Abstract
Human genetic diseases and mouse knockouts illustrate that the maintenance of central nervous system myelin requires connexin expression by both astrocytes and oligodendrocytes. Because these cell types express nonoverlapping sets of connexins, the intercellular channels formed between them must be asymmetric with regard to connexin content, defined as heterotypic. Here, we show that oligodendrocyte Cx47 can form heterotypic channels with astrocyte Cx43 or Cx30 but not Cx26, whereas oligodendrocyte Cx32 can functionally interact with astrocyte Cx30 or Cx26 but not Cx43. Thus, as many as four types of intercellular channels could be formed between astrocytes and oligodendrocytes.
Keywords: gap junction, connexin, myelin, glia
INTRODUCTION
In the central nervous system, gap junctions are established between neighboring astrocytes, between neighboring oligodendrocytes, and between astrocytes and oligodendrocytes (Massa and Mugnaini, 1982; Maglione et al., 2010). In addition, astrocytes and oligodendrocytes establish “reflexive” or “autologous” gap junctions within the same cell. In oligodendrocytes, reflexive junctions between the layers of myelin and between successive paranodal loops (Kamasawa et al., 2005) markedly shorten the pathway for diffusion between periaxonal cytoplasm and cell body. Together, these gap junctions are thought to link cells in a network that facilitates absorption and removal of extracellular K1 released during neuronal activity (Orkand et al., 1966; Kettenmann and Ransom, 1988; Menichella et al., 2006) and provide a critical pathway for the supply of glucose to neurons (Rouach et al., 2008). Their importance is underscored by the association of mutations in several human glial connexins with white matter abnormalities (Taylor et al., 2003; Loddenkemper et al., 2002; Uhlenberg et al., 2004).
Oligodendrocytes express three connexins; Cx29, Cx32, and Cx47 (Altevogt et al., 2002; Scherer et al., 1995; Menichella et al., 2003; Odermatt et al., 2003) although Cx29 does not localize to gap junctional plaques and thus may not form intercellular channels (Altevogt and Paul, 2004). Astrocytes express a different set of three; Cx26, Cx30, and Cx43 (Nagy et al., 2001; Kunzelmann et al., 1999; Giaume et al., 1991) although there are conflicting reports regarding levels and distribution of Cx26 (Mercier and Hatton, 2001; Altevogt and Paul, 2004; Filippov et al., 2003). Because oligodendrocytes and astrocytes express nonoverlapping sets of connexins, the intercellular channels formed between them are asymmetric with regard to connexin content, defined as heterotypic. Several models for these heterotypic interactions have been proposed. In one, Cx47-Cx43 and Cx32-Cx30 heterotypic pairs provide two independent parallel pathways for communication between oligodendrocytes and astrocytes (Nagy et al., 2003b). This model is supported by studies of connexintransfected N2A cells (Orthmann-Murphy et al., 2007) in which Cx47-Cx43 and Cx32-Cx30 pairings were functional but Cx47-Cx30 pairing was not. A second model based on connexin co-localization in vivo proposes that Cx47 forms heterotypic channels with both Cx43 and Cx30 while Cx32 forms channels with Cx26 (Altevogt and Paul, 2004). A recent study of junctional communication in connexin knockout (KO) mouse lines (Maglione et al., 2010) suggested a third model. In brain slices, oligodendrocyte-astrocyte communication was not affected by loss of either Cx32 or Cx43 but was essentially eliminated by loss of Cx47 or loss of Cx43 and Cx30 together but not Cx43 alone. One interpretation is that oligodendrocyte-astrocyte communication relies principally on heterotypic channels composed of oligodendrocyte Cx47 and astrocyte Cx30.
To explore these models, we evaluated the heterotypic interactions of glial connexins using HeLa cells and communication-negative primary astrocytes. Using neurobiotin transfer as a measure of junctional coupling, we found that oligodendrocyte Cx47 can form heterotypic channels with astrocyte Cx43 or Cx30 but not Cx26, whereas oligodendrocyte Cx32 can form channels with astrocyte Cx30 or Cx26 but not Cx43. Thus, four types of heterotypic intercellular channels, Cx47-Cx43, Cx47-Cx30, Cx32-Cx30, and Cx32-Cx26, could contribute to oligodendrocyte-astrocyte communication in vivo.
MATERIALS AND METHODS
HeLa Cell Lines
HeLa cell lines were obtained from ATCC (CCL-2; Manassas, Virginia) and from John Blenis (Harvard Medical School, Boston, MA), Dan Goodenough (Harvard Medical School, Boston, MA), Felix Bukauskas (Albert Einstein College of Medicine, Bronx, NY), David Corey (Harvard Medical School, Boston, MA), and Junying Yuan (Harvard Medical School, Boston, MA). Except for CCL-2, the origin and history of the lines were unknown. Only the cells obtained from the Yuan laboratory were completely communication-negative in our assay. All cells were cultured in high glucose Dulbecco's Modified Eagle Media (dMEM; Invitrogen, Carlsbad, CA) with sodium pyruvate containing 10% FBS (HyClone, Logan, UT) and 1% penicillin/streptomycin (Invitrogen).
