Abstract
The proper level of proliferation and differentiation along the proximodistal axis is crucial for lung organogenesis. Elucidation of the factors that control these processes will therefore provide important insights into embryonic lung development and regeneration. Eya1 is a transcription factor/protein phosphatase that regulates cell lineage specification and proliferation. Yet its functions during lung development are unknown. In this paper we show that Eya1-/- lungs are severely hypoplastic with reduced epithelial branching and increased mesenchymal cellularity. Eya1 is expressed at the distal epithelial tips of branching tubules as well as in the surrounding distal mesenchyme. Eya1-/- lung epithelial cells show loss of progenitor cell markers with increased expression of differentiation markers and cell cycle exit. In addition, Eya1–/– embryos and newborn mice exhibit severe defects in the smooth muscle component of the bronchi and major pulmonary vessels with decreased Fgf10 expression. These defects lead to rupture of the major vessels and hemorrhage into the lungs after birth. Treatment of Eya1-/- epithelial explants in culture with recombinant Fgf10 stimulates epithelial branching. Since Shh expression and activity are abnormally increased in Eya1-/- lungs, we tested whether genetically lowering Shh activity could rescue the Eya1-/- lung phenotype. Indeed, genetic reduction of Shh partially rescues Eya1-/- lung defects while restoring Fgf10 expression. This study provides the first evidence that Eya1 regulates Shh signaling in embryonic lung, thus ensuring the proper level of proliferation and differentiation along the proximodistal axis of epithelial, mesenchymal and endothelial cells. These findings uncover novel functions for Eya1 as a critical upstream coordinator of Shh-Fgf10 signaling during embryonic lung development. We conclude, therefore, that Eya1 function is critical for proper coordination of lung epithelial, mesenchymal and vascular development.
Keywords: lung development, Eya1, proliferation, differentiation, Shh, Fgf10
INTRODUCTION
The development of mammalian lung begins when two primary buds consisting of an inner epithelial layer surrounded by mesenchyme arise from the larygotracheal groove in the ventral floor of the primitive foregut. These buds undergo stereotypic rounds of branching and outgrowth to give rise to a tree-like respiratory organ which contains different specialized epithelial cell types organized along the proximodistal axis (Cardoso, 2000; Metzger et al, 2009; Warburton et al., 2000, 2008). Controlled and rapid proliferation of lung epithelial progenitors, in association with the outgrowth and branching of the epithelial tubes, is essential for producing an alveolar gas diffusion surface sufficient to sustain extra-uterine life. The undifferentiated epithelial cells leave the progenitor pool to give rise first to lineage-committed precursor cells and eventually to fully differentiated cell lineages (Cardoso and Lü, 2006; Rawlins and Hogan, 2006). Developmental defects in this progression lead to defective differentiation and postnatal respiratory distress (i.e. pulmonary hypoplasia), a hallmark of congenital lung diseases.
Epithelial-mesenchymal interactions are crucial to the formation and patterning of the mammalian lung. The pulmonary mesenchyme gives rise to several different cell types, including smooth muscle cells of the upper airways during branching morphogenesis. Moreover, the mesenchyme produces many important growth factors and signaling molecules required for airway epithelial branching and development, e.g. members of the fibroblast growth factor (Fgf) family (reviewed by Warburton et al., 2000, 2005, Morrisey and Hogan, 2010). In turn, the epithelium produces signaling molecules essential for mesenchymal proliferation and differentiation including sonic hedgehog (Shh) and bone morphogenetic protein 4 (BMP4) (Bellusci et al., 1996, 1997a,b; Litingtung et al., 1998; Pepicelli et al., 1998).
In mammals, Eya (Eya1-4) and sine oculis (Six) genes are co-expressed and exhibit synergistic genetic interactions to regulate the development of multiple organs by controlling cell cycle regulators and inhibition of apoptosis (Xu et al., 1997b; Ford et al., 1998; Coletta et al., 2004). Both Eya1-/- and Six1-/- mouse embryos have defects in the proliferation and survival of the precursor cells of multiple organs and die at birth (Xu et al., 1999, 2002; Li et al., 2003; Zou et al., 2004). Mutations in the Eya1 protein, which is endowed with a phosphatase activity (Li et al., 2003; Jemc and Rebay, 2007) are responsible for Branchio-Oto-Renal and Oto-Facio-Cervical syndromes (Abdelhak et al., 1997; Estefania et al., 2005). Eya1 encodes a transcription coactivator containing a divergent N-terminal activation domain and a conserved C-terminal Eya domain that mediates protein–protein interactions with Six proteins (Xu et al., 1997a,b; Pignoni et al., 1997). The phosphatase function of Eya1 switches Six1 function from repression to activation in the nucleus causing transcriptional activation through recruitment of co-activators providing a mechanism for activation of specific gene targets including those regulating precursor cell proliferation/survival during organogenesis (Li et al., 2003). Yet, the specific functions of Eya1 in lung development are unknown.
Herein, we identify a functional role for mammalian Eya1 in lung epithelial development. The lungs of Eya1-/- mice are severely hypoplastic with greatly reduced epithelial branching and increased mesenchymal cellularity and show extensive hemorrhage at birth. Eya1-/- lung epithelial cells show loss of progenitor cell markers, with increased expression of differentiation markers and cell cycle exit. In addition, Eya1-/- embryos and neonates show vascular smooth muscle defects, which lead to vessel rupture and hemorrhage. Eya1-/- lung defects are partly attributed to increased expression and activity of Shh signaling. Furthermore, partial inactivation of the Shh gene in Eya1-/- lungs partially rescues the epithelial, mesenchymal and endothelial defects.
MATERIALS AND METHODS
Animals and genotyping
Eya1 knockout (KO) mice on the 129/SvEv background and Fgf10LacZ/+ mice have been published previously (Xu et al., 1999, De Moerlooze et al., 2000, Mailleux et al., 2005). Shh f/f mice were from JAX and Spc-rtTA+/- mice were provided by Dr J. Whitsett (Perl et al., 2002). Eya1+/-;Shhf/WT female mice were generated by intercrossing Eya1+/- mice with the Shh f/f mouse line. Eya1+/--Spc-rtTA+/--tet(o) Cre+/- mice were generated by intercrossing Eya1+/- mice with the Spc-rtta+/--tet(o) Cre+/+ mouse line previously generated in our laboratory. The resulting Eya1+/--Spc-rtTA+/--tet(o) Cre+/- mouse males were intercrossed with Eya1+/-;Shhf/WT females to decrease 50% of Shh activity in the distal epithelium by generating Eya1-/-; Spc-rtTA+/--tet(o) Cre+/--ShhΔ/WT mutant mice in a mixed 129/C57BL6 background for analysis. Pregnant Eya1+/-;Shhf/WT females were maintained on doxycycline containing food (Rodent diet with 0.0625% Doxycycline, Harlan Teklad, TD01306) from E6.5 until sacrificed. Ten compound mutant embryos were generated at expected Mendelian ratios and examined at different stages. Genotyping of mice and embryos was performed as previously described (Xu et al., 1999, 2002, Perl et al., 2002). Wildtype littermates were used as controls.
Phenotype analysis, in situ hybridization (ISH) and Real-time PCR
Histological staining and ISH using Eya1 probe were performed as previously described (Xu et al., 1997a; De Langhe et al., 2006). Real-time PCR and semi-Quantitative PCR were performed in triplicate as previously described (del Moral et al. 2006a,b). PCR primers used in this study are described in Table 1 in the Supplemental Data.
Antibody/X- gal staining, BrdU labeling and Western blot
Antibody/X-gal staining on paraffin sections or fixed MLE-15 cells and bromodeoxyuridine (BrDU) labeling were performed using standard protocols as described previously (Tefft et al., 2005; El-Hashash et al., 2005; del Moral et al. 2006a,b). Preparations of cell lysates and Western blot were performed as described (Tefft et al., 2005). Antibodies used in this study are described in Table 2 in the Supplemental Data. Staining and Western blot were performed in triplicate. For all immunohistochemistry, mutant sections were mounted on the same slides of wildtype control sections and processed for antibody staining under the same conditions.
Isolated epithelial explant cultures
Lungs from E12.5 embryos were treated with dispase (BD Biosciences) for 5 min at 4 °C then transferred to Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12 (DMEM:F12) with 10% fetal bovine serum (FBS; Invitrogen) to block the enzymatic reaction. Epithelial buds were separated from the mesenchyme, embedded in 200 μl of Matrigel diluted 1:1 with DMEM:F12 serum-free media containing 250 ng/ml recombinant Fgf10 (R&D Systems) then grown in culture for 48 hours as described previously (Carraro et al., 2009).
Cell culture and transfection
Lung epithelial cells MLE15 were grown in culture then treated and processed for transfection as described by Tefft (Tefft et al., 2002). To insure transfection MLE15 cells were seeded in eight chamber slides (BD Biosciences) at 50 % confluency. The next day these cells were transfected with either 200 ng of expression vector for mouse Eya1 or a luciferase-expressing vector (control) using lipofectamine LTX (Invitrogen). Fresh medium was added 6 hour later. Cells were fixed with 4%PFA three days after transfection as above. We used an expression vector encoding a VP16 fusion protein, and the transfection efficiency was monitored by immunostaining using anti-VP16 antibody.
Data and statistical analysis
Data are presented as means ± SD. Statistical significance between wildtype control and mutant lungs was calculated using Student's t-test. For counting PHH3-, Caspase-3 or SP-C positive cells, labeled mesenchymal cells or epithelial cells and total cell number per section were counted. Three random fields were chosen, 400–800 cells per field, from three different embryos of each indicated genotype. The Mitotic index was determined as the ratio of mitotic cells to the total cell number, and the percent of labeled cells was reported. Analyses were done using GraphPad Prism software (GraphPad Software, Inc., San Diego). Protein quantification was produced by the software Image J.
RESULTS
Eya1 expression pattern in embryonic lung
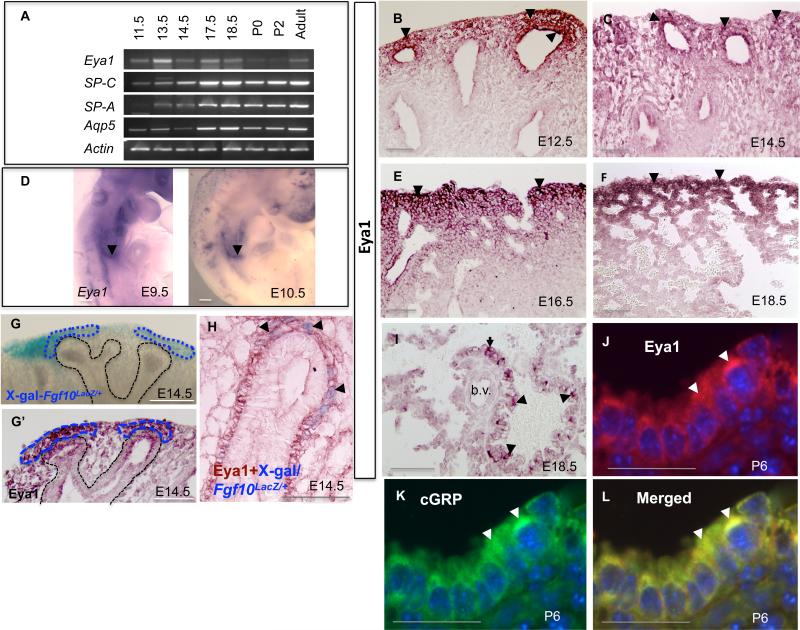
RT-PCR showed that levels of Eya1 transcripts are highest during initiation and branching phases of lung development at E9.5-E11.5 and E11.5-E17.5 respectively in mouse then decline significantly during the differentiation phase (from E18.5) and then continue to be expressed in the adult lung (Fig. 1A). Eya1 transcripts were detected in the lung bud from E9.5 (Fig. 1D) and in the pharyngeal region, ear and somites (Fig. 1D and Xu et al., 1999; Zoe et al., 2006). Eya1 protein was more specifically expressed in distal lung mesenchymal and epithelial cells from around E12.5 to E18.5 (Fig. 1B,C,E,F). Eya1 protein was seen within both the cytoplasm and the nucleus as reported in other organs (Fig. 1G’,H; Fougerousse et al., 2002; Rayapureddi et al., 2003). The specificity of Eya1 antibody was confirmed by Western blotting of lung proteins that detected a proper expected protein band corresponding to Eya1 protein size (data not shown).
