Abstract
The execution of apoptosis is critical for proper development of the nervous system. However, it is equally important that neurons strictly inhibit apoptosis after development to ensure their survival throughout the lifetime of the organism. Here we show that a microRNA, miR-29b, is markedly induced with neuronal maturation and functions as a novel inhibitor of neuronal apoptosis. The prosurvival function of miR-29b is mediated by targeting genes in the proapoptotic BH3-only family. Our results identify a unique strategy evolved by maturing neurons that uses a single microRNA to inhibit the multiple, redundant BH3-only proteins that are key initiators of apoptosis.
Keywords: apoptosis, neurons, miRNAs, BH3-only, miR-29, Bcl-2 family
During normal development of the nervous system, a period of massive neuronal apoptosis occurs to precisely match neurons to their respective target cells (Oppenheim 1991). However, once appropriate neuronal connections are in place, it is imperative that neurons strictly inhibit their apoptotic program, since these cells do not divide, have limited capability for regeneration, and must survive for the lifetime of the organism (Benn and Woolf 2004). In mammalian cells, apoptosis is triggered when cells encounter cytotoxic stresses, such as nutrient withdrawal, DNA damage, or endoplasmic reticulum (ER) stress. These insults initiate signaling cascades that activate proapoptotic BH3-only members of the Bcl-2 family of proteins and cause the release of cytochrome c from the mitochondrial intermembrane space into the cytoplasm (Wang 2001). The release of cytochrome c from mitochondria is a key event that triggers the rapid activation of caspases, the key cellular proteases that ultimately execute cell death (Hengartner 2000). Although some changes in apoptotic machinery have been identified during neuronal maturation (Tsui-Pierchala and Ginty 1999; Putcha et al. 2000; Walsh et al. 2004; Wright et al. 2007), it is unclear whether other mechanisms exist in mature neurons to strictly disable apoptosis.
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression (Bartel 2009). While misexpression of some miRNAs has been linked with apoptosis and cancer (Kent and Mendell 2006), very little is known about how miRNAs regulate cell death during normal development. Here, we examined whether miRNAs function as critical regulators of survival in mature neurons. We show that miR-29b becomes induced with neuronal maturation and potently inhibits apoptosis in normal, healthy neurons. Importantly, we report that miR-29b mediates its function by targeting multiple members of the BH3-only family of proapoptotic genes. Together, these data identify miR-29b as the first mammalian miRNA capable of inhibiting neuronal apoptosis.
Results and Discussion
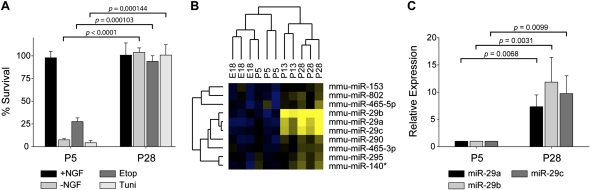
In contrast to developing P5 (postnatal day 5) sympathetic neurons, mature P28 neurons are strikingly resistant to nerve growth factor (NGF) deprivation, DNA damage, and ER stress (Fig. 1A; Easton et al. 1997; Wright et al. 2007). To determine whether miRNAs may have a role in restricting apoptosis, we profiled their expression during neuronal maturation and reasoned that miRNAs that are most highly expressed in mature neurons would likely function to prevent cell death. Sympathetic neurons were obtained from the superior cervical ganglia (SCG) of mice at four developmental stages: embryonic day 18 (E18), P5, P13, and P28. Each of these stages occurs after post-mitotic differentiation, thereby focusing our study on a time period when increasing restriction of apoptosis is a major known event occurring in these neurons (Glebova and Ginty 2005). Using significance analysis of microarrays (SAM) (Tusher et al. 2001) to compare young neurons (E18 and P5) with mature neurons (P13 and P28), we found that the expression of only one miRNA family, miRNA-29 (miR-29), was significantly increased in mature neurons (Fig. 1B; Supplemental Table S1).
