Abstract
Ginsenosides are the main bioactive components in American ginseng, a commonly used herb. In this study, we showed that the ginsenoside Rh2 exhibited significantly more potent cell death activity than the ginsenoside Rg3 in HCT116 and SW480 colorectal cancer cells. Cell death induced by Rh2 is mediated in part by the caspase-dependent apoptosis and in part by the caspase-independent paraptosis, a type of cell death that is characterized by the accumulation of cytoplasmic vacuoles. Treatment of cells with Rh2 activated the p53 pathway and significantly increased the levels of the pro-apoptotic regulator, Bax, while decreasing the levels of anti-apoptosis regulator Bcl-2. Removal of p53 significantly blocked Rh2 induced cell death as well as vacuole formation, suggesting that both types of cell death induced by Rh2 are mediated by p53 activity. Furthermore, we show that Rh2 increased ROS levels and activated the NF-κB survival pathway. Blockage of ROS by NAC or catalase inhibited the activation of NF-κB signaling and enhanced Rh2-induced cell death, suggesting that the anticancer effect of Rh2 can be enhanced by antioxidants.
Keywords: Ginsenoside, apoptosis, ROS, NF-κB, antioxidant
1. Introduction
Colon cancer is the third leading cause of cancer-related deaths in the western world[1]. Current treatment of this cancer generally employs surgical resection combined with chemotherapy using cytotoxic drugs and radiation therapy. Because this therapy is only moderately successful, novel approaches to the treatment of colorectal cancer are required.
Natural products are potentially valuable source for the development of new anti-cancer drugs[2; 3]. American ginseng (Panax quinquefolius) is a very popular herb in the United States, and its main effective components are ginsenosides that have been reported to have a wide variety of biological activities including immunomodulatory effects, anti-inflammatory and anti-tumor activity[4; 5; 6]. We recently reported that steamed American ginseng extract potently killed colorectal cancer cells and that Rg3 and Rh2, derivatives of protopanaxadiol (PPD), are the main ginsenosides in the extract[7; 8]. Interestingly, it is reported that Rg3 can be metabolized by human intestinal bacteria to Rh2 and further to PPD[9]. In this report, we characterized the effects of Rg3 and Rh2 on the colorectal cancer cell lines, HCT116 and SW480.
Apoptosis is programmed cell death involving the activation of caspases through either a mitochondria-dependent cell intrinsic or mitochondria–independent cell extrinsic pathway[10; 11]. In addition to apoptosis, several types of caspase independent programmed cell death have been identified including autophagy, paraptosis, mitotic catastrophe, and necroptosis[12; 13]. Autophagy is characterized by the sequestration of bulk cytoplasm and/or organelles in double membrane autophagic vesicles and can be visualized by the localization of Atg8/LC3 to the membrane of pre-autophagosome[14]. Paraptosis is characterized by cytoplasmic vacuolization. It lacks apoptotic morphology and does not respond to caspases inhibitors. However paraptosis does require new protein synthesis and MAP kinase activation[15; 16; 17]. Necroptosis is a form of programmed necrosis that is caspase-independent and have been reviewed recently (reviewed in [13]). It should be pointed out that a dying cell may exhibit characteristics of several death pathways. It is postulated that the dominant death phenotype is determined by the relative speed of the available death programs [12].
In this study, we show that Rh2 exerts significantly more potent colorectal cancer cell killing activities than Rg3. We show that Rh2 induced cell death is partially dependent on caspase-3 activation. Interestingly, we find that Rh2 induces a significant level of cytoplasmic vacuole formation, which is characteristic of paraptosis. Similar to our studies of the steamed ginseng extracts, we show that Rh2 induces ROS generation in colorectal cancer cells, which in turn activates the NF-κB signaling and partially counteracts the cancer cell killing activities of Rh2. Consistent with this, inhibition of ROS or the NF-κB pathway increases the toxicity of Rh2 to colorectal cancer cells. Furthermore, we show that p53 transcription activity is induced by Rh2 and that inactivation of p53 significantly decreases Rh2-induced vacuole formation and cell death.
2. Methods and Materials
2.1. Chemicals and reagents
N-Acetyl-L-cysteine (NAC) and PS1145 were obtained from Sigma. Rh2 and Rg3 were obtained from National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China; and were of biochemical reagent grade and at least 95% pure as determined by HPLC. NAC was dissolved in the growth medium. PS1145, a specific inhibitor of NF-κB pathway, was dissolved in DMSO as a 20 mM stock buffer. Luciferase assay kits were purchased from Promega. Anti-Bad and monoclonal anti-β-actin was obtained from Cell Signaling Technology. Anti-Bcl-2, anti-Bcl-XL and anti-Bax were obtained from Santa Cruz. Annexin V Kit was purchased from BD Biosciences, and 5-(and-6)-chloromethyl-2′7′-dichlorodihydrofluorescein diacetate acetyl ester (H2DCFDA) was obtained from Invitrogen. Experiments were carried out at least three times to obtain the mean and the standard deviation.