Generating Glial Connexin Expression Constructs
Rat connexin32 (rCx32) cDNA (Paul, 1986) was subcloned into the EcoRI site of pIRES2-eGFP, and rat connexin26 (rCx26) cDNA (Zhang and Nicholson, 1989) was subcloned into the BglII site of pIRES2-eGFP. The coding regions of mouse Cx47 and mouse Cx30 were amplified by PCR and subcloned into the XhoI and XmaI sites in the same vector. Plasmids containing human connexin47 (hCx47), human connexin43 (hCx43), and human connexin30 (hCx30) were kindly provided by Dr. Steve Scherer (University of Pennsylvania, Philadelphia, PA) in the pIRES2-eGFP vector (Clontech, Mountain View, CA) as described in Orthmann-Murphy et al., 2007. The lentiviral expression vector pRRLSIN.cPPT.PGK-GFP.WPRE (Addgene, Cambridge, MA) was modified by replacing the PGK-GFP cassette with CMV-IRES2-Venus or -Cerulean. Finally, connexins were removed from pIRES2-eGFP by NheI/BamHI digestion and transferred into pRRLSIN.cPPT.CMV-IRES2-Venus/Cerulean.WPRE. For virus production, 1 μg of the transfer construct, 1 μg of the psPAX2 packaging plasmid (Addgene), and 1 μg of the pMD2.G envelop-encoding plasmid (Addgene) were co-transfected into 293FT cells at 80% confluence in a 6-cm dish using Lipofectamine and the PLUS reagent (Invitrogen). The medium was collected after 48 h, filtered through a 0.45-μm pore size filter and stored at −80°C until use. All experiments described in this study involving Cx47 and Cx30 used the human iso-forms because the mouse counterparts did not express well in our system for unknown reasons.
Generating HeLa cell lines expressing glial connexins
HeLa cells were seeded into 6-well plates at a density of 1 × 105 cells per well. One milliliter of viral supernatant was added to 1 ml fresh medium supplemented with 4 μg/ml Polybrene (Sigma-Aldrich, St. Louis, MO), and the cells were incubated overnight. The next day, the medium was replaced with fresh medium. Expression levels were analyzed 48-72 hours post infection. Depending on the connexin, between 5 and 50% of the HeLa cells were infected as determined by expression of the fluorescent protein. Where necessary, cultures were expanded and sorted by fluorescence activated cell sorting to obtain populations of HeLa cells that were >90% positive.
Dye Transfer
Three days before the dye transfer assay, cells were plated on 35-mm glass bottom Fluorodishes (World Precision Instruments, Sarasota, FL) and incubated in culture media as described above plus 400 ng/μl unlabeled avidin (Sigma). Cells were grown to confluence and transferred to a 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffered solution containing 150 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 10 mM D-glucose, pH 7.4 immediately before dye transfer assay. The nonfluorescent tracer neurobiotin (MW 5 287, +1 charge; Vector Laboratories, Burlingame, CA) was dissolved at 10% along with 3% dextran-Cascade Blue, (MW = 10,000; Invitrogen) in a 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffered solution of 140 mM KCl, 2 mM MgCl2, 6 mM EGTA, and 5 mM CsCl. Pipettes were backfilled with tracer solution and iontophoretic injection was carried out by applying alternating currents of −1 nA and +1 nA at 200 ms each for a total of 1 min. Ten injections were performed per dish and the total time of the experiment never exceeded 15 min. A minimum of 3 dishes were used for each cell type for a total of 30 injections. For neurobiotin detection, cells were fixed in 4% paraformaldehyde for 15 min, blocked with 1% fish skin gelatin in phosphate buffered saline (PBS) containing 0.1% Tween-20 for 30 min, and incubated in NeutrAvidin, tetramethylrhodamine conjugated (1:1000; Invitrogen) for 1 hour at room temperature.
RT-PCR
Total RNA was isolated using the RNA Easy Mini Kit (Qiagen, Hilden, Germany). Five microgram of RNA was DNase treated and first strand cDNA was synthesized using random primers and the Superscript-II preamplification system (Invitrogen). Primers were designed to detect a ~500-bp fragment in the coding region of each connexin. Primer sequences are listed in Table 1. cDNA synthesis was confirmed by primers amplifying GAPDH (Clontech, Mountain View, CA).
TABLE 1.
