Abstract
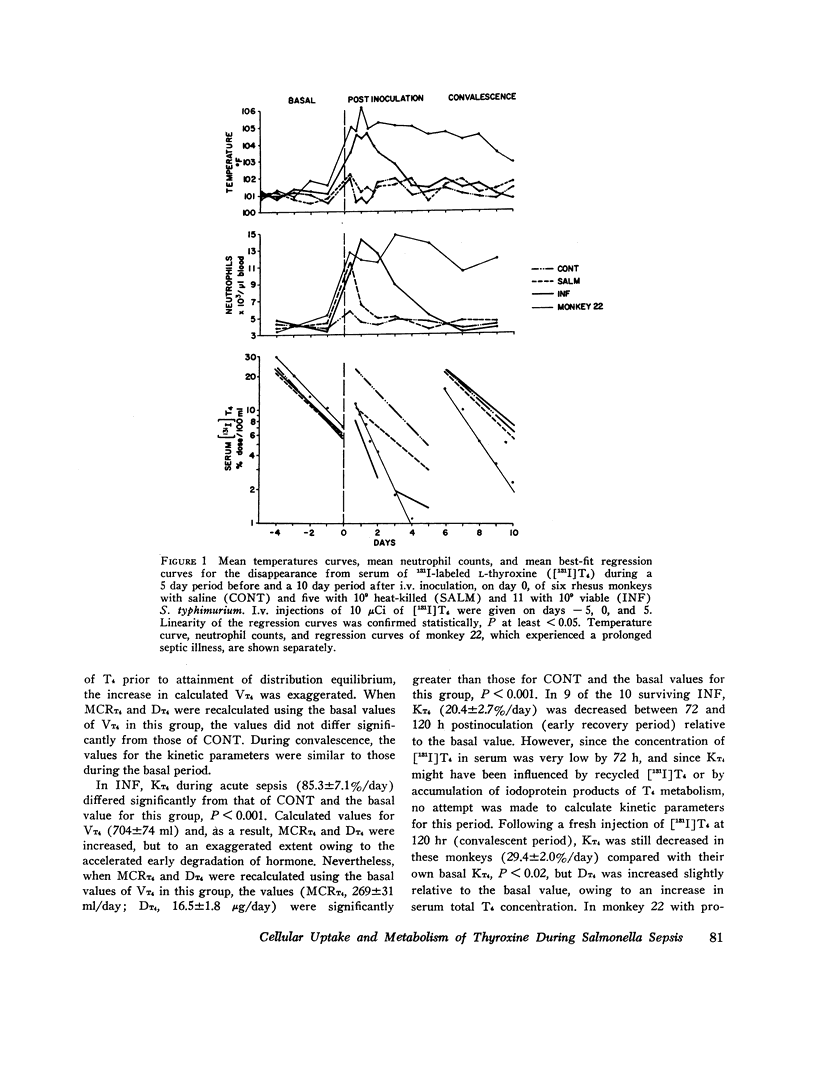
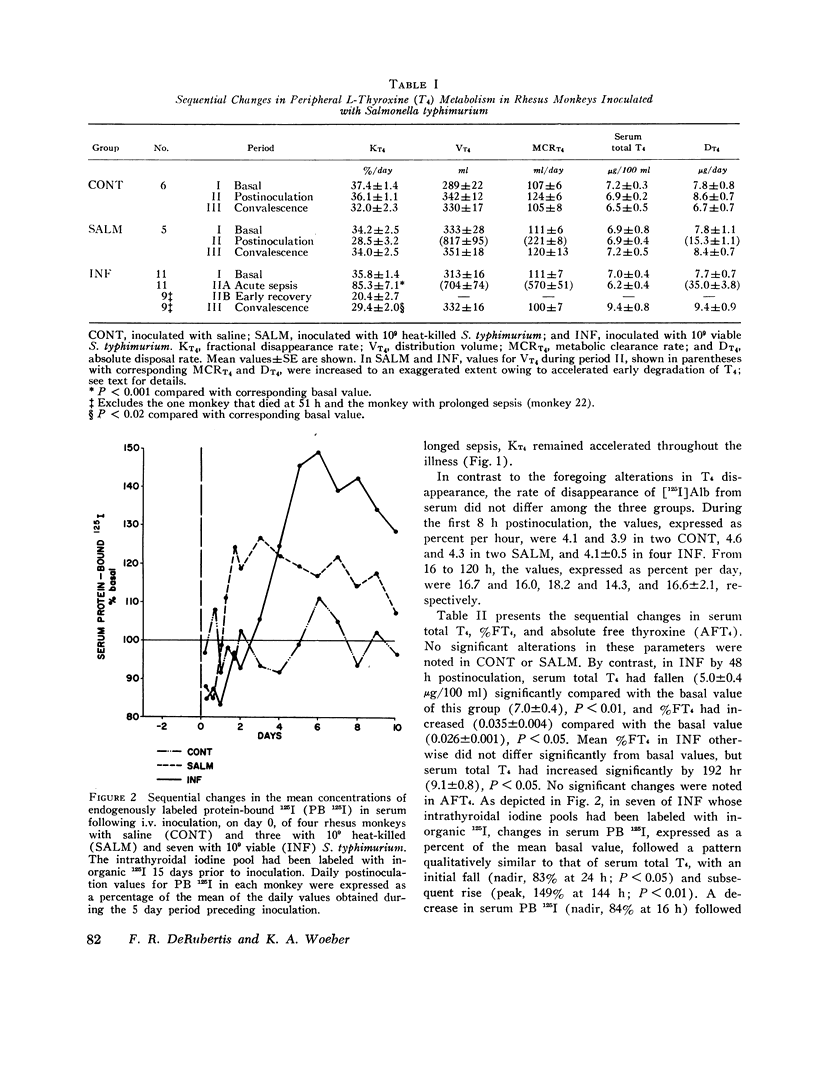
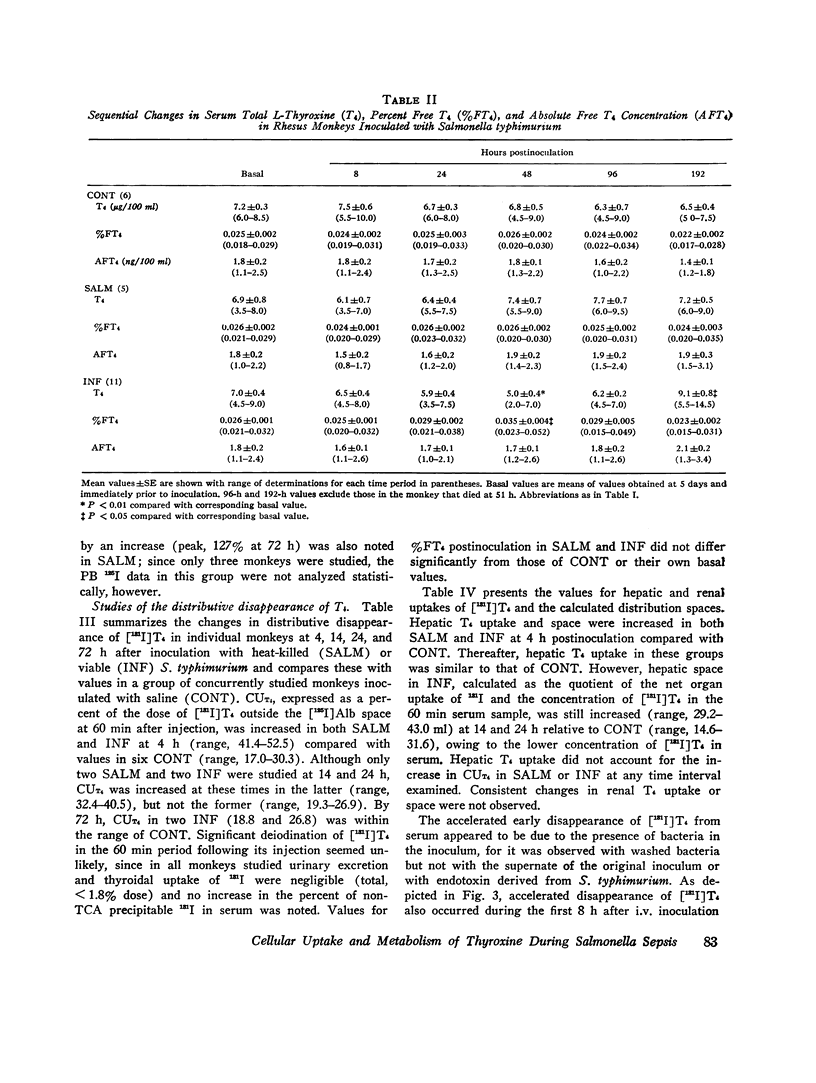
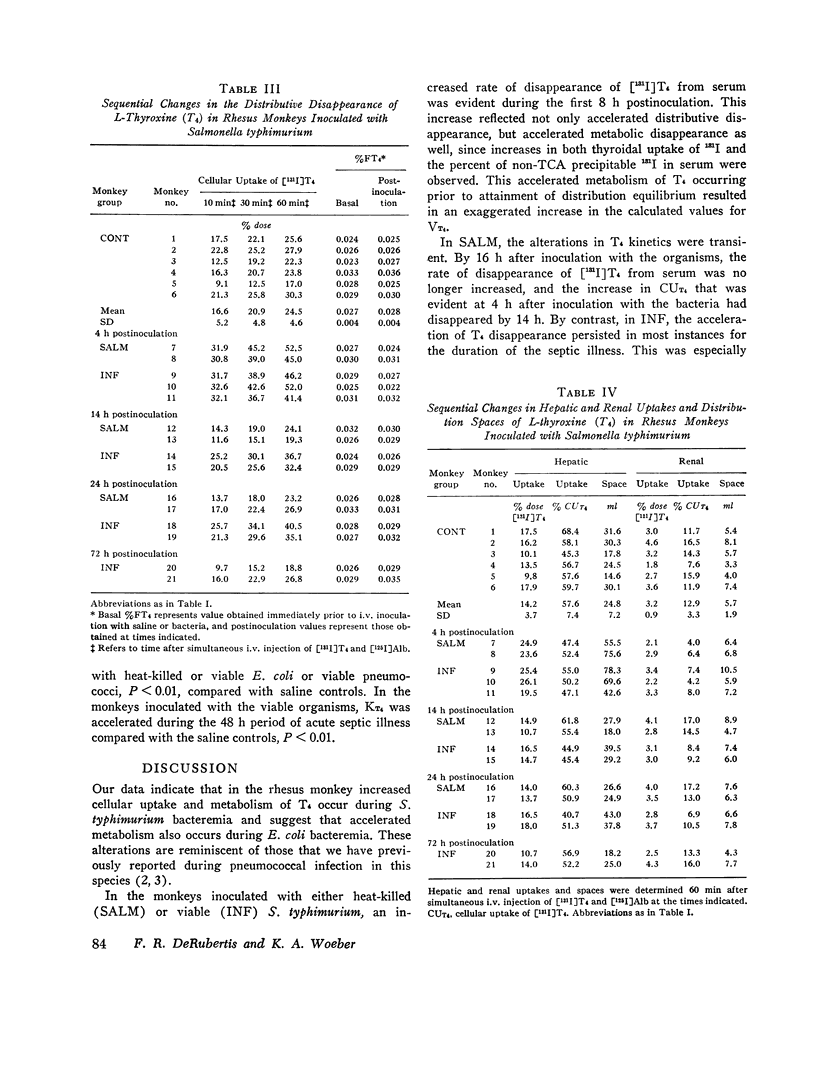
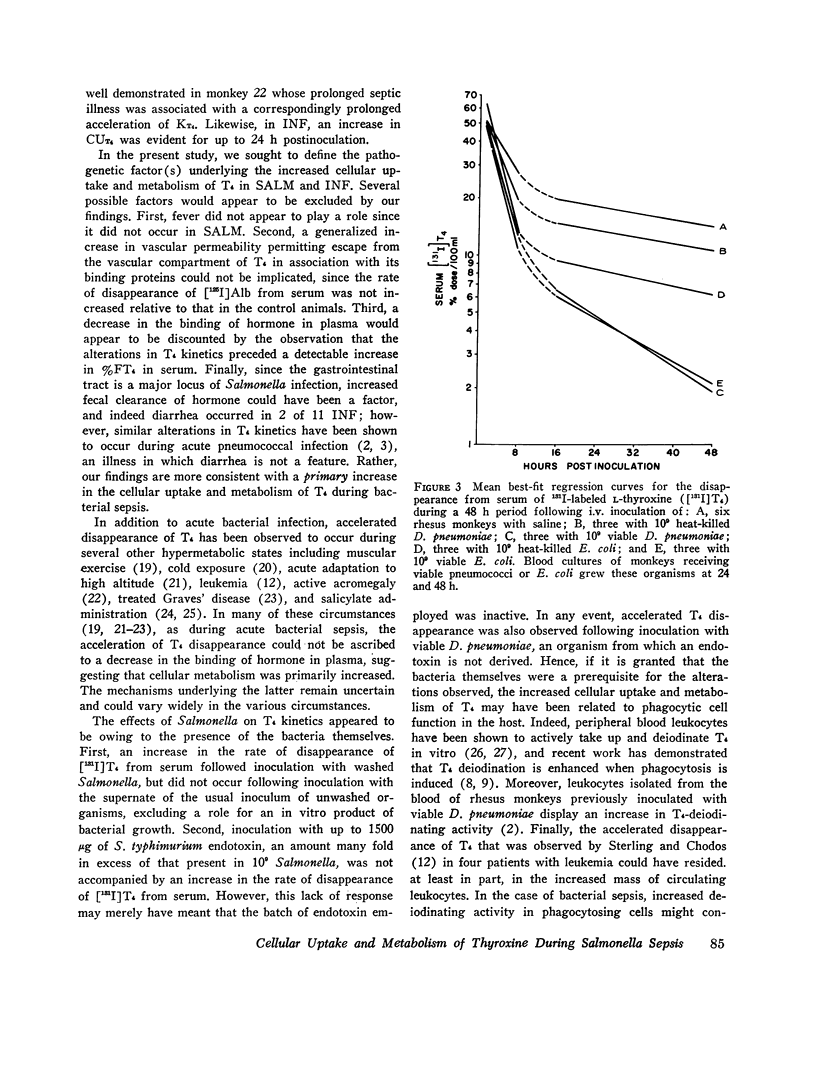
The effects of acute Salmonella typhimurium sepsis on the kinetics of peripheral L-thyroxine (T4) distribution and metabolism and on serum total and free T4 concentrations were studied in rhesus monkeys inoculated i.v. with either heat-killed or viable organisms. The rate of disappearance of labeled T4 from serum was increased within 8 h after inoculation of monkeys with either heat-killed or viable Salmonella. The effects of the heat-killed organisms were transient and no longer evident by 16 h postinoculation. The monkeys inoculated with the viable Salmonella experienced a 2-3 day febrile, septic illness that was accompanied by an increase in the absolute rate of T4 disposal. In the infected monkeys, serum total T4 and endogenously labeled protein-bound iodine concentrations fell significantly during the period of acute sepsis and then rose during convalescence to values that exceeded the preinoculation values, suggesting that thyroidal secretion of hormone had increased in response to a primary depletion of the peripheral hormonal pool. Total cellular and hepatic uptakes of T4 were enhanced by 4 h after inoculation of monkeys with either heat-killed or viable Salmonella, but the increase in total cellular uptake persisted for 24 h only in the monkeys inoculated with the viable organisms. These alterations in T4 kinetics could neither be correlated with changes in the binding of T4 in plasma nor attributed to an increase in vascular permeability. Moreover, they could not be ascribed to an in vitro product of bacterial growth, suggesting that the presence of the organisms themselves was required. An acceleration of T4 disappearance was also observed during Escherichia coli and Diplococcus pucumoniae bacteremias. Our findings are consistent with a primary increase in the cellular uptake and metabolism of T4 during bacterial sepsis, possibly related to phagocytic cell function in the host.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTEN F. K., RUBINI M. E., MERONEY W. H., WOLFF J. Salicylates and thyroid function. I. Depression of thyroid function. J Clin Invest. 1958 Aug;37(8):1131–1143. doi: 10.1172/JCI103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelberg H. M., Siemsen J. K., Jung R. C., Nicoloff J. T. Scintigraphic detection of pulmonary bacterial infections with labeled thyroid hormones and pertechnetate. Radiology. 1971 Apr;99(1):141–146. doi: 10.1148/99.1.141. [DOI] [PubMed] [Google Scholar]
- BECK G. E., BERAUD T., PASQUIER J. La fonction thyroïdienne dans les états fébriles. Schweiz Med Wochenschr. 1956 Nov 17;86(46):1300–1302. [PubMed] [Google Scholar]
- DeRubertis F. R., Woeber K. A. Evidence for enhanced cellular uptake and binding of thyroxine in vivo during acute infection with Diplococcus pneumoniae. J Clin Invest. 1972 Apr;51(4):788–795. doi: 10.1172/JCI106873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREINKEL N., LEWIS D. The effect of lowered environmental temperature on the peripheral metabolism of labelled thyroxine in the sheep. J Physiol. 1957 Feb 15;135(2):288–300. doi: 10.1113/jphysiol.1957.sp005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahimi H. D. The fine structural localization of endogenous and exogenous peroxidase activity in Kupffer cells of rat liver. J Cell Biol. 1970 Oct;47(1):247–262. doi: 10.1083/jcb.47.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALTON V. A., INGBAR S. H. ROLE OF PEROXIDASE AND CATALASE IN THE PHYSIOLOGICAL DEIODINATION OF THYROXINE. Endocrinology. 1963 Nov;73:596–605. doi: 10.1210/endo-73-5-596. [DOI] [PubMed] [Google Scholar]
- Gee J. B., Vassallo C. L., Bell P., Kaskin J., Basford R. E., Field J. B. Catalase-dependent peroxidative metabolism in the alveolar macrophage during phagocytosis. J Clin Invest. 1970 Jun;49(6):1280–1287. doi: 10.1172/JCI106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregerman R. I., Solomon N. Acceleration of thyroxine and triiodothyronine turnover during bacterial pulmonary infections and fever: implications for the functional state of the thyroid during stress and in senescence. J Clin Endocrinol Metab. 1967 Jan;27(1):93–105. doi: 10.1210/jcem-27-1-93. [DOI] [PubMed] [Google Scholar]
- HAUSMANN W., KARLISH A. J. Acute myxoedema precipitated by pneumonia. Report of five cases. Br Med J. 1961 Oct 21;2(5259):1063–1065. doi: 10.1136/bmj.2.5259.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGBAR S. H., FREINKEL N. Studies of thyroid function and the peripheral metabolism of I 131-labeled thyroxine in patients with treated Graves disease. J Clin Invest. 1958 Nov;37(11):1603–1614. doi: 10.1172/JCI103753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada M., Sterling K. Thyroxine turnover and transport in active acromegaly. J Clin Endocrinol Metab. 1967 Jul;27(7):1019–1027. doi: 10.1210/jcem-27-7-1019. [DOI] [PubMed] [Google Scholar]
- Irvine C. H. Effect of exercise on thyroxine degradation in athletes and non-athletes. J Clin Endocrinol Metab. 1968 Jul;28(7):942–948. doi: 10.1210/jcem-28-7-942. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., Mitchell G. W., Jr, Strauss R. R., Paul B. B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. XXXI. Serum and cellular iodine levels and their relationship to the hyperbactericidal activity of pregnancy. Am J Obstet Gynecol. 1971 Aug 1;110(7):911–918. doi: 10.1016/0002-9378(71)90543-6. [DOI] [PubMed] [Google Scholar]
- KURLAND G. S., KROTKOV M. V., FREEDBERG A. S. Oxygen consumption and-thyroxine deiodination by human leukocytes. J Clin Endocrinol Metab. 1960 Jan;20:35–46. doi: 10.1210/jcem-20-1-35. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Bernstein G., Hasen J. Estimation of rapidly exchangeable cellular thyroxine from the plasma disappearance curves of simultaneously administered thyroxine-131-I and albumin-125-I. J Clin Invest. 1967 May;46(5):762–777. doi: 10.1172/JCI105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. B., Jacobs A. A., Strauss R. R., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXIV. Aldehyde Generation by the Myeloperoxidase-H(2)O(2)-Chloride Antimicrobial System: a Possible In Vivo Mechanism of Action. Infect Immun. 1970 Oct;2(4):414–418. doi: 10.1128/iai.2.4.414-418.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Function of h(2)o(2), myeloperoxidase, and hexose monophosphate shunt enzymes in phagocytizing cells from different species. Infect Immun. 1970 Apr;1(4):338–344. doi: 10.1128/iai.1.4.338-344.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S. H., Klebanoff S. J. Quantitative leukocyte iodination. N Engl J Med. 1971 Apr 8;284(14):744–750. doi: 10.1056/NEJM197104082841402. [DOI] [PubMed] [Google Scholar]
- ROGERS D. E. Host mechanisms which act to remove bacteria from the blood stream. Bacteriol Rev. 1960 Mar;24(1):50–66. doi: 10.1128/br.24.1.50-66.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL E., SACHS B. A. IN VITRO LEUKOCYTE UPTAKE OF 131-I LABELED IODIDE, THYROXINE AND TRIIODOTHYRONINE, AND ITS RELATION TO THYROID FUNCTION. J Clin Endocrinol Metab. 1964 Apr;24:313–318. doi: 10.1210/jcem-24-4-313. [DOI] [PubMed] [Google Scholar]
- STERLING K., CHODOS R. B. Radiothyroxine turnover studies in myxedema, thyrotoxicosis, and hypermetabolism without endocrine disease. J Clin Invest. 1956 Jul;35(7):806–813. doi: 10.1172/JCI103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambaugh G. E., 3rd, Beisel W. R. Early alterations in thyroid hormone physiology during acute infection in man. J Clin Endocrinol Metab. 1967 Dec;27(12):1667–1673. doi: 10.1210/jcem-27-12-1667. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Beckwitt H. J., Chidsey C. A. Changes in plasma thyroxine concentration and metabolism, catecholamine excretion and basal oxygen consumption in man during acute exposure to high altitude. J Clin Endocrinol Metab. 1967 Jun;27(6):789–799. doi: 10.1210/jcem-27-6-789. [DOI] [PubMed] [Google Scholar]
- WISWELL J. G., CORONHO V. Disappearance of I-131-triiodo-thyronine from the plasma in the presence of fever. J Clin Endocrinol Metab. 1962 Jun;22:657–659. doi: 10.1210/jcem-22-6-657. [DOI] [PubMed] [Google Scholar]
- WOEBER K. A., INGBAR S. H. THE EFFECTS OF NONCALORIGENIC CONGENERS OF SALICYLATE ON THE PERIPHERAL METABOLISM OF THYROXINE. J Clin Invest. 1964 May;43:931–942. doi: 10.1172/JCI104979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeber K. A. Alterations in thyroid hormone economy during acute infection with Diplococcus pneumoniae in the rhesus monkey. J Clin Invest. 1971 Feb;50(2):378–387. doi: 10.1172/JCI106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeber K. A., Doherty G. F., Ingbar S. H. Stimulation by phagocytosis of the deiodination of L-thyroxine in human leukocytes. Science. 1972 Jun 2;176(4038):1039–1041. doi: 10.1126/science.176.4038.1039. [DOI] [PubMed] [Google Scholar]


