Abstract
Repair of adult liver, like many tissues, involves the coordinated response of a number of different cell types. In adult livers, fibroblastic cells, ductular cells, inflammatory cells, and progenitor cells contribute to this process. Our studies demonstrate that the fates of such cells are dictated, at least in part, by Hedgehog, a fetal morphogenic pathway that was once thought to be active mainly during embryogenesis. Studies of injured adult human and rodent livers demonstrate that injury-related activation of the Hedgehog pathway modulates several important aspects of repair, including the growth of hepatic progenitor populations, hepatic accumulation of myofibroblasts, repair-related inflammatory responses, vascular remodeling, liver fibrosis and hepatocarcinogenesis. These findings identify the Hedgehog pathway as a potentially important target for biomarker development and therapeutic manipulation, and emphasize the need for further research to advance knowledge about how this pathway is regulated by and interacts with other signals that regulate adult liver repair.
Keywords: Cholangiocyte, epithelial-to-mesenchymal transition, fibrosis, hepatic stellate cell, myofibroblast
INTRODUCTION
The adult liver is comprised largely of epithelial cells and mesenchymal cells similar to the skin, intestine, lung, and glandular tissues like the pancreas. In all of these organs, the ultimate outcome of epithelial injury is dictated by repair. Successful liver repair results in replacement of dead or damaged hepatic epithelial cells with healthy new epithelial cells, i.e., liver regeneration. Regenerative responses differ depending on the severity and chronicity of liver injury. For example, it has long been believed that residual mature hepatocytes and cholangiocytes proliferate to restore liver mass after acute partial hepatectomy (Grisham, 1962). However, liver progenitors are thought to play a critical role in the repair of chronically injured livers (Falkowski et al., 2003). Repair of liver injury also variably involves changes in mesenchymal cells. Presumably, alterations in hepatic “stromal” cells in some way contribute to epithelial repair. However, they may also lead to hepatic inflammation, vascular remodeling, and fibrosis, and result in hepatic architectural distortion and liver dysfunction, eventually culminating in cirrhosis (Wynn, 2008).
Signal transduction pathways, such as wnt/beta catenin and notch/jagged, that control the viability, proliferation, and differentiation of progenitor cells are known to regulate fetal liver development (Cavard et al., 2008, Lemaigre and Zaret, 2004). These pathways also become activated during various types of liver injury in adults, and are presumed to modulate adult liver repair (Kordes et al., 2009). Consistent with this concept, dysregulation of wnt/beta catenin signaling has been documented in primary liver cancer and is believed to contribute to its pathogenesis (de La Coste et al., 1998, Huang et al., 1999, Suzuki et al., 2002). Our group has been examining another morphogenic signaling pathway, Hedgehog. Although relatively little is known about the role of Hedgehog in fetal liver development, we became interested in this pathway because it controls the viability and fate of progenitors in many tissues, and it was reported that Sonic hedgehog ligand is expressed by endoderm that has undergone hepatic specification (Bort et al., 2006, Deutsch et al., 2001). Our work in adult liver has proven that several types of cells that reside in healthy adult livers are capable of producing and/or responding to Hedgehog ligands, shown that the pathway is activated in many types of acute and chronic liver injury, and demonstrated roles for Hedgehog pathway activation in several of the tissue responses that occur during adult liver repair, including expansion of liver progenitor populations, myofibroblast accumulation and fibrogenesis, repair-related inflammation, vascular remodeling, liver regeneration and carcinogenesis. The goal of this review is to summarize this new evidence and suggest a new model that helps to explain how various aspects of liver repair are coordinated by Hedgehog-regulated signals.
OVERVIEW OF THE HEDGEHOG PATHWAY
The Hedgehog (Hh) Pathway, originally identified in Drosophila (Hooper and Scott, 2005, Lee et al., 1992, Schuske et al., 1994), is a highly conserved signaling pathway which orchestrates multiple aspects of embryogenesis, development and tissue remodeling in a wide spectrum of systems (Beachy et al., 2004, Berman et al., 2003, Ingham and McMahon, 2001, van den Brink, 2007). This usually occurs by autocrine/paracrine signaling and aims to control the size and localization of Hh-responsive cell populations in response to local/long distance signals (Ingham and McMahon, 2001, Ingham and Placzek, 2006). Hh pathway activation typically enhances the growth and viability of Hh-responsive cells, whereas abrogating Hh signal transduction usually triggers apoptosis in such cells, unless other locally available differentiating factors expedite cellular differentiation to a more mature phenotype that no longer requires Hh viability signals (Beachy et al., 2004, Ingham and Placzek, 2006). Thus, depending upon the context, up-regulation and down-regulation of the Hh pathway can provide selective growth advantages for cell types that are capable of responding to Hh ligands, compared to neighboring cells that lack Hh receptors. This then leads to either expansion or contraction, respectively, of Hh-responsive cells, thereby orchestrating the cellular composition of several tissues (Beachy et al., 2004, Ingham and McMahon, 2001, van den Brink, 2007).
