Abstract
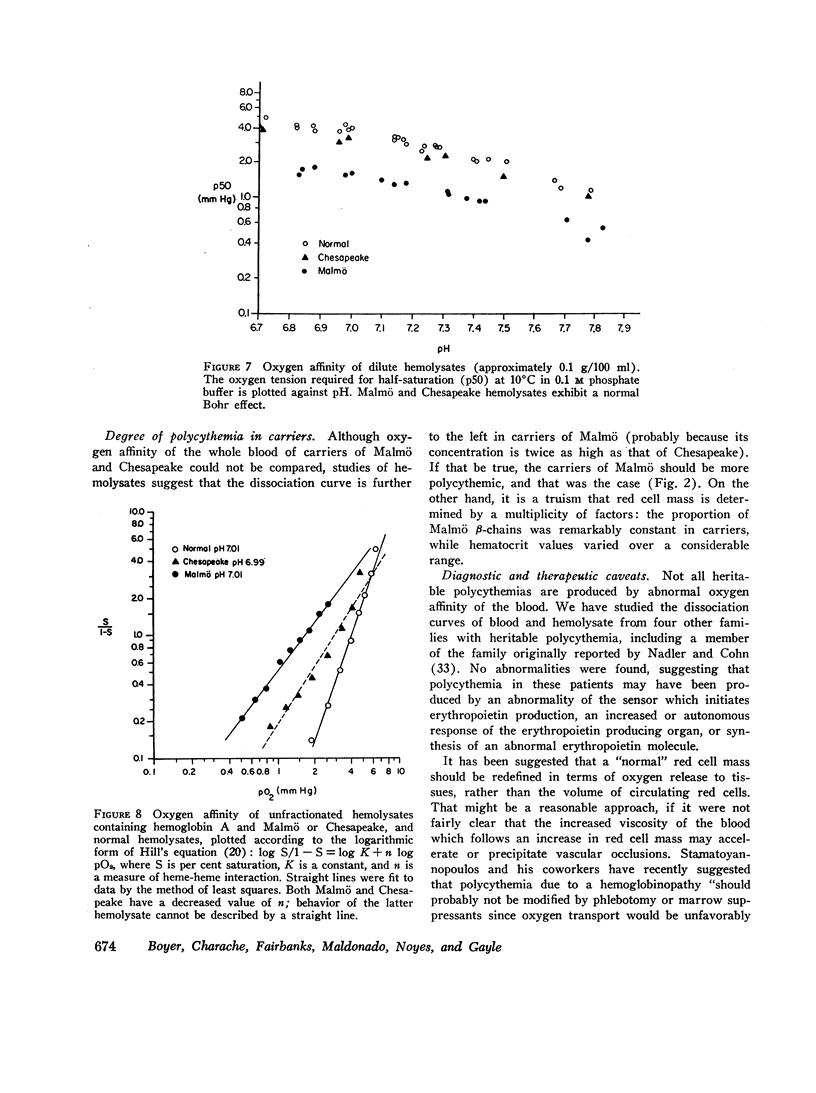
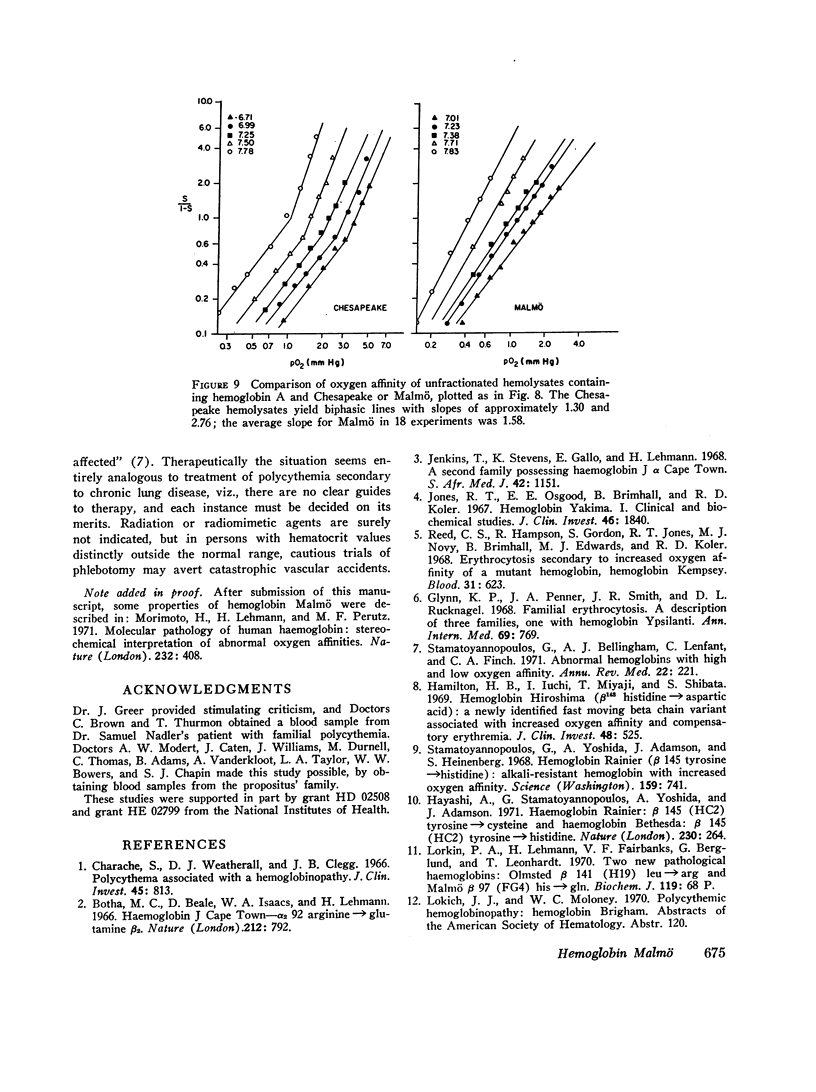
A striking history of familial polycythemia led to a search for an abnormal hemoglobin. None could be demonstrated by routine electrophoretic methods, but the propositus' hemolysate had increased oxygen affinity. Manipulation of the conditions of electrophoresis, and chromatographic methods, permitted identification of hemoglobin Malmö. Studies of hemolysates demonstrated a normal Bohr effect, decreased heme-heme interaction (n=1.58), and a p50 of 1.3 mm Hg at 10°C and pH 7.2. The amino acid substitution occurs in the same position (FG-4) as that of hemoglobin Chesapeake, but in the β-chain rather than the α-chain. The two types of hemolysate have different pathophysiologic properties, and carriers of hemoglobin Malmö exhibit more striking hematologic abnormalities.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYER S. H., FAINER D. C., NAUGHTON M. A. Myoglobin: in herited structural variation in man. Science. 1963 Jun 14;140(3572):1228–1231. doi: 10.1126/science.140.3572.1228-a. [DOI] [PubMed] [Google Scholar]
- Bellingham A. J., Huehns E. R. Compensation in haemolytic anaemias caused by abnormal haemoglobins. Nature. 1968 Jun 8;218(5145):924–926. doi: 10.1038/218924a0. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Tyuma I. Subunit exchange and ligand binding. II. The mechanism of the allosteric effect in hemoglobin. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1268–1274. doi: 10.1073/pnas.56.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Botha M. C., Beale D., Isaacs W. A., Lehmann H. Hemoglobin J Cape Town-alpha-2 92 arginine replaced by glutamine beta-2. Nature. 1966 Nov 19;212(5064):792–795. doi: 10.1038/212792a0. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Hathaway P., Pascasio F., Bordley J., Orton C., Naughton M. A. Differences in the amino acid sequences of tryptic peptides from three sheep hemoglobin beta chains. J Biol Chem. 1967 May 10;242(9):2211–2232. [PubMed] [Google Scholar]
- Bunn H. F. Subunit dissociation of certain abnormal human hemoglobins. J Clin Invest. 1969 Jan;48(1):126–138. doi: 10.1172/JCI105961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Jenkins T. Oxygen equilibrium of hemoglobin J Cape Town. J Clin Invest. 1971 Jul;50(7):1554–1555. doi: 10.1172/JCI106642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Dozy A. M., Kleihauer E. F., Huisman T. H. Studies on the heterogeneity of hemoglobin. 13. Chromatography of various human and animal hemoglobin types on DEAE-Sephadex. J Chromatogr. 1968 Feb 20;32(4):723–727. doi: 10.1016/s0021-9673(01)80551-3. [DOI] [PubMed] [Google Scholar]
- Glynn K. P., Penner J. A., Smith J. R., Rucknagel D. L. Familial erythrocytosis. A description of three families, one with hemoglobin Ypsilanti. Ann Intern Med. 1968 Oct;69(4):769–776. doi: 10.7326/0003-4819-69-4-769. [DOI] [PubMed] [Google Scholar]
- Hamilton H. B., Iuchi I., Miyaji T., Shibata S. Hemoglobin Hiroshima (beta-143 histidine--aspartic acid): a newly identified fast moving beta chain variant associated with increased oxygen affinity and compensatory erythremia. J Clin Invest. 1969 Mar;48(3):525–535. doi: 10.1172/JCI106010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A., Stamatoyannopoulos G., Yoshida A., Adamson J. Haemoglobin Rainier: beta-145 (HC2) tyrosine leads to cysteine and haemoglobin Bethesda: beta-145 (HC2) tyrosine leads to histidine. Nat New Biol. 1971 Apr 28;230(17):264–267. doi: 10.1038/newbio230264a0. [DOI] [PubMed] [Google Scholar]
- Imai K. Oxygen-equilibrium characteristics of abnormal hemoglobin Hiroshima (alpha-2 beta-2 143 Asp). Arch Biochem Biophys. 1968 Sep 20;127(1):543–547. doi: 10.1016/0003-9861(68)90260-9. [DOI] [PubMed] [Google Scholar]
