Abstract
Background
Autism spectrum disorders (ASDs) are frequently occurring disorders diagnosed by deficits in three core functional areas: social skills, communication, and behaviours and/or interests. Mental retardation frequently accompanies the most severe forms of ASDs, while overall ASDs are more commonly diagnosed in males. Most ASDs have a genetic origin and one gene recently implicated in the etiology of autism is the Deleted-In-Autism-1 (DIA1) gene.
Methodology/Principal Findings
Using a bioinformatics-based approach, we have identified a human gene closely related to DIA1, we term DIA1R (DIA1-Related). While DIA1 is autosomal (chromosome 3, position 3q24), DIA1R localizes to the X chromosome at position Xp11.3 and is known to escape X-inactivation. The gene products are of similar size, with DIA1 encoding 430, and DIA1R 433, residues. At the amino acid level, DIA1 and DIA1R are 62% similar overall (28% identical), and both encode signal peptides for targeting to the secretory pathway. Both genes are ubiquitously expressed, including in fetal and adult brain tissue.
Conclusions/Significance
Examination of published literature revealed point mutations in DIA1R are associated with X-linked mental retardation (XLMR) and DIA1R deletion is associated with syndromes with ASD-like traits and/or XLMR. Together, these results support a model where the DIA1 and DIA1R gene products regulate molecular traffic through the cellular secretory pathway or affect the function of secreted factors, and functional deficits cause disorders with ASD-like symptoms and/or mental retardation.
Introduction
Autism spectrum disorders (ASDs) encompass a variety of syndromes, diagnosed on the basis of three core symptoms: deficits in social skills, impaired use of language, and restricted and stereotypic behaviours and/or interests [1]. Classical ‘autistic disorder’ (AD) falls under the ASD umbrella and, in addition to the three core symptoms, is frequently accompanied by mental retardation, as indicated by an intelligence quotient (IQ) of <70 [2]–[11]. By contrast, presence of the core ASD symptoms accompanied by an average (or above) IQ, is typically classified as Asperger syndrome or high-functioning autism [6], [12], [13]. The term pervasive development disorder (PDD) is often used as a broader diagnostic descriptor, to encompass not only all ASDs, but also PDD-not-otherwise-specified (PDD-NOS or atypical autism), Rett disorder and childhood disintegrative disorder [14]. ASDs cause morbidity in as many as 1∶60 people, with the incidence being as high as 1∶40 in males [15]–[19].
ASDs have an almost exclusive genetic origin [20]–[25]. The concordance rate for monozygotic twins, from various studies, ranges from 36% to as high as 100% in one study on female twins, while concordance rates of dizygotic twins are reported to vary from 0% to 31%, with values as high as 40% for male-male twin pairs, and heritability estimates of ∼90% have been calculated [21], [25]–[35]. In twin studies, when a broader definition of ASD is used, higher concordance rates are found; while lower rates are determined when a stricter definition of autistic disorder is used. These findings indicate that the severity of ASD symptoms (e.g. AD versus AS/HFA or PDD-NOS) and the age of diagnosis can differ between monozygotic twins diagnosed with ASD [26], [28]–[30], [34]. As a result, epigenetic influences have been suggested to contribute to the overall ASD phenotype [36]–[39]. Recent data also indicate a further ∼10% of ASD cases are caused by spontaneous genetic mutations [40]–[43]. Currently, more than 25 different loci encode ASD-susceptibility genes, with many more being investigated [44]. Some, but not all, of the genes implicated in ASD overlap with those implicated in MR [45]–[48]. Nonetheless, no genetic susceptibility locus accounts for more than a small percentage of ASD cases [49]. While these findings demonstrate an etiological heterogeneity for ASDs, many of the genes appear to interact at the level of molecular pathways, making it likely that mutations of one ASD-implicated gene may affect the function of others [50]. These findings suggest a common pathway(s) for the etiology of ASDs and raise hope that common treatments may be developed.
It is rare for those with ASDs to lead independent lives in adulthood, and those with severe intellectual disability (IQ<50) have the poorest outcomes [51]–[55]. By contrast, the outcome is improved for those with an ASD who lack accompanying mental retardation (MR), and 10–15% of those with Asperger syndrome/high-functioning ASD achieve independence in adulthood [55]–[57]. While these studies highlight the debilitating life-long effects of ASDs, they emphasize the additional problems conferred by the presence of intellectual disability. As with ASDs, X-linked mental retardation (XLMR) is more common in males and also has a heterogenous genetic etiology [58]. MR affects around 2% of the population and accounts for 5–10% of the health care budget in developed countries [58], with XLMR representing between 5–15% of all MR [59]. Mutations in approximately 10% of the 2,000 genes on the human X chromosome can cause XLMR, although the cellular role of most of these genes remains uncharacterized [60].
We report the identification of a human gene related to the recently identified Deleted In Autism 1 (DIA1) gene. The DIA1 gene was identified in a genetic study for ASD genes [61], where it was found that hemizygous DIA1 deletions were asymptomatic, while a homozygous DIA1 deletion coincided with a classical autism diagnosis. Using a bioinformatics-based approach, we have identified a DIA1-related gene, we term DIA1R, which localizes to the human X chromosome at position Xp11.3. Deletion and/or mutation of human DIA1R is associated with ASD-like syndromes and/or XLMR.
Results
Identification of a human DIA1-related gene
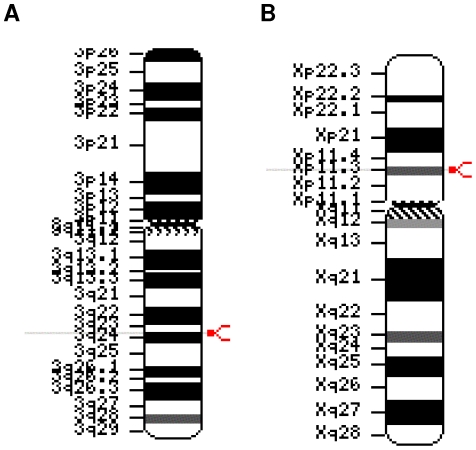
The human c3orf58 gene, found at chromosome position 3q24, has recently been renamed DIA1 on the basis of its deletion in ASD [61]. Human DIA1 has a known orthologue in both the mouse (Mus musculus) and rat (Rattus norvegicus) genomes, where it is alternatively called 1190002N15Rik, Ab2-095 or GoPro49 [62]. A search of the HomoloGene database [63], revealed further DIA1 orthologues in the chimpanzee (Pan troglodytes), dog (Canis familiaris), cow (Bos taurus), mouse (Mus musculus), chicken (Gallus gallus), and zebrafish (Danio rerio) genomes (data not shown; [64]). Unexpectedly, a Basic Local Alignment Search Tool (BLAST) search [65] of the non-redundant (NCBI) database [63], [66], using the sequence of the human DIA1 gene product, revealed a significantly similar human gene product, LOC79742. This gene product was encoded by the cXorf36 gene (for accession numbers and identifiers, see Table 1) and localized to the human X chromosome at position Xp11.3 (Figure 1). We have renamed this gene DIA1R (DIA1-Related). A search of the HomoloGene database [63] revealed orthologues of DIA1R in the cow, mouse, chicken, and zebrafish genomes (data not shown).
Table 1. Accession numbers and identifiers for human DIA1 and DIA1R.
| Database | DIA1 identifier or accession number | DIA1R identifier or accession number |
| HUGO gene symbol* | c3orf58 | cXorf58 |
| RefSeq** protein | NP_775823 | NP_789789 |
| RefSeq mRNA | NM_173552 | NM_176819 |
| RefSeq chromosome | NC_000003 | NC_000023 |
| GenInfo (GI) | 27734895 | 193804854 |
| ENSEMBL protein | ENSP00000320081 | ENSP00000381086 |
| ENSEMBL transcript | ENST00000315691 | ENST00000398000 |
| ENSEMBL gene | ENSG00000181744 | ENSG00000147113 |
| UniProt | Q8NDZ4 | Q9H7Y0 |
| UniGene | Hs.288954 | Hs.98321 |
| Entrez gene GeneID | 205428 | 79742 |
| HGNC*** | 28490 | 25866 |
| Aliases | LOC205428MGC33365GoPro49Ab2-095DIA1 | LOC79742DKFZp313K0825EPQL1862FLJ55198FLJ14103PRO3743bA435K1.1FLJ55198 |
*Human genome organization (HUGO) official gene name.
**NCBI non-redundant reference sequence [63].
***HUGO gene nomenclature committee (HGNC) identifier.
Figure 1. Chromosomal ideogram representation of the location of human DIA1 (c3orf58) and DIA1R (cXorf36).
(A) DIA1 localization on human chromosome 3 at position 3q24. (B) DIA1R localization on the human X chromosome at position Xp11.3. Gene localization is indicated by a modified red arrowhead on the right-hand side, and a light-grey line extending through the text on the left-hand side.
Comparison of the human DIA1 and DIA1R gene products
The human DIA1 and DIA1R genes encode gene products of 430 and 433 residues, respectively, with predicted molecular weights of 49.5 and 48.6 kDa. To compare the human DIA1 and DIA1R proteins with each other, we used two methods: BLAST analyses and amino acid alignments. Pair-wise protein BLAST analyses generate ‘expect values’ (E-values), where values less than one are considered significantly similar, and the smaller the E-value, the greater the similarity [65]. E-values of 2e-42 and 1e-42 were obtained for reciprocal BLAST searches, comparing the human DIA1 gene product with that of DIA1R, or vice versa. Next, amino acid alignments were employed to compare the human DIA1 and DIA1R proteins at the amino acid level (Figure 2). Amino acid alignments revealed human DIA1 and DIA1R are 62% similar overall (28% identical, and 34% similar, residues), with similarity greater in the central portion of the two proteins, compared to the amino- or carboxy-terminal regions. This similarity suggests that the central region of DIA1 and DIA1R encodes a biological function common to the two gene products.
Figure 2. Amino acid sequence comparison of human DIA1 and human DIA1R.