2.2. Cell culture
Normal human colon epithelial cells, FHC, and human colorectal cancer cells HCT116 and SW480 were obtained from the American Type Culture Collection. HCT116 and SW480 cells were maintained in McCoy’s 5A medium supplemented with 5% fetal bovine serum (FBS, Hyclone Laboratories), 50 IU of penicillin/streptomycin (Gemini Bio-Products) and 2 mmol/L of L-glutamine (Invitrogen) in a humidified atmosphere with 5% CO2 at 37°C. FHC cells were maintained in the ATCC suggested complete growth medium (DMEM:F12 medium with 25 mM HEPES supplemented with 10% FCS, 10 ng/ml cholera toxin, 0.005 mg/ml insulin, 0.005 mg/ml transferrin, and 100 ng/ml hydrocortisone).
2.3. FACS analysis, Trypan blue staining, and quantification of vacuolization
For the cell death assay, 25×104 cells/well were seeded into 6-well plates. Samples were prepared based on the instruction provided together with Annexin V Apoptosis Kit. Briefly, after treatment as indicated in the result section, the adherent and detached cells were collected and washed twice with binding buffer containing 10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl, and then 1×105 cells were resuspended in 100 μl of binding buffer. 5 μl of Annexin V-FITC and 10 μl of propidium iodide (50 μg/ml, stocking concentration) were added to the cell suspension. After gently mixing, the cells were incubated for 15 min at room temperature, and then 400 μl of binding buffer was added to get the sample ready. Quantification of cell death was performed using a FACScan (BD Biosciences). Annexin V-positive and/or PI-positive cells were considered cell death.
For Trypan Blue Staining, after treatment as indicated in the result section, the adherent and detached cells were collected and stained with Trypan Blue dye for 5 min at room temperature. Cell death is determined as the percent of cells that are stained.
For quantification of vacuolization, cells were treated as described in the result section and observed under microscope to determine the fraction of cells with obvious cytoplasmic vacuoles.
Intracellular ROS production was monitored by the permeable fluorescence dye, H2DCFDA. H2DCFDA can readily react with ROS to form the fluorescent product 2,7-dichlorofluorescein (DCF)[18]. The intracellular fluorescence intensity of DCF is proportional to the amount of ROS generated by the cells[19]. After the indicated treatment, the cells were incubated with 10 μM of H2DCFDA for thirty minutes and then cells were harvested and resuspended in PBS (106 cells/mL). The fluorescence intensity of intracellular DCF (excitation 488 nm, emission 530nm) was measured using FACScan. All the data analyses were performed using FlowJo analysis software, version 6.0 (Tree Star).
2.4. Western blot and luciferase activity assay
For western blots, after desired treatments as specified in the Results section, cells were washed twice with PBS, lysed in buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EDTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium vanadate, 1 μg/ml leupeptin, 1mM phenylmethylsulfonylfluoride]. Equal amounts of protein were loaded. Western detection was carried out using a Li-Cor Odyssey image reader. The goat anti-mouse IgG (680 nm) and goat anti-rabbit IgG (800 nm) secondary antibodies were obtained from Li-Cor.
For luciferase assay, the plasmids containing luciferase reporter gene with or without a NF-κB response element and phRL-TK plasmid for the transfection control were gifts from Liao’s lab (Ben May Department for Cancer Research, University of Chicago). 104 cells were seeded into 48-well plates for 24h and were co-transfected with 0.5 μg of plasmid containing reporter construct and 10 ng of phRL-TK using transfection reagent Effectene (Qiagen). At 24h post-transfection, the cells were treated as desired. Luciferase activity was measured with a commercial kit (Promega Dual luciferase II) on a Monolight luminometer (Becton Dickinson).
2.5. Lentivirus generation and infection
Human catalase cDNA (gift from Dr. Jian Wu, UC Davis Medical Center), and human Bcl-XL cDNA (gift from Dr. Kay Macleod, University of Chicago) were subcloned into the lentiviral expressing vector pCDH-CMV-EF1-puro (System Biosciences). Viral packaging was done according to the previously described protocol[20]. Briefly, expressing plasmid pCDH-catalse-myc or pCDH-Bcl-XL-myc, pCMV-dR8.91, and pCMV-VSV-G were co-transfected into 293T cells using the Calcium Phosphate method at 20:10:10 μg (for a 10-cm dish). The transfection medium containing calcium phosphate and plasmid mixture was replaced with fresh complete medium after incubation for 5 hours. Media containing virus was collected 48 h after transfection and then concentrated using 20% sucrose buffer at 20000g for 4 hours. The virus pellet was re-dissolved in the proper amount of complete growth medium and stocked at −80°C. Colorectal cancer cells were infected with the viruses at the titer of 100% infection in the presence of Polybrene (5μg/ml) for 48 hours, and were treated as desired.