Screening Primers for Mouse Connexins
| Mouse connexin | 5′ PCR primer (5′→3′) | 3′ PCR primer (5′→3′) | Size |
|---|---|---|---|
| cx26 | CCCCAAAGCTCCCAAACAAAGG | CACGCCAGTGATGAATACAATAGGTG | 520 bp |
| cx29 | ATCTGTGCTGTGCTATTTGGAGT | GAAGGAAGCGCCCCACAGG | 350 bp |
| cx30 | GCCAGGGTGCAAGAACGTCTGC | GGCATGGTTGGGGTGGTTTCTC | 550 bp |
| cx30.2 | CGCTTCTGGCTCTTCCACATCC | GACGGCGAAGTAGAAGACCACG | 365 bp |
| cx30.3 | TTCCGGGTGCTGGTGTATGTGG | ACACTCCCTTCGCCCATTTTCC | 750 bp |
| cx31 | TCGGCCCACGGAGAAGAAGG | AGAGCCCAGCACCCACAAATACC | 412 bp |
| cx31.1 | AGCGGGGTGGACTCTGGTGG | TTGCTCATCGGTGCCTTCGTG | 734 bp |
| cx32 | GTGGCGTGAATCGGCACTCTAC | CTCCGCCACGTTGAGGATAATG | 750 bp |
| cx33 | GTGCGCCTCTGGCTCCTC | CCTCCGCTTTTCTCATACGATTC | 511 bp |
| cx36 | GCGGAGGGAGCAAACGAGAAG | CTGCCGAAATTGGGAACACTGAC | 560 bp |
| cx37 | AGAGCGGTTGCGGCAGAAAGAGG | TGGATGAGAGCCCGTTGTAGGTG | 551 bp |
| cx39 | GTGGTGGTCTTGGCAGGGTCACC | CCCACTGCGTGGTCCTCAGTGTC | 517 bp |
| cx40 | AAGCTAAAGAGGCCCACCGCACTG | TTCCGGGAGCCCATGTTATTACTG | 535 bp |
| cx43 | CACGGCAAGGTGAAGATGAGAGG | CTCCACGGGAACGAAATGAACAC | 500 bp |
| cx45 | GAGGTGGGCTTTCTAATAGGGCAG | ATGGGGGTTGTTTTGGTGATGG | 528 bp |
| cx46 | TCTACGCCCACCCTCATCTATCTG | GGCTTGCTGGCTGGAGTCTGC | 782 bp |
| cx47 | ACGGCCGCGCACAGCATC | AGGGCCCCGGAGAAGACACG | 460 bp |
| cx50 | TCATCGTGGGCCATTACTTCCTG | TTCTGGGGCCACCTTCTCTGC | 584 bp |
| cx57 | TCCAGGCCCACAGAGAAGAC | TGGGGCCAAAGACAGAATG | 475 bp |
| GAPDH | TGAAGGTCGGTGTGAACGGATTTGGC | CATGTAGGCCATGAGGTCCACCAC | 1 kb |
Mice
The generation of Cx43flx/flx mice (Liao et al., 2001) as been described previously. The hGFAP-Cre transgenic line was obtained from The Jackson Laboratory (Bar Harbor, ME). Primary astrocyte cultures used for this study were obtained from mice that were positive for the hGFAP-Cre transgene and heterozygous for the floxed Cx43 allele (GFAP-Cre+, Cx43flx/+; control cultures) or positive for the hGFAP-Cre transgene and homozygous for floxed Cx43 (GFAP-Cre1, Cx43flx/flx; KO cultures). Animals were maintained as approved by the Institutional Animal Care and Use Committee at Harvard Medical School.
Primary Astrocyte Cultures
Primary astrocytes were purified from P5-P6 mouse cerebella by selective preplating. Cerebella were excised, and the meninges were removed in Dulbecco's PBS (Invitrogen), under a dissecting microscope. The tissue was cut into small pieces and incubated in 1% trypsin (Invitrogen) and 0.02% deoxyribonuclease (DNase; Worthington, Freehold, NJ) in PBS for 20 min at 37 C. The trypsin was inactivated by addition of high glucose dMEM without sodium pyruvate containing 10% FBS, and the tissue was gently triturated using fire-polished glass Pasteur pipettes of successively decreasing bore size. Cells were centrifuged at 750g for 5 min and resuspended in fresh medium. To separate out astroglia, cells were plated onto a noncoated 100 mm tissue culture dish and incubated at 37°C for 30 min to allow fibroblasts to attach. Unattached cells (neurons and glia) were removed and plated onto a poly-D-lysine (0.5 μg/ml; Sigma-Aldrich) -coated dish. After incubation for 30 min at 37°C, unattached cells (primarily neurons) were removed, and the dish was washed gently with dMEM to remove loosely adherent cells. The remaining cells, primarily glia, were incubated in astrocyte media (high glucose dMEM, 10% FBS, and 2 mM L-glutamine). The cells were passaged 5-6 days later, a step that removed any remaining neurons, producing a glial culture >95% GFAP-positive. All cells were incubated at 37°C with 5% CO2.
Immunostaining
Cells were grown on glass bottom dishes (MatTek, Ashland, MA) or coverslips, fixed with 4% paraformaldehyde for 15 min at room temperature, blocked with 10% FBS in PBS containing 0.2% Tween-20, and incubated for 1 hour at room temperature in appropriate combinations of the following primary antibodies: rabbit anti-Cx43 (1:10,000 dilution; Sigma C-6219), mouse anti-Cx43 (1:100, Zymed 13-8300), rabbit anti-Cx47 (1:1000, Menichella et al., 2003), rabbit anti-Cx32 (1:100; Zymed 34-5700), mouse anti-Cx32 (1:100, Zymed 13-8200), rabbit anti-Cx26 (1:50; Zymed 71-0500), mouse anti-Cx26 (1:100, Zymed 33-5800), rabbit anti-Cx30 (1:1000; Zymed 71-2200), or rabbit anti-GFAP(1:250 dilution; Dako, Carpinteria, CA). After incubation with primary antibodies, cells were washed and incubated with appropriate secondary antibodies (rhodamine-labeled goat anti-rabbit (1:200, Chemicon, Temecula, CA); Alexa568-labeled goat anti-mouse, (1:500, Invitrogen); Alex647-labeled goat anti-rabbit (1:1000, Invitrogen); Cy5-labeled donkey anti-goat (1:500, Jackson ImmunoResearch, West Grove, PA); or Cy3-labeled donkey anti-rabbit (1:500, Jackson ImmunoResearch). Because both anti-Cx47 and anti-Cx30 primary antibodies were raised in rabbit, to perform a double labeling, we first applied one of the primary antibodies, blocked it with goat anti-rabbit IgG Fab fragments (AffiniPure, 1:500, Jackson ImmunoResearch), then labeled with the second primary followed by anti-rabbit and anti-goat secondary antibodies.