The sequence alignment was generated using CLUSTALX [164]. Identical amino acids are highlighted in red font and indicated below the alignment with an asterisk (*). Strongly similar amino acids are highlighted in green font and indicated below the alignment with a colon (:). Weakly similar amino acids are highlighted in blue font and indicated below the alignment with a full stop (.). Dissimilar amino acids are in black font. Amino acid numbering is provided above the alignment. Human DIA1 is 430, and DIA1R 433, amino acids in their immature forms (before signal peptide cleavage). Gaps required for optimal alignment are indicated by dashes. The two proteins have 28% identical, 21% strongly similar, and 13% weakly similar, residues. Standard single-letter amino acid abbreviations are used.
Signal peptides in human DIA1 and DIA1R
Most proteins transported to the endoplasmic reticulum (ER) have a stretch of hydrophobic amino acids at their amino-terminus, known as the signal peptide (SP), which are recognized by the signal recognition particle (SRP) to facilitate ER import [67]. The length of signal peptides usually falls within a range of 5–60 residues. Subsequent to SRP recognition, SPs are typically cleaved from the imported pre-protein by signal peptide peptidase activity [68]. Most imported proteins are then transported from the ER to the Golgi apparatus [69]. As determined above, the most disparate region found when DIA1 and DIA1R are aligned (Figure 2) is the extreme amino-terminus. In a previous assessment of the rat DIA1 gene product, the presence of a predicted signal peptide was detected, and functionally supported by localization of DIA1 to the lumen of the Golgi apparatus [62]. We therefore analyzed the human DIA1 and DIA1R gene products for the presence of amino-terminal signal peptides.
There are a variety of algorithms designed to predict the presence of signal peptides, and we used three of the best-performing algorithms [70], [71] to both analyze the DIA1 and DIA1R gene products for signal peptides, and to predict the signal peptide cleavage sites. The methodologies used were: (i) the neural network (NN) algorithm of SignalP v3.0 [72], (ii) the hidden Markov model (HMM) of SignalP v3.0 [72], and (iii) the SigCleave algorithm [73] at EMBOSS [74]. No trans-membrane domains were predicted, nor ER-retrieval or retention motifs, in either DIA1 or DIA1R (data not shown).
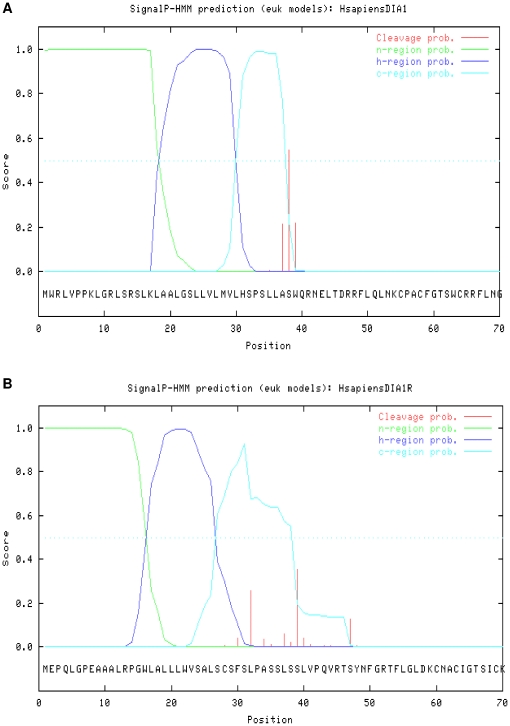
Our analyses revealed SPs in human DIA1 and DIA1R, using all three prediction methods (Figures 3 and S1; data not shown). However, while all methods predicted a SP in both DIA1 and DIA1R, some methods used had varying success in pin-pointing the exact cleavage site used to generate the mature protein. The HMM algorithm of SignalP confidently (score of 0.544, where 0.5 is considered significant) predicted the signal peptide cleavage site of human DIA1 (Figure 3A) as between amino acids 37 and 38 (SLLA-SWQR using single-letter amino-acid code, where the hyphen indicates the site of cleavage). The same site was also the highest-scoring cleavage site predicted by SigCleave (score 7.79, where a score over 3.50 is considered significant), providing confidence that this is the site of human DIA1 SP cleavage. By contrast, the NN algorithm did not predict a DIA1 cleavage site with a significant score (Figure S1A). For human DIA1R, two cleavage sites were suggested by the HMM algorithm (Figure 3B), but neither score passed into the significant range (0.26 for cleavage between amino acids 31 and 32, 0.35 for between amino acids 38 and 39, where 0.5 is considered significant). The NN algorithm (Figure S1B) only predicted a single DIA1R cleavage site (between amino acids 31 and 32) with a Y-score close to significance (a Y-score of 0.44, where a Y-max value of 0.5 is considered significant). SigCleave analysis of DIA1R (data not shown) provided a highly significant score for cleavage between amino acids 31 and 32 (CSFS-LPAS, using single-letter amino-acid code, where the hyphen indicates the site of cleavage) of 10.23 (where values of greater than 3.5 are considered significant), suggesting this may represent the true site of SP cleavage for human DIA1R. Therefore, while all methods indicate amino-terminal SPs in both DIA1 and DIA1R, the exact SP cleavage sites will need to be determined experimentally. Together, our data indicate that both the DIA1R and DIA1 gene products will be translocated into the ER, and transported to the Golgi apparatus.
Figure 3. Prediction of signal peptides in human DIA1 and DIA1R.
The amino acid sequence of (A) human DIA1 or (B) human DIA1R, was evaluated for amino-terminal signal peptides using the hidden Markov model of SignalP v3.0 [72]. C-region (cleavage-recognition region) scores are in aqua, H-region (hydrophobic region) scores are in dark blue, N-region (N-terminal signal peptide sequence) scores are in green, and the cleavage probability (that a given amino acid is the first in the mature protein) indicated in red. The significance cut-off value for all probability scores is 0.5, and is indicated by a blue dotted line. Standard single-letter amino acid abbreviations and numbering is provided below the graph.
Ubiquitous tissue expression of human DIA1 and DIA1R
To determine the cell types and tissues in which human DIA1 and DIA1R are expressed, we explored the relevant expressed sequence tag (EST) profiles via UniGene [63] and searched two microarray databases: the GeneNote normal human expression database at the Weizmann Institute [75] and human Gene Atlas [76] at BioGPS [77].
EST profile data detected DIA1 expression at levels of 61 transcripts per million (TPM) isolated from brain tissue (Table 2) and DIA1R at 25 TPM from brain tissue (Table 3). Of note, a wide analysis of human gene expression levels, in 14 different tissues, selected cut-off values for gene expression, with ‘low’ being ≤37 TPM and ‘high’ being ≥134 TPM [78]. Another human expression study indicated the majority (64%) of genes are expressed at between 3–50 TPM, 6% at levels between 100–5,000 TPM, and only 0.03% expressed at levels above 10,000 TPM [79]. Using these values as a guide, DIA1R can be described as being expressed at low-to-intermediate levels. By contrast, while DIA1 is frequently expressed at low-to-intermediate levels, expression is high in mammary gland (135 TPM), ‘mouth’ (223 TPM; where libraries included in this tissue category are often derived from head or tongue), and vascular tissue (135 TPM). Ear expression of DIA1 was the highest of those tissues falling in the intermediate range, and is close (123 TPM) to the ‘high expression’ category. These four tissue types, apparently expressing high levels of human DIA1, are all tissues with a high secretory output. One explanation is that Golgi apparatus-localized DIA1 has a role in an aspect of secretion common to these tissues. Abnormalities in secreted levels of vascular growth factors and soluble vascular receptors have been reported in patients with ASD [80], as have abnormal levels of the endothelial cell protein PECAM-1 (platelet endothelial cell adhesion molecule-1) [81]. Defects in secretion or secreted factors are also considered major risk factors for otitis media [82], a condition occurring 2 to 3 times more frequently in those with ASD, than those without ASD [83]–[85]. Therefore, EST profiling results indicate widespread expression of human DIA1 and DIA1R at moderate levels, including in brain tissue, with higher levels of DIA1 in some tissues with a high secretory load.
Table 2. Expressed sequenced tag (EST) profiles showing DIA1 gene expression in human tissues.
| cDNA library source* | TPM** | EST/total*** |
| adipose tissue | 0 | 0/13105 |
| adrenal gland | 0 | 0/33195 |
| ascites | 0 | 0/40013 |
| bladder | 0 | 0/29757 |
| blood | 0 | 0/123476 |
| bone | 13 | 1/71655 |
| bone marrow | 81 | 4/48798 |
| brain | 59 | 66/1100969 |
| cervix | 41 | 2/48171 |
| connective tissue | 6 | 1/149254 |
| ear | 123 | 2/16212 |
| embryonic tissue | 23 | 5/215722 |
| esophagus | 0 | 0/20208 |
| eye | 37 | 8/211052 |
| heart | 33 | 3/89625 |
| intestine | 17 | 4/234477 |
| kidney | 56 | 12/211769 |
| larynx | 41 | 1/24144 |
| liver | 19 | 4/207739 |
| lung | 17 | 6/336969 |
| lymph | 0 | 0/44269 |
| lymph node | 54 | 5/91607 |
| mammary gland | 135 | 20/153267 |
| mouth | 223 | 15/67053 |
| muscle | 9 | 1/107711 |
| nerve | 0 | 0/15768 |
| ovary | 68 | 7/102050 |
| pancreas | 4 | 1/214811 |
| parathyroid | 48 | 1/20540 |
| pharynx | 0 | 0/41328 |
| pituitary gland | 0 | 0/16584 |
| placenta | 42 | 12/280828 |
| prostate | 21 | 4/189352 |
| salivary gland | 0 | 0/20155 |
| skin | 4 | 1/210574 |
| spleen | 0 | 0/53953 |
| stomach | 10 | 1/96622 |
| testis | 39 | 13/330449 |
| thymus | 36 | 3/81130 |
| thyroid | 0 | 0/81130 |
| tonsil | 0 | 0/16999 |
| trachea | 38 | 2/52412 |
| umbilical cord | 0 | 0/13680 |
| uterus | 12 | 3/232876 |
| vascular | 135 | 7/51779 |
*The EST profiles show approximate gene expression patterns, as inferred from EST counts from cDNA library sources, reported by sequence submitters to Unigene at the NCBI [63]. Libraries known to be normalized, subtracted, or otherwise biased are not included.