3. Results
3.1. Ginsenoside Rh2 is significantly more potent than Rg3 in killing colorectal cancer cells
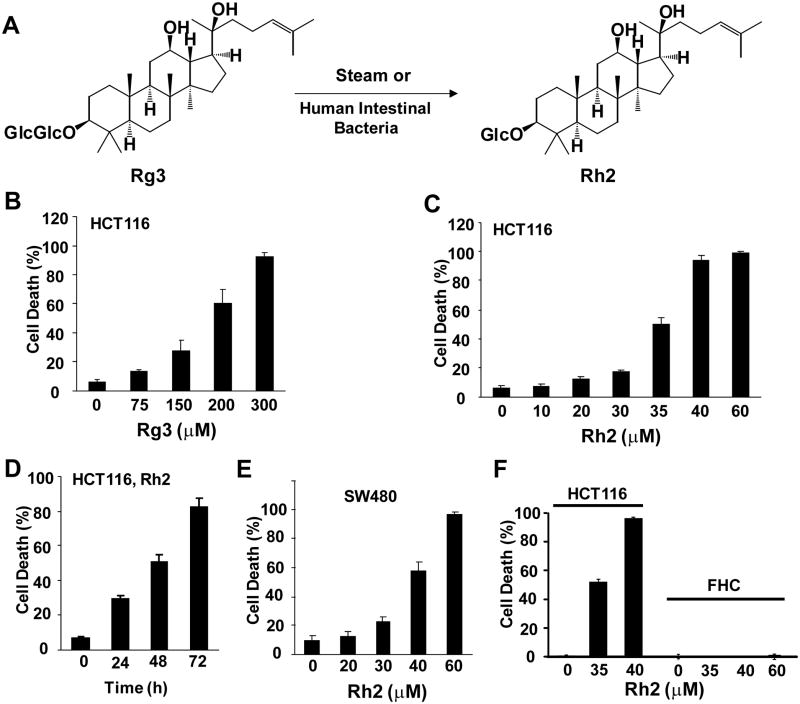
We previously reported that the steamed American ginseng root extract (S4h) induced cell death in colorectal cancer cell lines, and that ginsenoside Rg3 is the major constituent in S4h[7]. Recent analysis revealed that Rg3 as well as another ginsenoside, Rh2, is significantly enriched in S4h after the steaming process[8]. Interestingly, a recent report suggests that Rg3 may be converted to Rh2 by human intestinal bacteria[9] (Figure 1A). To evaluate the contribution of Rg3 and Rh2 in S4h induced cell death, we tested the effects of adding the purified ginsenosides Rh2 and Rg3 directly to colorectal cancer cells. Both ginsenosides exhibited a time-dependent and concentration-dependent killing of HCT116 colorectal cancer cells (Fig. 1B-D and data not shown). Rh2 had a significantly more potent toxic effect with IC50 values of around 35 μM compared to Rg3 which had an IC50 value of greater than 150 μM. Similar results were obtained for another colorectal cancer cell line, SW480 (Fig. 1E and data not shown and reference 8). In contrast, even at the highest concentration used, Rh2 did not lead to significant killing of normal human colon epithelial cells (Fig. 1F). Therefore, the ginsenoside Rh2 can specifically kill colorectal cancer cells and it is significantly more potent than Rg3. It should be pointed out that since Rg3 and Rh2 have different structure (Fig. 1A), it is possible that the apparent difference of their in vitro cytotoxic activity could be affected by how much of the drug is internalized by the cells in culture.
Figure 1. The effects of ginsenosides on colorectal cancer cell lines.
A) The structures of Rg3 and Rh2. B and C) HCT116 cells were treated with different concentrations of Rg3 or Rh2 for 48h, and the cell death was quantified. D) HCT116 cells were treated with 35 μM of Rh2 for 0–72 hours, and the cell death was determined. E) SW480 cells were treated with different concentrations of Rh2 for 48h, and cell death was measured. F) HCT116 and FHC cells were treated with different concentrations of Rh2 for 48h and cell death was determined by staining with Trypan Blue.
3.2. Treatment with the Ginsenoside, Rh2, induced increased ROS levels
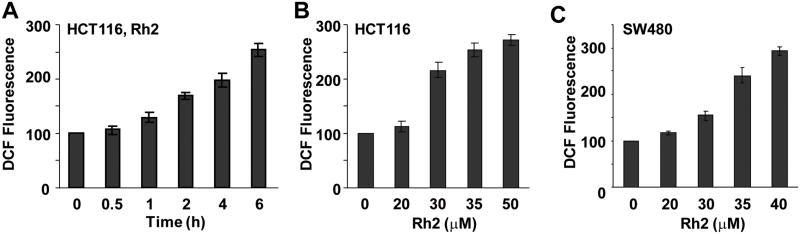
Our previous results indicated that steamed American ginseng root extract induced ROS generation in addition to cell death in colorectal cancer cells[7]. Although ROS is often reported to induce cell death, S4h-induced ROS in colorectal cancer cells protects cells from undergoing cell death by activation of the NF-κB pathway. To determine if Rh2 can also induce ROS in colorectal cancer cells, we measured the ROS levels in HCT116 and SW480 cells after they were treated with Rh2. As shown in Fig. 2, both HCT116 and SW480 cells treated with Rh2 showed time-dependent and concentration-dependent induction of ROS (Figure 2A–C and Data not shown). These results show that treatment with Rh2 induced increased ROS levels in colorectal cancer cells.
Figure 2. Ginsenoside Rh2 induced ROS generation in colorectal cancer cells.
(A) HCT116 cells were treated with 35 μM of Rh2, and then ROS level was measured at the indicated times. (B) HCT116 cells were treated with different concentrations of Rh2 for 6h, and then ROS level was determined. (C) SW480 cells were treated with different concentrations of Rh2 for 6h, and then ROS was determined.