RESULTS
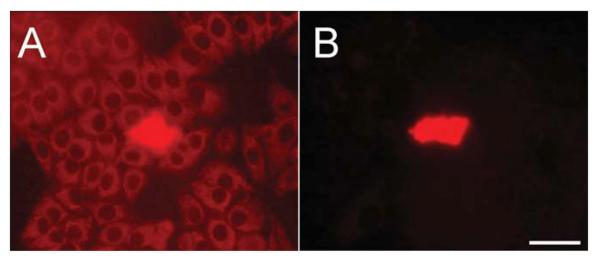
To assess intercellular communication, we performed microinjection of neurobiotin, a low molecular mass (287 Da) compound known to permeate homotypic gap junction channels comprised of each of the five glial connexins considered in this study (Elfgang et al., 1995; Orthmann-Murphy et al., 2007; Manthey et al., 2001). After injection, neurobiotin was aldehyde fixed and detected with fluorescently labeled avidin. As an expression system for the glial connexins, we used HeLa cells, which have been reported to be communication negative and have been widely used for analysis of connexin function (Elfgang et al., 1995). However, many HeLa strains have been established over the years (Masters, 2002) and they can differ strikingly in their behavior. Therefore, we tested eight HeLa strains for endogenous coupling. Seven of eight lines were coupled, as defined by transfer of neurobiotin to two or more adjacent cells (data not shown). Only one line consistently showed no neurobiotin transfer (Fig. 1). In the course of screening the cell lines, we observed a faint but consistent avidin-dependent signal in all cells (Fig. 1A) possibly reflecting the presence of biotin-containing compounds in mitochondria (Hollinshead et al., 1997). Because the sole source of cellular biotin is the fetal bovine serum (FBS) added to the culture media, we used unlabeled avidin to absorb it. After 3 days of culture using absorbed media, a major increase in signal-to-noise was achieved (Fig. 1B—photographic exposure equal to Fig. 1A) with no effects on cell growth or morphology. Thus, absorption significantly improved the sensitivity of our assay.
Fig. 1.
Incubation of HeLa cells with unlabeled avidin reduces background for neurobiotin injections. A) This HeLa strain does not exhibit gap junctional coupling as the junction-permeant tracer neurobiotin was detected only in the injected cell. However, a fine granular cytoplasmic background, likely reflecting biotin-containing compounds in HeLa mitochondria, reduces the sensitivity of the assay. B) Three days after the addition of unlabeled avidin to the culture media, a dramatic decrease in background was observed. Camera exposure time was identical to A. Scale bar: 50 μm.
All Glial Connexins form Homotypic Junctions but only Certain Combinations of Heterotypic Interactions Are Functional in HeLa Cells
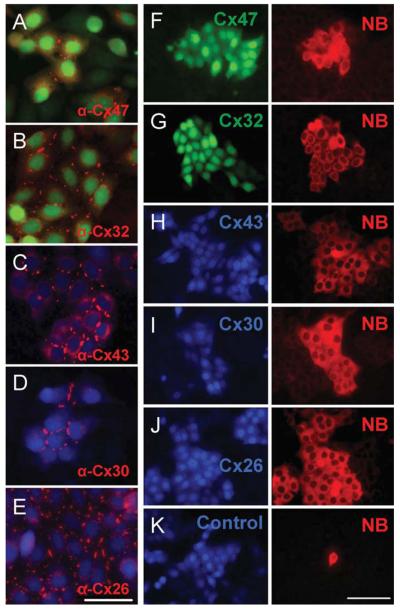
We used a lentiviral expression system (Zufferey et al., 1998) to generate cell lines expressing a glial connexin or an empty vector control. To identify cells expressing connexins, we included an internal ribosome entry site (IRES) to drive expression of a fluorescent marker. Thus, the connexins were not modified by fusion to a marker protein, which can affect trafficking and assembly (Hunter et al., 2005). Lines in which >90% of the cells expressed both the marker and the connexin were obtained. In each case, immunostaining revealed an orthodox pattern of connexin distribution that included puncta localized to apposing membranes of adjacent cells (Fig. 2A-E). Furthermore, HeLa cells expressing any of the glial connexins were able to transfer neurobiotin (Fig. 2F-J), whereas an empty vector control did not (Fig. 2K). Thus, all the glial connexins can form functional homotypic intercellular channels in our HeLa strain.
Fig. 2.
All glial connexins can form functional homotypic channels in HeLa cells. Lentiviral transduction was used to generate lines expressing specific glial connexins. Immunostaining (A-E) reveals an orthodox distribution including puncta localized to apposing membranes of adjacent cells (Scale bar: 30 μm). HeLa cells expressing individual glial connexins were able to transfer neurobiotin (F-J), whereas an empty vector control was not (K). Scale bar: 50 μm.