**TPM = transcripts per million.
***EST/total = number of DIA1 ESTs in the total EST pool.
Table 3. EST profiles of DIA1R gene expression in human tissues.
| cDNA library source* | TPM** | EST/total*** |
| adipose tissue | 76 | 1/13105 |
| adrenal gland | 0 | 0/33195 |
| Ascites | 0 | 0/40013 |
| Bladder | 0 | 0/29757 |
| Blood | 0 | 0/123476 |
| Bone | 0 | 0/71655 |
| bone marrow | 0 | 0/48798 |
| Brain | 25 | 28/1100969 |
| Cervix | 0 | 0/48171 |
| connective tissue | 40 | 6/149254 |
| Ear | 0 | 0/16212 |
| embryonic tissue | 0 | 0/215722 |
| esophagus | 0 | 0/20208 |
| Eye | 0 | 0/211052 |
| Heart | 78 | 7/89625 |
| Intestine | 8 | 2/234477 |
| Kidney | 9 | 2/211769 |
| Larynx | 0 | 0/24144 |
| Liver | 4 | 1/207739 |
| Lung | 5 | 2/336969 |
| Lymph | 0 | 0/44269 |
| lymph node | 0 | 0/91607 |
| mammary gland | 32 | 5/153267 |
| Mouth | 29 | 2/67053 |
| Muscle | 27 | 3/107711 |
| Nerve | 0 | 0/15768 |
| Ovary | 0 | 0/102050 |
| pancreas | 4 | 4/214811 |
| parathyroid | 0 | 0/20540 |
| Pharynx | 0 | 0/41328 |
| pituitary gland | 0 | 0/16584 |
| Placenta | 60 | 17/280828 |
| Prostate | 36 | 7/189352 |
| salivary gland | 0 | 0/20155 |
| Skin | 0 | 0/210574 |
| Spleen | 0 | 0/53953 |
| Stomach | 10 | 1/96622 |
| Testis | 0 | 0/330449 |
| Thymus | 0 | 0/81130 |
| Thyroid | 21 | 1/81130 |
| Tonsil | 0 | 0/16999 |
| Trachea | 19 | 1/52412 |
| umbilical cord | 73 | 1/13680 |
| Uterus | 17 | 4/232876 |
| Vascular | 57 | 3/51779 |
*The EST profiles show approximate gene expression patterns, as inferred from EST counts from cDNA library sources, reported by sequence submitters to Unigene at the NCBI [63]. Libraries known to be normalized, subtracted, or otherwise biased are not included.
**TPM = transcripts per million.
***EST/total = number of DIA1 ESTs in the total EST pool.
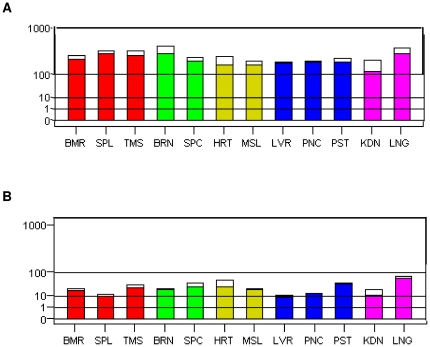
Microarray data from two sources (Figures 4, S2, and S3) supported the findings of the EST profiling results, as ubiquitous expression of both DIA1 and DIA1R was found, including expression in brain tissue. Microarray expression levels of human DIA1 were strikingly similar in all tissue types tested, but no confirmation of higher levels in ‘mouth’ tissues (tonsil, trachea, salivary gland, and tongue, being the comparable microarray tissue data), vascular tissue (CD105+ circulating endothelial cells being the closest microarray data), or mammary gland (no comparable microarray data) detected by EST profiling was found by microarray analysis. By microarray analysis, the highest level of DIA1 was found in BDCA-4 (blood dendritic cell antigen 4 or neuropilin-1 or CD304) -positive plasmacytoid dendritic cells (Figure S2), which secrete high levels of type I interferons [86]. By contrast to the DIA1 microarray data, microarray expression levels of DIA1R were less uniform (Figures 4 and S3). However, the highest DIA1R expression level found in the Gene Atlas microarray, which was found in liver (Figure S3), was not supported by the GeneNote microarray data (Figure 4). Within brain tissue, the highest expression level of DIA1R was in the cerebellar peduncles (Figure S3), a region of the brain abnormal in those with ASD [87]–[89]. However, microarray studies are plagued by a lack of reproducibility and accuracy [90]–[93], and we can not place too much emphasis on differences between gene expression levels, but must focus on overall expression profiles. While quantitative reverse-transcriptase polymerase chain reactions (RT-PCR) can provide more accurate and reproducible results [94], and can be used in conjunction with commercially available human tissue panels, analysis of data from this approach relies on the choice of reference gene for normalization [95]. Changes in the comparable housekeeping gene can lead to changes in the significance and expression of the target gene using RT-PCR approaches to quantifying gene expression [96], [97]. We can therefore only reliably conclude that human DIA1 and DIA1R are both ubiquitously expressed genes.
Figure 4. Microarray gene expression data for DIA1 and DIA1R.
Graphical presentation of microarray expression data for (A) DIA1 or (B) DIA1R in normal, healthy human tissues. The intensity values (shown on the root-scale y-axis) are average values, where variation in the range of measurements is represented by a white box above the coloured minimal-measurement bar. Tissues are grouped according to their origin and the groups coloured: red = immune system; green = nervous system; yellow = muscle; blue = secretory glands; pink = ‘other’. Tissue abbreviation (on the x-axis) legend: BMR = bone marrow; SPL = spleen; TMS = thymus; BRN = brain; SPC = spinal cord; HRT = heart; MSL = skeletal muscle; LVR = liver; PNC = pancreas; PST = prostate; KDN = kidney; LNG = lung. Data were obtained from the GeneNote database of human genes at the Weizmann Institute [75], and are MAS5.0 normalized data obtained using the Affymetrix HG-U95 set A–E.
Expression of human DIA1 and DIA1R during development
To examine expression of DIA1 and DIA1R during human development, microarray data from the Gene Atlas [76] at the BioGPS [77] database (Figure S2 and S3) and EST profile data from UniGene [63], were examined (Tables 4 and 5). As EST data was restricted by the depth at which each developmental stage could be sampled, absences of expression cannot be assumed to indicate a lack of expression. Despite the limitations of EST profiling, the data revealed that both DIA1 and DIA1R are expressed in fetal tissue, with DIA1 expression also detected in the blastocyst (Tables 4 and 5). Microarray data confirmed expression of DIA1 and DIA1R in fetal brain (Figure S2 and S3). Therefore, human DIA1 and DIA1R are expressed in both adult and fetal tissues, including adult and fetal brain.
Table 4. EST profiles of DIA1 gene expression during human development.
| cDNA library source* | TPM** | EST/total*** |
| embryoid body | 42 | 3/70760 |
| blastocyst | 32 | 2/62318 |
| fetus | 44 | 25/564004 |
| neonate | 0 | 0/31097 |
| infant | 0 | 0/23620 |
| juvenile | 0 | 0/55555 |
| adult | 31 | 62/1939097 |
*The EST profiles show approximate gene expression patterns, as inferred from EST counts from cDNA library sources, reported by sequence submitters to Unigene at the NCBI [63]. Libraries known to be normalized, subtracted, or otherwise biased are not included.
**TPM = transcripts per million.
***EST/total = number of DIA1 ESTs in the total EST pool.
Table 5. EST profiles of DIA1R gene expression during human development.
| cDNA library source* | TPM** | EST/total*** |
| embryoid body | 0 | 0/70760 |
| blastocyst | 0 | 0/62318 |
| Fetus | 33 | 19/564004 |
| Neonate | 0 | 0/31097 |
| Infant | 0 | 0/23620 |
| Juvenile | 0 | 0/55555 |
| Adult | 23 | 45/1939097 |
*The EST profiles show approximate gene expression patterns, as inferred from EST counts from cDNA library sources, reported by sequence submitters to Unigene at the NCBI [42]. Libraries known to be normalized, subtracted, or otherwise biased are not included.
**TPM = transcripts per million.
***EST/total = number of DIA1 ESTs in the total EST pool.
Discussion
The DIA1 gene, recently identified in a genetic study detecting autism genes by tracing recent shared ancestry, localizes to chromosome 3 at position 3q24 [61]. Using a bioinformatics approach, we have identified a DIA1-related gene, DIA1R, which localizes to the human X chromosome at position Xp11.3. As has been reported for mouse DIA1 [64], we find both human DIA1 and DIA1R are ubiquitously expressed, suggesting each gene has a generic cellular role. Furthermore, human DIA1R, like DIA1 [62], encodes a signal peptide for targeting to the secretory pathway. Our comparison of human DIA1 and DIA1R at the amino acid level found conservation greatest between the central portions of the proteins, indicating this region may contribute to a biological role shared by each protein. The presence of DIA1R in the human genome may therefore explain why a homozygous deletion of a ubiquitously-expressed gene such as DIA1 is not lethal [61]. However, human DIA1R, only has 62% similarity overall to human DIA1, and presumably cannot fully compensate for loss of DIA1, which would explain why DIA1 deletion is associated with the neurological deficits of autism [61].
Role of the DIA1R locus in autism and X-linked mental retardation
Around 10% of genes on the X chromosome cause XLMR if inactivated [60]. Human DIA1R is an X-linked gene at position Xp11.3, and is therefore part of the wider Xp11.3-Xp11.23 region. This wider region is implicated in a growing number of genetic diseases, including several eye disorders, XLMR, X-linked neuromuscular diseases, and susceptibility loci for schizophrenia and type 1 diabetes, with the Xp11.3 locus implicated specifically in XLMR [98], [99]. However, in addition to 19 characterized genes, there are at least four uncharacterized genes in this region of the X chromosome, including DIA1R, whose contribution to the disease phenotypes mapped to this region have not been assessed.