3.3. Blocking ROS generation increased Ginsenoside-induced cell death
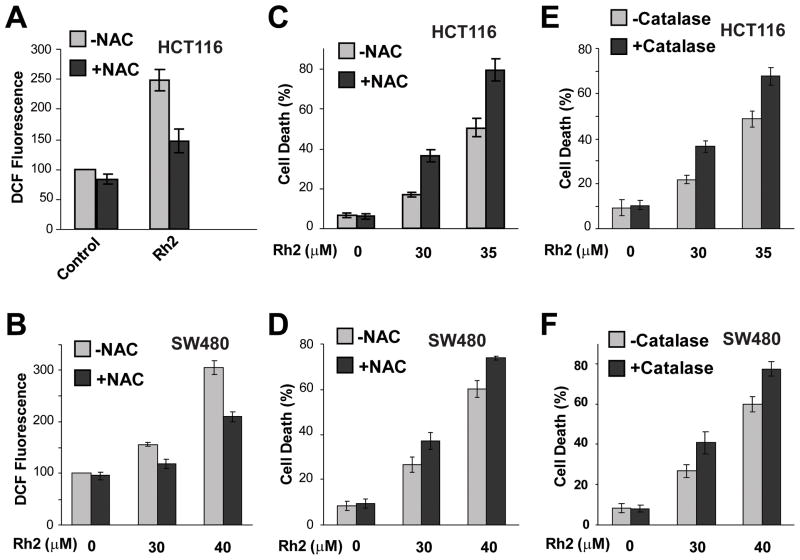
Since ROS induced by S4h protects colorectal cancer cells from cell death[7], we tested the effect of Rh2-induced ROS on colorectal cancer cells. Addition of the antioxidant, NAC, significantly reduced ROS levels in both the HCT116 and the SW480 colorectal cancer cells (Fig. 3A and B). The decreased levels of ROS correlated with significantly enhanced Rh2-induced cell death in both colorectal cancer cell lines (Figure 3C and D). Additionally, expression of catalase, a ROS scavenger enzyme, also significantly increased Rh2 induced cell death in both HCT116 and SW480 cells (Fig. 3E and F). Therefore, at the level of Rh2 used, ginsenoside Rh2-induced ROS in colorectal cancer cells helps prevent induction of cell death.
Figure 3. Antioxidants increased Rh2-induced cell death of colorectal cancer cells.
(A) HCT116 cells were treated with 35 μM of Rh2 in the presence or absence 10 mM of NAC, an antioxidant, for 6h, and then ROS was determined. (B) SW480 cells were treated with 30 or 40 μM of Rh2 in the presence or absence 10 mM of NAC for 6h. (C and D) HCT116 cells or SW480 cells were treated with Rh2 in the presence or absence 10 mM of NAC for 48h, and the cell death was measured. (E and F) HCT116 (E) or SW480 (F) cells with or without Catalase expression were treated with Rh2 for 48h and the levels of cell death were determined.
3.4. NF-κB pathway is activated by Rh2-induced ROS and contributes to cell survival
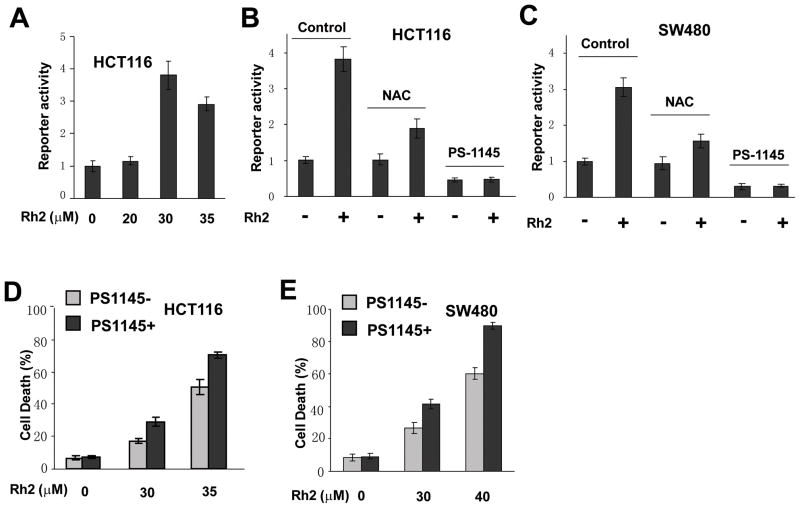
ROS promotes cell survival in colorectal cancer cells via activation of the NF-κB pathway [7]. To determine whether Rh2 induced ROS also leads to activation of the NF-κB pathway, we measured NF-κB reporter activity after treating cells with Rh2. A significant level of NF-κB reporter activity was induced by Rh2 treatment at the same concentration that led to high levels of ROS (Fig. 4A and Fig. 2B). If the increased level of ROS is required for the induction of NF-κB signaling, then decreasing the level of ROS in the cell should lead to a decrease in NF-κB reporter activity. Indeed, addition of NAC significantly blocked the Rh2-mediated induction of NF-κB transcriptional activity in both HCT116 and SW480 colorectal cancer cells (Figure 4B and C), suggesting that Rh2-induced ROS contributes to the activation of the NF-κB pathway. Since a decrease in ROS led to a decrease in NF-κB transcriptional activity we expected that the activated NF-κB pathway would counteract Rh2 induced cell death in colorectal cancer cells. To test this hypothesis, we inhibited the NF-κB pathway by adding PS-1145, a specific inhibitor of NF-κB pathway, to Rh2 treated HCT116 and SW480 cells and measured the cell death. PS-1145 significantly inhibited both the basal as well as the Rh2-induced NF-κB transcriptional activity (Figure 4B and C) and increased Rh2-induced cell death in both HCT116 and SW480 cells (Figure 4D and E). These results indicated that Rh2-induced ROS contributed to the survival of colorectal cancer cells via activation of the NF-κB pathway.