In certain conditions, Hh-producing cells (which may or may not be Hh-responsive themselves) release Hh ligands into the extracellular environment. Hh ligands (Sonic, Shh; Indian, Ihh; Desert, Dhh) are soluble, lipid-modified morphogens (Chamoun et al., 2001, Lee and Treisman, 2001, Pepinsky et al., 1998, Porter et al., 1996, Varjosalo and Taipale, 2008) that may be secreted in two different form: a short-range acting (poorly diffusible) type, and a second form for long-range transport, “packed” in membranous structures (Ingham and McMahon, 2001, Porter et al., 1995, Varjosalo and Taipale, 2008). Hh proteins are able to interact with Patched (Ptc), a membrane-spanning receptor on the surface of Hh-responsive cells (Carpenter et al., 1998). In the absence of Hh ligands, Ptc keeps the co-receptor Smoothened (Smo) in its inactive form, and silences the Smo-dependent down-stream intracellular signaling (Ingham and McMahon, 2001, Varjosalo and Taipale, 2008). Hence, when Smo-signaling is inhibited by “free”-Ptc, Hh-regulated transcription factors (which typically reside in the cytosol) undergo phosphorylation by glycogen synthase kinase 3 (GSK3), protein kinase A (PKA) and casein kinase (CSK); the phosphorylated (inactive) forms become target for proteasome degradation, and their nuclear translocation is prevented (Pan et al., 2006, Pan et al., 2008). In contrast, when the extracellular microenviroment is enriched with soluble Hh ligands, ligand-receptor interaction de-represses Smo. Activation of Smo, in turn, inhibits Hh transcription factor phosphorylation, leading to an intracellular signaling cascade that ultimately drives the activation and nuclear translocation of Glioblastoma (Gli) family zinc-finger transcription factors (Pan et al., 2006, Pan et al., 2008). In vertebrates, the latter consist of three distinct Gli proteins (Gli1, Gli2, and Gli3) (Hui et al., 1994). The binding of Gli proteins to their cognate cis-acting elements regulates the expression of Hh target genes. The latter include several components of the Hh pathway itself, such as Ptc, Smo and Glis. Gli1 and Gli2 are mostly responsible for providing prolonged cellular responses to Hh ligands, while Gli3 primarily acts as signaling repressor (Ingham and McMahon, 2001, van den Brink, 2007, Varjosalo and Taipale, 2008). Thus Hh activity is auto-regulated by complex positive and negative feedback mechanisms that are tightly conserved across species (Hooper and Scott, 2005, Ingham and McMahon, 2001, van den Brink, 2007, Varjosalo and Taipale, 2008).
Despite the conservation of the Hh pathway between invertebrates and vertebrates, Hh pathway regulation seems to diverge and differentiate at some point (Varjosalo et al., 2006, Varjosalo and Taipale, 2008). Only vertebrates, have an additional trans-membrane protein, Hh-interacting protein (Hhip, also a Hh target gene), that competes with Ptc for binding to Hh soluble ligands (Chuang and McMahon, 1999, Jeong and McMahon, 2005). Thus, in vertebrates, when levels of Ptc exceed those of the Hh ligands, or when Hhip sequesters Hh ligands and subtracts activating signals to Ptc, the Hh pathway is turned off.
HEDGEHOG SIGNALING IN ADULT LIVER REPAIR: GENERAL CONCEPTS
Adult hepatic damage evokes an intricate wound-healing response aimed to reconstitute the normal structure and function of injured livers. As in many other tissues, this complex repair process involves the post-natal reactivation of mechanisms that regulate tissue construction during development. Several types of cells in adult livers are capable of producing and/or responding to Hh ligands. However, expression of Hh ligands and Hh-target genes, such as Gli1 or Gli2, is barely detectable in healthy adult livers (Sicklick et al., 2006b). Various types of acute and chronic liver injury, on the other hand, increase mRNA and protein levels of Hh ligands and target genes. The level of Hedgehog pathway activation appears to be proportional to the severity and duration of liver injury in both rodents and humans (Fleig et al., 2007). This is explained, at least in part, by recent evidence that pro-apoptotic stimuli trigger mature hepatocytes to produce Sonic hedgehog (Shh) and Indian hedgehog (Ihh) ligands (Jung et al., 2010). Mature hepatocytes themselves do not express all of the proteins that are necessary to transduce Hh ligand-initiated signals, and thus, are not capable of responding to the Hh ligands that they produce (Sicklick et al., 2006b). However, many neighboring cells are Hh-responsive, including resident hepatic cell populations that are most engaged in liver remodeling (e.g., liver myofibroblasts, hepatic progenitors, hepatic stellate cells, immature cholangiocytes, endothelial cells, and T lymphocytes) (Fleig et al., 2007, Jung et al., 2008, Jung et al., 2007, Lowrey et al., 2002, Omenetti et al., 2008a, Omenetti et al., 2008b, Omenetti et al., 2007, Sicklick et al., 2005, Sicklick et al., 2006b, Stewart et al., 2002, Witek et al., 2008, Yang et al., 2008, Choi et al., 2009). In response to the increased Hh ligands generated by neighboring injured hepatocytes, these cells activate Hh pathway signaling.
Many of these Hh-responsive cells are also capable of producing Hh ligands in response to other injury-associated factors. For example, Platelet-Derived Growth Factor-BB (PDGF-BB), Transforming Growth Factor (TGF)-β1, Epidermal Growth Factor (EGF) have been demonstrated to stimulate immature ductular cells and/or hepatic stellate cells to up-regulate their synthesis and release of Hh ligands (Jung et al., 2008, Omenetti et al., 2008a, Omenetti et al., 2008b, Omenetti et al., 2007, Witek et al., 2009, Yang et al., 2008). This further enriches the injured liver with Hh ligands, and amplifies the stimulus for Hh pathway activation. The proximity of various types of Hh producing and Hh responding cell types in damaged livers suggests that Hh pathway activation coordinates complex autocrine and paracrine signaling loops that help to orchestrate remodeling and re-construction of the injured liver.
Because mature hepatocytes are not able to respond to Hh ligands, enrichment of the microenvironment with these factors provides a selective survival advantage for cell types that are Hh-responsive, leading to the outgrowth of these populations as long as injury persists. However, when the insult abates and injury subsides, the Hh pathway turns off (Omenetti et al., 2008a), and other factors promote the differentiation of the progeny of Hh-responsive cells toward one cell population or another. Because PDGF-BB, EGF, and IGF-1 activate AKT-dependent post-translational mechanisms that stabilize Gli transcription factors in Hh-responsive cells (Riobo et al., 2006) (in addition to stimulating production of Hh ligands), Hh signaling may modulate the actions of multiple growth factors, and vice versa. Thus, variations in tissue remodeling during liver injury probably ultimately reflects differences in: 1) local cytokine/growth factor accumulation, 2) the dose and duration of Hh ligand exposure, 3) the balance between Hh-responsive/-unresponsive cell types, and 4) the presence/absence of poorly understood factors that regulate cell differentiation when these injury-related signals wane.
HEDGEHOG PATHWAY ACTIVATION AND LIVER PROGENITORS
Progenitor populations in many tissues are enriched with Hh-responsive cells and Hh ligands generally enhance the growth of such progenitors by inhibiting apoptosis and/or enhancing proliferative activity (Sicklick et al., 2006b, Jung et al., 2007, Yang et al., 2008). We reported that human embryonic stem cells that had been specified to undergo hepatic differentiation, as well as more-differentiated EpCam-expressing liver progenitors from human fetal livers, were Hh-responsive. Both cell types relied upon Hh pathway activity to retain optimal viability (Sicklick et al., 2006b). Progenitor populations in adult rodent and human livers, including oval cells, immature ductular cells and small hepatocytic cells, are also Hh responsive. Cell culture studies demonstrate that Hh ligands inhibit apoptosis and enhance proliferation in such cells (Omenetti et al., 2007). More recent studies that used a highly specific pharmacologic inhibitor of the Hh pathway (cyclopamine) to manipulate Hh signaling in regenerating livers after 70% partial hepatectomy (PH) suggest that Hh signaling also plays a major role in promoting progenitor growth in intact liver tissue (Ochoa et al., 2010). This concept is supported by other evidence in various rodent models of chronic liver injury. For example, in wild type mice and rats, the numbers of cells expressing various progenitor markers increase in parallel with the level of Hh ligand production during liver injury, and regress in parallel with the disappearance of Hh ligands as injury abates (Fleig et al., 2007). In addition, liver injury-related accumulation of Hh-responsive progenitor cells is enhanced in transgenic mice with an impaired ability to silence Hh signaling (Omenetti et al., 2007, Syn et al., 2009a). A similar relationship between the hepatic progenitor content and level of Hh pathway activity has been documented in different human liver diseases, including primary biliary cirrhosis (Jung et al., 2007, Omenetti et al., 2008b), alcoholic steatohepatitis (Jung et al., 2008), nonalcoholic fatty liver disease (Syn et al., 2009a), chronic hepatitis B, and chronic hepatitis C (Pereira et al., 2010).
HEDGEHOG PATHWAY ACTIVATION AND REPAIR-RELATED INFLAMMATION
Hh pathway activation stimulates immature ductular cells to produce various chemokines, including chemoattractants for monocytes, neutrophils, and various types of T lymphocytes, including Natural killer T (NKT) cells (Omenetti et al., 2009). In order to evaluate the potential significance of this response, we have examined Hh-regulation of CXCL16, the chemokine that recruits NKT cells to the liver. We proved that ductular cell expression of CXCL16 is Hh-dependent and showed that the resultant increase in CXCL16 recruits NKT cells towards the ductular epithelia. NKT cells respond to glycolipid antigens that are presented by CD1d molecules on antigen presenting cells (Brossay et al., 1997, Roark et al., 1998). Several types of resident liver cells, namely hepatocytes, bile ductular cells, and various sinusoidal lining cells, including HSC, are capable of presenting CD1d-associated glycolipids to NKT cells (de Lalla et al., 2004, Geissmann et al., 2005). Hence, it is not surprising that lymphocytes populations in both murine and human livers are particularly enriched with such cells (Ajuebor, 2007, Bendelac et al., 2007, Eberl et al., 1999, Emoto and Kaufmann, 2003, Klugewitz et al., 2004, Matsuda et al., 2000, Wingender and Kronenberg, 2008). Many types of liver cells that express CD1d are also capable of producing Hh ligands, particularly when the liver is injured. This is intriguing because we discovered that Hh ligands function as potent anti-apoptotic factors for NKT cells. In addition, we demonstrated some NKT cells that are isolated from adult livers or peripheral blood produce Hh ligands (Syn et al., 2009b). Therefore, activation of the Hh pathway in injured livers provides stimuli that not only recruit circulating NKT cells to the liver, but also that enhance their intra-hepatic survival and exposure to potential antigen-presenting cells. Consistent with this concept, we demonstrated that NKT cells accumulate in human liver diseases in which the hedgehog pathway is strongly activated, such as primary biliary cirrhosis (Jung et al., 2007, Omenetti et al., 2008b), and cirrhosis related to chronic hepatitis C or nonalcoholic steatohepatitis (Syn et al., 2010). It is conceivable that NKT cell accumulation also contributes to the progession of liver fibrosis in these conditions because NKT cells produce Hh ligands, which stimulate the growth of myofibroblasts (see below). In addition, Hh ligands simulate NKT cells to produce pro-fibrogenic cytokines, such as IL13 and IL4 (Syn et al., 2009b). We recently showed that conditioned medium from activated liver NKT cells stimulated quiescent hepatic stellate cells to transition to a myofibroblastic phenotype and produce collagen matrix (Syn et al., 2010). Therefore, immune-mediated events appear to be involved in hepatic repair responses that are regulated by the Hh pathway.