Fig. 1. Expression of Eya1 during lung development.
(A) RT-PCR shows Eya1 gene expression compared to the expression of epithelial differentiation cell markers (SP-C, SP-A and Aqp5). Immunoperoxidase staining (B,C,E,F) and in situ hybridization (D) show Eya1 expression in lung buds, and in the distal epithelial tips and distal mesenchyme (arrowheads). Both X-gal staining of Fgf10LacZ/+ lungs (blue; G) and Eya1 immuno-peroxidase staining (brown; G’) show expression of Fgf10 and Eya1 in the distal mesenchyme (blue circled areas). (H) Double staining of X-gal-stained Fgf10LacZ/+lungs with Eya1 antibody shows expression of Eya1 and Fgf10 in distal mesenchyme (arrowheads; 5 μm section). (I) Expression of Eya1 in presumed PNE airway epithelial cells (arrowheads) and at the sharp boundary between the terminal bronchioles and the future alveoli (arrow). Eya1 is absent in blood vessels (b.v). (J-L) Immunolocalization of Eya1 (J; arrowheads) in cGRP-expressing NEB cells (K; arrowhead) on day 6 after birth. (L) Merged images of Eya1, DAPI (blue) and cGRP, with co-staining in yellowish green. Scale bars: 50 μm.
LacZ expression in Fgf10LacZ/+ mouse lung can be used as a reporter for Fgf10 expression (Mailleux et al., 2005). Interestingly, X-gal staining of E14.5 Fgf10LacZ/+ lungs and Eya1 antibody staining show a striking similarity in the focal expression pattern of Fgf10 and Eya1 in the distal mesenchyme adjacent to distal tips of branching tubules (Fig. 1G; G’,H). Double staining of X-gal-stained Fgf10LacZ/+ lungs with Eya1 antibody further confirmed the expression of both Eya1 and Fgf10 in the distal mesenchyme (Fig. 1H).
At E18.5, Eya1 protein was seen along the proximal to distal axis of lung epithelium in presumed pulmonary neuroendocrine cells (PNE; Fig. 1I) and at the sharp boundary between the terminal bronchioles and the future alveoli that corresponds to the location of the future bronchio-alveolar duct junction (BADJ; Fig. 1I). Eya1 expression pattern in the PNE cells was also observed postnatally where it was co-expressed with cGRP (Fig. 1J-L). Thus, Eya1 protein continues to be expressed postnatally in cells with putative lung epithelial progenitor potential: neuroendocrine bodies (NEB; Pan et al., 2000; Reynolds et al., 2000; Hong el al., 2001; Giangreco et al., 2004; Fig. 1J-L).
Eya1–/– embryos die quickly after birth due to respiratory failure and display lung hypoplasia
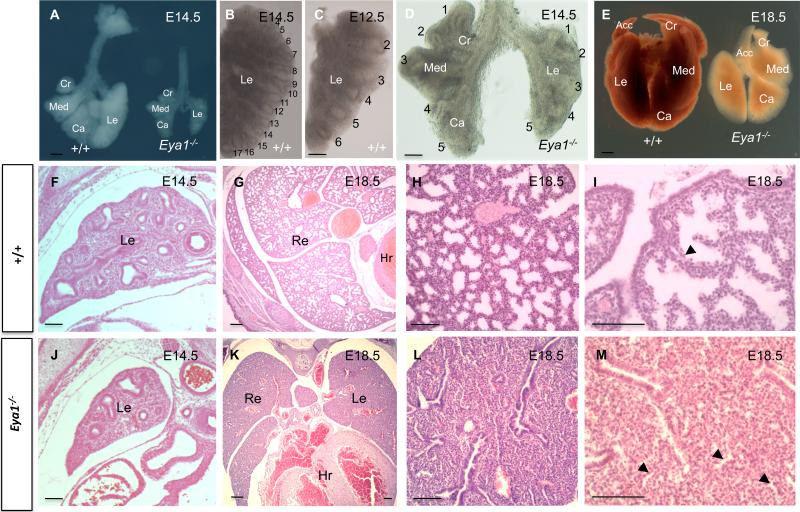
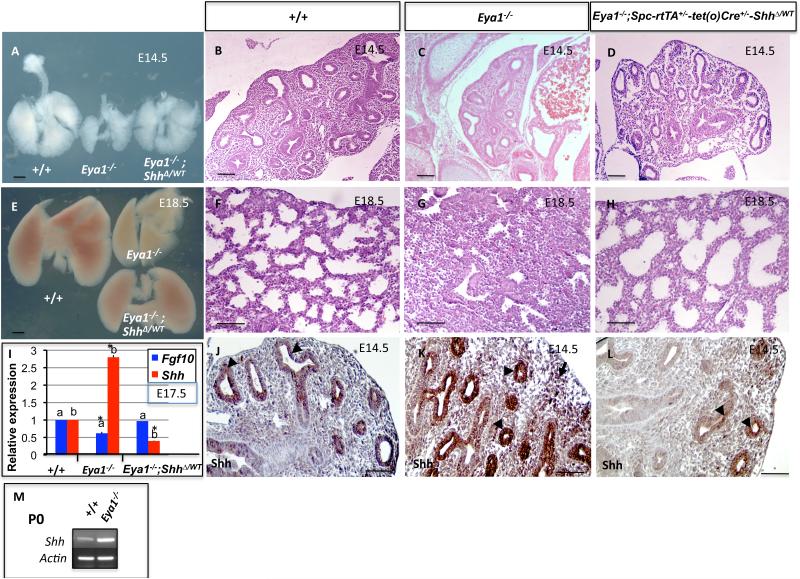
Eya1–/– embryos die quickly after birth, have defects in several organs and are relatively smaller in size than wildtype littermates (Xu et al., 1999, 2002). Observation of the newborn pups revealed that 25% of them, those genotyped as Eya1–/– mice, gasped for breath and appeared cyanotic. When compared with the lungs of wildtype littermates, the lungs of E18.5 Eya1–/– embryos were significantly smaller in appearance (Fig. 2E) but had normal lobation (the accessory lobe was deleted during dissection; Fig. 2A,D). Hematoxylin and Eosin stained sections from wildtype versus Eya1–/– mice showed that, as expected, E18.5 wildtype lungs were properly fluid inflated (Fig. 2G,H). However, Eya1–/– lungs were not inflated and had a smaller, collapsed appearance (Fig. 2K,L,E). Furthermore, Eya1-/- lungs were severely hypoplastic and morphogenesis of E18.5 mutant lungs appeared to be arrested in the pseudoglandular stage of development (Fig. 2G,H, K,L). Eya1-/- lungs showed an increase in the ratio of interstitial mesenchyme to epithelial tubules. Also, expansion of epithelial tubules did occur in mutant lungs but was greatly reduced compared to wildtype lungs (compare Fig. 2K,L,M with G,H,I). Primitive pre-alveoli were also greatly reduced in Eya1-/- lungs (Fig. 2L,M) with different degrees of severity (compare Figs. 2K-M and 4B,F). Thus, E18.5 mutant lungs depicted the histopathological features of pulmonary hypoplasia including small sized lungs, greatly reduced branching morphogenesis, narrow bronchi, arrested expansion of epithelial tubules and dense mesenchymal cellularity as well as the apparent failure of normal lung maturation (interstitial condensation), which should occur in late lung development (Fig. 2L,M). In addition, Eya1–/– mice exhibited pulmonary hemorrhage particularly around the large blood vessels (Figs. 2K, 8B,F). These results suggest that Eya1–/– mice die quickly after birth because of respiratory failure and the lungs of these mice display several severe hypoplastic defects including lack of inflation and pulmonary hemorrhage.
Fig. 2. Eya1-/- lungs are hypoplastic.
External appearance (A-E) and histology (F-M) of control and mutant lungs show severely hypoplastic lung phenotype in Eya1-/- embryos at E14.5 and E18.5. (B-D) Reduction of lung size and branching in E14.5 mutant lungs, which have fewer epithelial branches (number) than E14.5 and E12.5 control lungs. (F,J). Hematoxylin and Eosin staining shows reduced branching in E14.5 Eya1-/- lungs. (G-I,K-M). Low and high magnifications show histology of wildtype and Eya1-/- lungs at E18.5. Note greatly reduced primitive pre-alveoli in mutant (M; arrowheads) compared to wildtype lungs (I; arrowhead). (Cr) Cranial lobe, (Med) Medial lobe, (Ca) Caudal lobe, (Acc) Accessory lobe, (Le) Left lobe, (Re) right lobe, (Hr) heart. Scale bars: panels A-E, 0.25 mm, panels F-M, 50 μm.
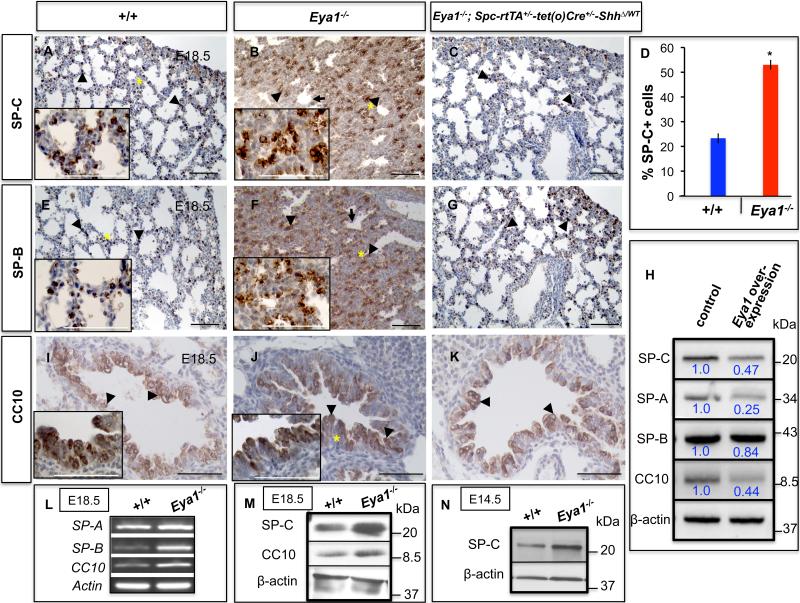
Fig. 4. Increased epithelial cell differentiation in Eya1-/- lungs.
(A-C,E-G,I-K) Immunohistochemistry shows expression of lung epithelial cell differentiation markers SP-C, SP-B, and CC10 in E18.5 Eya1-/- lungs (B,F,J; arrowheads) and control lungs (A,E,I; arrowheads). Note increased SP-C and SP-B expression in mutant lungs, which was restored in Eya1-/-; Spc-rtTA+/--tet(o)Cre+/--ShhΔ/WT compound mutant lungs (C,G; arrowheads). Arrows in B,F refer to primitive pre-alveoli. Insets in A,B,E,F, I,J represent high magnifications of the area marked with an asterisk in the same panel, and show no change in the expression levels of SP-C, SP-B or CC10 inside the cells in mutant compared to control lungs. (D) Quantification of experiments shown in A,B for SP-C. *Significantly different from control lungs (n=3; p < 0.05; Student's t-test). (L) RT-PCR shows increased expression of SP-A, SP-B and CC10 in E18.5 Eya1-/- lungs. (M,N) Western blot shows increased expression of SP-C and CC10 proteins in E18.5 Eya1-/- lungs (M), and SP-C protein in E14.5 Eya1-/- lungs (N). (H) Decreased expression levels of epithelial differentiation markers after Eya1 overexpression in MLE-15 cells in culture. Blue numbers in H represent relative band intensity. Scale bars: 50 μm.