We next highlight a number of publications that support the hypothesis that deletion of the Xp11.3 region encompassing DIA1R causes ASD-like symptoms and/or XLMR. Essentially, we have carried out a retrospective genotype-phenotype analysis based on previously published studies which characterized the phenotypic traits of patients with deletions encompassing DIA1R. Genotype-phenotype mapping is a concept first introduced in 1991 [100], and genotype-phenotype correlation is widely used to examine the contribution of a certain mutation (genotype) to the resulting clinical traits (phenotype) [101]–[106]. Deletions encompassing a certain chromosomal region have variable phenotypes, depending on the size and position of the deleted region. However, features common to patients with deletions overlapping a locus of interest provide evidence for the specific effect of the loss of that gene or genes. The findings outlined below suggest DIA1R is joining an increasing number of genes able to cause both ASD and/or MR [45]–[48].
In the first study, examination of a patient with developmental delay, no expressive speech, mental retardation, and microcephaly, revealed a 4.0-Mb heterozygous deletion at Xp11.3–p11.4. This region included 5 candidate genes, including DIA1R and CASK, the latter of which was suggested to be causative due to its known role in synaptic function [107]. We suggest DIA1R deletion contributes to the phenotype of this patient.
Secondly, a linkage study of a four-generation family, implicated chromosomal region Xp11.3–11.21 in a novel neurological syndrome [108]. The syndrome is characterized by mild mental retardation, poor verbal skills, aggressive and/or agitated behaviour, and involuntary movements referred to as choreoathetosis. These symptoms overlap with those of ASD, and choreoathetosis is associated with Rett syndrome [109]. Reyniers and colleagues [108] discussed the possible role of 11 known genes with roles in neural signalling and neurotransmission, and how they might contribute to patient symptoms. The region does not include the CASK locus, but does include DIA1R, which can now also be considered a candidate gene for this novel syndrome.
A third study, by Zhang and colleagues [110], linked the region Xp11.3–Xq22.2 of male members of a large, cross-generational family group to poor verbal skills, mental retardation, and microcephaly. Zhang and coworkers discussed OPHN1 as a candidate gene in this region, as it had previously been implicated in XLMR [110]. Nonetheless, the authors acknowledged that uncharacterized genes in this region may contribute to patient symptoms, and this may include DIA1R.
A fourth study further strengthens the argument that deletion of the Xp11.3 region, including DIA1R, is implicated in ASD-like symptoms and XLMR. This study identified a 5 Mb deletion at Xp11.3–11.4 in Turner syndrome (TS) patients [111], a region that does not involve OPHN1. TS is a relatively common genetic disorder, caused by X chromosome monosomy or other X chromosome defects. TS does not typically affect intellectual function or expressive verbal abilities, although it can be associated with reduced visual-spatial and executive skills, and demonstrable impairments in social skills [112]–[113]. Overall, TS is associated with a substantially increased risk of ASD [114]. Good and colleagues [111] found that deletion of Xp11.3–11.4 is associated with the development of ASD-like symptoms in TS patients, including poor eye contact, poor recognition of facial expression, and impaired social skills. Deletion of this region was also associated with increased amygdala volumes in TS patients, to levels typically associated with normal males [111]. Of note, many studies have also reported increased amygdala volumes in patients with ASD [115]–[118], but the phenomenon is not universal and the finding may be age-dependent [119].
Good and co-workers [111] hypothesized that there is a critical gene in the Xp11.3–11.4 region that is expressed in two copies in normal (46,XX) females, and that full dosage compensation does not occur in normal (46,XY) males or in Turner syndrome females (45,X), resulting in the increased amygdala volume found in normal males [120] and TS females [111]. They further suggested that this unknown gene has implications for autism and may contribute to the increased male susceptibility to ASD [111]. This gene cannot be CASK, as CASK undergoes X-inactivation [111]. As human DIA1R has recently been shown to escape X inactivation [121], DIA1R is a good candidate within this locus. Together, these findings demonstrate that DIA1R is a candidate gene for ASD-like symptoms and/or XLMR.
A more direct link between DIA1R and XLMR comes from two independent studies, where point mutations in DIA1R were implicated in XLMR [60], [122]. It should be noted that any ASD-like symptoms were not commented on in either of these studies, and may not have been evaluated or specifically ruled-out. In the first study, a mutation affecting the DIA1R signal peptide (a change of serine at position 24 to proline or ‘S24P’, using single amino acid abbreviations) was reported in a single XLMR patient, while the same mutation was absent in controls [122]. Our analyses reveal that the mutant DIA1R gene product (DIA1R-S24P) is still expected to have a functional signal peptide, but the mutation is predicted to change the signal peptide cleavage site (data not shown). Therefore, the DIA1R-S24P mutant is still expected to translocate to the secretory pathway, but the mutation may affect the size of the mature protein, with resulting impact on structure and function. In the absence of knowledge about ASD-like symptoms in this patient, the data currently suggest that mutation of DIA1R can cause XLMR, but complete deletion of DIA1R causes ASD-like symptoms with or without MR.
In the second study, a systematic large-scale screen for mutations implicated in XLMR found recurrent mutations in DIA1R [60]. In 67 XLMR patients a 383G>A mutation in DIA1R was found [60], where the mutation is part of an arginine-coding residue (codon: AGA), resulting in an arginine to lysine (mutated codon: AAA) change in the DIA1R gene product. The amino acid mutation (R128K) falls within the amino-terminal one-third of the DIA1R protein. While we are currently investigating the conservation of this amino acid in the wider DIA1 and DIA1R family, the findings of Tarpey and colleagues [60] suggest an R128K mutation alters DIA1R function in the human brain. These two studies demonstrate that independent DIA1R mutations are specifically associated with human XLMR, while the deletion studies implicate DIA1R deletion in autism-like syndromes and/or XLMR.
Overall, multiple lines of evidence now support a role for DIA1R in ASD and/or mental retardation: (i) the genotype-phenotype correlation data provided above; (ii) the high degree of amino acid conservation between DIA1 and DIA1R, where DIA1 is implicated in ASD [61]; (ii) targeting of both gene products to the Golgi apparatus, due to the conservation of signal peptide sequence in DIA1 and DIA1R; (iii) the ubiquitous tissue expression of both genes, including in brain tissue; (iv) and data from our accompanying paper which indicates an origin of DIA1R from a DIA1 gene duplication early in the vertebrate lineage [123]. Each of these findings indicate conserved gene function. Therefore, it is not surprising that mutation of DIA1R, appears to cause a similar phenotype to deletion DIA1. (How these genes might function within the Golgi apparatus to cause this phenotype will be discussed below.)
Disorders associated with the human 3q24 locus
In addition to the publication originally describing the homozygous deletion of DIA1 in autism [61], wider deletions encompassing 3q24 (the region of human chromosome 3 encoding the DIA1 gene), have also been reported. While each patient with a deletion encompassing 3q24 has unique features, presumably related to differences in deletion breakpoints between patients, several common features are found: developmental delay, mental retardation, and growth delay including microcephaly [124]–[126]. These three common symptoms overlap those described in a subset of PDD patients: females with Rett syndrome (where MECP2 is mutated), patients with Angelman syndrome, and male Rett syndrome-like patients in which the MECP2 gene is not implicated [127], [128].
By contrast, a patient with a deletion mapping cytogenetically to 3q25, showed developmental delay, but lacked the delayed growth and microcephaly of larger deletions in this chromosomal area [129]. Another patient with a deletion around 3q25 was specifically described as having autistic traits, severe learning difficulties, hypogonadism, and dysmorphic features [130]. These latter two deletions [129], [130] have only been characterized cytogenetically, which is a relatively imprecise method [131], and it would be beneficial to map the exact deletion boundaries to confirm whether the deleted regions encompass DIA1.
Therefore, while a single publication links homozygous DIA1 deletion to autism [61], numerous studies report that deletion of portions of the 3q24 region encompassing DIA1 are associated with developmental delay, ASD-like traits, and/or mental retardation [124]–[126]. Together, these publications provide further support for a role for DIA1 in autism etiology, as first proposed by Morrow and colleagues in 2008 [61].
Why does DIA1 or DIA1R mutation cause autism-like syndromes?
We have demonstrated that human DIA1 and DIA1R both encode signal peptides for targeting to the secretory pathway. Indeed, the DIA1 gene product has been localized, using immunofluorescence microscopy, to the lumen of the Golgi apparatus [62]. Surprisingly, for genes with roles in neurological diseases, we find both DIA1 and DIA1R are ubiquitously expressed, which indicates they fulfil basic, non-specialist cellular roles. However, despite ASDs being diagnosed on the behavioural manifestations of neurological deficits [132]–[135], many co-morbid non-neuronal conditions occur in ASD patients, suggesting widespread physiological and biochemical abnormalities in ASD patients [136]–[139]. Secretory pathway malfunction is a plausible explanation for the wide-ranging deficits in ASD and, as both DIA1 and DIA1R are predicted to play ubiquitous roles in some aspect of the cellular secretion pathway, they may be involved in a common cellular pathway deficient in those with ASDs.
The secretory pathway plays a key role in all cells, with essential roles in neuron development and function. Secretion in neurons, while having many factors in common to that in non-neuronal cells, has specific challenges. Recently, dendrites have been found to have satellite Golgi-like cisternal stacks, known as Golgi outposts, which have no membranous connection with the somatic Golgi [140]. What triggers Golgi outpost formation is not known, and only proteins common to the somatic Golgi have been identified in Golgi outposts [141]. Therefore, despite using the same secretory compartments as other cells, the dependence of neurons on the secretory pathway for synaptic transmission, outgrowth and remodelling of dendrites and axons, combined the vast distances involved, means the impact of mutation in many secretory pathway genes has a greater impact on neuronal, rather than non-neuronal, cell function [142]. Indeed, aberrations in cell secretion are being detected in increasing numbers of patients with ASD and/or mental retardation [143]–[149]. While large numbers of genes have been implicated as causative of ASD, we next discuss data suggesting that a common theme in ASD etiology may be alterations in cellular secretory pathway(s).
