Abstract
The essential trace element selenium has long been considered to exhibit anti-diabetic and insulin-mimetic properties, but recent epidemiological studies indicated supranutritional selenium intake and high plasma selenium levels as possible risk factors for development of type 2 diabetes, pointing to adverse effects of selenium on carbohydrate metabolism in humans. However, increased plasma selenium levels might be both a consequence and a cause of diabetes. We summarize current evidence for an interference of selenium compounds with insulin-regulated molecular pathways, most notably the phosphoinositide-3-kinase/protein kinase B signaling cascade, which may underlie some of the pro- and anti-diabetic actions of selenium. Furthermore, we discuss reports of hyperinsulinemia, hyperglycemia and insulin resistance in mice overexpressing the selenoenzyme glutathione peroxidase 1. The peroxisomal proliferator-activated receptor gamma coactivator 1α represents a key regulator for biosynthesis of the physiological selenium transporter, selenoprotein P, as well as for hepatic gluconeogenesis. As proliferator-activated receptor gamma coactivator 1α has been shown to be up-regulated in livers of diabetic animals and to promote insulin resistance, we hypothesize that dysregulated pathways in carbohydrate metabolism and a disturbance of selenium homeostasis are linked via proliferator-activated receptor gamma coactivator 1α.
Keywords: selenoprotein, glutathione peroxidase, hyperglycemia, insulin, PGC-1α, Akt
Introduction
The essential trace element selenium is believed to exert beneficial influence on human health, mainly based on the antioxidant capacity of selenoproteins such as glutathione peroxidases (GPx) and thioredoxin reductases (TrxR) containing the 21st proteinogenic amino acid, selenocysteine, in their active center.(1) Potential selenium-mediated health benefits include prevention of cardiovascular and neurodegenerative diseases, delay of aging, functioning of the immune system, and prevention of certain forms of cancer.(1–6) A wide range of dietary selenium sources comprise cereals, garlic, brazil nuts, meat and fish. Even though overt selenium deficiency is observed rarely, consumers in industrialised countries habitually ingest high amounts of selenium-enriched dietary supplements. However, it has long been known that the therapeutic window of selenium is narrow, and adverse health effects may occur due to supranutritional selenium intake even below the levels required for intoxication.(7–9)
In this regard, an ongoing discussion on the safety of dietary selenium supplementation has arisen from a coincidental and unexpected finding of the Nutritional Prevention of Cancer (NPC) trial: participants of the trial, who received a daily dose of 200 µg selenium over 12 years, were more likely to develop type 2 diabetes mellitus than those assigned to placebo.(10) Moreover, the diabetes risk of the participants increased with higher baseline plasma selenium levels.(10) Since then several epidemiological studies have reported that high plasma selenium levels were associated with increased prevalence of type 2 diabetes as well as hyperglycemia and enhanced plasma levels of total and low-density lipoprotein (LDL) cholesterol and triacylglycerols in the selenium-replete US-American population.(11–15) On the other hand, the outcome of similar recent studies in Europe was rather ambiguous, ranging from adverse to slightly beneficial effects of selenium on carbohydrate and/or lipid metabolism.(16–18) These divergent results might be explained by a generally lower selenium intake in most European countries in comparison to the USA, but differences in lifestyle and genotype between US-American and European populations as well as varied dietary selenium sources may also contribute.(9,19) Given the rising numbers of patients suffering from morbid obesity and diabetes as well as increasing world-wide trends in dietary selenium supplementation, the molecular mechanisms underlying a potential adverse effect of selenium compounds on carbohydrate and/or lipid metabolism need to be addressed. An important unresolved issue, which cannot be answered by epidemiological studies, is the cause-and-effect-relationship of those associations: does selenium oversupply contribute to development of type 2 diabetes by disturbing insulin signalling and/or secretion, or conversely, may a dysregulated carbohydrate metabolism influence selenium homeostasis? Experimental evidence on these issues is available, and to make the picture more complex and somewhat paradoxical, selenium may act as an insulin-mimetic under certain circumstances.