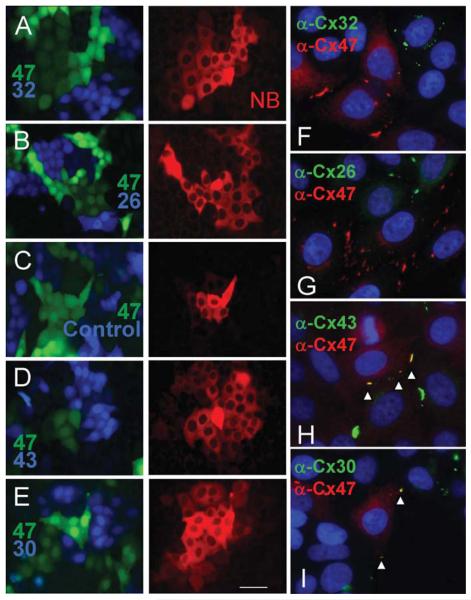
The ability of oligodendrocyte Cx47 to form functional heterotypic channels with other glial connexins was tested by plating Cx47-positive cells with those expressing a different connexin and a different fluorescent marker. Cells expressing Cx47 (Fig. 3A-E, Venus) were plated with cells expressing Cx32 (Fig. 3A-Cerulean), Cx26 (Fig. 3B-Cerulean), or an empty vector control (Fig. 3C-Cerulean). When a Cx47-positive cell was injected, neurobiotin was detected only in Cx47-positive cells. Complementary results were obtained when Cx32- or Cx26-positive cells were injected (data not shown). Furthermore, double-label immunofluorescence microscopy did not reveal significant co-localization of Cx47 and Cx32 (Fig. 3F) or Cx47 and Cx26 (Fig. 3G) consistent with the notion that heterotypic intercellular channels were not formed. In contrast, when cells expressing Cx47 were plated with Cx43- or Cx30-positive cells (Fig. 3D, E-Cerulean), injection of Cx47-positive cells resulted in neurobiotin transfer to all connexin-positive cell types. Complementary results were obtained when Cx43- or Cx30-positive cells were injected (data not shown). As expected, co-localization of Cx47 with Cx43 (Fig. 3H) or Cx30 (Fig. 3I) was robust. Thus, Cx47 forms functional heterotypic channels with either Cx43 or Cx30. This result conflicts with an earlier report in which communication between Cx47- and Cx30-expressing cells was not observed (Orthmann-Murphy et al., 2007).
Fig. 3.
Oligodendrocyte Cx47 cannot form functional heterotypic channels with Cx32 or Cx26 but does so readily with Cx43 or Cx30. Cells expressing Cx47 (A-E, green) were plated with cells expressing Cx32 (A, blue), Cx26 (B, blue), an empty vector control (C, blue), Cx43 (D, blue) and Cx30 (E, blue). Neurobiotin injection revealed heterotypic transfer only for Cx47-Cx43 and Cx47-Cx30 pairs. Consistent with these data, no co-localization of Cx47 with either Cx32 (F) or Cx26 (G) was evident, whereas co-localization of Cx47 with Cx43 (H) or Cx30 (I) was robust (arrowheads). Scale bar: 50 μm.
The ability of Cx32, the other major oligodendrocyte connexin, to support heterotypic communication was tested in a similar fashion. Cells expressing Cx32 (Fig. 4A-D, Venus) were mixed with cells expressing Cx43 (Fig. 4A, Cerulean) or empty vector control cells (Fig. 4B, Cerulean). Neurobiotin injection into Cx32-positive cells resulted in transfer only to other Cx32-positive cells. In addition, no significant co-localization of Cx32 and Cx43 (Fig. 4E) was observed, indicating that heterotypic channels were not formed. In contrast, mixed populations of cells expressing Cx32 or Cx30 (Fig. 4C) and Cx32 or Cx26 (Fig. 4D) exhibited neurobiotin transfer between all cells and co-localization was observed in both cases (Fig. 4F,G) consistent with the formation of heterotypic pairs.
Fig. 4.
Oligodendrocyte Cx32 can form functional heterotypic channels with Cx30 or Cx26. Cells expressing Cx32 (A-D, green) were mixed with cells expressing Cx43 (A, blue), empty vector controls (B, blue), Cx30 (C, blue), or Cx26 (D, blue). Neurobiotin injection revealed heterotypic transfer only in Cx32-Cx30 and Cx32-Cx26 pairs (scale bar: 50 lm. Consistent with these data, Cx32 (E-G, red) did not co-localize with Cx43 (E, green) but did overlap (arrowheads) with Cx30 (F, green) and with Cx26 (G, green) when adjacent cells expressed those combinations of connexins. Scale bar: 50 μm.
Primary Astrocytes Derived from Mice Lacking Cx43 Are Uncoupled
As our results differed significantly from those of Orthmann-Murphy et al. (2007), we developed a second expression system to assess connexin interactions consisting of primary astrocyte cultures derived from Cx43KO mice. Because constitutive loss of Cx43 is peri-natal lethal due to cardiac abnormalities (Reaume et al., 1995), we achieved a nervous system-specific deletion of Cx43 by crossing a conditional Cx43 line (Liao et al., 2001) and a transgenic line in which the glial fibrillary acidic protein (GFAP) promoter drives Cre (Zhuo et al., 2001). Astrocytes derived from a similar cross were reported to be communication-negative as assessed by injection of the fluorescent dye Lucifer yellow (Theis et al., 2004).
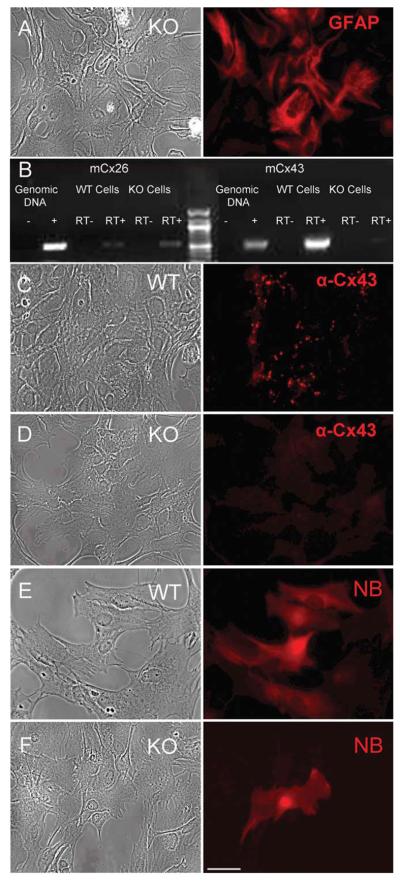
Primary astrocyte cultures were 95% pure as demonstrated by expression of GFAP (Fig. 5A). A reverse transcription-polymerase chain reaction (RT-PCR) screen (Fig. 5B) revealed that at 18 days in vitro (DIV), control astrocytes expressed abundant Cx43, whereas astrocytes derived from the conditional Cx43 KO exhibited very weak expression of Cx43, likely reflecting contamination of the culture with nonastrocytic cells and/or a small population of astrocytes, which escaped recombination. A Cx26 signal was consistently found in both wildtype (WT) and Cx43 KO astrocyte cultures but at levels below one mRNA copy per cell on average. Cx30 was detected in neither WT nor KO cells (data not shown), consistent with the late onset of its expression in vivo (~P21) and in primary cultures (21-28 DIV) (Kunzelmann et al., 1999). Similarly, RT-PCR screens for the remaining 17 murine connexins did not reveal consistent expression for any (data not shown).
Fig. 5.
Primary astrocytes lacking Cx43 are communication negative. A) Primary astrocyte cultures were 95% GFAP-positive. B) RTPCR screen of control astrocytes reveals abundant Cx43 while astrocytes derived from a conditional Cx43KO showed very weak expression of Cx43, likely reflecting contamination of the culture with non-astrocytic cells. Both WT and KO cells express very low levels of Cx26 signal (< one copy per cell on average). No other connexin transcript was consistently observed (data not shown). C) Control astrocytes displayed multiple Cx43-positive puncta reminiscent of gap junctions. D) Most KO astrocytes lacked any detectable signal. E) Control astrocytes displayed extensive transfer of neurobiotin. F) Cx43KO astrocytes exhibited no transfer.
The general loss of Cx43 was confirmed by immuno-staining astrocyte cultures. Although control astrocytes displayed multiple puncta reminiscent of gap junctions (Fig. 5C), most KO astrocytes lacked any detectable signal (Fig. 5D). We also tested both control and KO astrocytes for endogenous coupling by neurobiotin injection. Control astrocytes showed extensive transfer of neurobiotin (Fig. 5E), whereas KO astrocytes exhibited none (Fig. 5F). Therefore, we conclude that most primary astrocytes from the conditional Cx43KO do not express any connexin at significant levels and do not exhibit detectable levels of endogenous coupling.
Heterotypic Pairings that Were Functional in HeLa Cells Are also Functional in Primary Astrocytes
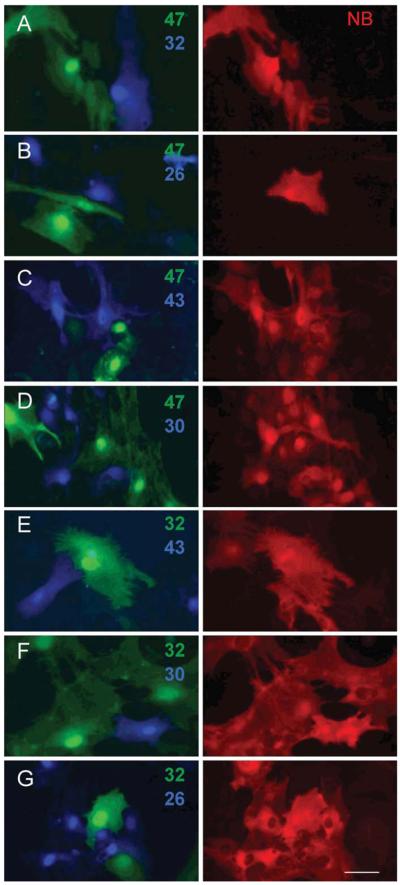
Lentiviral transduction of astrocytes was at least as efficient as in HeLa cells, which allowed experiments to be completed well before (≤15 DIV) the appearance of endogenous Cx30 (21-28 DIV). Expression of the glial connexins was verified by immunohistochemistry (Supp. Info. Fig. 1A-E) and each glial connexin induced homotypic coupling in the previously uncoupled KO astrocytes (Supp. Info. Fig. 1F-K). We first mixed astrocytes expressing Cx47 (Fig. 6A-D, Venus) with astrocytes expressing Cx32, Cx26, Cx43 and Cx30 (Fig. 6A-D, Cerulean). Neurobiotin transfer was not observed in Cx47-Cx32 (Fig. 6A) or Cx47-Cx26 pairs (Fig. 6B) but was readily detected in Cx47-Cx43 (Fig. 6C) and Cx47-Cx30 (Fig. 6D) pairs. Thus, Cx47 can form heterotypic channels with either Cx43 or Cx30 in both HeLa cells and primary astrocytes. Next, we mixed astrocytes expressing Cx32 (Fig. 6E-G, Venus) with astrocytes expressing Cx43, Cx30, or Cx26 (Fig. 6E-G, Cerulean). As in HeLa cells, Cx32-Cx43 pairs (Fig. 6E) were unable to transfer neurobiotin while Cx32-Cx26 (Fig. 6F) and Cx32-Cx30 (Fig. 6G) pairs did so robustly. Thus, regulation of heterotypic channel formation is the same in astrocyte primary cultures as in HeLa cells.
Fig. 6.
The pattern of heterotypic communication in primary astrocytes is identical to HeLa cells. Lentiviral transduction of astrocytes was at least as efficient as in HeLa cells allowing experiments to be completed well before (≤15 DIV) the appearance of endogenous Cx30 (21-28 DIV). As with HeLa cells, neurobiotin (NB) transfer was not observed in pairings of Cx47-Cx32 (A), Cx47-Cx26 (B), or Cx32-Cx43 (C) but was readily detected in pairings of Cx47-Cx43 (D), Cx47-Cx30 (E), Cx32-Cx30 (F), and Cx32-Cx26 (G). Scale bar: 50 μm.
DISCUSSION
Our studies establish the types of interactions between connexins expressed in oligodendrocytes and astrocytes that produce functional intercellular channels. We show that in two different cell types, Cx47 can form heterotypic channels with either Cx43 or Cx30 but not Cx26, whereas Cx32 can do so with either Cx30 or Cx26 but not Cx43. Thus, as many as four parallel pathways for gap junctional communication between oligodendrocytes and astrocytes are theoretically possible.
Our data differ from those of a similar study in which functional channels between Cx47 and Cx30 were not observed (Orthmann-Murphy et al., 2007). The difference in our results could reflect a cell-type specific regulation of intercellular channel assembly, as the expression system used by Orthmann-Murphy et al. was based on the N2A neuroblastoma cell line. However, we obtained identical results with two different cell types, HeLa cells and primary astrocytes. Another difference between the two studies was the assay for coupling; intercellular movement of a small, microinjected tracer in ours and dual whole-cell patch clamping in Orthmann-Murphy et al. (2007). Dual whole-cell voltage clamping is considered a very sensitive method because it is capable of revealing the activity of single intercellular channels in real time. In contrast, tracer injection is often considered a low sensitivity method, in part because the most widely used tracer, Lucifer yellow, is relatively large (443 Da) and permeates gap junctions comprised of some connexins poorly (Beltramello et al., 2003) or not at all (Teubner et al., 2000). However, neurobiotin permeates most if not all junctional channels. Ultimately, the sensitivity will depend on the rate of permeation by the solute, the rate of loss from recipient cells and the signal-to-noise of the detection system. In our study, rate of permeation was optimized by using a fixable tracer of low molecular mass and signal-to-noise was improved by preincubation of the cultures with unlabeled avidin to eliminate background due to endogenous biotin. It remains unclear why Cx47-Cx30 channels were not detected in the previous study. In whole-cell mode, soluble components of the cytoplasm are rapidly lost, which could conceivably affect activity of those channels. Regardless, it is more difficult to explain the presence of junctional coupling as an artifact of methodology than its absence.
Although it is clear that functional Cx47-Cx30 heterotypic channels can form in vitro, there is conflicting evidence regarding their formation in vivo. By light microscopic immunolabeling, Cx43 and Cx30 co-localize at junctions between neighboring astrocytes, whereas Cx43, Cx30, Cx47, Cx32 all robustly co-localize in puncta on gray matter oligodendrocytes somata presumed to include astrocyte-oligodendrocyte junctions. (Rash et al., 2001; Nagy et al., 2003b; Altevogt and Paul, 2004; Li et al., 2004; Kamasawa et al., 2005). However, Cx47 and Cx32 display a lower degree of coincidence in white matter than in gray, providing an opportunity to identify preferentially co-localizing astrocyte connexins. In white matter, Altevogt and Paul (2004) described preferential associations of Cx47 with Cx43 and Cx30, consistent with the functional heterotypic pairing we report here. Furthermore, recent studies of astrocyte-oligodendrocyte coupling in the corpus callosum using slice preparations (Maglione et al., 2010) showed that loss of Cx43 had little effect on coupling, whereas loss of Cx47 or loss of Cx43 and Cx30 together dramatically reduced it. This suggests that astrocyte-oligodendrocyte coupling is largely mediated by heterotypic junctions containing oligodendrocyte Cx47 and either astrocyte Cx30 alone or Cx30 and Cx43 together. In contrast, it was reported that Cx30 is no longer detected at oligodendrocyte somata in Cx32 KO mice (Nagy et al., 2003b; Altevogt and Paul, 2004) and that Cx43 is similarly absent in Cx47 KO mice (Li et al., 2008). The simplest interpretation of these immunolocalization studies is that the preferential heterotypic interactions at oligodendrocyte somata are Cx32-Cx30 and Cx47-Cx43. However, it is possible that connexin KOs provoke compensatory changes excluding particular connexins from junctional plaques at oligodendrocyte somata. Thus, it is difficult to reconcile the conflicting evidence regarding in vivo interactions of Cx47 and Cx30.
The functions of Cx29, the third oligodendrocyte connexin, remain unclear. Although abundantly expressed in both oligodendrocytes and Schwann cells, Cx29 does not associate with junctional plaques or co-localize with other connexins (Nagy et al., 2003a; Kleopa et al., 2004; Altevogt and Paul, 2004; Ahn et al., 2008). Furthermore, astrocyte-oligodendrocyte coupling in the murine corpus callosum is not affected by loss of Cx29 (Maglione et al., 2010). Cx29 has a diffuse localization consistent with a role in reflexive communication but Cx29 does not form functional channels when expressed in Xenopus oocytes or HeLa cell pairs (Altevogt et al., 2002; Ahn et al., 2008). However, Cx29 KO mice develop a defect in Schwann cell myelin specifically restricted to somata of spiral ganglion neurons, leading to hearing loss (Tang et al., 2006) and Cx29 mutations have also been associated with hearing loss in humans (Yang et al., 2007). Thus, Cx29 might also have subtle effects in oligodendrocytes, which could affect interpretation of studies of glial connexin function and interaction.
It is not clear why glial cells express so many connexins and why astrocytes and oligodendrocytes express nonoverlapping sets. Initial studies of KO mice suggested that oligodendrocyte Cx47 and Cx32 provide redundant functionality because single KOs do not exhibit clinical phenotypes (Scherer et al., 1998; Odermatt et al., 2003), whereas the double KO dies by the 5th or 6th postnatal week (Menichella et al., 2003). However, both Cx47 and Cx32 KO mice develop subtle ultrastructural abnormalities of myelin (Odermatt et al., 2003; Sargiannidou et al., 2009). Furthermore, human mutations in Cx32, which cause the peripheral neuropathy X-linked Charcot-Marie-Tooth disease, have been associated with transient central nervous system symptoms (Kleopa et al., 2002; Paulson et al., 2002; Hanemann et al., 2003; Takashima et al., 2003) and Cx47 mutations cause Pelizaeus-Merzbacher-Like disease, a hypomyelinating leukodystrophy with severe clinical correlates (Uhlenberg et al., 2004). Taken together, it seems likely that oligodendrocyte connexins have nonredundant functions. Whether these nonredundant functions reflect a requirement for the presence of heterotypic channels to achieve particular biophysical or regulatory properties remains to be determined.
ACKNOWLEDGMENTS
We thank for the introduction to microelectrode technique by Friso Postma and the expert technical assistance of Yaqiao Li.
Grant numbers: NIH RO1 GM37751 (to D.L.P.), RO1 GM18974 (to D.A.G.), and P30-HD18655 (to the IDDRC at Children's Hospital, Boston).
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Ahn M, Lee J, Gustafsson A, Enriquez A, Lancaster E, Sul JY, Haydon PG, Paul DL, Huang Y, Abrams CK, Scherer SS. Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J Neurosci Res. 2008;86:992–1006. doi: 10.1002/jnr.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL. Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J Neurosci. 2002;22:6458–6470. doi: 10.1523/JNEUROSCI.22-15-06458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. J Neurosci. 2004;24:4313–4323. doi: 10.1523/JNEUROSCI.3303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, D'Andrea P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem Biophys Res Commun. 2003;305:1024–1033. doi: 10.1016/s0006-291x(03)00868-4. [DOI] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Fraté H, Butterweck A, Traub O, Klein RA, Hülser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov MA, Hormuzdi SG, Fuchs EC, Monyer H. A reporter allele for investigating connexin 26 gene expression in the mouse brain. Eur J Neurosci. 2003;18:3183–3192. doi: 10.1111/j.1460-9568.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- Giaume C, Fromaget C, El Aoumari A, Cordier J, Glowinski J, Gros D. Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron. 1991;6:133–143. doi: 10.1016/0896-6273(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Hanemann CO, Bergmann C, Senderek J, Zerres K, Sperfeld AD. Transient, recurrent, white matter lesions in X-linked Charcot-Marie-Tooth disease with novel connexin 32 mutation. Arch Neurol. 2003;60:605–609. doi: 10.1001/archneur.60.4.605. [DOI] [PubMed] [Google Scholar]
- Hollinshead M, Sanderson J, Vaux DJ. Anti-biotin antibodies offer superior organelle-specific labeling of mitochondria over avidin or streptavidin. J Histochem Cytochem. 1997;45:1053–1057. doi: 10.1177/002215549704500803. [DOI] [PubMed] [Google Scholar]
- Hunter AW, