Abstract
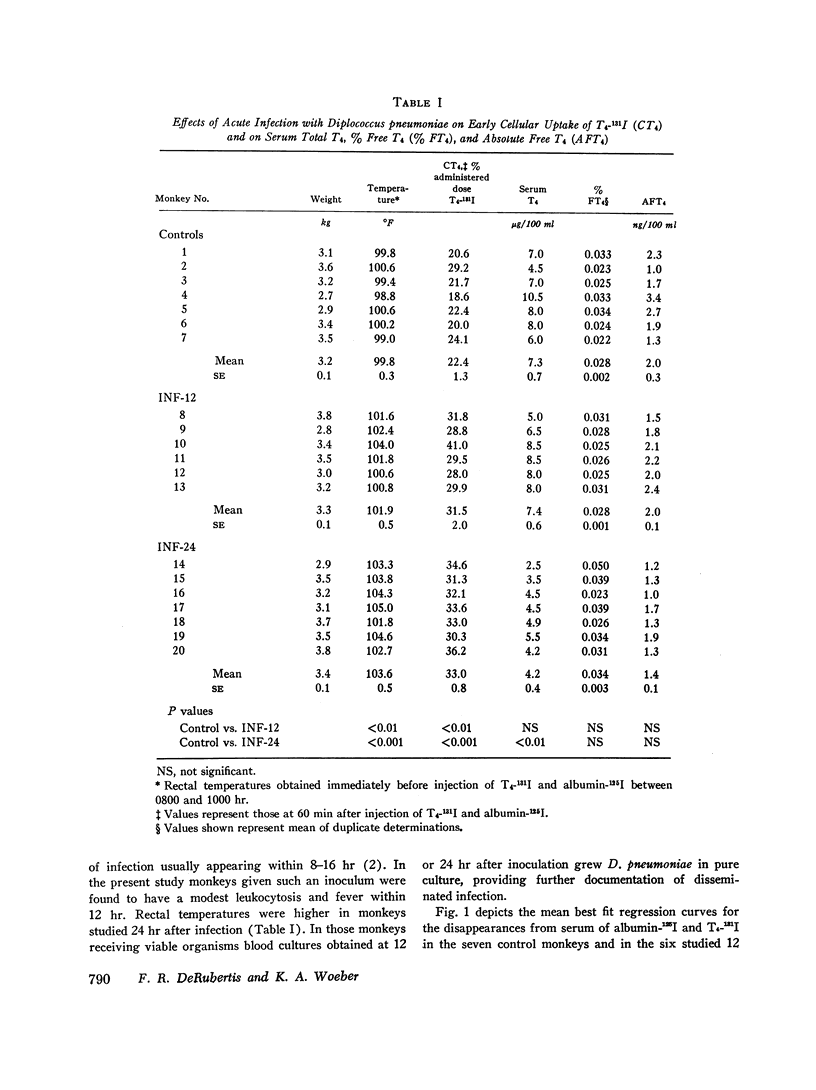
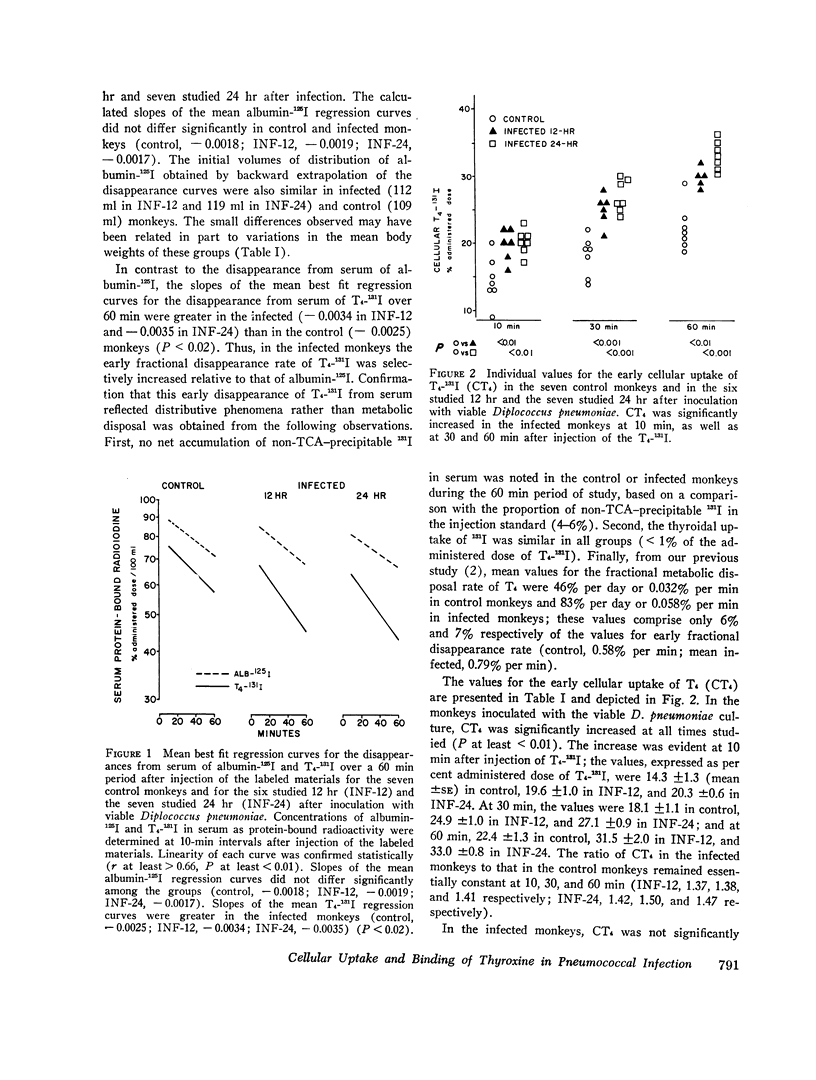
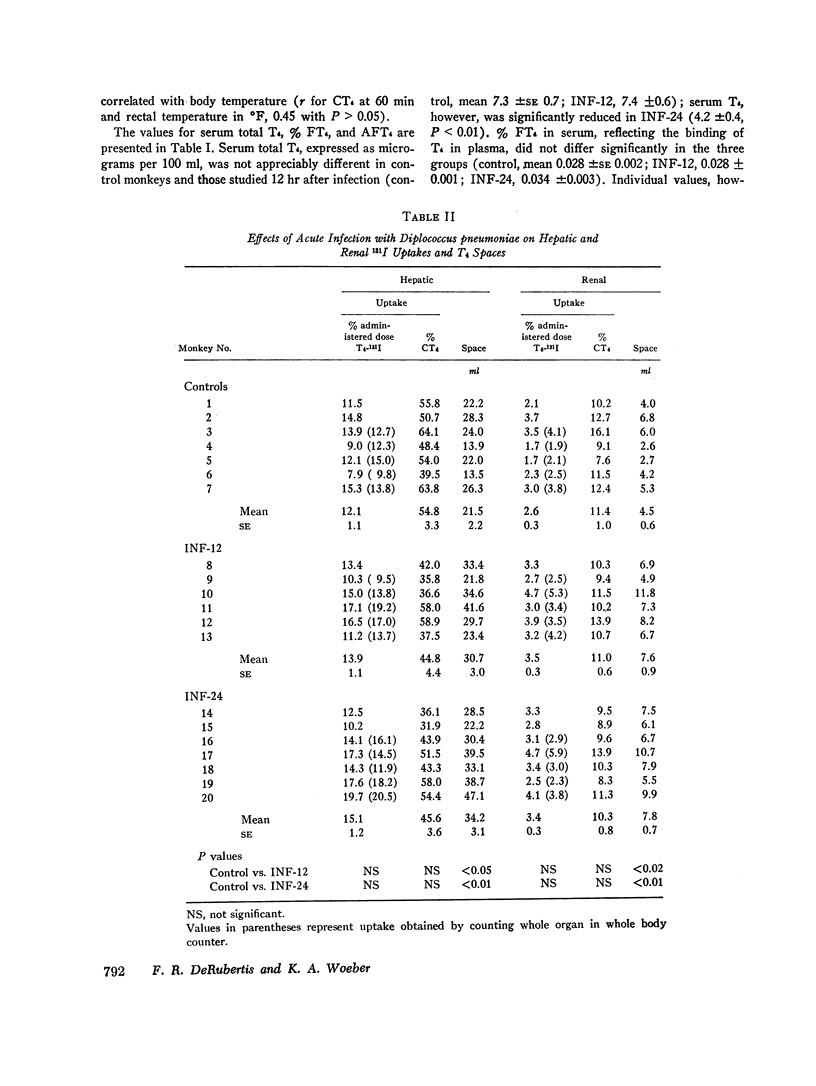
Previous work has demonstrated that acute pneumococcal infections in man and in the rhesus monkey are accompanied by accelerated metabolic disposal of L-thyroxine (T4). In order to study the influence of acute pneumococcal infection on the kinetics of hormone distribution, the early cellular uptake of T4 (CT4), reflecting the net effect of plasma and cellular binding factors, was assessed in rhesus monkeys from the differences in instantaneous distribution volumes of T4-131I and albumin-125I during the first 60 min after their simultaneous injection. Hepatic and renal uptakes of 131I were also determined. Plasma binding of T4 was assessed by measuring the per cent of free T4 (% FT4) in serum. Six monkeys were studied 12 hr (INF-12) and seven 24 hr (INF-24) after intravenous inoculation with Diplococcus pneumoniae; seven controls were inoculated with a heat-killed culture. CT4 at 60 min as per cent administered dose was 31.5 ±2.0 (mean ±SE) in INF-12 and 33.0±0.8 in INF-24, values significantly greater than control (22.4±1.3). By contrast, mean% FT4 was identical in control and INF-12 (0.028 ±0.002 and 0.028 ±0.001) and variably increased in INF-24 (0.034 ±0.003). Thus, in the infected monkeys CT4 and% FT4 were not significantly correlated. The increased CT4 in the infected monkeys could not be ascribed to an increase in vascular permeability and did not correlate with the magnitude of fever. Although the increased CT4 could not be accounted for by increased hepatic or renal uptake of hormone, hepatic and renal T4 spaces were increased, results consistent with increased binding by these tissues. Our data indicate that the cellular uptake of T4 is increased early in acute pneumococcal infection and suggest that this results from a primary enhancement of cell-associated binding factors for T4.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT H. S., LUFT J. H., HAMPTON J. C. Morphological classifications of vertebrate blood capillaries. Am J Physiol. 1959 Feb;196(2):381–390. doi: 10.1152/ajplegacy.1959.196.2.381. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. R., Searle G. L. The kinetics of distribution between plasma and liver of 131-I-labeled L-thyroxine in man: observations of subjects with normal and decreased serum thyroxine-binding globulin. J Clin Invest. 1966 Jun;45(6):939–949. doi: 10.1172/JCI105409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregerman R. I., Solomon N. Acceleration of thyroxine and triiodothyronine turnover during bacterial pulmonary infections and fever: implications for the functional state of the thyroid during stress and in senescence. J Clin Endocrinol Metab. 1967 Jan;27(1):93–105. doi: 10.1210/jcem-27-1-93. [DOI] [PubMed] [Google Scholar]
- Hasen J., Bernstein G., Volpert E., Oppenheimer J. H. Analysis of the rapid interchange of thyroxine between plasma and liver and plasma and kidney in the intact rat. Endocrinology. 1968 Jan;82(1):37–46. doi: 10.1210/endo-82-1-37. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY B. E., PATTEE C. J. DETERMINATION OF THYROXINE UTILIZING THE PROPERTY OF PROTEIN-BINDING. J Clin Endocrinol Metab. 1964 Feb;24:187–196. doi: 10.1210/jcem-24-2-187. [DOI] [PubMed] [Google Scholar]
- Musa B. U., Kumar R. S., Dowling J. T. Effects of salicylates on the distribution and early plasma disappearance of thyroxine in man. J Clin Endocrinol Metab. 1968 Oct;28(10):1461–1464. doi: 10.1210/jcem-28-10-1461. [DOI] [PubMed] [Google Scholar]
- Nicoloff J. T., Dowling J. T. Estimation of thyroxine distribution in man. J Clin Invest. 1968 Jan;47(1):26–37. doi: 10.1172/JCI105712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SQUEF R., SURKS M. I., HAUER H. BINDING OF THYROXINE BY SERUM PROTEINS EVALUATED BY EQUILIBRUM DIALYSIS AND ELECTROPHORETIC TECHNIQUES. ALTERATIONS IN NONTHYROIDAL ILLNESS. J Clin Invest. 1963 Nov;42:1769–1782. doi: 10.1172/JCI104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Bernstein G., Hasen J. Estimation of rapidly exchangeable cellular thyroxine from the plasma disappearance curves of simultaneously administered thyroxine-131-I and albumin-125-I. J Clin Invest. 1967 May;46(5):762–777. doi: 10.1172/JCI105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Surks M. I., Schwartz H. L. The metabolic significance of exchangeable cellular thyroxine. Recent Prog Horm Res. 1969;25:381–422. doi: 10.1016/b978-0-12-571125-8.50011-9. [DOI] [PubMed] [Google Scholar]
- Schussler G. C., Plager J. E. Effect of preliminary purification of 131-I-thyroxine on the determination of free thyroxine in serum. J Clin Endocrinol Metab. 1967 Feb;27(2):242–250. doi: 10.1210/jcem-27-2-242. [DOI] [PubMed] [Google Scholar]
- Woeber K. A. Alterations in thyroid hormone economy during acute infection with Diplococcus pneumoniae in the rhesus monkey. J Clin Invest. 1971 Feb;50(2):378–387. doi: 10.1172/JCI106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeber K. A., Ingbar S. H. The contribution of thyroxine-binding prealbumin to the binding of thyroxine in human serum, as assessed by immunoadsorption. J Clin Invest. 1968 Jul;47(7):1710–1721. doi: 10.1172/JCI105861. [DOI] [PMC free article] [PubMed] [Google Scholar]