Anti-Diabetic and Insulin-Mimetic Actions of Selenium
Diabetes mellitus is affecting over 170 million people world-wide with more than 90% of the patients suffering from type 2 diabetes.(20) The onset of type 2 diabetes is hallmarked by resistance of liver, skeletal muscle and fat tissue to insulin, thereby causing dyslipidemia, hyperglycemia and a reactive increase in insulin secretion by pancreatic beta cells for compensation of the poor insulin response of major target tissues.(21) Binding of insulin to its receptor initiates the intracellular insulin signalling cascade, whose components have been reviewed comprehensively elsewhere.(21–23) Among them, the insulin receptor substrate (IRS)-2, the protein tyrosine phosphatase (PTP)-1B and the protein kinase B (serine/threonine kinase Akt) as well as the forkhead box class (Fox) O1a transcription factor and its coactivator peroxisomal proliferator-activated receptor gamma coactivator (PGC)-1α have received particular attention in diabetes research. At present, it is evident from in vitro and in vivo studies that dysregulated expression, localisation and/or activity of one or more of those proteins may result in insulin resistance.(24–28)
Besides selenium, a number of metal ions (e.g., vanadium, copper, zinc and cadmium) are capable of eliciting insulin-mimetic effects by activation of Akt and other kinases of the insulin signaling cascade such as p70 S6 kinase. The insulin-like phosphorylation of Akt upon exposure of cells to micromolar (10 µM, 100 µM) doses of heavy metal ions at oxidation number +II (Cu2+, Zn2+, Cd2+) is interpreted primarily as a stress response, because signaling through phosphoinositide-3-kinase (PI3K) and Akt also promotes anti-apoptotic and cytoprotective pathways.(29) With regard to regulation of carbohydrate metabolism, insulin-mimetic properties of selenium compounds at oxidation numbers +IV (sodium selenite) and +VI (sodium selenate) have been reported in close resemblance to such effects of vanadium at oxidation number +IV (vanadyl sulphate).(30–32) Early studies have been performed in isolated rat adipocytes, and found that sodium selenate stimulated glucose uptake through translocation of glucose transporters to the plasma membrane and activated serine/threonine kinases including the p70 S6 kinase.(31,33) As these insulin-like actions were observed only at the very high dose of 1 mM sodium selenate, an anti-diabetic application in humans appears to be difficult or impossible. The results of animal studies are somewhat conflicting: A cautious view is corroborated by a study in genetically obese Zucker rats, whose glucose tolerance was transiently improved during acute selenate exposure, rapidly followed by progressive development of hyperglycemia indicating toxicity of high selenate doses.(34) On the other hand, whole-body insulin sensitivity was improved in type 2 diabetic db/db mice by dietary supplementation with supranutritional sodium selenate doses.(35) Moreover, sodium selenate effectually improved glucose homeostasis in streptozotocin-treated rodents.(36,37) Streptozotocin causes necrosis of pancreatic beta cells through DNA alkylation and, to a minor extent, generation of nitric oxide and reactive oxygen species (ROS), resulting in insulin deficiency and hyperglycemia.(38) The anti-diabetic effects of selenate in streptozotocin-treated rats were attributed to partial reversal of abnormal expression and activity of glycolytic and gluconeogenic liver enzymes, whereas plasma insulin levels did not increase upon selenate administration.(37)
Similar to heavy metal ions, sodium selenite at low micromolar doses induced a cytoprotective response in vitro, thereby counteracting apoptotic cell death following serum withdrawal or exposure to hydrogen peroxide (H2O2); survival of both Huh7 hepatoma cells and HT1080 fibrosarcoma cells was mediated through selenite-induced Akt activation.(39,40) An insulin-like action of selenite on carbohydrate metabolism was observed in the isolated perfused rat liver, where glucagon-stimulated glycogen breakdown was inhibited by infusion of 10 µM sodium selenite.(32) Consistent with the narrow therapeutic range of selenium, higher doses of selenite (500 µM) severely impaired the metabolic function of the liver, causing degeneration and necrosis of periportal hepatocytes.(32) In vivo, oral selenite administration failed to improve insulin sensitivity in type 2 diabetic db/db mice, presumably due to formation of different intermediary selenium metabolites in peripheral organs compared to sodium selenate.(35)