Figure 4. Gisenosides activated NF-κB pathway via ROS.
(A) Twenty four hours after transient transfection of NF-κB reporter plasmids, HCT116 cells were treated with different concentrations of Rh2 for 24 h before the luciferase reporter activity was determined. (B and C) After HCT116 cells or SW480 cells were treated with 30 μM Rh2 for 24h in the presence or absence of NAC (10 mM) or PS1145 (50 μM), NF-κB reporter activity was determined. (D and E) HCT116 or SW480 cells were treated with PS1145 (50 μM) for 48 hours and cell death was measured.
3.5. Rh2-induces both caspase-dependent apoptosis and caspase-independent papraptosis
Apoptosis is a type of programmed cell death that is caspase-dependent[10]. In order to determine whether Rh2-induced cell death was caspase-dependent, we pre-treated HCT116 cells with a pan-inhibitor of caspases, Z-VAD-fmk, and measured the effects of Rh2 on cell death induction. As shown in Fig. 5A, 100 μM of Z-VAD-fmk partially inhibited Rh2-induced cell death in HCT116 cells, indicating that Rh2 induced cell death is partially caspase-dependent apoptotic cell death. To determine whether the cell death was dependent or independent of mitochondria in Rh2-induced apoptosis, the levels of pro and anti apoptotic mitochondria proteins Bad, Bax, Bcl-2, and Bcl-XL were visualized by western blot from protein extracts from cells treated with Rh2. A time-dependent increase in the levels of Bax and Bad were observed in HCT116 cells when treated with Rh2 (Fig. 6A). Rh2 treatment also led to reduced levels of the anti-apoptotic proteins Bcl-2 and Bcl-XL (Figure 6A). In support of the hypothesis that the Bcl-2 family of pro and anti apoptotic proteins are important for Rh2-induced cell death, the overexpression of Bcl-XL partially protected HCT116 cells against Rh2-indcued cell death (Figure 6B). These results indicate that Rh2-induced cell death is mediated in part by mitochondria-dependent apoptosis.
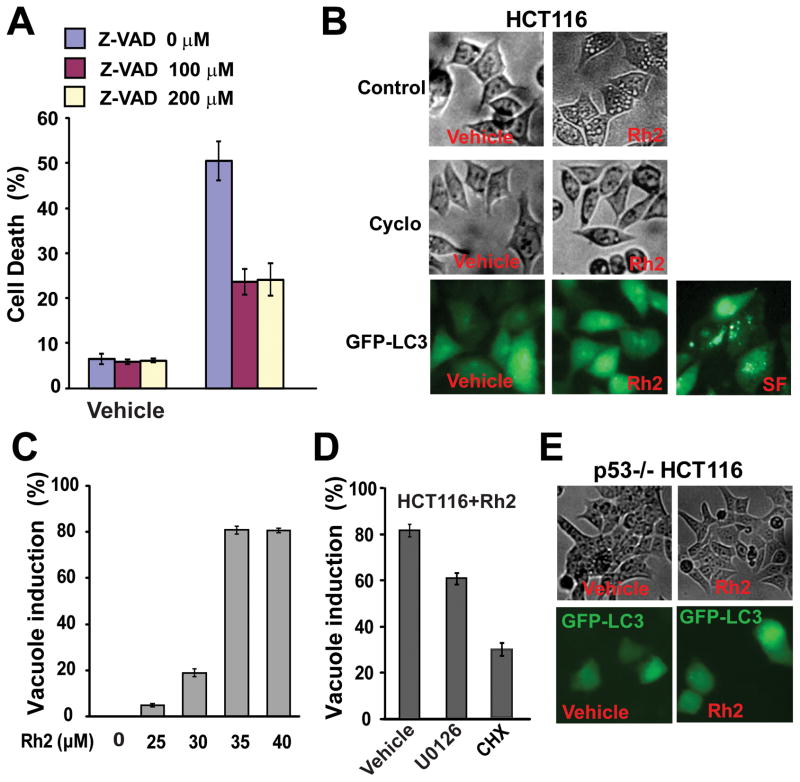
Figure 5. Ginsenoside Rh2 induced apoptosis and paraptosis-like cell death.
(A) HCT116 cells were treated with 35 μM of Rh2 with or without the indicated amount of the pan-inhibitor of caspases, Z-VAD, for 48h, and then the cell death was determined. (B) HCT116 cells were treated with 35 μM of Rh2 with or without 10μg/ml of cycloheximide for 6 hours and were imaged (top and middle). HCT116 cells expressing EGFP-LC3 were treated 35 μM of Rh2 for 6 hours and were imaged with a fluorescence microscope (bottom). The serum-free (SF) treatment for 48h induced autophagy, and was taken as the positive control. (C) HCT116 cells were treated with the indicated concentration of Rh2 for 6 hours and the fraction of cells with cytoplasmic vacuoles were determined. (D) HCT116 cells were treated with 35 μM of Rh2 with either vehicle control, 10 μM U0126, or 10μg/ml cycloheximide for 6 hours. The fraction of cells with cytoplasmic vacuoles were determined. (E) HCT116/p53−/− cells were treated with 35 μM of Rh2 for 6h and were imaged (top). HCT116/p53−/− cells expressing EGFP-LC3 were treated 35 μM of Rh2 for 6h and imaged with fluorescence microscope (bottom)
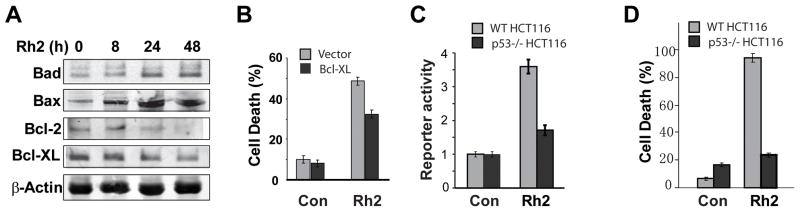
Figure 6. Rh2 treatment resulted in altered levels of apoptosis-associated proteins and activated transcriptional activity of p53.
(A) HCT116 cells were treated with 35 μM of Rh2, and then protein samples were collected at different times for Western blot analysis. (B) HCT116 cells expressed Bcl-XL or empty vector were treated with 35 μM of Rh2 for 48h, and the cell death was determined. (C) Twenty four hour after transient transfection of p53 reporter plasmids and treatment with 35 μM of Rh2, the luciferase reporter activity was measured. (D) Cells were treated with 35 μM Rh2 for 48h, and then cell death was quantified.
Z-VAD only partially blocked Rh2-induced cell death (Fig. 5A). Further increasing the concentrations of Z-VAD from 100 μM to 200 μM did not lead to further inhibition of Rh2-induced cell death (Fig. 5A). These results suggested that Rh2-induced cell death is partially caspase-independent (non-apoptosis). Paraptosis is a type of programmed cell death that is characterized by the visible cytoplasmic vacuolization. Induction of paraptosis has been shown to require new protein synthesis but is resistant to inhibition by caspase inhibitors[16]. While HCT116 cells treated with vehicle controls did not show any vacuoles, increasing the level of Rh2 induced an increasing fraction of cells that exhibited cytoplasmic vacuolization (Fig. 5B top, Fig. 5C). Moreover, the pre-treatment of HCT116 cells with cycloheximide (CHX), a protein synthesis inhibitor, blocked Rh2-induced vacuole formation (Fig. 5B middle, Fig. 5D). In addition, MAP kinase activation has been shown to be important for paraptosis[17]. To determine the involvement of MAP kinase in Rh2 induced vacuole formation, U0126, a specific inhibitor of MEK1 and 2, was used. As shown in Fig. 5D, U0126 significantly reduced the fraction of cells with vacuole formation (P<3×10−16).
The microtubule-associated light chain 3 (LC3) is incorporated into autophagosomes upon autophagy induction, which can be visualized by the foci formation of a GFP tagged LC3 (GFP-LC3)[14]. To determine if Rh2-induced vacuolization is associated with autophagy, we examined GFP-LC3 foci formation following Rh2 treatment. We found that Rh2 did not induce GFP-LC3 foci formation even though significant level of GFP-LC3 foci was observed with serum-free treatment, a treatment which induces autophagy (Fig. 5B bottom). Furthermore, chloroquine, an inhibitor of autophagy, did not affect Rh2-induced cell death or vacuole formation (data not shown). Therefore, these results indicate that Rh2 does not induce autophagy. Taken together, our results suggest that Rh2-induced cell death in colorectal cancer cells is mediated in part by the caspase-dependent apoptosis and in part by the caspase-independent paraptosis-like cell death.
Bax is a key target of the p53 transcription factor in apoptosis[21]. The increased levels of Bax protein induced by treatment of the cells with Rh2 raised the possibility that Rh2 treatment activates the p53 tumor suppressor. To test this idea, HCT116 cells were treated with Rh2 and the expression of a p53 reporter was quantified. Strong activation of the p53 reporter gene expression by Rh2 was observed in WT HCT116 cells but not in HCT116 cells when p53 was knocked out (Fig. 6C). To further determine the effect of p53 on Rh2 induced cell death, we compared the effect of Rh2 on cell death induction in WT and p53 mutant HCT116 cells. Interestingly, knockout of p53 dramatically decreased Rh2 induced cell death, indicating that p53 is involved in Rh2 induced cell death (Fig. 6D). Removal of p53 significantly inhibited Rh2-induced vacuole formation without affecting GFP-LC3 foci formation (Fig. 5E). Only 10.1±0.7% of p53−/− HCT116 cells showed vacuoles as compared to 81±2.3% of the WT HCT116 cells. These results suggest that p53 contributes to both apoptosis as well as paraptosis-like cell death induced by Rh2 in colorectal cancer cells (Fig. 7).
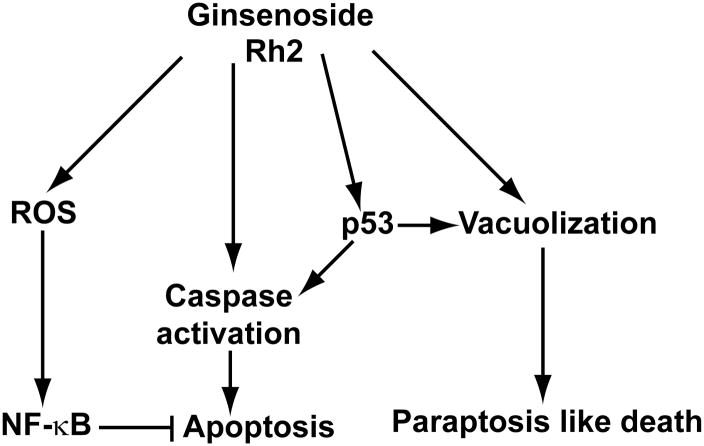
Figure 7. A working model for the action of ginsenoside Rh2 on colorectal cancer cells.
Ginsenoside Rh2 induces both apoptosis and paraptosis in colorectal cancer cells. In addition, Rh2 also activates ROS-NF-κB pathway, which counteracts cell death induction.
4. Discussion
Rg3 and Rh2 are the main bioactive components in steamed American ginseng extracts, which exhibited potent ability to kill colorectal cancer cells. In this report, we showed that Rh2 is significantly more potent at inducing cell death in colorectal cancer cells than Rg3 and that Rh2-induced cell death in colorectal cancer cells is mediated partially by caspase-dependent apoptosis and caspase-independent paraptosis-like cell death. Furthermore, we show that both types of cell death induced by Rh2 in colorectal cancer cells are p53-dependent.
Apoptosis and paraptosis are two different types of programmed cell death. Both are accompanied by the phosphatidylserine translocation from the inner (cytoplasmic) leaflet of the plasma membrane to the outer (cell surface) leaflet and can be detected by Annexin V staining[22]. Besides morphological differences, a key difference between the two types of cell death is that apoptosis is generally caspase-dependent while paraptosis is caspase-independent. Our results that Rh2 significantly alters levels of the Bcl-2 family of pro and anti apoptotic proteins in conjunction with the observation that inhibition of caspases by Z-VAD partially inhibited Rh2-induced cell death in colorectal cancer cells indicate that a mitochondria-dependent apoptosis contributes to Rh2-induced cell death. Consistent with this, overexpression of Bcl-XL significantly suppressed Rh2-induced cell death.
In addition to inducing apoptosis, Rh2 also induced extensive cytosolic vacuolization in HCT116 cells, suggesting the involvement of paraptosis. Rh2 induced vacuolization was significantly inhibited by cycloheximide and MEK1/2 specific inhibitor U0126, indicating the requirements for protein translation and MAP kinase signaling, which are also consistent with parapotosis. In addition, Rh2 induced cytosolic vacuolization is not due to autophagy since Rh2 did not induce autophagosome formation in these colorectal cancer cells. The pan-caspases inhibitor Z-VAD can only partially inhibit Rh2 induced cell death and further increasing the level of Z-VAD did not further decrease Rh2-induced cell death or decrease the level of cytosolic vacuolization suggesting that there is another type of cell death being induced which exhibits vacuolization. Taken together these results suggest that Rh2 also induces a paraptosis-like cell death in colorectal cancer cells. Interestingly, although 48 hour treatment of HCT116 cells with CHX led to 29.6% cell death, similar level of cell death was observed when HCT116 cells were treated with Rh2 alone or Rh2+CHX (52.2±1.8% and 53.0±1.9%, P=0.6). It is likely that the effect of CHX on paraptosis is compensated by the cytotoxic effect of CHX alone. It is worth noting that although the effectiveness of chemotherapeutic drugs generally depend on their ability to induce cell death in cancer cells, there are forms of chemotherapy-induced cell death that cannot readily be classified as apoptosis or necrosis but fit more in the apoptosis-like/necrosis-like programmed cell death model[12]. It has been suggested that multiple cell death program can be activated in the same cells and the dominant cell death phenotype is determined by the relative speed of those death programs [12; 23]. As cancer cells often developed some resistance to undergoing apoptosis, it is possible that such alternative forms of programmed cell death play important roles in cancer therapy. In support of this notion, chemotherapeutic agents such as Paclitaxel, Arsenic trioxide, Doxorubicin, etc can all induce caspase-independent cell death[12].
Consistent with previous reports that Rh2 can induce ROS in some cell lines [24], we found that Rh2 increased ROS level in HCT116 cells. ROS has been reported either to induce cell death or to activate some survival pathways to protect cells from death. The exact effect of ROS on a particular cell type is likely depends on the cell type involved and the nature and levels of ROS induced. Similar to our observation with steamed ginseng extracts, we found that ROS scavengers, NAC and catalase, block ROS generation and enhance Rh2-induced cell death in colorectal cancer cells. We showed that this survival effect of ROS in colorectal cancer cells is mediated by the activation of the NF-κB survival pathway.
Given the results presented here we propose a model in which Rh2 treatment activates the NF-κB pathway to protect the cells from cell death while simultaneously activates two different cell death pathways. Rh2 treatment likely activates the p53 pathway, which contributes to the induction of both apoptosis and parapotosis-like cell death.
Since Rg3 can be metabolized to Rh2 and to PPD by human intestinal bacteria, it will be interesting to determine the effect of PPD on colorectal cancer cells in comparison to Rh2 and Rg3. Furthermore our results about the effects of anti oxidants on Rh2-induced cell death in colorectal cancer cells suggest that steamed American ginseng or its ginsenosides in combination with the safe antioxidants can potentially be used as chemoprevention agents to prevent the development of colorectal cancers.
Acknowledgments
We would like to thank Drs. Jian Wu for the Human catalase cDNA, Richard Hiipakka and John Kokontis for help with the Luciferase activity assay, Kay Macleod for the Bcl-XL and pEGFP-LC3 plasmid. This work was supported in part by grants from the NIH/NCCAM AT004418, NIH GM074197, NIH CA106569.
Abbreviations
- DCF
2,7-dichlorofluorescein
- H2DCFDA
5-(and-6)-chloromethyl-2′7′-dichlorodihydrofluorescein diacetate acetyl ester
- NAC
N-Acetyl-L-cysteine
- PCD
programmed cell death
- ROS
reactive oxygen species
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.da Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol. 2001;1:364–369. doi: 10.1016/s1471-4892(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 3.Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- 4.Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–674. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo X, Wang CZ, Chen J, Song WX, Luo J, Tang N, He BC, Kang Q, Wang Y, Du W, He TC, Yuan CS. Characterization of gene expression regulated by American ginseng and ginsenoside Rg3 in human colorectal cancer cells. Int J Oncol. 2008;32:975–983. [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Wang CZ, He TC, Yuan CS, Du W. Antioxidants potentiate American ginseng-induced killing of colorectal cancer cells. Cancer Lett. 2009;289:62–70. doi: 10.1016/j.canlet.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CZ, Li XL, Wang QF, Mehendale SR, Fishbein AB, Han AH, Sun S, Yuan CS. The mitochondrial pathway is involved in American ginseng-induced apoptosis of SW-480 colon cancer cells. Oncol Rep. 2009;21:577–584. doi: 10.3892/or_00000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae EA, Han MJ, Choo MK, Park SY, Kim DH. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 10.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 11.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 12.Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 13.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 14.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 16.Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci U S A. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperandio S, Poksay K, de Belle I, Lafuente MJ, Liu B, Nasir J, Bredesen DE. Paraptosis: mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004;11:1066–1075. doi: 10.1038/sj.cdd.4401465. [DOI] [PubMed] [Google Scholar]
- 18.Robinson JP, Carter WO, Narayanan PK. Oxidative product formation analysis by flow cytometry. Methods Cell Biol. 1994;41:437–447. doi: 10.1016/s0091-679x(08)61733-1. [DOI] [PubMed] [Google Scholar]
- 19.Huang RF, Huang SM, Lin BS, Hung CY, Lu HT. N-Acetylcysteine, vitamin C and vitamin E diminish homocysteine thiolactone-induced apoptosis in human promyeloid HL-60 cells. J Nutr. 2002;132:2151–2156. doi: 10.1093/jn/132.8.2151. [DOI] [PubMed] [Google Scholar]
- 20.al Yacoub N, Romanowska M, Haritonova N, Foerster J. Optimized production and concentration of lentiviral vectors containing large inserts. J Gene Med. 2007;9:579–584. doi: 10.1002/jgm.1052. [DOI] [PubMed] [Google Scholar]
- 21.Selvakumaran M, Lin HK, Miyashita T, Wang HG, Krajewski S, Reed JC, Hoffman B, Liebermann D. Immediate early up-regulation of bax expression by p53 but not TGF beta 1: a paradigm for distinct apoptotic pathways. Oncogene. 1994;9:1791–1798. [PubMed] [Google Scholar]
- 22.Wang Y, Li X, Wang L, Ding P, Zhang Y, Han W, Ma D. An alternative form of paraptosis-like cell death, triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell Sci. 2004;117:1525–1532. doi: 10.1242/jcs.00994. [DOI] [PubMed] [Google Scholar]
- 23.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–581. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 24.Ham YM, Lim JH, Na HK, Choi JS, Park BD, Yim H, Lee SK. Ginsenoside-Rh2-induced mitochondrial depolarization and apoptosis are associated with reactive oxygen species- and Ca2+-mediated c-Jun NH2-terminal kinase 1 activation in HeLa cells. J Pharmacol Exp Ther. 2006;319:1276–1285. doi: 10.1124/jpet.106.109926. [DOI] [PubMed] [Google Scholar]