HEDGEHOG PATHWAY ACTIVATION AND LIVER FIBROSIS
Myofibroblasts are the major source of fibrous matrix that accumulates during chronic liver injuries that result in cirrhosis. Liver myofibroblasts may be derived from several sources, including circulating bone marrow-derived monocytes/fibrocytes, epithelial-to-mesenchymal transition (EMT) of certain types of liver epithelial cells, and myofibroblastic transformation of resident hepatic stellate cells. The latter process is generally believed to be the predominant source of myofibroblasts in most types of adult liver injury (Friedman, 2008).
In healthy adult livers most hepatic stellate cells (HSC) are quiescent (Q) and exhibit a fat-storing, non-myofibroblastic (MF) phenotype. However, factors released during liver injury stimulate Q-HSC to undergo transition to MF-HSC; other injury-related factors then promote hepatic accumulation of MF-HSC by enhancing proliferation and/or inhibiting apoptosis of MF-HSC (Friedman, 2008). We discovered that Q–HSC produce large amounts of the Hh inhibitor, Hhip, but quickly down-regulate Hhip when exposed to conditions that promote their transition to MF-HSC (Choi et al., 2009). These situations also activate PI3K/AKT-dependent mechanisms that induce HSC production of Hh ligands (Yang et al., 2008). HSC-derived Hh ligands, in turn, result in increased expression of Hh-target genes, such as Gli2 (Choi et al., 2009). Hh neutralzing antibodies or specific pharmacologic inhibitors of smoothened that abrogate downstream Hh signaling drastically reduce MF-HSC viability and virtually eliminate the proliferative effects of known MF-HSC mitogens, such as PDGF-BB (Yang et al., 2008). In addition, pharmacologic inhibition of Hh pathway with the Smoothened antagonist, cyclopamine, caused surviving MF-HSC to revert to a less myofibroblastic and more quiescent phenotype (Choi et al., 2009, Choi et al., 2010, Sicklick et al., 2005).
Hh pathway activation also may promote hepatic accumulation of myofibroblasts that are derived from “non-conventional” sources, such as bone marrow and EMT. We demonstrated that activating Hh signaling in immature ductular cells caused such cells to produce MCP-1, which is a known chemokine for circulating monocytes/fibrocytes (Omenetti et al., 2009). Moreover, Hh pathway activation increases local production of IL-13 (see above) and IL-13 has been shown to promote the differentiation of monocytes into fibrocytes (Shao et al., 2008). Finally, Hh signaling induces EMT in immature ductular cells (Omenetti et al., 2008b). Thus, the aggregate findings predict that injury-related activation of the Hh pathway would play a major role in hepatic accumulation of myofibroblasts.
The concept that Hh pathway activation promotes hepatic accumulation of myofibroblasts is supported by several lines of experimental evidence. First, myofibroblast numbers and matrix accumulation in regenerating livers after PH parallel Hh pathway activation and are virtually abolished by pharmacologic inhibition of Hh signaling (Ochoa et al., 2010). Second, hepatic accumulation of myofibroblasts and collagen matrix increase in parallel with cholestatic liver injury following chronic bile duct ligation, and resolve in parallel with the progressive down-regulating of Hh signaling that occurs once biliary obstruction is relieved (Omenetti et al., 2008a). Third, myofibroblast accumulation and liver fibrogenic activity are enhanced transgenic mice with an overly-active Hh pathway following bile duct ligation (Omenetti et al., 2008a) or exposure to hepatotoxic diets (Syn et al., 2009a). Fourth, myofibroblast accumulation and the severity of liver fibrosis parallel the level of Hh pathway activity in patients with different types of liver disease, including chronic viral hepatitis (Pereira et al., 2010) and nonalcoholic fatty liver disease (Syn et al., 2009a).
HEDGEHOG PATHWAY ACTIVATION AND VASCULAR REMODELING
Cirrhosis is characterized by changes in hepatic sinusoidal architecture together with extrahepatic vasculature rearrangement (Friedman, 2008). Several types of cells that reside near sinusoidal endothelial cells are capable of generating Hh ligands, including injured hepatocytes, activated HSC, liver progenitors, and certain types of resident lymphocytes. The Hh pathway is a key regulator of vascular remodeling during development (Vokes et al., 2004), while PDGF-BB (which activates Hh signaling in adult liver cell populations)(Omenetti et al., 2008a, Yang et al., 2008) has also been demonstrated to regulate hepatic vascular structure and function (Semela et al., 2008).