Recent work has found that four independent gene rearrangements, all previously implicated in ASD, induce the same morphological and functional aberrations in the large dense core granules of platelets [149]. It is also well-established that aberrant serotonin secretion from platelets is common in those with ASD [150]–[151], along with aberrant levels of other secreted factors, including neuropeptides and neurotrophins [137], [143], [144]. Furthermore, two research groups have found evidence for defective enzymatic activity in the secretory pathway of those with autism. The more recent of these two studies, found a functional deficit in a secretory pathway-localized kinase by assaying substrates in the saliva of patients with ASD [152]. The activity of this ubiquitously expressed kinase can affect the traffic and/or function of its substrates [153]–[157]. The second research group found a deficit in the activity of tyrosylprotein sulfotransferase (TPST) in platelets of patients with ASD [158], [159]. TPST is a ubiquitious sulfotransferase, which acts on substrates in the lumen of the Golgi apparatus [160]. Protein tyrosine sulfation is again known to affect the function of the target protein [161]. Therefore, an emerging theme in autism is defects in the delivery or function of secreted, or cell-surface, molecules. The location of DIA1 and DIA1R suggests they may also affect such processes.
Further evidence supporting a role of secretory pathway deficits in ASD etiology, are studies identifying genes related to glycobiology in autism [48], [162]. As the Golgi apparatus is the main site of glycosylation [163], this finding further implicates deficits in Golgi apparatus function in ASD etiology. Therefore, a unifying hypothesis is that genes involved in the pathogenesis of autism are involved in regulation of secretion, the expression of secreted proteins, or the optimal function of proteins trafficking via the secretory pathway. We propose a model where DIA1 and DIA1R play a role in regulation of cargo secretion or modification of cargo molecules, which is required for optimal cargo functionality. We conclude that defects in DIA1 or DIA1R function within the lumen of the Golgi apparatus of human cells causes autism and/or mental retardation. However, in the absence of known protein motifs and domains (data not shown), ongoing studies are required to determine the exact cellular roles of human DIA1 and DIA1R, particularly the effects of mutation on brain function.
Materials and Methods
Detection and chromosome mapping of human DIA1R
The human DIA1 amino acid sequence [61], [62] was used in a BLAST search, using the BLASTP [65] program, and this identified the related human sequence, DIA1R, on the non-redundant protein database at the NCBI [63]. Chromosomal location and chromosome ideograms were obtained using MapViewer at NCBI [63].
Alignments, protein analysis, and homologue identification
Protein sequence alignments were generated with CLUSTALX version 1.8 [164]. The ExPASy Compute pI/MW tool [165] was used to calculate theoretical molecular weights. Three trans-membrane prediction methods were used to analyze protein sequences: TMpred [166], TMAP [167], and HMMTOP version 2.0 [168]. Signal peptides were evaluated using SignalP version 3.0 [72] or the SigCleave algorithm [73] which is part of the EMBOSS software suite [74]. Amino acid motifs and domains were investigated using the following resources: MOTIF at GenomeNet [169]; PSORT-II [170]; the Conserved Domain Database at the NCBI, which also contains data from Pfam, SMART and COG [171]; and the Eukaryotic Linear Motif resource [172]. Homologs of human DIA1 and DIA1R were identified using the NCBI HomoloGene resource [63].
Human tissue expression
EST profiles were obtained via the UniGene database at NCBI [63] and were accessed on 12th November 2010. Microarray data was accessed from the GeneNote normal human expression database at the Weizman Institute [75] and the Gene Atlas database [76] via the BioGPS gene portal server [7].
Supporting Information
Neural network prediction of signal peptides in human DIA1 and DIA1R. The amino acid sequence of (A) human DIA1 or (B) human DIA1R, was evaluated for amino-terminal signal peptides using the neural network (NN) algorithm of SignalP v3.0 [72]. The C-score (cleavage score for each amino acid) is indicated in red, the S-score (signal peptide score) is indicated in green, and the Y-score (derived from the C-score and S-score, and can give a better indication of the cleavage site) is indicated in blue. The significance cut-off value for all probability scores is 0.5, and is indicated by a pink dotted line. Standard single-letter amino acid abbreviations and numbering are provided below the graph.
(0.02 MB PDF)
BioGPS microarray expression data for DIA1. Graphical presentation of expression data for DIA1 in a variety of normal, and cancerous human tissues and cells. Expression values were obtained from an Affymetrix U133A microarray and relate to fluorescence intensity. Multiple probes were used for each transcript on the microarray and these intensity values have been normalized, background-subtracted, and summarized using the data-processing algorithm GCRMA (GeneChip Robust Multi-array Average). The identifier of the Affymetrix probe set used is indicated above the graph. Data were obtained from the Gene Atlas database of human genes [76] at the BioGPS gene portal server [77].
(0.02 MB PDF)
BioGPS microarray expression data for DIA1R. Graphical presentation of expression data for DIA1R in a variety of normal, and cancerous human tissues and cells. Expression values were obtained from an Affymetrix U133A microarray and relate to fluorescence intensity. Multiple probes were used for each transcript on the microarray and these intensity values have been normalized, background-subtracted, and summarized using the GCRMA (GeneChip Robust Multi-array Average) data-processing algorithm. The identifier of the Affymetrix probe set used is indicated above the graph. Data were obtained from the Gene Atlas database of human genes [76] at the BioGPS gene portal server [77].
(0.03 MB PDF)
Acknowledgments
We thank Cheryl Dissanayake and other researchers at the Olga Tennison Autism Research Centre, David Austin and Enzo Palombo at the Swinburne Autism Bio-Research Initiative, and Dennis Crowley of the Autism Victoria Board, for enthusiastic discussions about autism spectrum disorders. We are also indebted to all research scientists who are able to provide publicly-available high quality sequence databases, gene expression databases, and software for gene and gene-product analysis. Finally, we thank the reviewers for their thoughtful and constructive criticisms.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: NEB and SPH were supported by La Trobe University and AA by La Trobe University, the Malaysian Ministry of Higher Education, and the Islamic Science University of Malaysia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.O'Hare A. Autism spectrum disorder: diagnosis and management. Arch Dis Child Educ Pract Ed. 2009;94:161–168. doi: 10.1136/adc.2008.150490. [DOI] [PubMed] [Google Scholar]
- 2.Lotter V. Epidemiology of autistic conditions in young children: I. Prevalence. Soc Psychiatry Psychiatr Epidemiol. 1966;1:124–135. [Google Scholar]
- 3.Gillberg C, Steffenburg S, Schaumann H. Is autism more common now than ten years ago? Br J Psychiatry. 1991;158:403–409. doi: 10.1192/bjp.158.3.403. [DOI] [PubMed] [Google Scholar]
- 4.Fombonne E, du Mazaubrun C. Prevalence of infantile autism in four French regions. Soc Psychiatry Psychiatr Epidemiol. 1992;27:203–210. doi: 10.1007/BF00789007. [DOI] [PubMed] [Google Scholar]
- 5.Nordin V, Gillberg C. Autism spectrum disorders in children with physical or mental disability or both. II: Screening aspects. Dev Med Child Neurol. 1996;38:314–324. doi: 10.1111/j.1469-8749.1996.tb12097.x. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. JAMA. 2001;285:3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- 7.Mayes SD, Calhoun SL. Influence of IQ and age in childhood autism: Lack of support for DSM-IV Asperger's Disorder. J Dev Phys Disabil. 2004;16:257–272. [Google Scholar]
- 8.Bryson SE, Bradley EA, Thompson A, Wainwright A. Prevalence of autism among adolescents with intellectual disabilities. Can J Psychiatry. 2008;53:449–459. doi: 10.1177/070674370805300710. [DOI] [PubMed] [Google Scholar]
- 9.Nishiyama T, Taniai H, Miyachi T, Ozaki K, Tomita M, et al. Genetic correlation between autistic traits and IQ in a population-based sample of twins with autism spectrum disorders (ASDs). J Hum Genet. 2009;54:56–61. doi: 10.1038/jhg.2008.3. [DOI] [PubMed] [Google Scholar]
- 10.Hoekstra RA, Happé F, Baron-Cohen S, Ronald A. Association between extreme autistic traits and intellectual disability: insights from a general population twin study. Br J Psychiatry. 2009;195:531–536. doi: 10.1192/bjp.bp.108.060889. [DOI] [PubMed] [Google Scholar]
- 11.Hoekstra RA, Happé F, Baron-Cohen S, Ronald A. Limited genetic covariance between autistic traits and intelligence: findings from a longitudinal twin study. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:994–1007. doi: 10.1002/ajmg.b.31066. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Chapter V. Mental and behavioural disorders. 2007. International Statistical Classification of Diseases and Related Health Problems. Tenth Revision. Available: http://apps.who.int/classifications/apps/icd/icd10online/. Accessed April 2010.