Adverse Effects of Selenium on Insulin Secretion and Signalling
An anti-diabetic impact of dietary selenium supplementation would be expected, given both the long track record of selenium as insulin-mimetic micronutrient and its antioxidant capacity as constituent of ROS-detoxifying selenoenzymes, suggesting a protective role against oxidative stress-related chronic complications in the progression of diabetes.(1,19,41) Contrarily to those expectations, recent epidemiological and intervention studies revealed a surprising association between high plasma selenium levels and type 2 diabetes, hyperglycemia and dyslipidemia.(10–16) The clue to answer the pivotal question of whether and how selenium exerts adverse effects on insulin-regulated metabolic pathways in humans may lie in the apparent “redox paradox” of insulin signalling, a concept that refers to facilitated insulin action by insulin-stimulated reactive oxygen species.(42) Upon binding to its receptor at the plasma membrane of adipocytes, insulin elicits a transient burst of ROS (superoxide and H2O2).(43) Insulin activates the NAD(P)H oxidase (Nox) 4 to generate superoxide, which is subsequently converted to H2O2.(44) These insulin-stimulated small amounts of H2O2 serve as second messengers, which attenuate the activity of phosphatases with redox-sensitive cysteine residues and thereby enhance the phosphorylation of components downstream in the insulin signalling cascade.(42,45) Thus, high supranutritional doses of antioxidants may have the capability to impair insulin sensitivity, as it has recently been shown in humans administered a combination of vitamin C (1,000 mg/day) and vitamin E (400 IU/day).(46)
Inorganic and organic selenium compounds have been reported to induce expression and activity of several antioxidant selenoproteins; the most pronounced stimulation was obtained for the selenoenzyme cytosolic GPx1,(47–49) which degrades H2O2 and other hydroperoxides.(50) A high GPx1 activity has been hypothesized to interfere with insulin signaling. Indeed, pregnancy-associated mild insulin resistance was shown to be accompanied by increased erythrocyte GPx activity in humans,(51) and transgenic mice overexpressing GPx1 developed at older age a type 2 diabetes-like phenotype characterised by insulin resistance, hyperglycemia, hyperinsulinemia and obesity.(52) GPx1 overexpression affected both pancreatic insulin production and insulin sensitivity of target cells; insulin resistance of liver and/or skeletal muscle was obvious from impaired insulin receptor and Akt phosphorylation.(52) Intriguingly, obesity together with insulin resistance and hyperglycemia could be prevented in the GPx1-overexpressing mice by dietary restriction, whereas the chronic hyperinsulinemia persisted, even at dietary selenium deficiency.(53,54) The authors conclude that dysregulation of pancreatic insulin biosynthesis and secretion is the primary outcome of transgenic GPx1 overproduction in their experimental model.(54) Insulin-producing pancreatic beta cells are among the worst-endowed cells in terms of intrinsic enzymatic antioxidants: expression and activity of the H2O2-degrading enzymes catalase and GPx1 in beta cells reach only 1% of the values in hepatocytes.(55) For this reason, beta cells are very susceptible to damage caused by hyperglycemia or proinflammatory cytokines, and overexpression of antioxidant enzymes including GPx1 has been applied to protect insulinoma cell lines and pancreatic islets from oxidative injury.(56,57) On the other hand, development of hyperinsulinemia in GPx1 overexpressing mice points to detrimental effects of high GPx1 activity on beta cell function in vivo, impairing the tight control of insulin release.(52–54) An adverse effect of high GPx1 activity on components of the insulin signalling cascade has been further substantiated by an in vitro study in MCF-7 human breast cancer cells, where GPx1 overexpression was associated with decreased phosphorylation of p70 S6 kinase and Akt.(58) An alternative approach to increase GPx1 in a more physiological manner was done by dietary supplementation of rats with sodium selenate: the higher GPx1 activity in livers of selenium-supplemented rats was associated with increased activity of protein tyrosine phosphatase 1B (PTP-1B),(59) which antagonizes insulin-induced signaling by dephosphorylation of the insulin receptor (IR) and the IRS-1.(60)
Conversely and in good agreement with the experimental models of GPx1 overexpression, knock-out of GPx1 in mice resulted in improved insulin sensitivity due to increased ROS generation, causing oxidation (inactivation) of the dual specificity protein phosphatase PTEN.(61) PTEN dephosphorylates the product of PI3K, phosphatidylinositol-3,4,5-triphosphate (PIP3), thus counteracting insulin-induced PI3K/Akt signalling.(62) In line with elevated PI3K/Akt signaling, insulin-induced glucose uptake was increased in skeletal muscles of GPx−/− mice, and most compelling, knock-out of GPx1 protected the rodents from insulin resistance provoked by high-fat diet.(61) These results are supported by observations of increased site-specific phosphorylation of both Akt and p70 S6 kinase in transgenic mice with an overall decreased biosynthesis of selenoproteins, caused by a mutant form of selenocysteine transfer RNA (tRNA [Ser] Sec).(63)
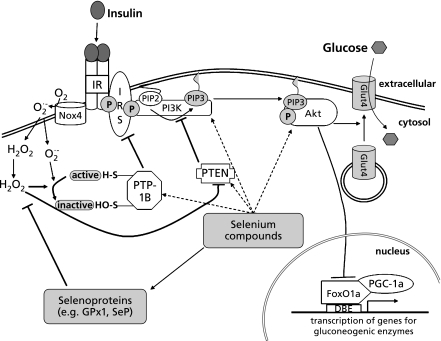
Despite the compelling evidence from transgenic animal models of GPx1 overexpression and knock-down, results from intervention studies with selenium supplements in several human populations argue against the idea that glutathione peroxidases are the only mediators of adverse effects of high dietary selenium intake under physiological conditions: plasma GPx activity in humans has been found to be saturated at selenium dietary supplement doses and total plasma selenium levels well below the values associated with increased risk for type 2 diabetes.(64–67) Human plasma contains selenium in form of the selenoenzyme GPx3, a low-molecular-weight selenium pool and most notably the selenium transporter selenoprotein P (SeP), which accounts for 50–60% of circulating selenium.(68) Compared to GPx activity, both SeP and the remaining non-selenoprotein plasma selenium pool require a higher dietary selenium intake for their optimization and saturation.(64–67) It is tempting to speculate that SeP and/or low-molecular-weight selenium compounds may affect insulin-induced signalling pathways related to carbohydrate and lipid metabolism. Fig. 1 schematically summarizes current experimental evidence and hypotheses concerning an influence of selenium on the insulin signalling cascade.
Fig. 1.
Scheme depicting a potential influence of selenium on components of the insulin signaling cascade. Selenoproteins and low molecular weight selenium compounds may interfere at different stages with insulin-induced signal transduction, eventually leading to dysregulation of carbohydrate metabolism. Please see text for details and explanation of the abbreviations.
PGC-1α: a Molecular Switch Linking Selenium and Carbohydrate Metabolism
The epidemiological association between high plasma selenium levels and hyperglycemia might also be explained by a disturbance of selenium homeostasis as side-effect of a dysregulated carbohydrate metabolism. The major fraction of total selenium in human plasma is present as SeP, which is mainly secreted by the liver and supplies peripheral tissues with selenium.(68,69) SeP represents a suitable biomarker for selenium status, because its plasma concentration increases in response to different dietary forms and to a wide range of doses in selenium supplementation studies.(64–67) This obvious importance of SeP for selenium homeostasis prompted us to investigate the regulation of hepatic SeP production by factors related to carbohydrate metabolism.
In the human SeP promoter, we identified a motif consisting of a binding site for the FoxO1a transcription factor, located in close proximity to a binding site for hepatocyte nuclear factor 4α (HNF-4α).(70,71) This motif is conserved in the SeP promoters of humans, rats and mice, and it mediates high-level expression of SeP in the liver as well as the hormonal regulation of hepatic SeP transcription. Both transcription factors are co-activated by the PGC-1α, which acts as “molecular switch” in response to hormones such as insulin, glucagon and glucocorticoids,(27,72,73) well-known for their control of hepatic glucose production and blood glucose levels. Insulin inhibited SeP transcription via the PI3K/Akt/FoxO1a axis,(70) whereas the PGC-1α-inducing glucocorticoid dexamethasone strongly enhanced SeP mRNA levels and protein secretion in cultured rat hepatocytes.(71) Oral administration of dexamethasone has been reported to give rise to a redistribution of selenium in mice, causing a decrease of liver GPx in favor of elevated plasma selenium levels;(74) these earlier results can be explained by enhanced hepatic secretion of SeP induced by dexamethasone treatment.