Figure 1.
Neuronal maturation is associated with a marked increase in miR-29 and restriction of apoptosis. (A) P0 sympathetic neurons were cultured for 5 d (P5) or 28 d (P28) in vitro and either maintained in NGF-containing media (+NGF), deprived of NGF (−NGF), treated with 20 μM etoposide to induce DNA damage (Etop), or treated with 2.5 μM tunicamycin to induce ER stress (Tuni) for 72 h. Cell survival was quantified by cell morphology and was expressed as a percentage of viable cells prior to cell treatment. (B) miRNA microarray expression data for sympathetic ganglia isolated from E18, P5, P13, and P28 mice. Yellow denotes high expression and blue denotes low expression. Complete expression data is included in Supplemental Table S1. (C) qRT–PCR for miR-29a, miR-29b, and miR-29c using RNA collected from P0 sympathetic neurons maintained in culture for 5 d (P5) or 28 d (P28). Expression of each miRNA is plotted relative to levels in P5 neurons. Data in A and C are mean ± SD of three independent experiments.
The miR-29 family consists of three members (miR-29a, miR-29b, and miR-29c) that map to two distinct genomic loci in clusters. Since these miRNAs have extensive sequence homology, especially at the 5′ seed region important for mRNA target recognition (Lewis et al. 2003), we focused on miR-29b, as it was the most highly expressed and is found at both genomic loci (Supplemental Fig. S1A). To confirm our microarray data, we performed quantitative RT–PCR (qRT–PCR) on isolated ganglia from P5 and P28 mice and found the levels of miR-29b to be increased dramatically in P28 ganglia (Supplemental Fig. S1B). In addition, using pure neuronal cultures, we found that miR-29 levels increased in P0 neurons cultured for 28 d in vitro versus neurons cultured for 5 d (Fig. 1C), indicating that the increase in miR-29 occurs specifically in neurons. The increase in miR-29b with neuronal maturation was not specific to sympathetic neurons, as a similar increase in expression was also observed during cerebellar and cortical maturation (Supplemental Fig. S1C,D). Together, these data indicate that miR-29b levels are induced at a time when neurons become increasingly resistant to apoptosis.
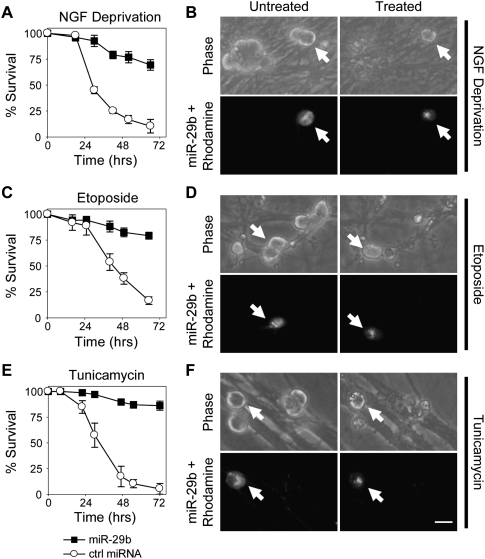
Since the marked increase in miR-29 expression during maturation correlates with a time when strict restrictions on neuronal apoptosis are engaged, we hypothesized that introducing miR-29b in young P5 neurons may provide enhanced resistance to apoptotic stimuli. miR-29b or a control miRNA (nonconserved Caenorhabditis elegans miRNA cel-miR-67) was microinjected into P3 neurons and, after 48 h, neurons were subjected to NGF deprivation. Remarkably, microinjection of miR-29b was sufficient to protect neurons from apoptosis, while cells injected with the control miRNA died at the expected rate (Fig. 2A,B). Treatment of P5 neurons with NGF deprivation alone did not have a significant effect on the endogenous expression of miR-29b (Supplemental Fig. S2). The ability of miR-29b to inhibit neuronal apoptosis was not specific to NGF deprivation, as miR-29b expression also effectively inhibited apoptosis in response to DNA damage (Fig. 2C,D) and ER stress (Fig. 2E,F). These data indicate that miR-29b is a potent inhibitor of neuronal apoptosis induced by multiple stimuli.
