains of 100 nm contain ≈104 lipids, which is the threshold between small domains and phase separation. Indeed, by this criterion, phase separation has been observed in the solid/Lo region of the DPPC/Chol phase diagram (Fig. 2 A), not only by fluorescence microscopy, but also by two-component 2H-NMR spectra (58).
In the Ld/Lo region, however, no such two-component spectra have been produced, except—but much less clearly—in DPPC/Chol 75:25, in a small region of the phase diagram, just 1–2°C above the three-phase, horizontal line (59). Other measurements largely rule out Ld/Lo phase separation (25). At 60°C, using a biradical spin-label, two-component spectra were observed by EPR in DPPC/Chol (52) and in sphingomyelin/Chol mixtures (60). But two-component spectra are expected in this case, even if only very small Ld and Lo domains exist, because in EPR the exchange is slow on the magnetic timescale (4).
The condensed complex model and ternary mixtures
McConnell and collaborators proposed to describe interactions between phospholipids and cholesterol by formation of condensed complexes, and provided a new interpretation for many experimental observations (15–18,26,61–64). However, the condensed complex model does not perform well in calculating ΔCp(T) of PC/Chol mixtures. It correctly yields freezing point depression and a broad high-temperature shoulder in ΔCp, which is interpreted as thermal dissociation of the complexes (17,63). But a very large transition is predicted at high cholesterol concentrations and low temperatures, which is not observed experimentally. Moreover, the shape of ΔCp(T) is quite different from that obtained by DSC. Furthermore, ΔCp was reported in arbitrary units (17), which precludes a quantitative comparison with experiment.
The free energies of interaction between lipids (ωAB) estimated from experiments are of the order of a few hundred calories per mole in absolute value (1). Typically, ωAB are positive (repulsive), which is normally expected (29). Remarkably, they are negative between PC and cholesterol in the Lo phase, indicating a favorable interaction (1,47,48). The condensed complex model is consistent with this favorable interaction, but the values of free energy and enthalpy used are approximately one order-of-magnitude too large. For example, formation of a PC/Chol 1:1 complex is associated with a Gibbs free energy change of ≈−2 to −3 kcal/mol (18,26,65), and an enthalpy of ≈−10 kcal/mol (18,65). This is larger than ΔH for the phase transition of DPPC from Ld to solid, where the acyl chains must be more ordered than in the complex. In addition, the experimental van 't Hoff enthalpy associated with ωAB in the Lo phase of DPPC/Chol is ΔHAB = −2 kcal/mol (47), not −10 kcal/mol.
The condensed complex model correctly predicts the existence of a closed loop in ternary phase diagrams of cholesterol with a high- and a low-melting phospholipid. However, a closed loop does not require the existence of complexes. Monte Carlo simulations of a simple lattice model with nearest-neighbor interactions demonstrated that phase separation occurs in this type of ternary mixture (27), but not in any of the three binary mixtures, provided that the Chol/Lo interaction be sufficiently attractive, and the Chol/Ld and Lo /Ld interactions be sufficiently repulsive.
None of the ωAB parameters that specify those interactions need be much larger than 300 cal/mol in absolute value (27). Subsequently, two other studies also demonstrated that the minimal requirements for the observation of phase separation in ternary mixtures, but not in binary mixtures, are sufficiently strong interactions of cholesterol, favorable with ordered and unfavorable with disordered phospholipids. This was shown by a combination of Monte Carlo simulations with mean-field theory (66), and by a simple, but very illuminating, phenomenological model that assumes first-order transitions and a free energy expanded in the acyl-chain order parameter (67).
Mean-field models
The interactions and phase behavior of DPPC/Chol mixtures have also been investigated using self-consistent mean-field approaches. Elliott et al. (68) modeled the lipid chain conformations using Flory's rotational isomers. The free energy is then determined by the area and orientation of the lipid chains. Cholesterol interacts more favorably with chain segments oriented perpendicular to the membrane, hence preferring ordered chains. If the interaction between cholesterol molecules is comparable to the interaction between phospholipids, the model generates a phase diagram with the same topology as that shown in Fig. 2 A; however, it is significantly displaced to high cholesterol concentrations, with first-order transitions between lipid phases.