The complex between FoxO1a and its coactivator PGC-1α is of crucial importance for transcriptional regulation of the gluconeogenic enzymes glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEP-CK).(27,72) Our observation that the selenium transporter SeP is regulated virtually like a gluconeogenic enzyme provides a rationale for the hypothesized link between selenium and carbohydrate metabolism.(71) Moreover, PGC-1α is elevated in livers of animal diabetes models,(72) and has been demonstrated to promote insulin resistance.(28) A vicious circle is observed when diabetes is not treated accurately: high glucose up-regulates expression of PGC-1α and gluconeogenic enzymes in the liver, resulting in overproduction of hepatic glucose and increased hyperglycemia.(72,75) We cultivated rat hepatocytes in the presence of high glucose (25 mM), and found an increase in SeP production paralleled by elevated PGC-1α mRNA levels.(76)
Thus, elevated hepatic PGC-1α may trigger not only hyperglycemia, but also a disturbance in selenium homeostasis. The anti-hyperglycemic drug metformin is widely described for treatment of type 2 diabetes, because it suppresses hepatic glucose production and improves peripheral insulin sensitivity.(77,78) In parallel with gluconeogenesis, metformin attenuated hepatic biosynthesis and secretion of SeP in vitro,(76) which might decrease selenium bioavailability in extrahepatic tissues and thereby impair expression and activity of selenoenzymes in vivo. This idea is supported by a study of Pavlovic et al.: A two-week metformin treatment resulted in decreased GPx activity in erythrocytes of obese patients with type 2 diabetes.(79)
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany (STE 1782/2-1, SFB 575/B4). H. Sies is a Fellow of the National Foundation for Cancer Research (NFCR), Bethesda, MD.
Abbreviations
- GPx
glutathione peroxidase
- TrxR
thioredoxin reductase
- NPC
Nutritional Prevention of Cancer
- LDL
low-density lipoprotein
- IRS
insulin receptor substrate
- PTP
protein tyrosine phosphatise
- FoxO
forkhead box class O
- PGC
peroxisomal proliferator-activated receptor gamma coactivator
- PI3K
phosphoinositide-3-kinase
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- NOX
NAD(P)H oxidase
- IR
insulin receptor
- PIP3
phosphatidylinositol-3,4,5-triphosphate
- SeP
selenoprotein P
- HNF-4α
hepatocyte nuclear factor 4α
- G6Pase
glucose-6-phosphatase
- PEP-CK
phosphoenolpyruvate carboxykinase
References
- 1.Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta. 2009;1790:1478–1485. doi: 10.1016/j.bbagen.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P. Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet. 1982;2:175–179. doi: 10.1016/s0140-6736(82)91028-5. [DOI] [PubMed] [Google Scholar]
- 3.Clark LC, Combs GF, Jr., Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 4.Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133:S1463–S1467. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Berry MJ. Selenium and selenoproteins in the brain and brain diseases. J Neurochem. 2003;86:1–12. doi: 10.1046/j.1471-4159.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- 6.Brenneisen P, Steinbrenner H, Sies H. Selenium, oxidative stress, and health aspects. Mol Aspects Med. 2005;26:256–267. doi: 10.1016/j.mam.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Yang G, Yin S, Zhou R, et al. Studies of safe maximal daily dietary Se-intake in a seleniferous area in China. Part II: Relation between Se-intake and the manifestation of clinical signs and certain biochemical alterations in blood and urine. J Trace Elem Electrolytes Health Dis. 1989;3:123–130. [PubMed] [Google Scholar]
- 8.Whanger P, Vendeland S, Park YC, Xia Y. Metabolism of subtoxic levels of selenium in animals and humans. Ann Clin Lab Sci. 1996;26:99–113. [PubMed] [Google Scholar]
- 9.Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008;100:254–268. doi: 10.1017/S0007114508939830. [DOI] [PubMed] [Google Scholar]
- 10.Stranges S, Marshall JR, Natarajan R, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 11.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in US adults. Diabetes Care. 2007;30:829–834. doi: 10.2337/dc06-1726. [DOI] [PubMed] [Google Scholar]
- 12.Bleys J, Navas-Acien A, Stranges S, Menke A, Miller ER, 3rd, Guallar E. Serum selenium and serum lipids in US adults. Am J Clin Nutr. 2008;88:416–423. doi: 10.1093/ajcn/88.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and diabetes in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect. 2009;117:1409–1413. doi: 10.1289/ehp.0900704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laclaustra M, Stranges S, Navas-Acien A, Ordovas JM, Guallar E. Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis. 2010;210:643–648. doi: 10.1016/j.atherosclerosis.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stranges S, Laclaustra M, Ji C, Cappuccio FP, et al. Higher selenium status is associated with adverse blood lipid profile in British adults. J Nutr. 2010;140:81–87. doi: 10.3945/jn.109.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbaraly TN, Arnaud J, Rayman MP, et al. Plasma selenium and risk of dysglycemia in an elderly French population: results from the prospective Epidemiology of Vascular Ageing Study. Nutr Metab (Lond) 2010;7:21. doi: 10.1186/1743-7075-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornhauser C, Garcia-Ramirez JR, Wrobel K, Pérez-Luque EL, Garay-Sevilla ME, Wrobel K. Serum selenium and glutathione peroxidase concentrations in type 2 diabetes mellitus patients. Prim Care Diabetes. 2008;2:81–85. doi: 10.1016/j.pcd.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Mueller AS, Mueller K, Wolf NM, Pallauf J. Selenium and diabetes: an enigma? Free Radic Res. 2009;43:1029–1059. doi: 10.1080/10715760903196925. [DOI] [PubMed] [Google Scholar]
- 20.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 21.Schinner S, Scherbaum WA, Bornstein SR, Barthel A. Molecular mechanisms of insulin resistance. Diabet Med. 2005;22:674–682. doi: 10.1111/j.1464-5491.2005.01566.x. [DOI] [PubMed] [Google Scholar]
- 22.Barthel A, Klotz LO. Phosphoinositide 3-kinase signaling in the cellular response to oxidative stress. Biol Chem. 2005;386:207–216. doi: 10.1515/BC.2005.026. [DOI] [PubMed] [Google Scholar]
- 23.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 24.Previs SF, Withers DJ, Ren JM, White MF, Shulman GI. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J Biol Chem. 2000;275:38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- 25.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 26.Gum RJ, Gaede LL, Koterski SL, et al. Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes. 2003;52:21–28. doi: 10.2337/diabetes.52.1.21. [DOI] [PubMed] [Google Scholar]
- 27.Puigserver P, Rhee J, Donovan J, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 28.Koo SH, Satoh H, Herzig S, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 29.Barthel A, Ostrakhovitch EA, Walter PL, Kampkötter A, Klotz LO. Stimulation of phosphoinositide 3-kinase/Akt signaling by copper and zinc ions: mechanisms and consequences. Arch Biochem Biophys. 2007;463:175–182. doi: 10.1016/j.abb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Shechter Y, Karlish SJ. Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl (IV) ions. Nature. 1980;284:556–558. doi: 10.1038/284556a0. [DOI] [PubMed] [Google Scholar]
- 31.Ezaki O. The insulin-like effects of selenate in rat adipocytes. J Biol Chem. 1990;265:1124–1128. [PubMed] [Google Scholar]
- 32.Roden M, Prskavec M, Fürnsinn C, et al. Metabolic effect of sodium selenite: insulin-like inhibition of glucagon-stimulated glycogenolysis in the isolated perfused rat liver. Hepatology. 1995;22:169–174. [PubMed] [Google Scholar]
- 33.Hei YJ, Farahbakhshian S, Chen X, Battell ML, McNeill JH. Stimulation of MAP kinase and S6 kinase by vanadium and selenium in rat adipocytes. Mol Cell Biochem. 1998;178:367–375. doi: 10.1023/a:1006819906820. [DOI] [PubMed] [Google Scholar]
- 34.Fürnsinn C, Leitner G, Roden M, Osterode W, Waldhäusl W. Improved glucose tolerance by acute vanadate but not by selenate exposure in genetically obese rats (fa/fa) Int J Obes Relat Metab Disord. 1995;19:458–463. [PubMed] [Google Scholar]
- 35.Mueller AS, Pallauf J. Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice. J Nutr Biochem. 2006;17:548–560. doi: 10.1016/j.jnutbio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 36.McNeill JH, Delgatty HL, Battell ML. Insulinlike effects of sodium selenate in streptozocin-induced diabetic rats. Diabetes. 1991;40:1675–1678. doi: 10.2337/diab.40.12.1675. [DOI] [PubMed] [Google Scholar]
- 37.Becker DJ, Reul B, Ozcelikay AT, Buchet JP, Henquin JC, Brichard SM. Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia. 1996;39:3–11. doi: 10.1007/BF00400407. [DOI] [PubMed] [Google Scholar]
- 38.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 39.Yoon SO, Kim MM, Park SJ, Kim D, Chung J, Chung AS. Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of PI3-K/Akt pathways. FASEB J. 2002;16:111–113. doi: 10.1096/fj.01-0398fje. [DOI] [PubMed] [Google Scholar]
- 40.Lee YC, Tang YC, Chen YH, Wong CM, Tsou AP. Selenite-induced survival of HuH7 hepatoma cells involves activation of focal adhesion kinase-phosphatidylinositol 3-kinase-Akt pathway and Rac1. J Biol Chem. 2003;278:39615–39624. doi: 10.1074/jbc.M304095200. [DOI] [PubMed] [Google Scholar]
- 41.Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.May JM, de Haën C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979;254:2214–2220. [PubMed] [Google Scholar]
- 44.Mahadev K, Motoshima H, Wu X, et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits proteintyrosine phosphatase 1B in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 46.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saito Y, Yoshida Y, Akazawa T, Takahashi K, Niki E. Cell death caused by selenium deficiency and protective effect of antioxidants. J Biol Chem. 2003;278:39428–39434. doi: 10.1074/jbc.M305542200. [DOI] [PubMed] [Google Scholar]
- 48.Steinbrenner H, Alili L, Bilgic E, Sies H, Brenneisen P. Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic Biol Med. 2006;40:1513–1523. doi: 10.1016/j.freeradbiomed.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 49.Steinbrenner H, Bilgic E, Alili L, Sies H, Brenneisen P. Selenoprotein P protects endothelial cells from oxidative damage by stimulation of glutathione peroxidase expression and activity. Free Radic Res. 2006;40:936–943. doi: 10.1080/10715760600806248. [DOI] [PubMed] [Google Scholar]
- 50.Brigelius-Flohé R. Tissue–specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Scholl TO, Leskiw MJ, Donaldson MR, Stein TP. Association of glutathione peroxidase activity with insulin resistance and dietary fat intake during normal pregnancy. J Clin Endocrinol Metab. 2003;88:5963–5968. doi: 10.1210/jc.2003-030544. [DOI] [PubMed] [Google Scholar]
- 52.McClung JP, Roneker CA, Mu W, et al. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 54.Pepper MP, Vatamaniuk MZ, Yan X, Roneker CA, Lei X. Impacts of Dietary Selenium Deficiency on Metabolic Phenotypes of Diet-Restricted GPX1-Overexpressing Mice. Antioxid. Redox Signal. 2010 doi: 10.1089/ars.2010.3295. DOI:10.1089/ars.2010.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 56.Lortz S, Tiedge M, Nachtwey T, Karlsen AE, Nerup J, Lenzen S. Protection of insulin-producing RINm5F cells against cytokine-mediated toxicity through overexpression of antioxidant enzymes. Diabetes. 2000;49:1123–1130. doi: 10.2337/diabetes.49.7.1123. [DOI] [PubMed] [Google Scholar]
- 57.Mysore TB, Shinkel TA, Collins J, et al. Overexpression of glutathione peroxidase with two isoforms of superoxide dismutase protects mouse islets from oxidative injury and improves islet graft function. Diabetes. 2005;54:2109–2116. doi: 10.2337/diabetes.54.7.2109. [DOI] [PubMed] [Google Scholar]
- 58.Nasr MA, Fedele MJ, Esser K, Diamond AM. GPx-1 modulates Akt and P70S6K phosphorylation and Gadd45 levels in MCF-7 cells. Free Radic Biol Med. 2004;37:187–195. doi: 10.1016/j.freeradbiomed.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 59.Mueller AS, Klomann SD, Wolf NM, et al. Redox regulation of protein tyrosine phosphatase 1B by manipulation of dietary selenium affects the triglyceride concentration in rat liver. J Nutr. 2008;138:2328–2336. doi: 10.3945/jn.108.089482. [DOI] [PubMed] [Google Scholar]
- 60.Cheng A, Dubé N, Gu F, Tremblay ML. Coordinated action of protein tyrosine phosphatases in insulin signal transduction. Eur J Biochem. 2002;269:1050–1059. doi: 10.1046/j.0014-2956.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 61.Loh K, Deng H, Fukushima A, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 63.Hornberger TA, McLoughlin TJ, Leszczynski JK, et al. Selenoprotein-deficient transgenic mice exhibit enhanced exercise-induced muscle growth. J Nutr. 2003;133:3091–3097. doi: 10.1093/jn/133.10.3091. [DOI] [PubMed] [Google Scholar]
- 64.Duffield AJ, Thomson CD, Hill KE, Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr. 1999;70:896–903. doi: 10.1093/ajcn/70.5.896. [DOI] [PubMed] [Google Scholar]
- 65.Xia Y, Hill KE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr. 2005;81:829–834. doi: 10.1093/ajcn/81.4.829. [DOI] [PubMed] [Google Scholar]
- 66.Hurst R, Armah CN, Dainty JR, et al. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91:923–931. doi: 10.3945/ajcn.2009.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia Y, Hill KE, Li P, et al. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr. 2010;92:525–531. doi: 10.3945/ajcn.2010.29642. DOI: 10.3945/ajcn.2010.29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burk RF, Early DS, Hill KE, Palmer IS, Boeglin ME. Plasma selenium in patients with cirrhosis. Hepatology. 1998;27:794–798. doi: 10.1002/hep.510270322. [DOI] [PubMed] [Google Scholar]
- 69.Schomburg L, Schweizer U, Holtmann B, Flohé L, Sendtner M, Köhrle J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J. 2003;370:397–402. doi: 10.1042/BJ20021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walter PL, Steinbrenner H, Barthel A, Klotz LO. Stimulation of selenoprotein P promoter activity in hepatoma cells by FoxO1a transcription factor. Biochem Biophys Res Commun. 2008;365:316–321. doi: 10.1016/j.bbrc.2007.10.171. [DOI] [PubMed] [Google Scholar]
- 71.Speckmann B, Walter PL, Alili L, et al. Selenoprotein P expression is controlled through interaction of the coactivator PGC-1alpha with FoxO1a and hepatocyte nuclear factor 4alpha transcription factors. Hepatology. 2008;48:1998–2006. doi: 10.1002/hep.22526. [DOI] [PubMed] [Google Scholar]
- 72.Yoon JC, Puigserver P, Chen G, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 73.Rhee J, Inoue Y, Yoon JC, et al. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci USA. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe C, Kim CY, Satoh H. Tissue-specific modification of selenium concentration by acute and chronic dexamethasone administration in mice. Br J Nutr. 1997;78:501–509. doi: 10.1079/bjn19970167. [DOI] [PubMed] [Google Scholar]
- 75.Massillon D, Barzilai N, Chen W, Hu M, Rossetti L. Glucose regulates in vivo glucose-6-phosphatase gene expression in the liver of diabetic rats. J Biol Chem. 1996;271:9871–9874. doi: 10.1074/jbc.271.17.9871. [DOI] [PubMed] [Google Scholar]
- 76.Speckmann B, Sies H, Steinbrenner H. Attenuation of hepatic expression and secretion of selenoprotein P by metformin. Biochem Biophys Res Commun. 2009;387:158–163. doi: 10.1016/j.bbrc.2009.06.143. [DOI] [PubMed] [Google Scholar]
- 77.Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hundal HS, Ramlal T, Reyes R, Leiter LA, Klip A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology. 1992;131:1165–1173. doi: 10.1210/endo.131.3.1505458. [DOI] [PubMed] [Google Scholar]
- 79.Pavlović D, Kocić R, Kocić G, et al. Effect of four-week metformin treatment on plasma and erythrocyte antioxidative defense enzymes in newly diagnosed obese patients with type 2 diabetes. Diabetes Obes Metab. 2000;4:251–256. doi: 10.1046/j.1463-1326.2000.00089.x. [DOI] [PubMed] [Google Scholar]