Figure 2.
miR-29b expression potently inhibits neuronal apoptosis induced by multiple stimuli. P3 sympathetic neurons were microinjected with miR-29b or cel-miR-67 (ctrl miRNA, each 30 μM) together with rhodamine to mark injected cells. (A,C,E) Forty-eight hours following injection, neurons were subjected to NGF deprivation (A), 20 μM etoposide (C), or 2.5 μM tunicamycin (E), and survival of injected cells was assessed at various time points following cell treatment. Survival was expressed as a percentage of viable cells prior to treatment. (B,D,F) Representative phase-contrast images of the exact field of sympathetic neurons before (untreated) or after 3 d of NGF deprivation (B), etoposide (D), or tunicamycin (F). Rhodamine marks cells injected with 30 μM miR-29b (arrows). Data in A, C, and E are mean ± SD of at least three independent experiments. Bar, 20 μm.
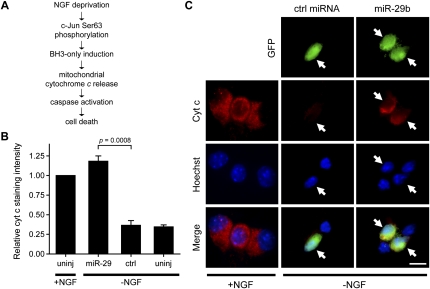
To determine precisely how miR-29b functions to inhibit apoptosis, we analyzed the effect of miR-29b expression on key steps in the pathway activated by NGF deprivation. Upon NGF withdrawal, neurons activate the transcription factor c-Jun by phosphorylation at Ser 63, causing the induction of proapoptotic BH3-only proteins in the Bcl-2 family (Eilers et al. 1998; Whitfield et al. 2001), which results in cytochrome c release, caspase activation, and cell death (Fig. 3A). We examined the phosphorylation status of c-Jun in neurons injected with miR-29b after NGF withdrawal. NGF deprivation induced robust nuclear staining for phospho-Ser 63-c-Jun in both control and miR-29b-expressing neurons, indicating that miR-29b expression did not affect c-Jun phosphorylation (Supplemental Fig. S3). Next, we tested the effect of miR-29b on the release of cytochrome c from mitochondria. Following NGF deprivation, while uninjected or control-injected neurons showed very faint cytochrome c staining, consistent with its release to the cytoplasm (Deshmukh and Johnson 1998), neurons injected with miR-29b maintained cytochrome c at the mitochondria (Fig. 3B,C; Supplemental Fig. S4). Thus, miR-29b expression potently inhibited apoptosis in neurons downstream from c-Jun phosphorylation but upstream of cytochrome c release. The identification that miR-29b acts at this step in the apoptotic pathway is consistent with the fact that mature neurons, which we found to express high levels of miR-29, also phosphorylate c-Jun but do not release cytochrome c after NGF deprivation (Easton et al. 1997; Putcha et al. 2000).
Figure 3.
Inhibition of apoptosis by miR-29b occurs upstream of cytochrome c release. (A) Schematic representation of the apoptosis pathway activated after NGF deprivation in P5 neurons. (B,C) P3 sympathetic neurons were either uninjected (uninj) or microinjected with a GFP-expressing plasmid and either miR-29b or cel-miR-67 (ctrl miRNA, each 30 μM). After 48 h, cells were left untreated (+NGF) or deprived of NGF (−NGF). Cells were fixed and immunostained 48 h after cell treatment. (B) Fluorescence intensity of cells after cytochrome c staining. (C) Representative photographs of cytochrome c staining in neurons; GFP expression indicates injected cells (arrows). Data in B are mean ± SEM of at least three independent experiments. Bar, 10 μm.
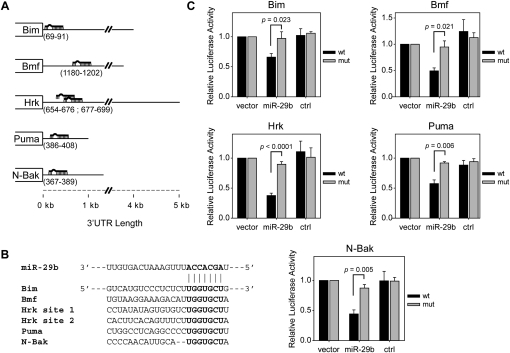
The BH3-only proteins are a family of proapoptotic regulators that are critical for inducing cytochrome c release from mitochondria. This gene family comprises at least eight members, many of which act redundantly (Giam et al. 2008). For example, although Bim and Hrk (also known as DP5) are transcriptionally induced and are important for NGF deprivation-induced apoptosis, the knockout of either gene has only a modest effect on survival (Putcha et al. 2001; Imaizumi et al. 2004). Thus, an effective block of apoptosis at this point would require inhibition of multiple BH3-only proteins. To assess whether miR-29b was capable of functioning in this manner, we used miRNA target prediction programs (TargetScan, MicroCosm Targets, and PicTar) to determine whether any BH3-only mRNAs were putative targets of miR-29b. To our surprise, we found that multiple BH3-only mRNAs had predicted miR-29b-binding sites in their 3′ untranslated regions (UTRs) (Fig. 4A,B).
Figure 4.
miR-29b targets multiple members of the BH3-only family. (A) Schematic representation of predicted miR-29b-binding sites in the 3′UTRs of multiple mouse BH3-only mRNAs. Nucleotides of the 3′UTR containing miR-29b-binding sites are listed in parentheses. (B) Sequence and alignment of the miR-29b-binding sites in the 3′UTRs of multiple BH3-only mRNAs. The predicted base-pairing of miR-29b with target recognition seed sequence is shown in bold. (C) Luciferase activity was measured 48 h after transfection of HEK293T cells with reporter plasmids in which regions of either wild-type (wt) or mutant (mut) 3′UTRs of genes listed in A were each fused downstream from the firefly luciferase gene. Reporter plasmids were transfected either alone (vector), together with 20 nM miR-29b (miR-29b), or together with 20 nM cel-miR-67 (ctrl). Expression was normalized by taking the ratio of firefly to renilla luciferase, and is plotted relative to vector alone. Data are mean ± SEM of at least three independent experiments.
To directly test whether miR-29b could target BH3-only mRNAs, we used a luciferase reporter assay in which the 3′UTRs of BH3-only genes, with or without the putative miR-29b-binding sites, were each fused downstream from the firefly luciferase gene. Each luciferase construct was cotransfected with either miR-29b or a control miRNA into HEK293T cells and luciferase activity was measured. Indeed, miR-29b was able to effectively reduce luciferase activity in cells transfected with constructs containing 3′UTRs of wild-type, but not mutant, Bim, Bmf, Hrk, and Puma (Fig. 4C). Furthermore, miR-29b was also able to target the 3′UTR of N-Bak, a BH3-only splice variant of Bak that is expressed exclusively in neurons (Fig. 4C; Ham et al. 2005). Together, these data identify miR-29b as a single molecule capable of targeting multiple BH3-only mRNAs.
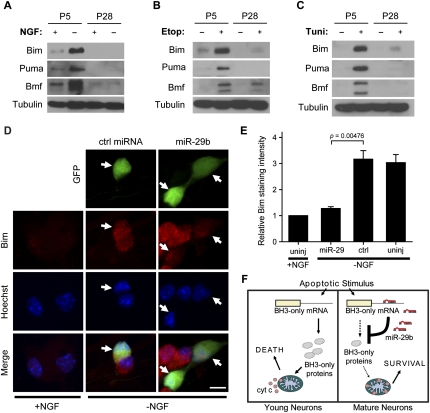
The observation that miR-29b is able to target the 3′UTRs of the BH3-only family of genes suggested to us that apoptosis is blocked in mature neurons due to the repression of BH3-only protein induction. Thus, we examined the status of several BH3-only proteins in P5 and mature P28 neurons after NGF deprivation or etoposide and tunicamycin treatment. Indeed, while Bim and Puma are induced after NGF deprivation in P5 neurons (Putcha et al. 2001), these proteins fail to be induced in P28 neurons (Fig. 5A). Equally important, while etoposide and tunicamycin treatment each robustly induced Bim and Puma in P5 neurons, an induction of these proteins was not seen in P28 neurons (Fig. 5B,C). In addition, we found that Bmf, a BH3-only protein whose function has not been well characterized in neurons, is also induced after all three treatments in P5 neurons, but not significantly in P28 neurons (Fig. 5A–C). miRNAs are known to suppress gene expression through a combination of mRNA cleavage and translational repression (Bartel 2009). Interestingly, although BH3-only mRNAs became induced in P28 neurons after each treatment, the amount of BH3-only mRNA that was detected in treated P28 neurons was as low as that seen in healthy P5 neurons (Supplemental Fig. S5). Taken together, our data show that, in mature neurons (which have high endogenous levels of miR-29), the induction of BH3-only proteins is effectively blocked after multiple apoptotic insults.
Figure 5.
miR-29b expression in neurons blocks induction of endogenous BH3-only proteins. (A–C) The protein levels of BimEL, Bmf, and Puma were determined by Western blot in P0 neurons cultured for 5 d (P5) or 28 d (P28) in vitro. Neurons were either left untreated (+NGF) or deprived of NGF (−NGF) (A), untreated (−Etop) or treated with 20 μM etoposide (+Etop) (B), or untreated (−Tuni) or treated with 2.5 μM tunicamycin (+Tuni) (C). All treatments were performed for 48 h in the presence of the caspase inhibitor Q-VD-OPh (25 μM) before lysates were collected. Representative Western blots are shown. Bmf isoforms were detected at ∼25 kDa and ∼30 kDa. (D,E) P3 sympathetic neurons were microinjected with a GFP-expressing plasmid and either miR-29b or cel-miR-67 (ctrl miRNA, each 30 μM). After 48 h, cells were left untreated (+NGF) or deprived of NGF (−NGF). Cells were fixed and stained 48 h after cell treatment. (D) Representative photographs of Bim staining in neurons; GFP expression indicates injected cells (arrows). Bar, 10 μm. (E) Fluorescence intensity of cells after Bim staining. Data are mean ± SEM of three independent experiments. (F) Proposed model showing that high miR-29b levels in mature neurons prevent induction of BH3-only proteins after apoptotic stimuli. Apoptotic stimuli cause cytochrome c release and death in young neurons, while mature neurons remain resistant.
A prediction of our model (Fig. 5F) is that expression of miR-29b should directly block the expression of BH3-only proteins in a situation where endogenous BH3-only proteins are induced. We tested this hypothesis in young P5 neurons and focused on Bim, since this is the best-characterized BH3-only protein shown to be induced after NGF deprivation. As expected, injection of the control miRNA had no effect on the induction of Bim following NGF deprivation in P5 neurons. Strikingly, however, P5 neurons expressing miR-29b showed a marked reduction in Bim staining after NGF deprivation (Fig. 5D,E). Together, these results show that miR-29b can block apoptosis in neurons by directly inhibiting the critical step of BH3-only protein induction.
While it is important for developing neurons to be sensitive to apoptotic stimuli for proper formation of the nervous system, the apoptotic pathway must be strictly inhibited after development to ensure that mature neurons can survive long-term. The observation that Bax remains inactive in the cytoplasm after NGF deprivation in mature neurons has been described, although the molecules responsible for this phenomenon were unknown (Putcha et al. 2000). Here, we identified miR-29b as a key molecule that is induced during neuronal maturation and functions to repress translation of the BH3-only family of proteins, thus preventing death in response to apoptotic stimuli. These results are the first to identify a mammalian miRNA that strictly inhibits apoptosis in normal, healthy neurons.
A recent study found that expression of the miR-29 family is reduced in sporadic Alzheimer's disease (AD) patients' brains (Hebert et al. 2008). This study identified β site APP-cleaving enzyme 1 (BACE1), a critical molecule in the release of β-amyloid peptides from APP, as a target of miR-29. Thus, loss of miR-29 expression in sporadic AD could lead to an increase in BACE1 expression and, ultimately, β-amyloid plaques, which are the characteristic protein aggregates of AD. Our results identifying miR-29b as an important inhibitor of apoptosis in neurons provide additional insight as to why loss of miR-29 expression may leave neurons more vulnerable to neurodegeneration, and emphasize the importance of miR-29 for long-term neuronal survival.
After cytotoxic stress, proapoptotic BH3-only proteins are crucial for triggering apoptosis by either inhibiting the anti-apoptotic proteins Bcl-2, Mcl-1, and Bcl-xL, or directly activating proapoptotic Bax and Bak (Willis and Adams 2005; Chipuk and Green 2008). In C. elegans, only a single BH3-only protein, EGL-1, is necessary for activating apoptosis during development (Conradt and Horvitz 1998). In contrast, mammals contain at least eight BH3-only proteins, distinct subsets of which are activated after different apoptotic stimuli (Giam et al. 2008). While this large repertoire of BH3-only proteins allows for increased regulation of apoptosis, it also leads to a redundancy in their function. In fact, loss of either Bim or Hrk alone in sympathetic neurons provides only a modest survival advantage over wild-type neurons (Putcha et al. 2001; Imaizumi et al. 2004). Thus, in order to efficiently inhibit apoptosis at the level of BH3-only activity, it is necessary to block multiple members of this pathway simultaneously. Indeed, we found that miR-29b is able to target at least five unique members of the BH3-only family. Interestingly, while we were unable to find predicted miR-29-binding sites in the 3′UTRs of Bid, Bad, or Noxa, evidence also shows that these BH3-only family members do not play a major role in sympathetic neurons (Putcha et al. 2002; Wyttenbach and Tolkovsky 2006).
Why would miR-29b evolve to inhibit apoptosis in neurons by repressing several BH3-only genes when, for example, targeting Bax alone would lead to similar, if not greater, resistance to apoptosis by intrinsic stimuli (Deckwerth et al. 1996)? One possibility is that targeting Bax may have undesirable consequences because of its nonapoptotic role in regulating mitochondrial fusion (Karbowski et al. 2006). Also, by targeting the BH3-only members of the Bcl-2 family, miR-29b may have evolved to fine-tune apoptosis regulation, as opposed to completely disengaging apoptotic signaling.
Intriguingly, miR-29 function in cancer cells appears to be complex. While miR-29 expression is elevated in some cancers where it appears to function as an oncogene (Gebeshuber et al. 2009; Han et al. 2010), others have found miR-29 to have tumor suppressor functions (Pekarsky et al. 2006; Wang et al. 2008), notably by indirectly activating p53 or targeting the anti-apoptotic protein Mcl-1 (Mott et al. 2007; Park et al. 2009). We examined levels of Mcl-1 in P5 versus P28 neurons and found that, paradoxically, Mcl-1 levels were down-regulated upon neuronal maturation, despite these neurons being strikingly resistant to apoptosis (Supplemental Fig. S6). Thus, although the consequence of miR-29 expression in various cancer cells may depend on cellular context, its ability to inhibit the BH3-only family proteins has a clear anti-apoptotic function in primary neurons.
miRNAs have been described to modulate a variety of cellular processes—including differentiation, proliferation, and apoptosis (Kent and Mendell 2006; Stefani and Slack 2008)—and may regulate nearly two-thirds of the entire mammalian genome (Friedman et al. 2009). Our results here identify miR-29b as being induced during the physiologically normal process of neuronal maturation, and demonstrate the ability of a single miRNA to inhibit apoptosis by targeting multiple members of a key proapoptotic gene family.
Materials and methods
Cell culture conditions, primers, plasmids, antibodies, and other standard methods are described in detail in the Supplemental Material.
RNA extraction and microarray analysis
For miRNA microarray and qRT–PCR analysis, total RNA was extracted using Trizol Reagent (Invitrogen) or the miRNeasy kit (Qiagen). miRNA microarray was performed essentially as described previously (Thomson et al. 2004). Normalized log2 data were clustered hierarchically by sample and gene and represented as a heat map using Cluster 3.0 and TreeView software programs, respectively (Michael Eisen, Stanford University).
Microinjection and quantification of cell survival
Cells were injected with 30 μM miR-29b or a control C. elegans miRNA (cel-miR-67) that is not conserved in mammalian cells (miRIDIAN mimics; Dharmacon) along with rhodamine dextran (8 μg/μL) and EGFP-expressing plasmid (50 ng/μL) in microinjection buffer containing 100 mM KCl and 10 mM KPi (pH 7.4) as described previously (Potts et al. 2003). This concentration of miR-29b was estimated to elevate miR-29b to approximately the levels seen in P28 neurons (see the Supplemental Material for more information). The number of viable rhodamine-positive cells with intact phase-bright cell bodies was counted prior to treatment with NGF deprivation, etoposide, or tunicamycin and then counted at indicated times after cell treatment. Cell survival was expressed as a percentage of the number of cells prior to treatment. This method of assessing survival correlates well with other cell survival assays such as trypan blue exclusion and calcein AM staining, and follows recent guidelines for assessment of death in neuronal cells (Potts et al. 2003; Galluzzi et al. 2009).
Acknowledgments
We thank members of the Deshmukh laboratory for critical review of this manuscript and helpful discussions. This work was supported by grant NS042197 to M.D. This research was also supported in part by a grant from the National Institute of Environmental Health Sciences (P30ES10126). A.J.K. was supported by grants T32GM008719 and F30NS068006.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1975411.
Supplemental material is available for this article.
References
- Bartel DP 2009. MicroRNAs: Target recognition and regulatory functions. Cell 136: 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn SC, Woolf CJ 2004. Adult neuron survival strategies—Slamming on the brakes. Nat Rev Neurosci 5: 686–700 [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR 2008. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18: 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt B, Horvitz HR 1998. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 93: 519–529 [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson EM Jr, Snider WD, Korsmeyer SJ 1996. Bax is required for neuronal death after trophic factor deprivation and during development. Neuron 17: 401–411 [DOI] [PubMed] [Google Scholar]
- Deshmukh M, Johnson EM Jr 1998. Evidence of a novel event during neuronal death: Development of competence-to-die in response to cytoplasmic cytochrome c. Neuron 21: 695–705 [DOI] [PubMed] [Google Scholar]
- Easton RM, Deckwerth TL, Sh PA, Johnson EM Jr 1997. Analysis of the mechanism of loss of trophic factor dependence associated with neuronal maturation: A phenotype indistinguishable from BAX deletion. J Neurosci 17: 9656–9666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers A, Whitfield J, Babij C, Rubin LL, Ham J 1998. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci 18: 1713–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP 2009. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH, Bazan NG, Blagosklonny MV, Blomgren K, Borner C, et al. 2009. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ 16: 1093–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebeshuber CA, Zatloukal K, Martinez J 2009. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep 10: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giam M, Huang DC, Bouillet P 2008. BH3-only proteins and their roles in programmed cell death. Oncogene 27: S128–S136 doi: 10.1038/onc.2009.50 [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD 2005. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci 28: 191–222 [DOI] [PubMed] [Google Scholar]
- Ham J, Towers E, Gilley J, Terzano S, Randall R 2005. BH3-only proteins: Key regulators of neuronal apoptosis. Cell Death Differ 12: 1015–1020 [DOI] [PubMed] [Google Scholar]
- Han YC, Park CY, Bhagat G, Zhang J, Wang Y, Fan JB, Liu M, Zou Y, Weissman IL, Gu H 2010. MicroRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J Exp Med 207: 475–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B 2008. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/β-secretase expression. Proc Natl Acad Sci 105: 6415–6420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO 2000. The biochemistry of apoptosis. Nature 407: 770–776 [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Benito A, Kiryu-Seo S, Gonzalez V, Inohara N, Lieberman AP, Kiyama H, Nunez G 2004. Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J Neurosci 24: 3721–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong S-Y, Youle RJ 2006. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443: 658–662 [DOI] [PubMed] [Google Scholar]
- Kent OA, Mendell JT 2006. A small piece in the cancer puzzle: MicroRNAs as tumor suppressors and oncogenes. Oncogene 25: 6188–6196 [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB 2003. Prediction of mammalian microRNA targets. Cell 115: 787–798 [DOI] [PubMed] [Google Scholar]
- Mott JL, Kobayashi S, Bronk SF, Gores GJ 2007. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 26: 6133–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW 1991. Cell death during development of the nervous system. Annu Rev Neurosci 14: 453–501 [DOI] [PubMed] [Google Scholar]
- Park SY, Lee JH, Ha M, Nam JW, Kim VN 2009. miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nat Struct Mol Biol 16: 23–29 [DOI] [PubMed] [Google Scholar]
- Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, et al. 2006. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res 66: 11590–11593 [DOI] [PubMed] [Google Scholar]
- Potts PR, Singh S, Knezek M, Thompson CB, Deshmukh M 2003. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J Cell Biol 163: 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Deshmukh M, Johnson EM Jr 2000. Inhibition of apoptotic signaling cascades causes loss of trophic factor dependence during neuronal maturation. J Cell Biol 149: 1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM 2001. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron 29: 615–628 [DOI] [PubMed] [Google Scholar]
- Putcha GV, Harris CA, Moulder KL, Easton RM, Thompson CB, Johnson EM Jr 2002. Intrinsic and extrinsic pathway signaling during neuronal apoptosis: Lessons from the analysis of mutant mice. J Cell Biol 157: 441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G, Slack FJ 2008. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9: 219–230 [DOI] [PubMed] [Google Scholar]
- Thomson JM, Parker J, Perou CM, Hammond SM 2004. A custom microarray platform for analysis of microRNA gene expression. Nat Methods 1: 47–53 [DOI] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Ginty DD 1999. Characterization of an NGF–P-TrkA retrograde-signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons. J Neurosci 19: 8207–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci 98: 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh GS, Orike N, Kaplan DR, Miller FD 2004. The invulnerability of adult neurons: A critical role for p73. J Neurosci 24: 9638–9647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X 2001. The expanding role of mitochondria in apoptosis. Genes Dev 15: 2922–2933 [PubMed] [Google Scholar]
- Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, et al. 2008. NF-κB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 14: 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J 2001. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron 29: 629–643 [DOI] [PubMed] [Google Scholar]
- Willis SN, Adams JM 2005. Life in the balance: How BH3-only proteins induce apoptosis. Curr Opin Cell Biol 17: 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Smith MI, Farrag L, Deshmukh M 2007. Chromatin modification of Apaf-1 restricts the apoptotic pathway in mature neurons. J Cell Biol 179: 825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyttenbach A, Tolkovsky AM 2006. The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons. J Neurochem 96: 1213–1226 [DOI] [PubMed] [Google Scholar]