In a different mean-field model (69,70) the bilayer was represented as a continuous field of DPPC acyl-chain order, onto which cholesterol molecules were superimposed. The order at each point depends on order at neighboring points and on interactions with cholesterol. The order parameter is derived from a library of acyl-chain conformations obtained from molecular dynamics simulations, instead of using Flory's isomeric states. The interaction between DPPC and cholesterol was considered anisotropic (70), reflecting the smooth and rough faces of the cholesterol molecule, the former interacting more favorably with DPPC. The model produces regions of low and high order, the latter increasing with cholesterol concentration. In some cases, a broad distribution of order parameters is observed, but no phase separation. A phase diagram representing the amounts of ordered states was generated, which is similar to that of Fig. 2 A, but no sharp transitions actually occur. The model also yields ΔCp(T) curves whose main features and magnitudes resemble those obtained experimentally by DSC (Fig. 1).
A simple thermodynamic model
The model proposed here to understand ΔCp(T) and the phase behavior of DPPC/Chol incorporates nearest-neighbor, lipid-lipid interactions and the phase transition of the phospholipid. The Lo state is considered a thermodynamic state of the phospholipid, characterized by enthalpy and entropy functions that are intermediate between those of the Ld and solid states, which is consistent with an intermediate acyl-chain order. In pure DPPC, the Lo state essentially does not exist, because its free energy is much larger than those of the solid and Ld states (Fig. 3 C). Cholesterol, however, stabilizes the Lo state by preferential nearest-neighbor interactions, thus increasing its population.
Monte Carlo simulations of the model, using interaction parameters consistent with experiment, reproduce the main features of ΔCp(T) of DPPC/Chol mixtures. Addition of cholesterol initially lowers the phase transition temperature, decreases ΔCp,max, and broadens the transition slightly. When Chol ≥ 20 mol %, the model correctly reproduces the broadening of the transition and its shift to higher temperatures. Quantitatively, however, the value of ΔCp,max in the simulations of DPPC/Chol mixtures is smaller than in experiment. In the simulations, ΔCp,max drops from 33 kcal/mol/K in pure DPPC to 1.6 kcal/mol/K in the presence of 10 mol % Chol, and to 0.43 kcal/mol/K in the presence 20% Chol. Experimentally, in MLVs containing 10 and 20 mol % Chol, ΔCp,max drops to ≈5 kcal/mol/K and ≈1 kcal/mol/K, respectively (6–8,10).
Two points may be noted. First, if the phase transition of DPPC is simulated using a simple two-state, solid/liquid model, inclusion of 10 mol % Chol strictly as an impurity (ωAB=0 between Chol and both phospholipid states) already decreases ΔCp,max to ≈2 kcal/mol/K.
Second, in the model proposed here the Chol/Lo interaction is isotropic, whereas its anisotropy appears to be important in the model of Pandit et al. (70). Even though our results are in good agreement with experiment without any anisotropy, its inclusion may improve the agreement—a possibility that will be explored in the future.
At low temperatures, solid/Lo phase separation clearly occurs in DPPC/Chol 80:20 and 70:30 mixtures (Figs. 6 A and 8, A and B). Thus, this Monte Carlo simulation result is in agreement with experiment (58). As noted above, the small local maximum in ΔCp(T) apparent at 25°C in the simulations of DPPC/Chol 70:30 (Fig. 4), which corresponds to a solid→Lo transition (Fig. 5 B), has never been observed by DSC. The phase diagram of Fig. 2 B, on the other hand, predicts the occurrence of this transition, as the system is heated across the slanted phase boundary from the solid/Lo region to the all-Lo region, at 28°C (8). This transition temperature is close to that observed in the simulations, although no particular effort was made to obtain a match by adjusting the solid→ Lo enthalpy change. The reason phase separation occurs in the solid/Lo region is the combination of the two unfavorable interactions, Chol/solid (ωAB = +340 cal/mol) and solid/Lo (ωAB = +330 cal/mol), with the favorable Chol/Lo interaction (ωAB = −340 cal/mol, at 20°C). This situation is similar to what occurred in the ternary mixture we simulated previously (27).
As the temperature increases, in the DPPC/Chol 80:20 mixture the solid state is progressively converted to Ld, whereas the amount of Lo state remains fairly constant, but all three phospholipid states are appreciably populated (Figs. 5 A and 6 B). Above 40°C, the solid disappears and the system enters the Ld/Lo coexistence region (Fig. 6, C and D). The DPPC/Chol 70:30 mixture had entered the all-Lo phase just above 25°C (Figs. 5 B and 8 C) and, on further increase in temperature, leaves this one-phase region and also enters the Ld/Lo coexistence region.
These results are in agreement with the phase diagram of Fig. 2, but with one important difference. In the simulations, only small Ld and Lo domains are observed in this coexistence region (Figs. 6, C and D, and 8 D). No massive phase separation actually occurs, in agreement with experiment (19,25). That is, the transition is of a continuous nature. The reason only small domains occur in the Ld/Lo region is that the favorable Chol/Lo interaction is weakened to ωAB = −200 cal/mol at high temperatures, and although the Ld/Lo interaction is still +330 cal/mol, the Chol/Ld interaction is essentially zero. Therefore, the combination of the three interactions does not result in phase separation.
Acknowledgments
I thank Ronald McElhaney and David Mannock for providing me with original heat capacity data.
This work was supported by Cottrell College Science Award No. 7622 from the Research Corporation.
Supporting Material
References
- 1.Almeida P.F.F. Thermodynamics of lipid interactions in complex bilayers. Biochim. Biophys. Acta. 2009;1788:72–85. doi: 10.1016/j.bbamem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz M.L. Detailed molecular dynamics simulations of model biological membranes containing cholesterol. Biochim. Biophys. Acta. 2009;1788:86–96. doi: 10.1016/j.bbamem.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Marsh D. Cholesterol-induced fluid membrane domains: a compendium of lipid-raft ternary phase diagrams. Biochim. Biophys. Acta. 2009;1788:2114–2123. doi: 10.1016/j.bbamem.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Marsh D. Liquid-ordered phases induced by cholesterol: a compendium of binary phase diagrams. Biochim. Biophys. Acta. 2010;1798:688–699. doi: 10.1016/j.bbamem.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Mannock D.A., Lewis R.N.A.H., McElhaney R.N. The effect of variations in phospholipid and sterol structure on the nature of lipid-sterol interactions in lipid bilayer model membranes. Chem. Phys. Lipids. 2010;163:403–448. doi: 10.1016/j.chemphyslip.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Estep T.N., Mountcastle D.B., Thompson T.E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978;17:1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- 7.Mabrey S., Mateo P.L., Sturtevant J.M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978;17:2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- 8.Huang T.-H., Lee C.W.B., Griffin R.G. A 13C and 2H nuclear magnetic resonance study of phosphatidylcholine/cholesterol interactions: characterization of liquid-gel phases. Biochemistry. 1993;32:13277–13287. doi: 10.1021/bi00211a041. [DOI] [PubMed] [Google Scholar]
- 9.Vist M.R., Davis J.H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990;29:451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- 10.McMullen T.P.W., McElhaney R.N. New aspects of the interaction of cholesterol with dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim. Biophys. Acta. 1995;1234:90–98. doi: 10.1016/0005-2736(94)00266-r. [DOI] [PubMed] [Google Scholar]
- 11.Ipsen J.H., Karlström G., Zuckermann M.J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta. 1987;905:162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 12.Ipsen J.H., Mouritsen O.G., Zuckermann M.J. Theory of thermal anomalies in the specific heat of lipid bilayers containing cholesterol. Biophys. J. 1989;56:661–667. doi: 10.1016/S0006-3495(89)82713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sankaram M.B., Thompson T.E. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 1990;29:10670–10675. doi: 10.1021/bi00499a014. [DOI] [PubMed] [Google Scholar]
- 14.Almeida P.F.F., Vaz W.L.C., Thompson T.E. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry. 1992;31:6739–6747. doi: 10.1021/bi00144a013. [DOI] [PubMed] [Google Scholar]
- 15.McConnell H.M., Radhakrishnan A. Condensed complexes of cholesterol and phospholipids. Biochim. Biophys. Acta. 2003;1610:159–173. doi: 10.1016/s0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 16.McConnell H.M., Vrljic M. Liquid-liquid immiscibility in membranes. Annu. Rev. Biophys. Biomol. Struct. 2003;32:469–492. doi: 10.1146/annurev.biophys.32.110601.141704. [DOI] [PubMed] [Google Scholar]
- 17.Anderson T.G., McConnell H.M. Condensed complexes and the calorimetry of cholesterol-phospholipid bilayers. Biophys. J. 2001;81:2774–2785. doi: 10.1016/S0006-3495(01)75920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radhakrishnan A., McConnell H. Condensed complexes in vesicles containing cholesterol and phospholipids. Proc. Natl. Acad. Sci. USA. 2005;102:12662–12666. doi: 10.1073/pnas.0506043102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veatch S.L., Keller S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feigenson G.W., Buboltz J.T. Ternary phase diagram of dipalmitoyl-PC/dilauroyl-PC/cholesterol: nanoscopic domain formation driven by cholesterol. Biophys. J. 2001;80:2775–2788. doi: 10.1016/S0006-3495(01)76245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samsonov A.V., Mihalyov I., Cohen F.S. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys. J. 2001;81:1486–1500. doi: 10.1016/S0006-3495(01)75803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veatch S.L., Keller S.L. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 2005;94:148101. doi: 10.1103/PhysRevLett.94.148101. [DOI] [PubMed] [Google Scholar]
- 23.Veatch S.L., Keller S.L. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J., Wu J., Feigenson G.W. Phase studies of model biomembranes: complex behavior of DSPC/DOPC/cholesterol. Biochim. Biophys. Acta. 2007;1768:2764–2776. doi: 10.1016/j.bbamem.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krivanek R., Okoro L., Winter R. Effect of cholesterol and ergosterol on the compressibility and volume fluctuations of phospholipid-sterol bilayers in the critical point region: a molecular acoustic and calorimetric study. Biophys. J. 2008;94:3538–3548. doi: 10.1529/biophysj.107.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McConnell H. Complexes in ternary cholesterol-phospholipid mixtures. Biophys. J. 2005;88:L23–L25. doi: 10.1529/biophysj.104.058834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frazier M.L., Wright J.R., Almeida P.F. Investigation of domain formation in sphingomyelin/cholesterol/POPC mixtures by fluorescence resonance energy transfer and Monte Carlo simulations. Biophys. J. 2007;92:2422–2433. doi: 10.1529/biophysj.106.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill T.L. Dover; New York: 1985. An Introduction to Statistical Thermodynamics. [Google Scholar]
- 29.de Gennes P.G. Cornell University Press; Ithaca, NY: 1979. Scaling Concepts in Polymer Physics. [Google Scholar]
- 30.Monod J., Wyman J., Changeux J.-P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 31.Koshland D.E., Jr., Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 32.Cevc G., editor. Phospholipids Handbook. Marcel Dekker; New York: 1993. [Google Scholar]
- 33.Koynova R., Caffrey M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta. 1998;1376:91–145. doi: 10.1016/s0304-4157(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 34.Mabrey S., Sturtevant J.M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA. 1976;73:3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krisovitch S.M., Regen S.L. Nearest-neighbor recognition in phospholipid membranes: a molecular-level approach to the study of membrane suprastructure. J. Am. Chem. Soc. 1992;114:9828–9835. [Google Scholar]
- 36.Davidson S.M.K., Regen S.L. Nearest-neighbor recognition in phospholipid membranes. Chem. Rev. 1997;97:1269–1280. doi: 10.1021/cr960381s. [DOI] [PubMed] [Google Scholar]
- 37.Sugár I.P., Thompson T.E., Biltonen R.L. Monte Carlo simulation of two-component bilayers: DMPC/DSPC mixtures. Biophys. J. 1999;76:2099–2110. doi: 10.1016/S0006-3495(99)77366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jerala R., Almeida P.F.F., Biltonen R.L. Simulation of the gel-fluid transition in a membrane composed of lipids with two connected acyl chains: application of a dimer-move step. Biophys. J. 1996;71:609–615. doi: 10.1016/S0006-3495(96)79261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugár I.P., Biltonen R.L., Mitchard N. Monte Carlo simulations of membranes: phase transition of small unilamellar dipalmitoylphosphatidylcholine vesicles. Methods Enzymol. 1994;240:569–593. doi: 10.1016/s0076-6879(94)40064-4. [DOI] [PubMed] [Google Scholar]
- 40.Ivanova V.P., Heimburg T. Histogram method to obtain heat capacities in lipid monolayers, curved bilayers, and membranes containing peptides. Phys. Rev. E. 2001;63:041914. doi: 10.1103/PhysRevE.63.041914. [DOI] [PubMed] [Google Scholar]
- 41.Hinderliter A., Almeida P.F.F., Biltonen R.L. Domain formation in a fluid mixed lipid bilayer modulated through binding of the C2 protein motif. Biochemistry. 2001;40:4181–4191. doi: 10.1021/bi0024299. [DOI] [PubMed] [Google Scholar]
- 42.Hinderliter A., Biltonen R.L., Almeida P.F.F. Lipid modulation of protein-induced membrane domains as a mechanism for controlling signal transduction. Biochemistry. 2004;43:7102–7110. doi: 10.1021/bi036334t. [DOI] [PubMed] [Google Scholar]
- 43.Heimburg T., Biltonen R.L. A Monte Carlo simulation study of protein-induced heat capacity changes and lipid-induced protein clustering. Biophys. J. 1996;70:84–96. doi: 10.1016/S0006-3495(96)79551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsamaloukas A., Szadkowska H., Heerklotz H. Nonideal mixing in multicomponent lipid/detergent systems. J. Phys. Condens. Matter. 2006;18:S1125–S1138. doi: 10.1088/0953-8984/18/28/S02. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez Y., Mezei M., Osman R. Association free energy of dipalmitoylphosphatidylserines in a mixed dipalmitoylphosphatidylcholine membrane. Biophys. J. 2007;92:3071–3080. doi: 10.1529/biophysj.106.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang J., Swanson J.E., Feigenson G.W. Nonideal mixing of phosphatidylserine and phosphatidylcholine in the fluid lamellar phase. Biophys. J. 1993;64:413–425. doi: 10.1016/S0006-3495(93)81382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Cao H., Regen S.L. Cholesterol-phospholipid association in fluid bilayers: a thermodynamic analysis from nearest-neighbor recognition measurements. Biophys. J. 2006;91:1402–1406. doi: 10.1529/biophysj.106.084152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugahara M., Uragami M., Regen S.L. Selective association of cholesterol with long-chain phospholipids in liquid-ordered bilayers: support for the existence of lipid rafts. J. Am. Chem. Soc. 2003;125:13040–13041. doi: 10.1021/ja038102n. [DOI] [PubMed] [Google Scholar]
- 49.Suurkuusk J., Lentz B.R., Thompson T.E. A calorimetric and fluorescent probe study of the gel-liquid crystalline phase transition in small, single-lamellar dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1976;15:1393–1401. doi: 10.1021/bi00652a007. [DOI] [PubMed] [Google Scholar]
- 50.Mountcastle D.B., Biltonen R.L., Halsey M.J. Effect of anesthetics and pressure on the thermotropic behavior of multilamellar dipalmitoylphosphatidylcholine liposomes. Proc. Natl. Acad. Sci. USA. 1978;75:4906–4910. doi: 10.1073/pnas.75.10.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S.C., Sturtevant J.M., Gaffney B.J. Scanning calorimetric evidence for a third phase transition in phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA. 1980;77:5060–5063. doi: 10.1073/pnas.77.9.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sankaram M.B., Thompson T.E. Cholesterol-induced fluid-phase immiscibility in membranes. Proc. Natl. Acad. Sci. USA. 1991;88:8686–8690. doi: 10.1073/pnas.88.19.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pink D.A., Green T.J., Chapman D. Raman scattering in bilayers of saturated phosphatidylcholines. Experiment and theory. Biochemistry. 1980;19:349–356. doi: 10.1021/bi00543a016. [DOI] [PubMed] [Google Scholar]
- 54.Pink D.A., Green T.J., Chapman D. Raman scattering in bilayers of saturated phosphatidylcholines and cholesterol. Experiment and theory. Biochemistry. 1981;20:6692–6698. doi: 10.1021/bi00526a026. [DOI] [PubMed] [Google Scholar]
- 55.Zuckermann M.J., Mouritsen O.G. The effects of acyl chain ordering and crystallization on the main phase transition of wet lipid bilayers. Eur. Biophys. J. 1987;15:77–86. [Google Scholar]
- 56.Almeida P.F.F., Pokorny A., Hinderliter A. Thermodynamics of membrane domains. Biochim. Biophys. Acta. 2005;1720:1–13. doi: 10.1016/j.bbamem.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Hill T.L. Dover; New York, NY: 1994. Thermodynamics of Small Systems (Parts I and II) [Google Scholar]
- 58.Veatch S.L., Soubias O., Gawrisch K. Critical fluctuations in domain-forming lipid mixtures. Proc. Natl. Acad. Sci. USA. 2007;104:17650–17655. doi: 10.1073/pnas.0703513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis J.H., Clair J.J., Juhasz J. Phase equilibria in DOPC/DPPC-d62/cholesterol mixtures. Biophys. J. 2009;96:521–539. doi: 10.1016/j.bpj.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collado M.I., Goñi F.M., Marsh D. Domain formation in sphingomyelin/cholesterol mixed membranes studied by spin-label electron spin resonance spectroscopy. Biochemistry. 2005;44:4911–4918. doi: 10.1021/bi0474970. [DOI] [PubMed] [Google Scholar]
- 61.Radhakrishnan A., McConnell H.M. Condensed complexes of cholesterol and phospholipids. Biophys. J. 1999;77:1507–1517. doi: 10.1016/S0006-3495(99)76998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radhakrishnan A., McConnell H.M. Chemical activity of cholesterol in membranes. Biochemistry. 2000;39:8119–8124. doi: 10.1021/bi0005097. [DOI] [PubMed] [Google Scholar]
- 63.McConnell H., Radhakrishnan A. Theory of the deuterium NMR of sterol-phospholipid membranes. Proc. Natl. Acad. Sci. USA. 2006;103:1184–1189. doi: 10.1073/pnas.0510514103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McConnell H., Radhakrishnan A. Molecular motion at the critical point in lipid membranes. Biophys. J. 2008;95:L22–L24. doi: 10.1529/biophysj.108.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radhakrishnan A. Phase separations in binary and ternary cholesterol-phospholipid mixtures. Biophys. J. 2010;98:L41–L43. doi: 10.1016/j.bpj.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reigada R., Buceta J., Lindenberg K. Phase separation in three-component lipid membranes: from Monte Carlo simulations to Ginzburg-Landau equations. J. Chem. Phys. 2008;128:025102. doi: 10.1063/1.2817333. [DOI] [PubMed] [Google Scholar]
- 67.Putzel G.G., Schick M. Phenomenological model and phase behavior of saturated and unsaturated lipids and cholesterol. Biophys. J. 2008;95:4756–4762. doi: 10.1529/biophysj.108.136317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elliott R., Szleifer I., Schick M. Phase diagram of a ternary mixture of cholesterol and saturated and unsaturated lipids calculated from a microscopic model. Phys. Rev. Lett. 2006;96:098101. doi: 10.1103/PhysRevLett.96.098101. [DOI] [PubMed] [Google Scholar]
- 69.Khelashvili G.A., Pandit S.A., Scott H.L. Self-consistent mean-field model based on molecular dynamics: application to lipid-cholesterol bilayers. J. Chem. Phys. 2005;123:34910. doi: 10.1063/1.1943412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandit S.A., Khelashvili G., Scott H.L. Lateral organization in lipid-cholesterol mixed bilayers. Biophys. J. 2007;92:440–447. doi: 10.1529/biophysj.106.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