- 13.American Psychiatric Association. Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. Fourth Edition. [Google Scholar]
- 14.Santangelo SL, Tsatsanis K. What is known about autism: Genes, brain, and behavior. Am J Pharmacogenomic. 2005;5:71–92. doi: 10.2165/00129785-200505020-00001. [DOI] [PubMed] [Google Scholar]
- 15.Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet. 2006;368:210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- 16.Honda H, Shimizu Y, Imai M, Nitto Y. Cumulative incidence of childhood autism: a total population study of better accuracy and precision. Dev Med Child Neurol. 2005;47:10–18. doi: 10.1017/s0012162205000034. [DOI] [PubMed] [Google Scholar]
- 17.Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66:S3–8. [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. Atlanta: Centers for Disease Control and Prevention; 2007. National Survey of Children's Health (USA). Available: http://www.cdc.gov/nchs/slaits/nsch.htm#2007nsch. Accessed April 2010. [Google Scholar]
- 19.ADDM Network Surveillance Year 2006 Principal Investigators. Prevalence of Autism Spectrum Disorders-Autism and Developmental Disabilities Monitoring Network, United States, 2006. Morb Mortal Wkly Rep. 2009;58:1–20. [PubMed] [Google Scholar]
- 20.Rutter M. Genetic studies of autism: from the 1970s into the millennium. J Abnorm Child Psychol. 2000;28:3–14. doi: 10.1023/a:1005113900068. [DOI] [PubMed] [Google Scholar]
- 21.Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 22.Veenstra-VanderWeele J, Cook EH., Jr Molecular genetics of autism spectrum disorder. Mol Psychiatry. 2004;9:819–832. doi: 10.1038/sj.mp.4001505. [DOI] [PubMed] [Google Scholar]
- 23.Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol. 2008;67:829–837. doi: 10.1097/NEN.0b013e318184482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- 26.Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 27.Ritvo ER, Freeman BJ, Mason-Brothers A, Mo A, Ritvo AM. Concordance for the syndrome of autism in 40 pairs of afflicted twins. Am J Psychiatry. 1985;142:74–77. doi: 10.1176/ajp.142.1.74. [DOI] [PubMed] [Google Scholar]
- 28.Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 29.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 30.Le Couteur A, Bailey A, Goode S, Pickles A, Robertson S, et al. A broader phenotype of autism: the clinical spectrum in twins. J Child Psychol Psychiatry. 1996;37:785–801. doi: 10.1111/j.1469-7610.1996.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 31.Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, et al. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatry. 2004;161:539–546. doi: 10.1176/appi.ajp.161.3.539. [DOI] [PubMed] [Google Scholar]
- 32.Ronald A, Happé F, Bolton P, Butcher LM, Price TS, et al. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry. 2006;45:691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- 33.Taniai H, Nishiyama T, Miyachi T, Imaeda M, Sumi S. Genetic influences on the broad spectrum of autism: study of proband-ascertained twins. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:844–849. doi: 10.1002/ajmg.b.30740. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, et al. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med. 2009;163:907–914. doi: 10.1001/archpediatrics.2009.98. [DOI] [PubMed] [Google Scholar]
- 35.Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 36.Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet. 2006;15:R138–150. doi: 10.1093/hmg/ddl213. [DOI] [PubMed] [Google Scholar]
- 37.Ptak C, Petronis A. Epigenetic approaches to psychiatric disorders. Dialogues Clin Neurosci. 2010;12:25–35. doi: 10.31887/DCNS.2010.12.1/cptak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grafodatskaya D, Chung B, Szatmari P, Weksberg R. Autism spectrum disorders and epigenetics. J Am Acad Child Adolesc Psychiatry. 2010;49:794–809. doi: 10.1016/j.jaac.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen A, Rauch TA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J. 2010;24:3036–3051. doi: 10.1096/fj.10-154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X, Leotta A, Kustanovich V, Lajonchere C, Geschwind DH, et al. A unified genetic theory for sporadic and inherited autism. Proc Natl Acad Sci U S A. 2007;104:12831–12836. doi: 10.1073/pnas.0705803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vorstman JA, van Daalen E, Jalali GR, Schmidt ER, Pasterkamp RJ, et al. A double hit implicates DIAPH3 as an autism risk gene. Mol Psychiatry. In press. 2010. [DOI] [PMC free article] [PubMed]
- 44.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalscheuer VM, FitzPatrick D, Tommerup N, Bugge M, Niebuhr E, et al. Mutations in autism susceptibility candidate 2 (AUTS2) in patients with mental retardation. Hum Genet. 2007;121:501–509. doi: 10.1007/s00439-006-0284-0. [DOI] [PubMed] [Google Scholar]
- 46.Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 47.Kooy RF. Distinct disorders affecting the brain share common genetic origins. F1000 Biol Rep. 2010;2:11. doi: 10.3410/B2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, et al. Funtional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev. 2009;19:271–278. doi: 10.1016/j.gde.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nordin V, Gillberg C. The long-term course of autistic disorders: update on follow-up studies. Acta Psychiatr Scand. 1998;97:99–108. doi: 10.1111/j.1600-0447.1998.tb09970.x. [DOI] [PubMed] [Google Scholar]
- 52.Howlin P. Autism and intellectual disability: diagnostic and treatment issues. J R Soc Med. 2000;93:351–355. doi: 10.1177/014107680009300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry. 2004;45:212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 54.Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev. 2004;10:234–247. doi: 10.1002/mrdd.20038. [DOI] [PubMed] [Google Scholar]
- 55.Cederlund M, Hagberg B, Billstedt E, Gillberg IC, Gillberg C. Asperger syndrome and autism: a comparative longitudinal follow-up study more than 5 years after original diagnosis. Autism Dev Disord. 2008;38:72–85. doi: 10.1007/s10803-007-0364-6. [DOI] [PubMed] [Google Scholar]
- 56.Engström I, Ekström L, Emilsson B. Psychosocial functioning in a group of Swedish adults with Asperger syndrome or high-functioning autism. Autism. 2003;7:99–110. doi: 10.1177/1362361303007001008. [DOI] [PubMed] [Google Scholar]
- 57.Renty JO, Roeyers H. Quality of life in high-functioning adults with autism spectrum disorder: The predictive value of disability and support characteristics. Autism. 2006;10:511–524. doi: 10.1177/1362361306066604. [DOI] [PubMed] [Google Scholar]
- 58.Ropers HH. X-linked mental retardation: Many genes for a complex disorder. Curr Opin Genet Dev. 2006;16:260–269. doi: 10.1016/j.gde.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Stevenson RE. Splitting and lumping in the nosology of XLMR. Am J Med Genet. 2000;97:174–82. doi: 10.1002/1096-8628(200023)97:3<174::AID-AJMG1034>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 60.Tarpey PS, Smith R, Pleasance E, Whibley A, Edkins S, et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet. 2009;41:535–543. doi: 10.1038/ng.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takatalo MS, Kouvonen P, Corthals G, Nyman TA, Rönnholm RH. Identification of new Golgi complex specific proteins by direct organelle proteomic analysis. Proteomics. 2006;6:3502–3508. doi: 10.1002/pmic.200500516. [DOI] [PubMed] [Google Scholar]
- 63.Sayers EW, Barrett T, Benson DA, Bolton E, Bryant SH, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2010;38:D5–16. doi: 10.1093/nar/gkp967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takatalo M, Järvinen E, Laitinen S, Thesleff I, Rönnholm R. Expression of the novel Golgi protein GoPro49 is developmentally regulated during mesenchymal differentiation. Dev Dyn. 2008;237:2243–2255. doi: 10.1002/dvdy.21646. [DOI] [PubMed] [Google Scholar]
- 65.Altshul SF, Madden, TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pruitt KD, Tatusova, T, Maglott DR. NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007;35:D61–65. doi: 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meacock SL, Greenfield JJ, High S. Protein targeting and translocation at the endoplasmic reticulum membrane—through the eye of a needle? Essays Biochem. 2000;36:1–13. doi: 10.1042/bse0360001. [DOI] [PubMed] [Google Scholar]
- 68.Halic M, Beckmann R. The signal recognition particle and its interactions during protein targeting. Curr Opin Struct Biol. 2005;15:116–125. doi: 10.1016/j.sbi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Teasdale RD, Jackson MR. Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the Golgi apparatus. Annu Rev Cell Dev Biol. 1996;12:27–54. doi: 10.1146/annurev.cellbio.12.1.27. [DOI] [PubMed] [Google Scholar]
- 70.Menne KM, Hermjakob H, Apweiler R. A comparison of signal sequence prediction methods using a test set of signal peptides. Bioinformatics. 2000;16:741–742. doi: 10.1093/bioinformatics/16.8.741. [DOI] [PubMed] [Google Scholar]
- 71.Klee EW, Ellis LB. Evaluating eukaryotic secreted protein prediction. BMC Bioinformatics. 2005;6:256. doi: 10.1186/1471-2105-6-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- 73.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rice P, Longden I, Bleasby A. EMBOSS: the European molecular biology open software suite. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 75.Shmueli O, Horn-Saban S, Chalifa-Caspi V, Shmoish M, Ophir R, et al. GeneNote: whole genome expression profiles in normal human tissues. C R Biol. 2003;326:1067–1072. doi: 10.1016/j.crvi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 76.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brandenberger R, Khrebtukova I, Thies RS, Miura T, Jingli C, et al. MPSS profiling of human embryonic stem cells. BMC Dev Biol. 2004;4:10. doi: 10.1186/1471-213X-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lercher MJ, Urrutia AO, Hurst LD. Clustering of housekeeping genes provides a unified model of gene order in the human genome. Nat Genet. 2002;31:180–183. doi: 10.1038/ng887. [DOI] [PubMed] [Google Scholar]
- 80.Emanuele E, Orsi P, Barale F, di Nemi SU, Bertona M, et al. Serum levels of vascular endothelial growth factor and its receptors in patients with severe autism. Clin Biochem. 2010;43:317–319. doi: 10.1016/j.clinbiochem.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Tsuchiya KJ, Hashimoto K, Iwata Y, Tsujii M, Sekine Y, et al. Decreased serum levels of platelet-endothelial adhesion molecule (PECAM-1) in subjects with high-functioning autism: a negative correlation with head circumference at birth. Biol Psychiatry. 2007;62:1056–1058. doi: 10.1016/j.biopsych.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 82.Lim DJ, Chun YM, Lee HY, Moon SK, Chang KH, et al. Cell biology of tubotympanum in relation to pathogenesis of otitis media - a review. Vaccine. 2000;19:S17–25. doi: 10.1016/s0264-410x(00)00273-5. [DOI] [PubMed] [Google Scholar]
- 83.Konstantareas MM, Homatidis S. Ear infections in autistic and normal children. J Autism Dev Disord. 1987;17:585–594. doi: 10.1007/BF01486973. [DOI] [PubMed] [Google Scholar]
- 84.Smith DE, Miller SD, Stewart M, Walter TL, McConnell JV. Conductive hearing loss in autistic, learning-disabled, and normal children. J Autism Dev Disord. 1988;18:53–65. doi: 10.1007/BF02211818. [DOI] [PubMed] [Google Scholar]
- 85.Rosenhall U, Nordin V, Sandström M, Ahlsén G, Gillberg C. Autism and hearing loss. J Autism Dev Disord. 1999;29:349–357. doi: 10.1023/a:1023022709710. [DOI] [PubMed] [Google Scholar]
- 86.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 87.Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, et al. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage. 2008;41:1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 88.Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Jr, Rodrigues Lde S, et al. Diffusion tensor imaging findings in school-aged autistic children. J Neuroimaging. 2009;19:337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- 89.Sivaswamy L, Kumar A, Rajan D, Behen M, Muzik O, et al. A diffusion tensor imaging study of the cerebellar pathways in children with autism spectrum disorder. J Child Neurol. 2010;25:1223–31. doi: 10.1177/0883073809358765. [DOI] [PubMed] [Google Scholar]
- 90.Shi L, Tong W, Goodsaid F, Frueh FW, Fang H, et al. QA/QC: challenges and pitfalls facing the microarray community and regulatory agencies. Expert Rev Mol Diagn. 2004;4:761–777. doi: 10.1586/14737159.4.6.761. [DOI] [PubMed] [Google Scholar]
- 91.Chen JJ, Hsueh HM, Delongchamp RR, Lin CJ, Tsai CA. Reproducibility of microarray data: a further analysis of microarray quality control (MAQC) data. BMC Bioinformatics. 2007;8:412. doi: 10.1186/1471-2105-8-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilkes T, Laux H, Foy CA. Microarray data quality - review of current developments. OMICS. 2007;11:1–13. doi: 10.1089/omi.2006.0001. [DOI] [PubMed] [Google Scholar]
- 93.Chiorino G, Mello Grand M, Scatolini M, Ostano P. From single gene to integrative molecular concept MAPS: pitfalls and potentials of microarray technology. J Biol Regul Homeost Agents. 2008;22:7–16. [PubMed] [Google Scholar]
- 94.Skrzypski M. Quantitative reverse transcriptase real-time polymerase chain reaction in translational oncology: lung cancer perspective. Lung Cancer. 2008;59:147–154. doi: 10.1016/j.lungcan.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 95.Ohl F, Jung M, Xu C, Stephan C, Rabien A, et al. Gene expression studies in prostate cancer tissue: which reference gene should be selected for normalization? J Mol Med. 2005;83:1014–1024. doi: 10.1007/s00109-005-0703-z. [DOI] [PubMed] [Google Scholar]
- 96.Ståhlberg A, Zoric N, Aman P, Kubista M. Quantitative real-time PCR for cancer detection: the lymphoma case. Expert Rev Mol Diagn. 2005;5:221–230. doi: 10.1586/14737159.5.2.221. [DOI] [PubMed] [Google Scholar]
- 97.Passmore M, Nataatmadja M, Fraser JF. Selection of reference genes for normalisation of real-time RT-PCR in brain-stem death injury in Ovis aries. BMC Mol Biol. 2009;10:72. doi: 10.1186/1471-2199-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lubs HA, Chiurazzi P, Arena JF, Schwartz C, Tranebjaerg L, et al. XLMR genes: update 1996. Am J Med Genet. 1996;64:147–157. doi: 10.1002/(SICI)1096-8628(19960712)64:1<147::AID-AJMG25>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 99.Thiselton DL, McDowall J, Brandau O, Ramser J, d'Esposito F, et al. An integrated, functionally annotated gene map of the DXS8026-ELK1 interval on human Xp11.3-Xp11.23: potential hotspot for neurogenetic disorders. Genomics. 2002;79:560–572. doi: 10.1006/geno.2002.6733. [DOI] [PubMed] [Google Scholar]
- 100.Alberch P. From genes to phenotype: dynamical systems and evolvability. Genetica. 1991;84:5–11. doi: 10.1007/BF00123979. [DOI] [PubMed] [Google Scholar]
- 101.Shapira SK. An update on chromosome deletion and microdeletion syndromes. Curr Opin Pediatr. 1998;10:622–627. doi: 10.1097/00008480-199810060-00015. [DOI] [PubMed] [Google Scholar]
- 102.Shaffer LG, Bejjani BA, Torchia B, Kirkpatrick S, Coppinger J, et al. The identification of microdeletion syndromes and other chromosome abnormalities: cytogenetic methods of the past, new technologies for the future. Am J Med Genet C Semin Med Genet. 2007;145C:335–345. doi: 10.1002/ajmg.c.30152. [DOI] [PubMed] [Google Scholar]
- 103.Shaffer LG, Theisen A, Bejjani BA, Ballif BC, Aylsworth AS, et al. The discovery of microdeletion syndromes in the post-genomic era: review of the methodology and characterization of a new 1q41q42 microdeletion syndrome. Genet Med. 2007;9:607–616. doi: 10.1097/gim.0b013e3181484b49. [DOI] [PubMed] [Google Scholar]
- 104.Feenstra I, Vissers LE, Orsel M, van Kessel AG, Brunner HG, et al. Genotype-phenotype mapping of chromosome 18q deletions by high-resolution array CGH: an update of the phenotypic map. Am J Med Genet A. 2007;143A:1858–1867. doi: 10.1002/ajmg.a.31850. [DOI] [PubMed] [Google Scholar]
- 105.Slavotinek AM. Novel microdeletion syndromes detected by chromosome microarrays. Hum Genet. 2008;124:1–17. doi: 10.1007/s00439-008-0513-9. [DOI] [PubMed] [Google Scholar]
- 106.Bonaglia MC, Marelli S, Novara F, Commodaro S, Borgatti R, et al. Genotype-phenotype relationship in three cases with overlapping 19p13.12 microdeletions. Eur J Hum Genet. 2010;18:1302–1309. doi: 10.1038/ejhg.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hayashi S, Mizuno S, Migita O, Okuyama T, Makita Y, et al. The CASK gene harbored in a deletion detected by array-CGH as a potential candidate for a gene causative of X-linked dominant mental retardation. Am J Med Genet. 2008;146A:2145–2151. doi: 10.1002/ajmg.a.32433. [DOI] [PubMed] [Google Scholar]
- 108.Reyniers E, Van Bogaert P, Peeters N, Vits L, Pauly F, et al. A new neurological syndrome with mental retardation, choreoathetosis, and abnormal behavior maps to chromosome Xp11. Am J Hum Genet. 1999;65:1406–1412. doi: 10.1086/302638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Al-Mateen M, Philippart M, Shields WD. Rett syndrome. A commonly overlooked progressive encephalopathy in girls. Am J Dis Child. 1986;140:761–765. doi: 10.1001/archpedi.1986.02140220043029. [DOI] [PubMed] [Google Scholar]
- 110.Zhang X, Liu Q, Chen B, Guo C, Li J, et al. A locus for nonspecific X-linked mental retardation mapped to a 22.3 cM region of Xp11.3-q22.3. Am J Med Genet. 2004;129A:286–289. doi: 10.1002/ajmg.a.30121. [DOI] [PubMed] [Google Scholar]
- 111.Good CD, Lawrence K, Thomas NS, Price CJ, Ashburner J, et al. Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain. 2003;126:2431–2446. doi: 10.1093/brain/awg242. [DOI] [PubMed] [Google Scholar]
- 112.Nijhuis-van der Sanden MW, Eling PA, Otten BJ. A review of neuropsychological and motor studies in Turner Syndrome. Neurosci Biobehav Rev. 2003;27:329–338. doi: 10.1016/s0149-7634(03)00062-9. [DOI] [PubMed] [Google Scholar]
- 113.Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Dev Disabil Res Rev. 2009;15:270–278. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cresswell CS, Skuse DH. Autism in association with Turner syndrome: Genetic implications for male vulnerability to pervasive developmental disorders. Neurocase. 1999;5:511–518. [Google Scholar]
- 115.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, et al. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Groen W, Teluij M, Buitelaar J, Tendolkar I. Amygdala and hippocampus enlargement during adolescence in autism. J Am Acad Child Adolesc Psychiatry. 2010;49:552–560. doi: 10.1016/j.jaac.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 118.Ortiz-Mantilla S, Choe MS, Flax J, Grant PE, Benasich AA. Associations between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. Neuroimage. 2010;49:2791–2799. doi: 10.1016/j.neuroimage.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 119.Verhoeven JS, De Cock P, Lagae L, Sunaert S. Neuroimaging of autism. Neuroradiology. 2010;52:3–14. doi: 10.1007/s00234-009-0583-y. [DOI] [PubMed] [Google Scholar]
- 120.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 121.Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jensen LR, Lenzner S, Moser B, Freude K, Tzschach A, et al. X-linked mental retardation: a comprehensive molecular screen of 47 candidate genes from a 7.4 Mb interval in Xp11. Eur J Hum Genet. 2007;15:68–75. doi: 10.1038/sj.ejhg.5201714. [DOI] [PubMed] [Google Scholar]
- 123.Aziz A, Harrop SP, Bishop NE. Characterization of the deleted in autism 1 protein family: implications for studying cognitive disorders. PLoS One. In press. 2010. [DOI] [PMC free article] [PubMed]
- 124.Alvarado M, Bocian M, Walker AP. Interstitial deletion of the long arm of chromosome 3: case report, review, and definition of a phenotype. Am J Med Genet. 1987;27:781–786. doi: 10.1002/ajmg.1320270406. [DOI] [PubMed] [Google Scholar]
- 125.Ko WT, Lam WF, Lo FM, Chan WK, Lam TS. Wisconsin syndrome in a patient with interstitial deletion of the long arm of chromosome 3: further delineation of the phenotype. Am J Med Genet. 2003;120A:413–417. doi: 10.1002/ajmg.a.20149. [DOI] [PubMed] [Google Scholar]
- 126.Zweier C, Guth S, Schulte-Mattler U, Rauch A, Trautmann U. 9 Mb deletion including chromosome band 3q24 associated with unsuspicious facial gestalt, persistent ductus omphaloentericus, mild mental retardation and tic. Eur J Med Genet. 2005;48:360–362. doi: 10.1016/j.ejmg.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 127.Jedele KB. The overlapping spectrum of Rett and Angelman syndromes: a clinical review. Semin Pediatr Neurol. 2007;14:108–117. doi: 10.1016/j.spen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 128.Santos M, Temudo T, Kay T, Carrilho I, Medeira A, et al. Mutations in the MECP2 gene are not a major cause of Rett syndrome-like or related neurodevelopmental phenotype in male patients. J Child Neurol. 2009;24:49–55. doi: 10.1177/0883073808321043. [DOI] [PubMed] [Google Scholar]