- Jenkins T., Stevens K., Gallo E., Lehmann H. A second family possessing haemoglobin J alpha Cape Town. S Afr Med J. 1968 Nov 2;42(42):1151–1154. [PubMed] [Google Scholar]
- Jones R. T., Osgood E. E., Brimhall B., Koler R. D. Hemoglobin Yakina. I. Clinical and biochemical studies. J Clin Invest. 1967 Nov;46(11):1840–1847. doi: 10.1172/JCI105674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H., Carrell R. W. Differences between alpha- and beta-chain mutants of human haemoglobin and between alpha- and beta-thalassaemia. Possible duplication of the alpha-chain gene. Br Med J. 1968 Dec 21;4(5633):748–750. doi: 10.1136/bmj.4.5633.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto H., Lehmann H., Perutz M. F. Moleuclar pathology of human haemoglobin: stereochemical interpretation of abnormal oxygen affinities. Nature. 1971 Aug 6;232(5310):408–413. doi: 10.1038/232408a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Mazzarella L., Crowther R. A., Greer J., Kilmartin J. V. Identification of residues responsible for the alkaline Bohr effect in haemoglobin. Nature. 1969 Jun 28;222(5200):1240–1243. doi: 10.1038/2221240a0. [DOI] [PubMed] [Google Scholar]
- Reed C. S., Hampson R., Gordon S., Jones R. T., Novy M. J., Brimhall B., Edwards M. J., Koler R. D. Erythrocytosis secondary to increased oxygen affinity of a mutant hemoglobin, hemoglobin Kempsey. Blood. 1968 May;31(5):623–632. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Bellingham A. J., Lenfant C., Finch C. A. Abnormal hemoglobins with high and low oxygen affinity. Annu Rev Med. 1971;22:221–234. doi: 10.1146/annurev.me.22.020171.001253. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Yoshida A., Adamson J., Heinenberg S. Hemoglobin Rainier (beta145 Tyrosine rarr Histidine): Alkali-Resistant Hemoglobin with Increased Oxygen Affinity. Science. 1968 Feb 16;159(3816):741–743. doi: 10.1126/science.159.3816.741. [DOI] [PubMed] [Google Scholar]