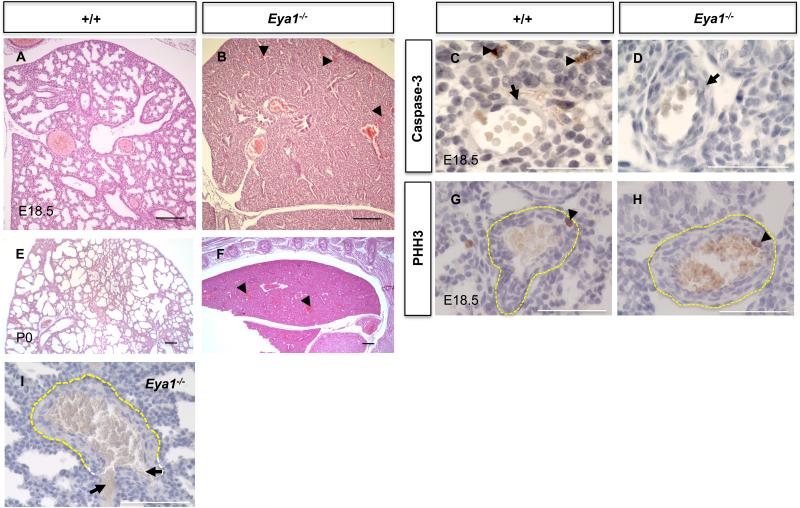
Fig. 8. Eya1-/- embryos and neonates exhibit pulmonary hemorrhage.
(A,B,E,F) Haematoxylin and Eosin staining shows increased pulmonary hemorrhage in Eya1-/- lungs at E18.5 (B; arrowheads) and at birth (F; arrowheads) compared to control littermates (A,E). (C,D,G,H) Immunoperoxidase staining with caspase-3 or PHH3 antibody shows no apparent cell death (C,D) and comparable cell proliferation (G,H; arrowheads) in the blood vessel walls between mutant and control lungs. Note more apoptotic cells in the mesenchyme (C; arrowheads), but not in the blood vessel walls (C,D; arrows). (I) Many large blood vessels show rupture of the smooth muscle layer with herniation of the intact endothelial cell layer in Eya1-/- lungs (arrows). Scale bars: 50 μm.
To determine the onset of phenotypic abnormalities during lung development, we examined lung development at E10-11.5 (Figure S1 in the Supplemental Data and data not shown). In Eya1-/- lungs, very early branching and development were comparable with control lungs wherein two primary buds arose in the ventrolateral foregut at E9.5 and underwent stereotypic rounds of branching and outgrowth in order to give rise to a tree like organ (Figs. S1A,B,D,E, and data not shown). However, both whole-mount and sections at E13-14.5 revealed that lung hypoplasia was noticeable as early as E13-13.5 of development, and was more severe from E14.5 (Fig. S1 C,F, Fig. 2 A-D, F,J). Consequently E13-13.5, E14.5 and E18.5 were used as the developmental stage of choice to analyze the Eya1-/- lung phenotype in this study. Together, these results suggest that the major impact of Eya1 abrogation is on lung branching morphogenesis subsequent to the first round(s) of domain branching.
Eya1 deletion changes cell proliferation of the lung mesenchyme and epithelium
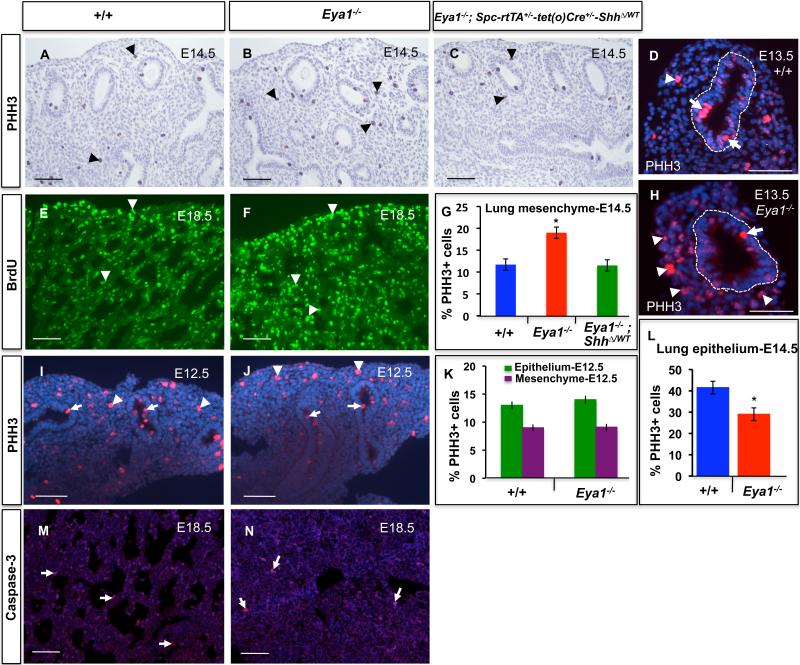
The abnormal mesenchymal phenotype of the Eya1-/- lungs further suggests that Eya1 deletion results in increased mesenchymal cell proliferation and/or cell survival. Staining with Phospho-histone 3 (PHH3), a specific marker of cells undergoing mitosis, is a simple and reliable method for identifying mitotic figures and determining mitotic index (Colman et al., 2006). When lung hypoplasia became noticeable at E13.5, PHH3 staining showed increased PHH-3-positives cells in Eya1-/- lung mesenchyme (Fig. 3D,H). At E14.5, a stage when the lung phenotype became severe, PHH3 staining demonstrated a 1.6 -fold increase in the number of mitotic cells per total cell number in the mesenchyme of Eya1-/- lungs (19.0±1.2% versus 11.7±1.0%, n=3, p < 0.05; Fig. 3A,B,G). In contrast, PHH3-positive mitotic cells were reduced at the most distal epithelium of Eya1-/- lungs at E13.5 (Fig. 3D,H) and at E14.5, compared to control lungs (29.0±1.2% versus 41.5±1.0%, n=3, p < 0.05; Fig. 3L). Interestingly, PHH3 staining at E12.5 showed a comparable cell proliferation for both epithelial and mesenchymal compartments between mutant and wildtype lungs (Fig. 3 I-K). This suggests that the mesenchymal proliferation defect is secondary to the epithelial defect, which was noticeable from E13-13.5 in Eya1-/- lungs.
Fig. 3. Cell proliferation and apoptosis in Eya1–/– lung mesenchyme.
Cell proliferation analysis by PHH3 (A-D,H) or BrdU (E,F) staining shows increased number of proliferative cells (arrowheads) in the Eya1–/– lung mesenchyme at E14.5 (B,G), E13.5 (H) and E18.5 (F) compared to wildtype littermates. Note the reduction of PHH3-positive cell number in the distal epithelium of E13.5 Eya1–/– lungs compared to wildtype lungs (D,H; arrows). (C,G) Mesenchymal cell proliferation reduced towards normal in Eya1-/-; Spc-rtTA+/--tet(o)Cre+/--ShhΔ/WT compound mutant lungs (C; arrowheads) compared to Eya1-/- lungs (B,F). (G) Quantification of experiments shown in A-C for the lung mesenchyme. (I,J) Staining with PHH3 antibody shows comparable cell proliferation between mutant and wildtype lungs at E12.5. (K) Quantification of experiments shown in I,J. (L) Mitotic index for distal epithelial cells of E14.5 Eya1-/- lungs. *Significantly different from control lungs for G,L (n=3; Student's t-test, p<0.05).(M,N) Caspase-3 antibody staining shows no change in apoptotic cell number (arrows) in mutant compared to control lungs. Scale bars: 50 μm.
Moreover, cell proliferation was further examined at later stages of development by exposing E18.5 embryos in utero to BrdU for 1 hour. The relative proportion of cells that had entered or passed through S phase was then determined by immunocytochemistry of lung sections, which showed a dramatic increase of BrdU-positive proliferative cells in Eya1-/- lungs (Fig. 3E,F). Conversely, Caspase-3 staining showed no obvious increase in cell death in E13.5-E18.5 mutant lung epithelium/mesenchyme (Fig. 3M,N and data not shown). Together, these observations suggest that Eya1 deletion affects cell proliferation in the embryonic lung.
Increased epithelial differentiation in Eya1-/- embryonic lungs
During lung development, epithelium cells differentiate during mid to late gestation resulting in distinct cell lineages being distributed along a proximodistal axis (Warburton et al., 2000, 2005, 2008). Eya1 and Six1 negatively regulate cell differentiation of placodal neuronal progenitors (Schlosser et al., 2008). To determine whether lung epithelial cell differentiation had occurred properly in Eya1-/- embryos, markers for proximal (CC10) and distal (SP-A, SP-B, and SP-C) epithelial differentiation were examined for E18.5 Eya1-/- and wildtype littermates. Transcripts for CC10, SP-A and SP-B were increased in E18.5 Eya1-/- lungs (Fig. 4L).
In wildtype lungs, late differentiation markers SP-C and SP-B are expressed in alveolar type II (AEC-2) cells of the distal airways, whereas CC10 expression is normally confined to non-ciliated Clara epithelial cells of the proximal airways (Fig. 4A,E,I). Immunoperoxidase staining showed that CC10 was expressed in mutant lungs (Fig. 4J) and that the relative number of epithelial cells positive for SP-A, SP-B and SP-C proteins was increased in Eya1-/- lungs (Fig. 4,B,F; and data not shown for SP-A). In particular, the percent of cells positive for SP-C showed a twofold increase in Eya1-/- mutant versus control lungs (positive cells were counts in 100× per field, n=3; Fig. 4D). Notably, no remarkable change in the expression levels of SP-C, SP-B or CC10 inside the cell was observed in mutant compared to control lungs as indicated by similar signal intensity levels (insets in A,B,E,F,I,J). Western Blot further confirmed that SP-C and CC10 protein levels increased in Eya1-/- lungs (Fig. 4M). The increase of both SP-B and SP-C expression was also evident in Eya1-/- lungs early on during the pseudoglandular stage at E14.5 (Fig. 4N and Figure S2 A,B in the Supplemental Data). In addition, ectopic expression of SP-C was observed in cells within both the proximal and distal airways of several Eya1-/- lungs suggesting defects in proximal-distal cell patterning (Fig. S2 C,D in the Supplemental Data).
To further investigate Eya1 control of epithelial cell differentiation in vitro, we used MLE15 lung epithelial cells, which expressed endogenous Eya1 as well as different epithelial differentiation and progenitor cell markers (Figs. 4H, 5J-M and data not shown). Upon Eya1 overexpression, MLE15 cells showed a decrease in the expression levels of lung epithelial differentiation markers (Fig. 4H), which further supports the inhibitory function of Eya1 on lung epithelial differentiation. Together, these data suggest that Eya1-/- embryos display lung epithelial defects that correlate with increased epithelial cell differentiation in both the proximal and distal airway.
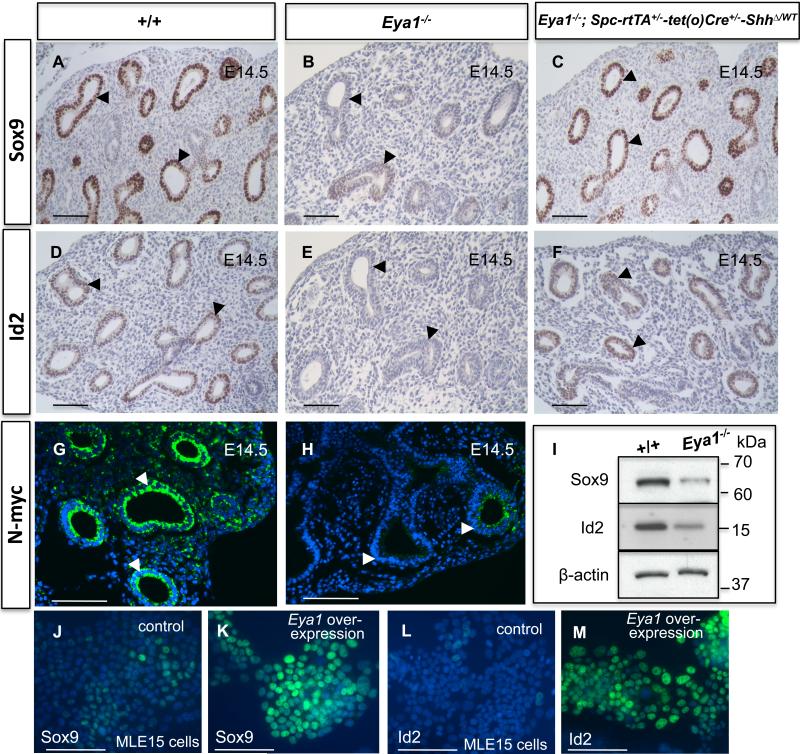
Fig. 5. Reduced expression of distal epithelial progenitor markers in Eya1-/- lungs.
Immunostaining shows marked reduction of the expression levels of epithelial progenitor markers Sox9, Id2 and N-myc at distal epithelial tips of E14.5 Eya1-/- lungs (B,E,H; arrowheads) compared to control lungs (A,D,G; arrowheads). (I) Western blotting shows decreased progenitor markers in Eya1-/- lungs. (C,F; arrowheads) Substantial rescue of progenitor cell marker expression in Eya1-/-; Spc-rtTA+/--tet(o)Cre+/--ShhΔ/WT compound mutant lungs. (J-M) Immunocytochemistry shows increased expression of Sox9 (K) and Id2 (M) in MLE-15 after Eya1 overexpression. Blue nuclear labeling with DAPI. Scale bars: 50 μm.
Reduction of distal epithelial progenitors in the Eya1-/- embryonic lungs
Eya1-/- mouse embryos have defects in the proliferation and survival of the progenitors of multiple organs (Xu et al., 1999, 2002). Therefore, we explored Eya1 effects on lung epithelial progenitors using antibodies against Sox9, Id2 and N-myc, which are highly expressed in lung distal epithelial progenitors (Fig. 5A, D,G; Okubo et al., 2005; Rawlins, 2008). In Eya1-/- lungs, signals of Sox9, Id2 and N-myc transcription factors were markedly reduced at E13.5 (Fig. 6B,D,F,H,J,L), a stage when lung hypoplasia became noticeable, and prematurely abolished in the distal epithelium at E14.5 (Fig. 5A,B,D,E,G,H). Furthermore, Western blot analysis of E14.5 mutant lungs confirmed greatly reduced expression of Sox9 and Id2 (Fig. 5I).
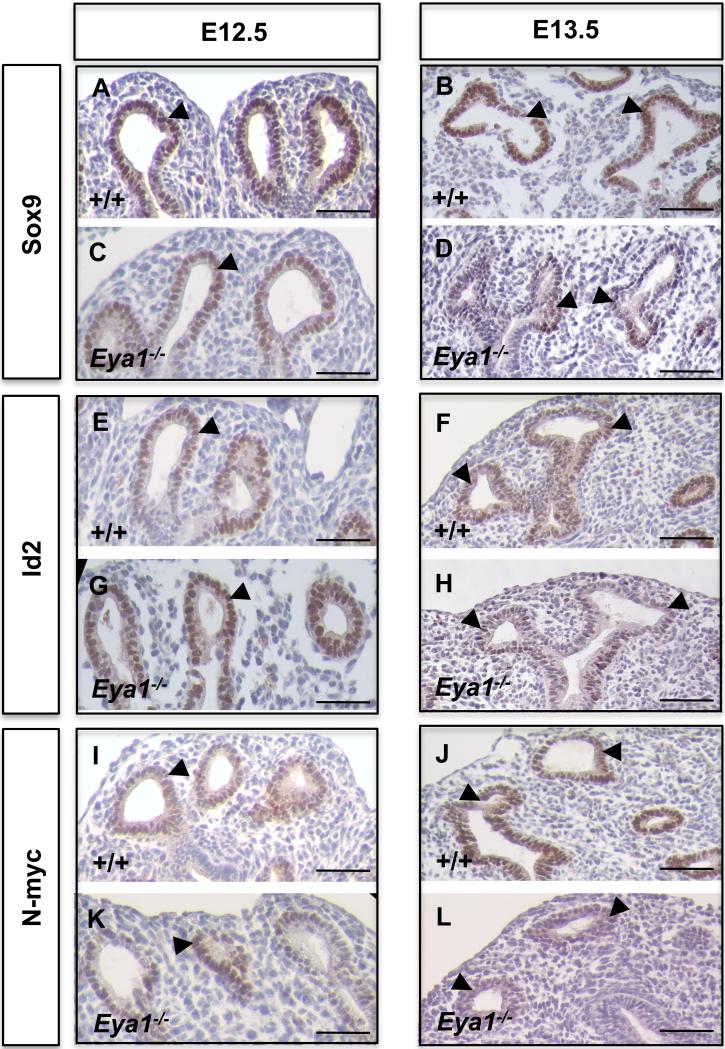
Fig. 6. Normal expression of distal epithelial progenitor markers in Eya1-/- lungs before E13-13.5 of development.
(A,C,E,G,I,K) Immunoperoxidase staining shows similar expression levels of Sox9, Id2 and N-myc at distal epithelial tips of Eya1-/- and wildtype lungs at E12.5 (arrowheads). (B,D,F,H,J,L) the expression levels of Sox9, Id2 and N-myc start to reduce at E13-13.5 in mutant compared to wildtype lungs (arrowheads). Scale bars: 50 μm.
To determine whether normal embryonic epithelial progenitors have ever formed in Eya1-/- lungs, we stained for progenitor cell markers at early embryonic stages. Indeed, Eya1-/- distal epithelial tips stained positively for Sox9, Id2 and N-myc and no apparent change was observed in the expression levels of these markers at E12-12.5 compared to wildtype littermates (Fig. 6A,C,E,G, I,K). Together, these data suggest that distal epithelial progenitors have formed normally but become rapidly depleted at the pseudoglandular phase of Eya1-/- lungs. Therefore, Eya1 must be critical for the maintenance of lung epithelial progenitors and for the correct expression of progenitor cell markers. This conclusion was further supported by our in vitro studies. Thus, overexpression of Eya1 in MLE15 lung epithelial cells in culture stimulated an increase in Sox9 and Id2 expression (Fig. 5J-M).
Increased cell cycle exit in Eya1-/- lung distal epithelial progenitor cells
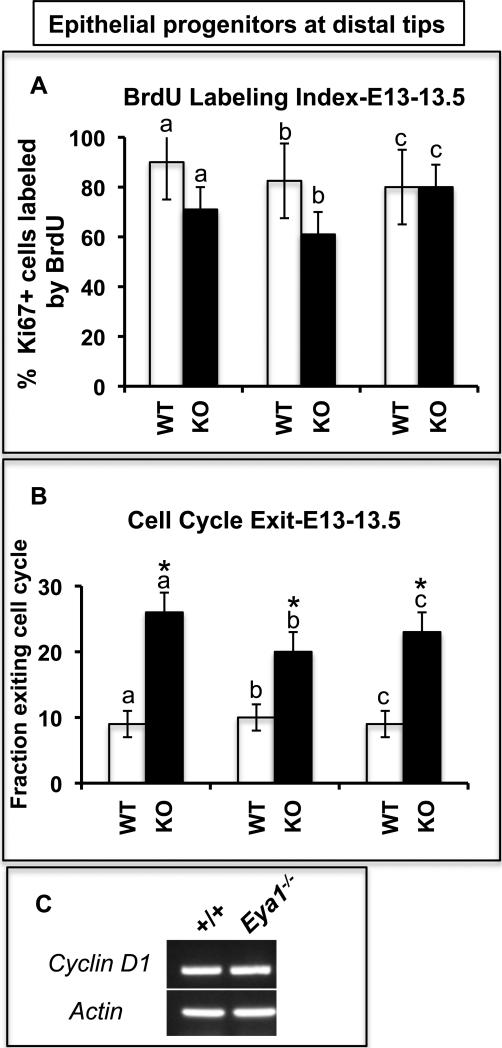
Because Eya1-/- lungs show enhanced epithelial differentiation, together with a great reduction in both number and mitosis of distal epithelial progenitors at E14.5, we thought that it is important to determine whether this phenotype is caused by shortening of the epithelial progenitor cell cycle or is the result of defective cell cycle withdrawal and thus increased differentiation that started before E14.5 (i.e. at E13-13.5). To reveal potential changes in the cell cycle of E13-13.5 Eya1-/- lung epithelium, we counted the proportion of lung epithelial tip cells labeled by a 30-min pulse of BrdU (Fig. 7A; Chenn and Walsh, 2002). Epithelial progenitors, which are a highly proliferative class of cells (Boers et al. 1998, 1999; Bishop, 2004), were identified in this experiment as those cells double-stained with Sox9 and Ki67 at the most distal epithelial tips. Because the length of S phase in mammalian cells remains constant (DiSalvo et al., 1995), the ratio between the BrdU-positive and Ki67-positive cells provides an estimation of the cell cycle length, so that a larger proportion of BrdU-positive epithelial progenitor cells would indicate shortening of the cell cycle. Quantitation of these data, i.e. random fields chosen from six lungs, indicated no significant change in the length of the cell cycle in the Eya1-/- distal lung epithelial cells compared to control lungs (n = 3 for each genotype; Fig. 7A). Similar results were shown after one hour labeling with BrdU (data not shown).
Fig. 7. Increased cell cycle exit in Eya1–/– distal epithelial progenitor cells.
(A) BrdU labeling index of distal epithelial progenitors determined as a percentage of epithelial progenitors (Sox9+Ki67+) incorporated BrdU (BrdU+) after 30-min pulse labeling. The ratio between the BrdU+ and Sox9+Ki67+ cells provides an estimation of the cell cycle length. (B) Cell cycle exit is determined as the ratio of the cells that exited the cell cycle (BrdU+/Ki67–) to all cells that incorporated the BrdU (BrdU+) after 24 hr labeling. For A,B, n=3; p < 0.05; Student's t-test. * Significantly different from control lungs. (C) RT-PCR shows similar Cyclin D1 expression levels in E14.5 Eya1-/- and control lungs.
Next, to determine whether loss of Eya1 causes changes in cell cycle withdrawal, we counted the proportion of cells that exit the cell cycle after 24 hr labeling with BrdU (Fig. 7B). The cell cycle exit is estimated as the ratio between the BrdU-positive/Ki67-negative cells and all BrdU-positive cells (Chenn and Walsh, 2002). Quantitation of random fields chosen for these experiments showed a two to three-fold increase in the number of cells that withdraw from the cell cycle at the most distal epithelial tips of E13-13.5 Eya1-/- lungs compared to control lungs (n = 3 for each genotype; Fig. 7B). Also in E13-13.5 Eya1-/- lungs, no apparent change was observed in the expression of Cyclin D1 (Fig. 7C), a key regulator of G1-to-S phase transition and cell cycle progression (Resnitzky et al., 1994). This further suggests that there is no major defect in the mutant lung cell cycle machinery. Taken together, these results indicate that Eya1-/- lung distal epithelial progenitors are less likely than control cells to self-renew and proliferate. Instead, they are more likely than control cells to withdraw from the cell cycle and differentiate. This can help explain the increase in epithelial cell differentiation in Eya1-/- lungs.
Pulmonary vascular defects of Eya1–/– embryos
Close inspection of Eya1–/– embryos showed hemorrhage surrounding the large pulmonary vessels and blood leakage at E18.5, suggesting a pulmonary vascular defect in these embryos (Figs. 8A,B, 2K and data not shown). Interestingly, this hemorrhage was not observed in any other region of the embryos or neonates (data not shown), suggesting a specific defect in the lung vasculature. Furthermore, close examination of the large pulmonary blood vessels showed no apparent change in the number of caspase-3-positive or PHH3-positive smooth muscle or endothelial cells (Fig. 8C,D,G,H).
At birth, increased severe hemorrhage was observed surrounding the large pulmonary vessels (Fig. 8E,F and data not shown) with many of them showing rupture of the vascular smooth muscle wall with herniation of the endothelial lining (Fig. 8I). Together, these data suggest that Eya1-/- lungs of late stage embryos/neonates exhibited vascular smooth muscle (VSMC)-specific defects and that the weakened pulmonary blood vessels of Eya1–/– embryos can rupture at birth, possibly due to the increased pulmonary blood flow or mechanical strain on the lungs, which occurs in association with diaphragm contractions at birth.
Eya1-/- lungs also display severely reduced vascular and bronchial smooth muscle α-actin expression
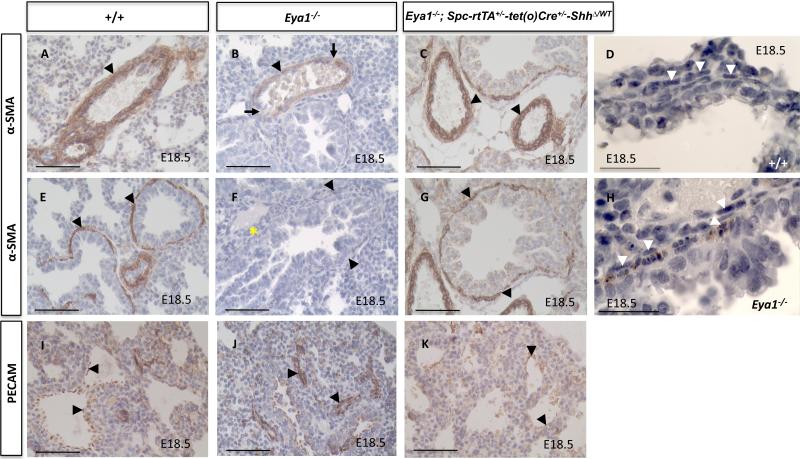
To determine whether smooth muscle had differentiated properly in Eya1–/– neonates, we immunostained for smooth muscle α-actin (α–SMA), which is normally mainly detected in cells surrounding blood vessels and the conducting airways (Mitchell et al., 1990; Low and White, 1998). In wildtype lungs, differentiated smooth muscle cells were normally seen surrounding all the pulmonary vasculature and the large upper bronchial airways in late development (Fig. 9A,E). In E18.5 Eya1-/- lungs, most of the hemorrhagic blood vessels contained noticeably less differentiated smooth muscle with frank breaches in the vessel wall while some vessels had almost undetectable smooth muscle α-actin staining (Fig. 9B and data not shown). Similarly, α–SMA expression was hardly detectable in Eya1–/– bronchial smooth muscles (Fig. 9F), although muscle cells appeared to morphologically exist with their characteristic spindle shape (Nie et al., 2010) compared to control lungs (Fig. 9D,H). Furthermore, these defects in vascular and bronchial smooth muscle were not correlated with increased smooth muscle cell death in E18.5 Eya1-/- lungs as indicated by caspase-3 staining (Figs 8C,D and data not shown). These results suggest that Eya1 is important for the differentiation of pulmonary bronchial and vascular smooth muscle during lung development.
Fig. 9. Defective smooth muscle integrity in Eya1-/- embryonic lungs.
Staining of Eya1-/- lungs with α-SMA antibody shows reduced staining surrounding blood vessels (B; arrowheads) and large bronchial airways (F; arrowheads) compared to control lungs (A,E; arrowheads). Note several breaches in the blood vessel wall in Eya1-/- lungs (B; arrows). (D,H) α-SMA/Haematoxylin staining shows that spindle-shaped smooth muscle cells present in Eya1-/- lungs (H; arrowheads) similar to control lungs (D; arrowheads). Note that α-SMA brown staining is removed in D to show cell morphology, and that H represents high magnification of the area marked with an asterisk in F. (I-K) Staining with PECAM antibody shows strong signals in both wildtype and Eya1-/- lungs (I,J; arrowheads). In Eya1-/-;Spc-rtTA+/--tet(o)Cre+/--ShhΔ/WT compound mutant lungs, both vascular (C) and bronchial (G) α-SMA as well as PECAM (K) stains are restored to control wildtype levels (arrowheads). Scale bars: 50 μm.
We next performed immunostaining with an antibody to platelet endothelial adhesion molecule (PECAM) to determine whether the smaller capillaries in the lung, which lack vascular smooth muscle, were affected in Eya1–/– embryos. Signals for PECAM, a marker for endothelial cells, were observed in E18.5 Eya1-/- and control lungs with the capillary plexus stained strongly in Eya1-/- lungs (Fig. 9I,J, n = 3 for each genotype). These results suggest that vascular endothelium and capillary network are normally formed in Eya1–/– lungs.
Genetic reduction of Shh activity in Eya1-/- lungs partially rescues the hypoplastic lung phenotype
To determine the possible mechanisms by which Eya1 deletion results in the phenotype described above, we examined the expression of Shh. Two lines of reasoning led us to examine Shh expression and its relationship with Eya1 in the lung. Firstly, similar to Eya1 (Fig. 1A,B,C), Shh expression is most intense in the distal epithelial tips of the lung and is downregulated during the differentiation phase of murine lung development (from E16.5; Urase et al., 1996; Bellusci et al., 1997b). Secondly, ectopic lung-specific overexpression of Shh from an Sp-c promoter/enhancer construct (Sp-c:Shh) in transgenic mice yields a phenotype that is very similar to the Eya1-/- lung phenotype (see above; and Bellusci et al., 1997b). Furthermore, both real-time PCR and immunohistochemistry showed a dramatic increase (1.7-fold) in Shh expression in E17.5 Eya1-/- versus control lungs (Fig. 10I) and in E14.5 Eya1-/- lungs (Fig. 10K versus J). Thus Shh expression in E14.5 Eya1-/- lungs was 1.3-fold higher than wildtype lungs (not shown), and continued to increase after E16.5, a time point where Shh expression starts to decrease progressively in wildtype lungs (Bellusci et al., 1997b), until birth at both gene and protein levels of expression (Fig. 10I,M and data not shown, n = 3 for each genotype).
Fig. 10. Genetic reduction of Shh activity substantially rescues the hypoplastic lung phenotype in Eya1-/- mice.
External appearance (A,E) and histological analysis (B-D, F-H) show properly inflated and well-grown Eya1-/-; Spc-rtTA+/--tet(o)Cre+/--ShhΔ/WT compound mutant lungs compared to lungs of Eya1-/- littermates (D,H versus C,G). (I) Real-time PCR shows a significant increase of Shh, but decrease of Fgf10 expression in E17.5 Eya1-/ - lungs. *Bars carrying the same letter (a,b) are significantly different from the wildtype control. (J-L) Immunohistochemistry shows increased Shh protein expression in the epithelium (arrowheads) and mesenchyme (arrows) of E14.5 Eya1-/ - lungs (K) compared to control lungs (J). (L) Shh expression reduces towards normal levels in compound mutant lungs (arrowheads). (M) Expression of Shh continues to increase at birth in Eya1-/- lungs. Scale bars: 50 μm.
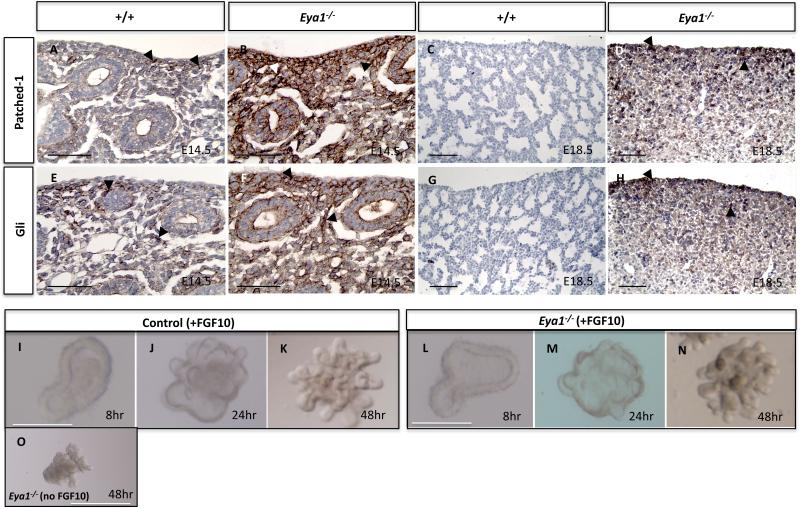
Hedgehog signaling activity is best reflected by measuring Shh receptor patched-1 (Ptc-1) and Gli1 expression levels, which are expressed in lung distal mesenchyme and are mediators of Shh activity (Grindley et al., 1997, Lees et al., 2005; Madison et al., 2005). Therefore, we investigated the expression of Ptc-1 and Gli1 (Fig. 11). Beginning around E16.5 of embryonic development, Shh, Ptc and Gli1/Gli2 RNA levels are normally declining in wildtype lungs and Shh overexpression results in increased levels of both Ptc and Gli mRNAs in the lung (Grindley et al., 1997; Bellusci et al., 1997b). In Eya1-/- lungs, Ptc-1 and Gli proteins were dramatically increased in the mesenchyme at E14.5 and E18.5 compared to control lungs (Fig. 11A-H). Together, these data suggest that Eya1 deletion causes an increase in both Shh expression and activity.
Fig. 11. Increased Shh signaling activity in Eya1-/- lungs.
(A-H) Immunoperoxidase staining with Patched-1 (A-D) or Gli (E-H) antibody shows increased expression levels of Patched-1 and Gli proteins (arrowheads) in the mesenchyme of E14.5 and E18.5 Eya1-/- lungs compared to control lungs. (I-N) Exogenous Fgf10 induces branching of Eya1–/– epithelial explants. E12.5 epithelial culture shows that Eya1–/– explants (n=8) continue to grow and branch in the presence of 250 ng/ml Fgf10 protein, but died in the absence of Fgf10 (O). Scale bars: 50 μm.
Next, we tested the hypothesis that excessive Shh pathway activity causes the pulmonary and vascular defects in Eya1-/- embryos by conditional genetic reduction of 50% of Shh activity in the distal epithelium of Eya1-/- lungs using Eya1-/-; Spc-rtTA+/--tet(o) Cre+/--ShhΔ/WT compound mutant mice (see Materials and Methods for details). As expected, real-time PCR/immunohistochemistry experiments showed that Shh expression was reduced in the lungs of these compound mutant embryos as compared to lungs of Eya1-/- littermates (Fig. 10I,L; n=3). No changes in the lung phenotype or gene expression were evident in DOX-fed Spc-rtta and Spc-rtta-tet(o) Cre mice (data not shown).
Eya1-/-; Spc-rtTA+/--tet(o) Cre+/--ShhΔ/WT compound mutant lungs well developed compared to Eya1-/- lungs, albeit smaller in size than control lungs (Fig. 10 A,E). They showed properly inflated lungs, restoration of epithelial branching, increased lung size, and expanded saccules and epithelial tubules, as well as reduced mesenchymal cellularity compared to corresponding lungs of Eya1-/- littermates (Fig. 10 A-D, E-H). This suggests partial rescue of the Eya1-/- hypoplastic lung phenotype after inactivation of 50% of the Shh gene dosage at distal epithelium. This conclusion was further confirmed by analysis of cell proliferation and progenitor cell markers. As expected, a 1.65-fold decrease in mesenchymal cell proliferation (11.5±1.0% versus 19.0±1.2%, n=3, p < 0.05; Fig. 3B,C,G) and a notable increase in the expression of epithelial progenitor markers Sox9 and Id2 (Fig. 5B,C,E,F) were observed in compound mutant lungs versus Eya1-/- lungs. Interestingly, the expression of distal epithelial differentiation markers (SP-C, SP-B) was rescued into the wildtype range in compound mutant lungs versus Eya1-/- lungs (Fig. 4B,C,F,G).
Shh signaling to lung mesenchyme positively regulates capillary network formation in the developing distal lung (White et al., 2007). Therefore, we investigated changes in vascular endothelium and capillary network formation after partial inactivation of the Shh gene in Eya1–/– lungs. As expected, lowering Shh activity generally reduced PECAM staining in compound mutant lungs versus Eya1-/- lungs (Fig. 9K,J). Conversely, α-SMA staining demonstrated well formed bronchial and vascular smooth muscle surrounding all of the large upper airways and blood vessels in compound mutant lungs versus Eya1-/- lungs (Fig. 9B,C,F,G). Together, our results demonstrated that the conditional genetic reduction of Shh activity in Eya1-/- deficient lungs can, at least partially, rescue different critical lung defects in the epithelial, mesenchymal and vascular compartments.
Exogenous Fgf10 induces branching morphogenesis in Eya1–/– lung epithelial explants
Overexpression of Shh inhibits Fgf10 expression in the lung (Bellusci et al., 1997b). Real-time PCR showed that Fgf10 expression was significantly reduced in parallel to increased Shh expression levels in Eya1-/- lungs (Fig. 10I). Therefore, we next tested the hypothesis that excessive Shh-mediated reduction of Fgf10 expression resulted in reduction of epithelial branching morphogenesis by using epithelial culture of Eya1-/- lungs. In the absence of Fgf10, neither the wildtype nor the Eya1-/- lung isolated airways showed signs of branching and died (n=3; Fig. 11O and data not shown). Addition of exogenous 250 ng/ml Fgf10 rescued Eya1-/- branching defects so that all Fgf10-treatedmutant epithelial explants continued to grow and branch in the presence of Fgf10 in a similar fashion to control explants (Fig. 11 I-N; n=8). Thus the number of branches was comparable between Fgf10-treated control and mutant explants after 48 hr in culture (18±3 versus 17±4; n=8; Fig. 11K,N). This suggests that Eya1-/- epithelial tip cells remain fully competent to respond to signals from lung mesenchyme and that Eya1-/- branching defects are therefore not an intrinsic property of the Eya1-/- epithelium. In addition, no change in Eya1 expression was evident in Fgf10 hypomorphic lungs (data not shown), which further suggests that Eya1 acts upstream of Fgf10 signaling.
DISCUSSION
Eya1 expression and phenotype in embryonic lung
Much less is known about putative transcription factors and signals originating from the epithelial cells and how these might affect lung development compared to well-known mesenchymal signals (reviewed in Warburton et al., 2000,2005, Morrisey and Hogan, 2010). Here, we demonstrate that Eya1 transcription coactivator coordinately regulates growth and differentiation of murine lung epithelium, mesenchyme and endothelium. We found that Eya1 is highly expressed in both distal epithelial and mesenchyme during lung development until E18.5. An epithelial-mesenchymal expression pattern of Eya1 has been described in other organs (Zou et al., 2006). Furthermore, Eya1-/- embryos exhibit severe pulmonary hypoplasia (Fig. 2), which is characterized by reduced branching morphogenesis, dense mesenchymal cellularity and severe bronchial/vascular smooth muscle defects as well as pulmonary hemorrhage.
Eya1 controls lung epithelial and mesenchymal proliferation
Our data indicate that Eya1 plays an essential role in proliferation of the lung epithelium and mesenchyme. Cell proliferation at distal epithelial tips, wherein Eya1 is highly expressed, is reduced from E13.5 in Eya1-/- lungs. In contrast, mesenchymal proliferation is unexpectedly greatly increased in the face of Eya1 deletion in Eya1-/- lungs at E13.5 and later in development.
Control of cell proliferation is a key function of Eya1, which together with Six1 transcription factor acts as an essential positive regulator of precursor cell proliferation in different organs (Li et al., 2003). Eya1 deletion reduces cell proliferation of otic epithelium (Xu et al., 2002; Zou et al., 2006) and may result in inappropriate cell proliferation leading to neural crest (Xu et al., 2002) and neuroblast progenitor (Zou et al., 2004) cell defects. Furthermore, high levels of Eya1 and Six1 inhibit neuronal differentiation but expand the pool of proliferative neuronal progenitors (Schlosser et al., 2008). Thus, whereas lack of Eya1 expression in Eya1-/- lung distal epithelium may account for reduced cell proliferation at the distal tips, the increased mesenchymal proliferation in Eya1-/- lungs is less likely to be a direct effect of Eya1 deletion. Rather, Eya1 may act indirectly on mesenchymal proliferation by controlling other key regulators of mesenchymal cell proliferation such as Shh signaling.
Eya1 regulates Shh expression and activity during lung development
Secreted diffusible factors are proposed to play important roles in controlling lung growth and morphogenesis. Of particular interest, Shh acts as a mitogen for lung mesenchyme and thus controls epithelial branching in the developing lung (Bellusci et al., 1997b). We found that embryonic/neonatal Eya1-/- lungs also exhibit failure of interstitial condensation, which is an important feature of prenatal fetal lung maturation that is most likely mediated through abnormally persistent high-level expression and activity of Shh(Figs. 2, 10,11). The latter may also explain the severe Eya1-/- hypoplastic lung phenotype (Fig. 2). Thus, we propose that Eya1 controls Shh signaling and is necessary for down-regulation of Shh expression/activity starting from E16.5 in the lung. Our current study provides several evidences to support this hypothesis. Firstly, the Eya1-/- lung phenotype is very similar to the phenotype of the Sp-C:Shh transgenic lungs (Fig. 2 and Bellusci et al., 1997b). Secondly, both Eya1 and Shh are involved in the regulation of lung branching morphogenesis through controlling mesenchymal development (Fig. 3, Litingtung et al., 1998; Pepicelli et al., 1998, Bellusci et al., 1997b).
Finally, our findings that genetically lowering Shh activity in Eya1-/- lungs substantially rescues the lung phenotypes in vivo (Figs. 3-5,9-10) provide strong evidence that Eya1 is indeed required for controlling Shh expression and activity to ensure appropriate mesenchymal/epithelial proliferation, normal epithelial branching, interstitial condensation/lung maturation and eventually alveoli formation. Furthermore, since Shh null mice also have pulmonary hypoplasia (Litingtung et al., 1998; Pepicelli et al., 1998), levels of Shh expression must be tightly regulated in order for normal lung development to occur. Therefore, our findings are crucial for understanding how Shh expression is tightly regulated and for identifying Eya1 as one of the epithelial transcription factors that putatively control it. Recently, a crosstalk between Eya1 and Shh has been suggested during inner ear development because Eya1-/- and Shh-/- have similar cochlear phenotypes (Zou et al., 2006).
Similar to Eya1, several other proteins also have an inhibitory effect on Shh signaling and co-ordinate Shh contribution to controlling lung bud size and shape as well as epithelial branching. For instance, Shh induces its own regulator, Hedgehog-binding protein (Hhip), in the lung mesenchyme. Hhip1 inhibits Shh signaling by ligand sequestration and thus releases Shh-mediated repression of Fgf10. Insufficient levels of Hhip result in reduced Fgf10 levels, insufficient growth and lung hypoplasia (Chuang et al., 2003; Cardoso and Lu, 2006). The precise functional relationship between Eya1, Hhip1 and Shh signaling during lung morphogenesis will be the subjects of future study.
Eya1 and lung epithelial branching and differentiation
Our findings that Fgf10 mRNAs are significantly decreased in Eya1-/- lungs combined with increased Shh expression during pseudoglandular stage and later in development (Fig. 10I) are also internally consistent with the known function of Shh as a modulator/negative regulator of Fgf10 signaling during lung epithelial branching. Shh overexpression reduces Fgf10, which is highly expressed in the mesenchyme at the very distal tips of branching tubules and is a critical regulator of lung bud formation and epithelial branching (Bellusci et al., 1997a,b). In addition, Shh and Fgf10 are thought to interact to induce bud formation/epithelial branching, based on the finding that Fgf10 expression is upregulated and delocalized in Shh mutant lungs (Pepicelli et al, 1998).
Similar to Shh-/- lungs (Pepicelli et al, 1998), our data show that Eya1 abrogation negatively impinges on lung branching morphogenesis, subsequent to the first round(s) of domain branching (Fig. S1). This occurs in parallel to increased Shh activity and reduced Fgf10 expression in Eya1-/- lungs. Thus, we suggest that critical interactions between these three molecules occur in an Eya1-Shh-Fgf10 pathway, which controls subsequent rounds of epithelial branching. In support of this hypothesis, we found that genetically lowering Shh activity in Eya1-/- lungs results in restored Fgf10 expression and increased epithelial branching while Fgf10 protein stimulates branching of Eya1-/- epithelial explants in vitro.
Furthermore, the available data from knockout mice and our results give cues to support the novel hypothesis that Eya1 is essential for the control of Fgf10 signaling in the lung. Similar to Eya1 functions (Figs. 1, 5-7), Fgf10 signaling is essential for the maintenance of epithelial progenitor cell proliferation (Ramasamy et al., 2007) and positively regulates the expression of epithelial progenitor marker Sox9 (Abler et al., 2009). Fgf10 controls lung epithelial progenitors and inhibits differentiation of the lung epithelium (Nyeng et al., 2008) similar to the functions of Eya1 (Fig. 4). Indeed, high levels of Eya1 and Six1 factors inhibit cell differentiation in other cell types (e.g. neuronal progenitors; Schlosser et al., 2008). Moreover, both epithelial branching (Abler et al., 2009) and bronchial smooth muscle cells (Mailleux et al., 2005, Ramasamy et al., 2007) are severely defective in Fgf10/ Fgfr2b inactivated or Fgf10 hypomorphic lungs, which are very similar to the phenotype of Eya1-/- lungs (Figs. 2, 9). In addition, Eya1 positively regulates Fgf10 expression during inner ear development (Zou et al., 2006).
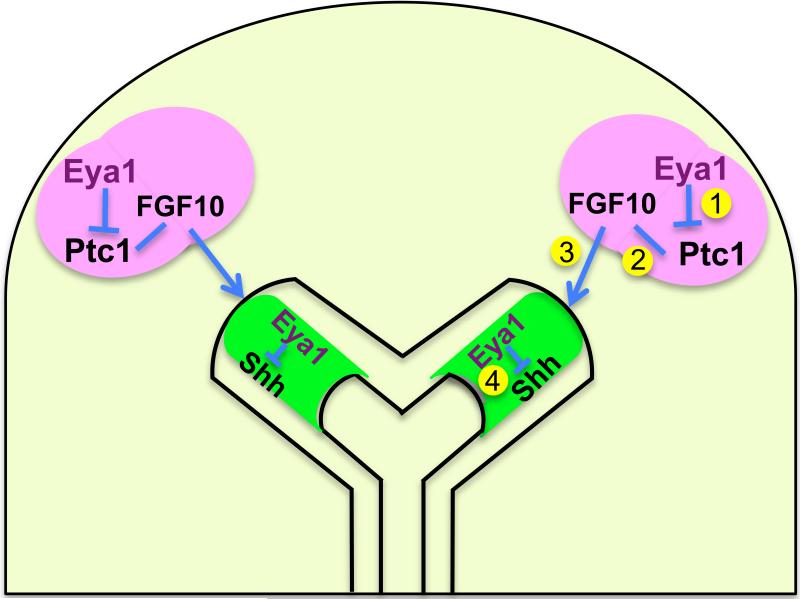
It is noteworthy that the localized Eya1 expression in the mesenchyme at the very distal tips of the branching buds (Fig. 1G,G’,H) is very similar to that of Fgf10 (Bellusci, et al., 1997a). Shh signaling has been suggested to facilitate proper Fgf10 localization during distal tip branching by inhibiting Fgf10 expression, mainly in the inter-bud regions after mesenchymal Fgf10 expression has been initiated. However, Shh expression is upregulated at new sites of branching, which is associated with higher levels of Fgf10 expression. This creates an apparent paradox as to how Fgf10 expression can be maintained in the presence of high levels of Shh expression (Chuang and McMahon, 2003). Based on our current results about Eya1 functions, we propose a model in which Eya1 is essential to control Shh/Fgf10 expression pattern and activity during lung branching (Fig. 12). We propose that Eya1 focally expressed in distal mesenchyme inhibits Ptc1 receptor expression and thus maintains focal Fgf10 expression by inhibiting local Shh activity (interactions 1,2; Fig. 12). The response of distal tip epithelial cells to Fgf10 is further proposed to be facilitated because epithelial Eya1 is also necessary to inhibit Shh expression and/or autocrine activity in these cells (interactions 3,4; Fig. 12). Indeed, recent studies show that Shh has autocrine functions during the development of multiple organs (McGlinn and Tabin, 2006; Yang et al., 2008; Handrigan and Richman, 2009) and Shh autocrine activities in the lung epithelium are currently under investigation.
Fig. 12. A schematic model showing how Eya1 coordinates interactions with Shh/Fgf10 signaling to control Shh/Fgf10 expression pattern and inductive activity during lung branching.
This model is based on our current results on Eya1 expression pattern and functions, and modifies the previous model of Weaver et al. (2000) and Warburton (2008). We suggest that Eya1 focally expressed in distal mesenchyme inhibits Patched 1 (Ptc1) receptor expression, and thus it maintains focal Fgf10 expression by inhibiting local Shh activity (interactions 1,2). The response of distal tip epithelial cells to Fgf10 is further proposed to be facilitated because epithelial Eya1 also inhibits Shh expression and/or autocrine activity in these cells (interactions 3,4).
Potential role of Eya1 in regulating lung progenitor cell self-renewal and other signal pathways
Our data about the loss of epithelial progenitors and increased epithelial differentiation in Eya1-/- lungs suggest an essential role for Eya1 in controlling epithelial progenitor cell self-renewal and cell fate determination in the lung. Indeed, Eya1 is a critical determination factor for acquiring progenitor cell fate in other cell types (Zou et al., 2004; Schlosser et al., 2008). Also, Eya1 and Six1/2 genes play critical roles in the proper expression of neuronal determination and differentiation genes in the olfactory, otic and epibranchial placodes (Zou et al., 2004; Friedman et al., 2005; Bricaud and Collazo, 2006; Ikeda et al., 2007) as well as in the proliferation and survival of sensory and neuronal progenitors (Li et al., 2003; Zheng et al., 2003; Bricaud and Collazo, 2006; Zou et al., 2006; Kriebel et al., 2007). In addition, it remains to be determined whether Eya1 controls other mesenchyme/epithelial-regulatory factors in the lungs such as BMP4 and Fgf9, which also interact with Shh-Fgf10 signaling (Hyatt et al., 2002; White et al., 2007; De Langhe et al., 2008). Indeed, Eya1 co-activator and Six1 transcription factor target several members of BMP, Fgf and Wnt signaling during development in different organs (Xu et al., 1999; Zou et al., 2006, and unpublished data). Interestingly, Eya1 expressed in lung distal mesenchyme can act as a co-activator to Six1 to control cell cycle regulators as reported for Six1 in other organs (Xu et al., 1997b; Ford et al., 1998; Coletta et al., 2004). Further work will be needed to determine Eya1 functions in epithelial cell fate and mesenchymal development of the early lung.
Eya1 and pulmonary vascular development
Our study suggests that Eya1 controls late development of smooth muscle differentiates from the mesenchyme in the lung because of reduced expression of α-SMA in Eya1-/- pulmonary VSMCs. Since Eya1 is undetectable in pulmonary VSMCs (Fig. 1I), Eya1 may be necessary for the expression of other auto- or paracrine factors in epithelial or mesenchymal cell lineages required for VSMC differentiation/growth. For instance, Eya1 can positively control the expression of Fgf10 (Fig. 10I), which regulates the growth of aortic VSMC in vitro (Onda et al., 2003). Another possible mechanism is that Eya1 deletion could result in defective differentiation of VSMCs, which could lead to degradation of the smooth muscle component of the vessel wall. The Eya1-/- pulmonary vascular defects seem less likely to be directly linked to lung hypoplasia because other mouse models that have severe lung hypoplasia, e.g. Fgf9-/- mice, exhibit normal vasculardevelopment (Colvin et al., 2001). Conversely, Eya1 is likely to play a role during the early events of pulmonary VSMC differentiation because Eya1 expression decreases during late gestation in the lung from E18.5 (Fig.1A). Thus, we propose that Eya1 expressed in the most distal mesenchyme may initiate a mesenchymal differentiation program that propagates from the distal to proximal regions as the lung grows, resulting in the proper differentiation of vascular smooth muscle, which occurs in parallel to distal-proximal progression of mesenchymal differentiation.
It remains to be determined what transcription factor(s) Eya1 could be binding to in order to carry out the effects in the lungs. Previous studies suggested that Eya1 functions in an evolutionarily conserved cassette downstream of Pax genes and upstream of Six genes during the development of pharyngeal pouch endoderm, otic vesicle and kidney (Xu et al., 1999, 2002). Several members of Sox, Pax, and Six genes are expressed in different endoderm-derived tissues including embryonic lung and thus can act as binding partners for Eya1. For example, Eya1 physically interacts with Sox2 transcription factor (Zou et al., 2008), which is important for lung branching morphogenesis and epithelial cell differentiation (Gontan et. al., 2008). Further studies are needed to identify genes that bind to Ey1 during lung development.
In our future studies, we will investigate Eya1-Shh-Fgf10 functional interactions. We will also use a conditional knockout approach to specifically delete Eya1 from lung epithelial component or mesenchyme to investigate its specific functions in epithelial versus mesenchymal compartments. Nonetheless, the Eya1 mutants provide a new mouse model for congenital lung hypoplasia and malformations and help us to understand the mechanisms that control lung morphogenesis, and to determine co-ordination of Shh-Fgf10 signaling pathways during lung development.
In conclusion, our results identify Eya1 as a critical upstream regulator of Shh-FgF10 signaling pathway which controls and coordinates key interlocking aspects of epithelial branching, mesenchymal and vascular development during lung organogenesis.
Supplementary Material
Legends for Supplementary Figures
Supplementary Figure 1 (S1). Eya1 is not required for the initiation of primary lung buds. Cross-sections from E10-E10.5 Eya1-/- embryos show apparently normal formation, and early branching of lung buds (D,E; arrowheads), compared to control littermates at E10-10.5 (A,B). Lung hypoplasia was noticeable as early as E13-E13.5 in Eya1-/- embryos (F versus C). b; bronchus. Scale bars: 50 μm.
Supplementary Figure 2 (S2). Immunoperoxidase staining shows increased expression of SP-B in E14.5 Eya1-/- lungs, compared to control littermates (A,B; arrowheads), and ectopic expression of SP-C in cells within the proximal airways in E18.5 Eya1-/- lungs (D; arrowheads). Scale bars: 50 μm.
ACKNOWLEDGMENTS
We thank Drs. C. Wigfall for editing of the manuscript, and C Wee for advice on statistical analysis. This study was funded by NIH-NHLBI P01 HL 60231, HL 44060 and HL44977 grants to DW as well as California Institute of Regenerative Medicine (CIRM) stem cell training grant to DW and AHE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Eya1 is expressed in lung distal epithelium and mesenchyme.
- Eya1-/- lungs are hypoplastic with reduced branching but increased mesenchymal cellularity
- Eya1 controls both epithelial and mesenchymal cell proliferation.
- Eya1 induces epithelial progenitor cell proliferation but inhibits differentiation.
- Eya1 coordinates Shh-Fgf10 signaling during lung development
REFERENCES
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, et al. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nature Genet. 1997;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Abler LL, Mansour SL, Sun X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev Dyn. 2009;238(8):1999–2013. doi: 10.1002/dvdy.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122(6):1693–702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997a;124(23):4867–78. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997b;124(1):53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- Bishop AE. Pulmonary epithelial stem cells. Cell Prolif. 2004;37:89–96. doi: 10.1111/j.1365-2184.2004.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers JE, Ambergen A, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal airway epithelium. Am. J. Respir. Crit. Care Med. 1998;157:2000. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- Boers JE, Ambergen A, Thunnissen FB. Number and proliferation of clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1999;159:1585. doi: 10.1164/ajrccm.159.5.9806044. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes haircell fate in the developing zebrafish (Danio rerio) inner ear. J Neurosci. 2006;26(41):10438–51. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso WV. Lung morphogenesis revisited: old facts, current ideas. Dev Dyn. 2000;219(2):121–30. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1053>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133(9):1611–24. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Carraro G, El-Hashash A, Guidolin D, Tiozzo C, Turcatel G, Young B, De Langhe S, Bellusci S, Shi W, Parnigotto PP, et al. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev Biol. 2009;333(2):238–250. doi: 10.1016/j.ydbio.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chuang P-T, McMahon AP. Branching morphogenesis of the lung: new molecular insights into an old problem. Trends Cell Biol. 2003;13(2):86–91. doi: 10.1016/s0962-8924(02)00031-4. [DOI] [PubMed] [Google Scholar]
- Chuang P-T, Kawcak T, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17:342–347. doi: 10.1101/gad.1026303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta RD, Christensen K, Reichenberger K, Lamb J, Micomonaco D, Wolf D, Müller-Tidow C, Golub T, Kawakami K, Ford HL. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci U S A. 2004;101(17):6478–83. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman H, Giannini C, Huang L, Gonzalez J, Hess K, Bruner J, Fuller G, Langford L, Pelloski C, Aaron J, Burger P, Aldape K. Assessment and prognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low and intermediate-grade infiltrating astrocytomas. Am J Surg Pathol. 2006;30(5):657–64. doi: 10.1097/01.pas.0000202048.28203.25. [DOI] [PubMed] [Google Scholar]
- Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128:2095–2106. doi: 10.1242/dev.128.11.2095. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Warburton D, Hajihosseini M, Bellusci S. Levels of mesenchymal FGFR2 signaling modulate smooth muscle progenitor cell commitment in the lung. Dev Biol. 2006;299:52–62. doi: 10.1016/j.ydbio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One. 2008;3(1):e1516. doi: 10.1371/journal.pone.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127(3):483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Del Moral PM, De Langhe S, Sala F, Veltmaat J, Tefft D, Wang K, Warburton D, Bellusci S. Differential role of FGF9 on epithelium and mesenchyme in mouse embryonic lung. Dev Biol. 2006a;293(1):77–89. doi: 10.1016/j.ydbio.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 2006b;290(1):177–88. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- DiSalvo CV, Zhang J, Jacobberger JW. Regulation of NIH-3T3 cell G1 phase transit by serum during exponential growth. Cell Prolif. 1995;28:511–524. doi: 10.1111/j.1365-2184.1995.tb00089.x. [DOI] [PubMed] [Google Scholar]
- El-Hashash AH, Esbrit P, Kimber SJ. PTHrP promotes murine secondary trophoblast giant cell differentiation through induction of endocycle, upregulation of giant-cell-promoting transcription factors and suppression of other trophoblast cell types. Differentiation. 2005;73(4):154–174. doi: 10.1111/j.1432-0436.2005.00013.x. [DOI] [PubMed] [Google Scholar]
- Estefania ER, Ramirez-Camacho M, Gomar A, Trinidad B, Arellano J, Verdaguer J, Vilches C. Point mutation of an Eya1-gene splice site in a patient with Otofacio-cervical syndrome. Ann. Hum. Genet. 2005;70:140–144. doi: 10.1111/j.1529-8817.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- Ford HL, Kabingu EN, Bump E, Mutter G, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc Natl Acad Sci U S A. 1998;95(21):12608–13. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougerousse F, Durand M, Lopez S, Suel L, Demignon J, Thornton C, Ozaki H, Kawakami K, Barbet P, Beckmann J, Maire P. Six and Eya expression during human somitogenesis and MyoD gene family activation. J Muscle Res Cell Motil. 2002;23:255–264. doi: 10.1023/a:1020990825644. [DOI] [PubMed] [Google Scholar]
- Friedman RA, Makmura L, Biesiada E, Wang X, Keithley EM. Eya1 acts upstream of Tbx1, Neurogenin 1, NeuroD and the neurotrophins BDNF and NT-3 during inner ear development. Mech Dev. 2005;122(5):625–34. doi: 10.1016/j.mod.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Giangreco A, Shen H, Reynolds SD, Stripp BR. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004:L624–L630. doi: 10.1152/ajplung.00149.2003. [DOI] [PubMed] [Google Scholar]
- Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol. 2008;317(1):296–309. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Bellusci S, Perkins D, Hogan BL. Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev Biol. 1997;188(2):337–348. doi: 10.1006/dbio.1997.8644. [DOI] [PubMed] [Google Scholar]
- Handrigan GR, Richman JM. Autocrine and paracrine Shh signaling are necessary for tooth morphogenesis, but not tooth replacement in snakes and lizards (Squamata). Dev. Biol. 2010;337:171–186. doi: 10.1016/j.ydbio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Yingling JM. Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr. Opin. Genet. Dev. 1998;8:481–486. doi: 10.1016/s0959-437x(98)80121-4. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory proteinexpressing cells of the airway neuroepithelial body micro-environment include label-retaining 33 subset and are critical for epithelial renewal after progenitor depletion. Amer J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Shangguan X, Shannon JM. BMP4 modulates fibroblast growth factor-mediated induction of proximal and distal lung differentiation in mouse embryonic tracheal epithelium in mesenchyme-free culture. Dev Dyn. 2002;225(2):153–65. doi: 10.1002/dvdy.10145. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ookawara S, Sato S, Ando Z, Kageyama R, Kawakami K. Six1 is essential for early neurogenesis in the development of olfactory epithelium. Dev Biol. 2007;311(1):53–68. doi: 10.1016/j.ydbio.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Jemc J, Rebay I. The eyes absent family of phosphotyrosine phosphatases: properties and roles in developmental regulation of transcription. Annu Rev Biochem. 2007;76:513–38. doi: 10.1146/annurev.biochem.76.052705.164916. [DOI] [PubMed] [Google Scholar]
- Kriebel M, Muller F, Hollemann T. Xeya3 regulates survival and proliferation of neural progenitor cells within the anterior neural plate of Xenopus embryos. Dev. Dyn. 2007;236:1526–1534. doi: 10.1002/dvdy.21170. [DOI] [PubMed] [Google Scholar]
- Lees C, Howie S, Sartor RB, Satsangi J. The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease. Gastroenterology. 2005;129(5):1696–1710. doi: 10.1053/j.gastro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Li X, Oghi K, Zhang J, Krones A, Bush K, Glass C, Nigam S, Aggarwal A, Maas R, Rose D, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426(6964):238–9. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hogan BL. Differential gene expression in the distal tip endoderm of the embryonic mouse lung. Gene Expr Patterns. 2002;2(3-4):229–33. doi: 10.1016/s1567-133x(02)00057-1. [DOI] [PubMed] [Google Scholar]
- Low RB, White SL. Lung smooth muscle differentiation. Int J Biochem Cell Biol. 1998;30:869–883. doi: 10.1016/s1357-2725(98)00049-1. [DOI] [PubMed] [Google Scholar]
- Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132(2):279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Kelly R, Veltmaat JM, De Langhe S, Zaffran S, Thiery J, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132(9):2157–66. doi: 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- McGlinn E, Tabin CJ. Mechanistic insight into how Shh patterns the vertebrate limb. Curr. Opin. Genet. Dev. 2006;16:426–432. doi: 10.1016/j.gde.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Kransow MA. The branching programme of mouse lung development. Nature. 2008;453(7196):745–50. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JJ, Reynolds S, Leslie K, Low R, Woodcock-Mitchell J. Smooth muscle cell markers in developing rat lung. Am J Respir Cell Mol Biol. 1990;3:515–523. doi: 10.1165/ajrcmb/3.6.515. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18(1):8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Sun J, Gordon R, Cai CL, Xu PX. SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation. Development. 2010;137(5):755–65. doi: 10.1242/dev.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev Biol. 2008;10(8):2. doi: 10.1186/1471-213X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Knoepfler P, Eisenman R, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132(6):1363–74. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- Onda M, Naito Z, Wang R, Fujii T, Kawahara K, Ishiwata T, Sugisaki Y. Expression of keratinocyte growth factor receptor (KGFR/FGFR2 IIIb) in vascular smooth muscle cells. Pathol Int. 2003;53(3):127–32. doi: 10.1046/j.1440-1827.2003.01445.x. [DOI] [PubMed] [Google Scholar]
- Pan J, Yeger H, Cutz E. Neuronal developmental marker FORSE-1identifies a putative progenitor of the pulmonary neuroendocrine cell lineage during lung development. J Histochem Cytochem. 2000;50:1567–1578. doi: 10.1177/002215540205001201. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wert S, Nagy A, Lobe C, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002;99(16):10482–7. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz K, Xiao J, Garrity P, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91(7):881–91. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Ramasamy SK, Mailleux A, Gupte V, Mata F, Sala F, Veltmaat J, Del Moral P, De Langhe S, Parsa S, Kelly L, et al. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol. 2007;307:237–247. doi: 10.1016/j.ydbio.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL. Lung epithelial progenitor cells: lessons from development. Proc Am Thorac Soc. 2008;5(6):675–81. doi: 10.1513/pats.200801-006AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133(13):2455–65. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136(22):3741–5. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayapureddi JP, Kattamuri C, Steinmetz B, Frankfort B, Ostrin E, Mardon G, Hegde RS. Eyes absent represents a class of protein tyrosine phosphatases. Nature. 2003;426(6964):238–9. doi: 10.1038/nature02093. [DOI] [PubMed] [Google Scholar]
- Resnitzky D, Gossen M, Bujard H, Reed SI. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14(3):1669–79. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G, Awtry T, Brugmann S, Jensen E, Neilson K, Ruan G, Stammler A, Voelker D, Yan B, Zhang C, Klymkowsky M, Moody SA. Eya1 and Six1 promote neurogenesis in the cranial placodes in a SoxB1-dependent fashion. Dev Biol. 2008;320(1):199–214. doi: 10.1016/j.ydbio.2008.05.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefft D, Lee M, Smith S, Crowe D, Bellusci S, Warburton D. mSprouty2 inhibits FGF10-activated MAP kinase by differentially binding to upstream target proteins. Am J Physiol Lung Cell Mol Physiol. 2002;283(4):L700–6. doi: 10.1152/ajplung.00372.2001. [DOI] [PubMed] [Google Scholar]
- Tefft D, De Langhe S, Del Moral P, Sala F, Shi W, Bellusci S, Warburton D. A novel function for the protein tyrosine phosphatase Shp2 during lung branching morphogenesis. Dev Biol. 2005;282(2):422–31. doi: 10.1016/j.ydbio.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Treisman JE. A conserved blueprint for the eye? Bioessays. 1999;21(10):843–50. doi: 10.1002/(SICI)1521-1878(199910)21:10<843::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Urase K, Mukasa T, Igarashi H, Ishii Y, Yasugi S, Momoi MY, Momoi T. Spatial expression of Sonic hedgehog in the lung epithelium during branching morphogenesis. Biochem Biophys Res Commun. 1996;225(1):161–6. doi: 10.1006/bbrc.1996.1147. [DOI] [PubMed] [Google Scholar]
- Warburton D. Developmental biology: Order in the lung. Nature. 2008;453(7196):73–5. doi: 10.1038/453733a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson K, Cardoso W. The molecular basis of lung morphogenesis. Mech Dev. 2000;92(1):55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Warburton D, Bellusci S, De Langhe S, Del Moral P, Fleury V, Mailleux A, Tefft D, Unbekandt M, Wang K, Shi W. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res. 2005;57(5 Pt 2):26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- Warburton D, Perin L, Defilippo R, Bellusci S, Shi W, Driscoll B. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc Am Thorac Soc. 2008;5(6):703–6. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawersik S, Maas RL. Vertebrate eye development as modeled in Drosophila. Hum Mol Genet. 2000;9(6):917–25. doi: 10.1093/hmg/9.6.917. [DOI] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127(12):2695–704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol. 2003;258(1):169–84. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- White AC, Lavine KJ, Ornitz DM. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development. 2007;134(20):3743–52. doi: 10.1242/dev.004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Dabbouseh N, Rebay I. Interactions with the abelson tyrosine kinase reveal compartmentalization of eyes absent function between nucleus and cytoplasm. Dev Cell. 2009;16(2):271–9. doi: 10.1016/j.devcel.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Woo I, Her H, Beier D, Maas RL. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997a;124(1):219–31. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- Xu PX, Cheng J, Epstein J, Maas RL. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc Natl Acad Sci U S A. 1997b;94:11974–9. doi: 10.1073/pnas.94.22.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown M, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23(1):113–7. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Laclef C, Maire P, Maas R, Peters H, Xu X. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development. 2002;129(13):3033–44. doi: 10.1242/dev.129.13.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J. Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei Z, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130(17):3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Fritzsch B, Xu P. Eya1/ Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–72. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Rodrigo-Blomqvist S, Enerbäck S, Xu PX. Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas in the inner ear. Dev Biol. 2006;298(2):430–41. doi: 10.1016/j.ydbio.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Erickson C, Kim EH, Jin D, Fritzsch B, Xu PX. Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet. 2008;17(21):3340–56. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Legends for Supplementary Figures
Supplementary Figure 1 (S1). Eya1 is not required for the initiation of primary lung buds. Cross-sections from E10-E10.5 Eya1-/- embryos show apparently normal formation, and early branching of lung buds (D,E; arrowheads), compared to control littermates at E10-10.5 (A,B). Lung hypoplasia was noticeable as early as E13-E13.5 in Eya1-/- embryos (F versus C). b; bronchus. Scale bars: 50 μm.
Supplementary Figure 2 (S2). Immunoperoxidase staining shows increased expression of SP-B in E14.5 Eya1-/- lungs, compared to control littermates (A,B; arrowheads), and ectopic expression of SP-C in cells within the proximal airways in E18.5 Eya1-/- lungs (D; arrowheads). Scale bars: 50 μm.