- 129.Robin NH, Magnusson M, McDonald-McGinn D, Zackai EH, Spinner NB. De novo interstitial deletion of the long arm of chromosome 3: 46,XX, del(3) (q25.1q26.1). Clin Genet. 1993;44:335–337. doi: 10.1111/j.1399-0004.1993.tb03911.x. [DOI] [PubMed] [Google Scholar]
- 130.Slavotinek AM, Huson SM, Fitchett M. Interstitial deletion of band 3q25. J Med Genet. 1997;34:430–432. doi: 10.1136/jmg.34.5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yen KH, Ho CL, Lee C. The analysis of inconsistencies between cytogenetic annotations and sequence mapping by defining the imprecision zones of cytogenetic banding. Bioinformatics. 2009;25:845–852. doi: 10.1093/bioinformatics/btn649. [DOI] [PubMed] [Google Scholar]
- 132.Pickett J, London E. The neuropathology of autism: a review. J Neuropathol Exp Neurol. 2005;64:925–935. doi: 10.1097/01.jnen.0000186921.42592.6c. [DOI] [PubMed] [Google Scholar]
- 133.Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 135.Sbacchi S, Acquadro F, Calò I, Calì F, Romano V. Functional annotation of genes overlapping copy number variants in autistic patients: focus on axon pathfinding. Curr Genomics. 2010;11:136–145. doi: 10.2174/138920210790886880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 137.McDougle CJ, Erickson CA, Stigler KA, Posey DJ. Neurochemistry in the pathophysiology of autism. J Clin Psychiatry. 2005;66:S9–18. [PubMed] [Google Scholar]
- 138.Castellani ML, Conti CM, Kempuraj DJ, Salini V, Vecchiet J, et al. Autism and immunity: revisited study. Int J Immunopathol Pharmacol. 2009;22:15–19. doi: 10.1177/039463200902200103. [DOI] [PubMed] [Google Scholar]
- 139.Coury D, Jones NE, Klatka K, Winklosky B, Perrin JM. Healthcare for children with autism: the Autism Treatment Network. Curr Opin Pediatr. 2009;21:828–832. doi: 10.1097/MOP.0b013e328331eaaa. [DOI] [PubMed] [Google Scholar]
- 140.Tang BL. Emerging aspects of membrane traffic in neuronal dendrite growth. Biochim Biophys Acta. 2008;1783:169–176. doi: 10.1016/j.bbamcr.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 141.Hanus C, Ehlers MD. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic. 2008;9:1437–1445. doi: 10.1111/j.1600-0854.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Horton AC, Ehlers MD. Secretory trafficking in neuronal dendrites. Nat Cell Biol. 2004;6:585–591. doi: 10.1038/ncb0704-585. [DOI] [PubMed] [Google Scholar]
- 143.Nelson KB, Grether JK, Croen LA, Dambrosia JM, Dickens BF, et al. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann Neurol. 2001;49:597–606. [PubMed] [Google Scholar]
- 144.Miyazaki K, Narita N, Sakuta R, Miyahara T, Naruse H, et al. Serum neurotrophin concentrations in autism and mental retardation: a pilot study. Brain Dev. 2004;26:292–295. doi: 10.1016/S0387-7604(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 145.Sadakata T, Kakegawa W, Mizoguchi A, Washida M, Katoh-Semba R, et al. Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci. 2007;27:2472–2482. doi: 10.1523/JNEUROSCI.2279-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chapleau CA, Larimore JL, Theibert A, Pozzo-Miller L. Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. J Neurodev Disord. 2009;1:185–196. doi: 10.1007/s11689-009-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sadakata T, Furuichi T. Developmentally regulated Ca2+-dependent activator protein for secretion 2 (CAPS2) is involved in BDNF secretion and is associated with autism susceptibility. Cerebellum. 2009;8:312–322. doi: 10.1007/s12311-009-0097-5. [DOI] [PubMed] [Google Scholar]
- 148.Sadakata T, Furuichi T. Ca(2+)-dependent activator protein for secretion 2 and autistic-like phenotypes. Neurosci Res. 2010;67:197–202. doi: 10.1016/j.neures.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 149.Castermans D, Volders K, Crepel A, Backx L, De Vos R, et al. SCAMP5, NBEA and AMISYN: three candidate genes for autism involved in secretion of large dense-core vesicles. Hum Mol Genet. 2010;19:1368–1378. doi: 10.1093/hmg/ddq013. [DOI] [PubMed] [Google Scholar]
- 150.Piven J, Tsai GC, Nehme E, Coyle JT, Chase GA, et al. Platelet serotonin, a possible marker for familial autism. J Autism Dev Disord. 1991;21:51–59. doi: 10.1007/BF02206997. [DOI] [PubMed] [Google Scholar]
- 151.Cook EH, Leventhal BL. The serotonin system in autism. Curr Opin Pediatr. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 152.Castagnola M, Messana I, Inzitari R, Fanali C, Cabras T, et al. Hypo-phosphorylation of salivary peptidome as a clue to the molecular pathogenesis of autism spectrum disorders. J Proteome Res. 2008;7:5327–5332. doi: 10.1021/pr8004088. [DOI] [PubMed] [Google Scholar]
- 153.Hendershot LM, Ting J, Lee AS. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol Cell Biol. 1988;8:4250–4256. doi: 10.1128/mcb.8.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bishop L, Dimaline R, Blackmore C, Deavall D, Dockray GJ, et al. Modulation of the cleavage of the gastrin precursor by prohormone phosphorylation. Gastroenterology. 1998;115:1154–1162. doi: 10.1016/s0016-5085(98)70086-1. [DOI] [PubMed] [Google Scholar]
- 155.Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- 156.Lee SN, Hwang JR, Lindberg I. Neuroendocrine protein 7B2 can be inactivated by phosphorylation within the secretory pathway. J Biol Chem. 2006;281:3312–3320. doi: 10.1074/jbc.M506635200. [DOI] [PubMed] [Google Scholar]
- 157.McMahon HE, Sharma S, Shimasaki S. Phosphorylation of bone morphogenetic protein-15 and growth and differentiation factor-9 plays a critical role in determining agonistic or antagonistic functions. Endocrinology. 2008;149:812–817. doi: 10.1210/en.2007-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Waring R, Phoenix J. TPST-assay for diagnosis of autism and related disorders. SHS International Ltd: International patent WO/2001/077681. 2001.
- 159.Waring R. Sulphation and autism – what are the links? 2001. Available: www.autismfile.com/papers/Rosemary_Waring_Sulphation.asp. Accessed April 2010.
- 160.Stone MJ, Chuang S, Hou X, Shoham M, Zhu JZ. Tyrosine sulfation: an increasingly recognised post-translational modification of secreted proteins. N Biotechnol. 2009;25:299–317. doi: 10.1016/j.nbt.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 161.Kehoe JW, Bertozzi CR. Tyrosine sulfation: a modulator of extracellular protein-protein interactions. Chem Biol. 2000;7:R57–61. doi: 10.1016/s1074-5521(00)00093-4. [DOI] [PubMed] [Google Scholar]
- 162.van der Zwaag B, Franke L, Poot M, Hochstenbach R, Spierenburg HA, et al. Gene-network analysis identifies susceptibility genes related to glycobiology in autism. PLoS One. 2009;4:e5324. doi: 10.1371/journal.pone.0005324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nilsson T, Au CE, Bergeron JJ. Sorting out glycosylation enzymes in the Golgi apparatus. FEBS Lett. 2009;583:3764–3769. doi: 10.1016/j.febslet.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 164.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins, DG The CLUSTALX windows interface: flexible strategies for multiple sequence alignments aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, et al. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–552. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
- 166.Hofmann K, Stoffel W. TMbase - A database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler. 1993;374:166–168. [Google Scholar]
- 167.Milpetz F, Argos P, Persson B. TMAP: a new email and WWW service for membrane-protein structural predictions. Trends Biochem Sci. 1995;20:204–205. doi: 10.1016/s0968-0004(00)89009-x. [DOI] [PubMed] [Google Scholar]
- 168.Tusnády GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17:849–850. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- 169.Kanehisa M. Linking databases and organisms: GenomeNet resources in Japan. Trends Biochem Sci. 1997;22:442–444. doi: 10.1016/s0968-0004(97)01130-4. [DOI] [PubMed] [Google Scholar]
- 170.Horton P, Nakai K. Better prediction of protein cellular localization sites with the k nearest neighbors classifier. Proc Int Conf Intell Syst Mol Biol. 1997;5:147–152. [PubMed] [Google Scholar]
- 171.Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, et al. CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 2009;37:D205–210. doi: 10.1093/nar/gkn845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gould CM, Diella F, Via A, Puntervoll P, Gemünd C, et al. ELM: the status of the 2010 eukaryotic linear motif resource. Nucleic Acids Res. 2010;38:D167–180. doi: 10.1093/nar/gkp1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neural network prediction of signal peptides in human DIA1 and DIA1R. The amino acid sequence of (A) human DIA1 or (B) human DIA1R, was evaluated for amino-terminal signal peptides using the neural network (NN) algorithm of SignalP v3.0 [72]. The C-score (cleavage score for each amino acid) is indicated in red, the S-score (signal peptide score) is indicated in green, and the Y-score (derived from the C-score and S-score, and can give a better indication of the cleavage site) is indicated in blue. The significance cut-off value for all probability scores is 0.5, and is indicated by a pink dotted line. Standard single-letter amino acid abbreviations and numbering are provided below the graph.
(0.02 MB PDF)
BioGPS microarray expression data for DIA1. Graphical presentation of expression data for DIA1 in a variety of normal, and cancerous human tissues and cells. Expression values were obtained from an Affymetrix U133A microarray and relate to fluorescence intensity. Multiple probes were used for each transcript on the microarray and these intensity values have been normalized, background-subtracted, and summarized using the data-processing algorithm GCRMA (GeneChip Robust Multi-array Average). The identifier of the Affymetrix probe set used is indicated above the graph. Data were obtained from the Gene Atlas database of human genes [76] at the BioGPS gene portal server [77].
(0.02 MB PDF)
BioGPS microarray expression data for DIA1R. Graphical presentation of expression data for DIA1R in a variety of normal, and cancerous human tissues and cells. Expression values were obtained from an Affymetrix U133A microarray and relate to fluorescence intensity. Multiple probes were used for each transcript on the microarray and these intensity values have been normalized, background-subtracted, and summarized using the GCRMA (GeneChip Robust Multi-array Average) data-processing algorithm. The identifier of the Affymetrix probe set used is indicated above the graph. Data were obtained from the Gene Atlas database of human genes [76] at the BioGPS gene portal server [77].
(0.03 MB PDF)