Furthermore, a potential role for Hh-mediated activation of other angiogenic factors that could impact vascular remodeling have been identified in other tissues (Yamazaki et al., 2008, Pola et al., 2001, Lavine et al., 2006, Ueda et al., 2010). Recently published evidence also suggests that activated HSC may enhance the vascular remodeling process by secreting angiogenic factors, such as angiopoeitin 1 (Taura et al., 2008). Hence, we postulated that Hh signaling might regulate cirrhosis-related vascular remodeling.
In support of this concept, biologically active Hh ligands were identified in exosomes that were released from myofibroblasts and immature cholangiocytes after these cells were exposed to PDGF-BB (Witek et al., 2009). Moreover, treatment of other cells that contained Hh-reporter constructs with exosomes purified from myofibroblast- or cholangiocyte- conditioned medium activated Hh transcriptional activity, proving that Hh-containing exosomes are capable of initiating Hh signaling in distant Hh-target cells (Witek et al., 2009). Consistent with the in vitro data, BDL-induced fibrosis/cirrhosis elicited the release of membrane-associated Hh ligands into both plasma and bile (Witek et al., 2009). Even more interestingly, when exposed to either plasma- or bile-derived exosome-enriched Hh-containing membrane particles, sinusoidal endothelial cells were stimulated to undergo phenotypic changes that are known to occur during the capillarization proccess that accompanies cirrhosis-related vascular remodeling (Witek et al., 2009). These findings identify a potentially novel mechanism for vascular remodeling during cirrhosis, namely, Hh-induced phenotypic changes in endothelial cells.
HEDGEHOG PATHWAY ACTIVATION AND CARCINOGENESIS
Hh pathway activation has been demonstrated in many types of cancer (Beachy et al., 2004). In some tumors, this results from activating mutations in smoothened or gli family members (Toftgard, 2000, Romer and Curran, 2005, Saldanha, 2001, Daya-Grosjean and Couve-Privat, 2005). However, in others enhanced Hh signaling is explained by epigenetic events that silence Hhip or that increase production of Hh ligands (Freeman et al., 2009). Beachy et al. (2004) demonstrated excessive Hh signaling in cholangiocarcinomas. Our group was the first to report increased Hh pathway activity in human hepatocellular carcinomas (HCC) and certain human HCC-derived hepatoma cell lines. Moreover, we showed that smoothened inhibitors significantly reduced the growth of Hh-responsive hepatoma cells (Sicklick et al., 2006a). These findings were quickly validated by several other research teams (Patil et al., 2006, Huang et al., 2006, Fu et al., 2008, Tada et al., 2008, Cheng et al., 2009). Subsequent microarray analyses of human HCC banks suggest that Hhip may be hypermethylated and silenced in as many as two-thirds of human HCCs (Wang et al., 2007, Villanueva et al., 2007), identifying the Hh pathway as a potentially important therapeutic target in hepatocarcinogenesis.
To date, however, little information has been published to clarify the cellular sources and targets of Hh ligand in HCC. We are using immunohistochemistry to address this issue. Preliminary results in an HCC that developed in a patient with HCV-related cirrhosis suggest that the malignant stroma is particularly-enriched with cells that produce Hh ligand, as well as Hh responsive cells. This finding is particularly intriguing given a recent report which demonstrated that a pharmacologic Hh inhibitor eliminated most of the tumor stroma and improved the outcomes in a mouse model of pancreatic cancer (Olive et al., 2009).
CONCLUSIONS
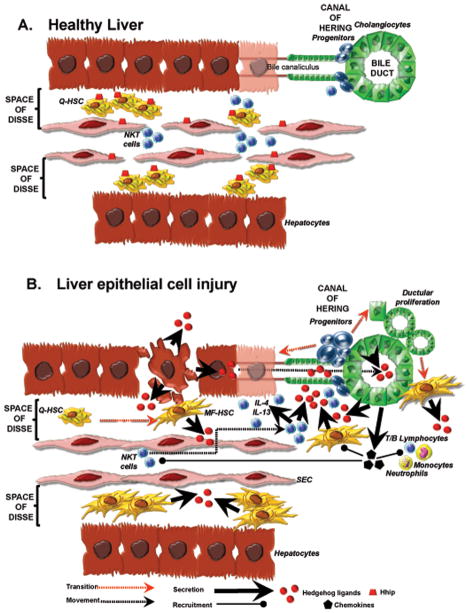
Recent evidence suggests that repair of adult liver, like many tissues, involves the coordinated response of a number of different cell types. In adult livers, fibroblastic cells, ductular cells, inflammatory cells, and progenitor cells contribute to this process. The fates of such cells are dictated, at least in part, by fetal morphogenic pathways which were once thought to be active mainly during embryogenesis, such as Hedgehog. Emerging data from studies of injured adult human and rodent livers demonstrate that injury-related activation of the Hedgehog pathway modulates several important aspects of repair, including the growth of hepatic progenitor populations, hepatic accumulation of myofibroblasts, repair-related inflammatory responses, vascular remodeling, liver fibrosis and hepatocarcinogenesis (Figure 1). These findings identify the Hedgehog pathway as a potentially important target for biomarker development and therapeutic manipulation, and emphasize the need for further research to advance knowledge about how this pathway is regulated by and interacts with other signals that regulate adult liver repair.
Figure 1.
A. Healthy liver. There is very little evidence of Hedgehog (Hh) pathway activity in healthy adult liver, although this tissue harbors a number of different cell types that are capable of producing and/or responding to Hedgehog ligands. Two mechanisms seem to account for these low basal levels of Hh activity: 1) relative lack of Hh ligand production and 2) high expression of the Hh ligand antagonist, Hhip, by sinusoidal lining cells, such as quiescent hepatic stellate cells (Q-HSC) and sinusoidal endothelial cells (SEC). B. Liver epithelial cell injury. Injury to liver epithelial cell unleashes a cascade that results in progressive activation of the Hh pathway and consequent expansion of cell populations that are involved in liver inflammation, regeneration, and fibrogenesis. 1) Injury stimulates mature hepatocytes and cholangiocytes to produce and release Hh ligands into the bile, space of Disse, and liver sinusoids. 2) Injury represses sinusoidal lining cell expression of Hhip. These two responses increase exposure of Hh-responsive cell types (e.g., HSC, SEC, liver progenitors, cholangiocytes, and liver lymphocytes) to Hh ligands. 3) Hh ligands stimulate Q-HSC to undergo transition to myofibroblastic (MF)-HSC; enhance MF-HSC proliferation and survival; and stimulate further production of Hh ligands by MF-HSC. 4) Hh ligands generated by injured liver epithelial cells and MF-HSC enhance the proliferation and survival of liver progenitors and cholangiocytes, both of which also produce Hh ligands. 5) Hh ligands stimulate cholangiocytes to undergo EMT, thereby providing another source of myofibroblasts in injured livers. 6) Hh-activated cholangiocytes also produce various chemokines that recruit different types of bone marrow-derived cells and immune cells to liver, such as fibrocytes, monocytes, neutrophils, T and B lymphocytes, and NKT cells. 7) Hh ligands are viability factors for lymphocytes, including NKT cells. Thus, they promote the accumulation of NKT cells within a microenvironment that is enriched with CD1d-expressing cells that function as antigen-presenting cells for NKT cells. 8) Hh ligands also stimulate NKT cells to produce pro-fibrogenic cytokines, including IL-4 and IL-13. These NKT cell-derived factors, as well as Hh ligands that are produced by the NKT cells themselves, further stimulate expansion of liver myofibroblasts by promoting growth of MF-HSC and inducing transition of bone marrow derived monocytes into fibrocytes. 9) The cumulative effects of Hh ligands on SEC promote SEC capillarization and alter liver blood flow by up-regulating SEC expression of CD31, adhesion factors, and nitric oxide. 10) Thus, injury-related activation of the Hh pathway contributes to many of the repair responses that typify chronic liver injury, including inflammation, fibrogenesis, vascular remodeling, and accumulation of progenitor populations that might provide the seeds for subsequent liver cancers.
Key Abbreviations
- BDL
Bile duct ligation
- BMP
bone morphogenic protein
- CCl4
carbon tetrachloride
- CSK
casein kinase
- Dhh
desert hedgehog
- EGF
epidermal growth factor
- EMT
epithelial-to-mesenchymal transition
- Gli
glioblastoma
- GSK3
glycogen synthase kinase 3
- Hh
hedgehog
- Hhip
hedgehog-interacting protein
- HSC
hepatic stellate cell
- HCC
hepatocellular carcinoma
- IGF-1
insulin-like growth factor 1
- Ihh
Indian hedgehog
- MET
mesenchymal-to-epithelial transition
- MF
myofibroblast
- NKT
natural killer T cell
- PH
partial hepatectomy
- Ptc
patched
- PDGF-BB
plateled-derived growth factor-BB
- PKA
protein kinase A
- Q
quiescent
- Smo
smoothened
- Shh
sonic hedgehog
- Id
inhibitor of differentiation
- TGF-β
transforming growth factor-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajuebor MN. Role of NKT cells in the digestive system. I. Invariant NKT cells and liver diseases: is there strength in numbers? Am J Physiol Gastrointest Liver Physiol. 2007;293:G651–656. doi: 10.1152/ajpgi.00298.2007. [DOI] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- Bort R, Signore M, Tremblay K, Martinez Barbera JP, Zaret KS. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev Biol. 2006;290:44–56. doi: 10.1016/j.ydbio.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Brossay L, Jullien D, Cardell S, Sydora BC, Burdin N, Modlin RL, Kronenberg M. Mouse CD1 is mainly expressed on hemopoietic-derived cells. J Immunol. 1997;159:1216–1224. [PubMed] [Google Scholar]
- Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, Rosenthal A, de Sauvage FJ. Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc Natl Acad Sci U S A. 1998;95:13630–13634. doi: 10.1073/pnas.95.23.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard C, Colnot S, Audard V, Benhamouche S, Finzi L, Torre C, Grimber G, Godard C, Terris B, Perret C. Wnt/beta-catenin pathway in hepatocellular carcinoma pathogenesis and liver physiology. Future Oncol. 2008;4:647–660. doi: 10.2217/14796694.4.5.647. [DOI] [PubMed] [Google Scholar]
- Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- Cheng WT, Xu K, Tian DY, Zhang ZG, Liu LJ, Chen Y. Role of Hedgehog signaling pathway in proliferation and invasiveness of hepatocellular carcinoma cells. Int J Oncol. 2009;34:829–836. doi: 10.3892/ijo_00000209. [DOI] [PubMed] [Google Scholar]
- Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA, Rojkind M, Diehl AM. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SS, Witek RP, Yang L, Omenetti A, Syn WK, Moylan CA, Jung Y, Karaca GF, Teaberry VS, Pereira TA, Wang J, Ren XR, Diehl AM. Activation of Rac1 promotes hedgehog-mediated acquisition of the myofibroblastic phenotype in rat and human hepatic stellate cells. Hepatology. 2010;52:278–290. doi: 10.1002/hep.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- Daya-Grosjean L, Couve-Privat S. Sonic hedgehog signaling in basal cell carcinomas. Cancer Lett. 2005;225:181–192. doi: 10.1016/j.canlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, Monno A, Nuti S, Colombo M, Callea F, Porcelli SA, Panina-Bordignon P, Abrignani S, Casorati G, Dellabona P. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol. 2004;173:1417–1425. doi: 10.4049/jimmunol.173.2.1417. [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003;24:364–369. doi: 10.1016/s1471-4906(03)00162-5. [DOI] [PubMed] [Google Scholar]
- Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, Thiese ND. Regeneration of hepatocyte “buds” in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357–364. doi: 10.1016/s0168-8278(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Fleig SV, Choi SS, Yang L, Jung Y, Omenetti A, VanDongen HM, Huang J, Sicklick JK, Diehl AM. Hepatic accumulation of Hedgehog-reactive progenitors increases with severity of fatty liver damage in mice. Lab Invest. 2007;87:1227–1239. doi: 10.1038/labinvest.3700689. [DOI] [PubMed] [Google Scholar]
- Freeman JW, Wang Y, Giles FJ. Epigenetic modulation and attacking the hedgehog pathway: potentially synergistic therapeutic targets for pancreatic cancer. Cancer Biol Ther. 2009;8:1340–1342. doi: 10.4161/cbt.8.14.8756. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Wang Q, Chen X, Huang X, Cao L, Tan H, Li W, Zhang L, Bi J, Su Q, Chen L. Expression patterns and polymorphisms of PTCH in Chinese hepatocellular carcinoma patients. Exp Mol Pathol. 2008;84:195–199. doi: 10.1016/j.yexmp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver: Autoradiographic studies. Cancer Res. 1962;22:842–849. [PubMed] [Google Scholar]
- Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795–1801. doi: 10.1016/s0002-9440(10)65496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, He J, Zhang X, Bian Y, Yang L, Xie G, Zhang K, Tang W, Stelter AA, Wang Q, Zhang H, Xie J. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis. 2006;27:1334–1340. doi: 10.1093/carcin/bgi378. [DOI] [PubMed] [Google Scholar]
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7:841–850. doi: 10.1038/nrg1969. [DOI] [PubMed] [Google Scholar]
- Jeong J, McMahon AP. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development. 2005;132:143–154. doi: 10.1242/dev.01566. [DOI] [PubMed] [Google Scholar]
- Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, Milton RJ, Hines IN, Rippe RA, Spahr L, Rubbia-Brandt L, Diehl AM. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–1543. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45:1091–1096. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, Guy CD, Diehl AM. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–665. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol. 2004;25:590–594. doi: 10.1016/j.it.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kordes C, Sawitza I, Haussinger D. Hepatic and pancreatic stellate cells in focus. Biol Chem. 2009 doi: 10.1515/BC.2009.121. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui CC, Ornitz DM. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Treisman JE. Sightless has homology to transmembrane acyltransferases and is required to generate active Hedgehog protein. Curr Biol. 2001;11:1147–1152. doi: 10.1016/s0960-9822(01)00323-2. [DOI] [PubMed] [Google Scholar]
- Lee JJ, von Kessler DP, Parks S, Beachy PA. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71:33–50. doi: 10.1016/0092-8674(92)90264-d. [DOI] [PubMed] [Google Scholar]
- Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14:582–590. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Lowrey JA, Stewart GA, Lindey S, Hoyne GF, Dallman MJ, Howie SE, Lamb JR. Sonic hedgehog promotes cell cycle progression in activated peripheral CD4(+) T lymphocytes. J Immunol. 2002;169:1869–1875. doi: 10.4049/jimmunol.169.4.1869. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, Zubiaga AM, Fresnedo O, Omenetti A, Zdanowicz M, Choi SS, Diehl AM. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712–1723. doi: 10.1002/hep.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, Schuppan D, Diehl AM. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008a;57:1275–1282. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008b;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, Yang L, Mayo MJ, Gershwin ME, Alpini G, Diehl AM. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–527. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, Choi S, Suzuki A, Diehl AM. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Wang C, Wang B. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil MA, Zhang J, Ho C, Cheung ST, Fan ST, Chen X. Hedgehog signaling in human hepatocellular carcinoma. Cancer Biol Ther. 2006;5:111–117. doi: 10.4161/cbt.5.1.2379. [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, Taylor FR, Wang EA, Galdes A. Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem. 1998;273:14037–14045. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- Pereira TA, Witek RP, Syn WK, Choi SS, Bradrick S, Karaca GF, Agboola KM, Jung Y, Omenetti A, Moylan CA, Yang L, Fernandez-Zapico ME, Jhaveri R, Shah VH, Pereira FE, Diehl AM. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010 doi: 10.1038/labinvest.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, Beachy PA. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995;374:363–366. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci U S A. 2006;103:4505–4510. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol. 1998;160:3121–3127. [PubMed] [Google Scholar]
- Romer J, Curran T. Targeting medulloblastoma: small-molecule inhibitors of the Sonic Hedgehog pathway as potential cancer therapeutics. Cancer Res. 2005;65:4975–4978. doi: 10.1158/0008-5472.CAN-05-0481. [DOI] [PubMed] [Google Scholar]
- Saldanha G. The Hedgehog signalling pathway and cancer. J Pathol. 2001;193:427–432. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH815>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Schuske K, Hooper JE, Scott MP. patched overexpression causes loss of wingless expression in Drosophila embryos. Dev Biol. 1994;164:300–311. doi: 10.1006/dbio.1994.1200. [DOI] [PubMed] [Google Scholar]
- Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135:671–679. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, Huang J, Zdanowicz M, Camp T, Torbenson MS, Rojkind M, Diehl AM. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest. 2005;85:1368–1380. doi: 10.1038/labinvest.3700349. [DOI] [PubMed] [Google Scholar]
- Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, Ludlow JW, Owzar K, Chen W, Torbenson MS, Diehl AM. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006a;27:748–757. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, Caballero M, Fair JH, Ludlow JW, McClelland RE, Reid LM, Diehl AM. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006b;290:G859–870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- Stewart GA, Lowrey JA, Wakelin SJ, Fitch PM, Lindey S, Dallman MJ, Lamb JR, Howie SE. Sonic hedgehog signaling modulates activation of and cytokine production by human peripheral CD4+ T cells. J Immunol. 2002;169:5451–5457. doi: 10.4049/jimmunol.169.10.5451. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yano H, Nakashima Y, Nakashima O, Kojiro M. Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol. 2002;17:994–1000. doi: 10.1046/j.1440-1746.2002.02774.x. [DOI] [PubMed] [Google Scholar]
- Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, Wang J, Witek RP, Fearing CM, Pereira TA, Teaberry V, Choi SS, Conde-Vancells J, Karaca GF, Diehl AM. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009a;137:1478–1488. e1478. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, Choi SS, Guy CD, Fearing CM, Teaberry V, Pereira FE, Adams DH, Diehl AM. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syn WK, Witek RP, Curbishley SM, Jung Y, Choi SS, Enrich B, Omenetti A, Agboola KM, Fearing CM, Tilg H, Adams DH, Diehl AM. Role for hedgehog pathway in regulating growth and function of invariant NKT cells. Eur J Immunol. 2009b;39:1879–1892. doi: 10.1002/eji.200838890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Kanai F, Tanaka Y, Tateishi K, Ohta M, Asaoka Y, Seto M, Muroyama R, Fukai K, Imazeki F, Kawabe T, Yokosuka O, Omata M. Down-regulation of hedgehog-interacting protein through genetic and epigenetic alterations in human hepatocellular carcinoma. Clin Cancer Res. 2008;14:3768–3776. doi: 10.1158/1078-0432.CCR-07-1181. [DOI] [PubMed] [Google Scholar]
- Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S, Brenner DA. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- Toftgard R. Hedgehog signalling in cancer. Cell Mol Life Sci. 2000;57:1720–1731. doi: 10.1007/PL00000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Takano H, Niitsuma Y, Hasegawa H, Uchiyama R, Oka T, Miyazaki M, Nakaya H, Komuro I. Sonic hedgehog is a critical mediator of erythropoietin-induced cardiac protection in mice. J Clin Invest. 2010;120:2016–2029. doi: 10.1172/JCI39896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Li SP, Taipale J. Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev Cell. 2006;10:177–186. doi: 10.1016/j.devcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Yatskievych TA, Heimark RL, McMahon J, McMahon AP, Antin PB, Krieg PA. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–4380. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- Wang F, Anderson PW, Salem N, Kuang Y, Tennant BC, Lee Z. Gene expression studies of hepatitis virus-induced woodchuck hepatocellular carcinoma in correlation with human results. Int J Oncol. 2007;30:33–44. [PubMed] [Google Scholar]
- Wingender G, Kronenberg M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1–8. doi: 10.1152/ajpgi.00437.2007. [DOI] [PubMed] [Google Scholar]
- Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM, Chen W, Diehl AM. Liver Cell-Derived Microparticles Activate Hedgehog Signaling and Alter Gene Expression in Hepatic Endothelial Cells. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM, Chen W, Diehl AM. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330. e322. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molelcular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Nakamura K, Mizukami Y, Ii M, Sasajima J, Sugiyama Y, Nishikawa T, Nakano Y, Yanagawa N, Sato K, Maemoto A, Tanno S, Okumura T, Karasaki H, Kono T, Fujiya M, Ashida T, Chung DC, Kohgo Y. Sonic hedgehog derived from human pancreatic cancer cells augments angiogenic function of endothelial progenitor cells. Cancer Sci. 2008;99:1131–1138. doi: 10.1111/j.1349-7006.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]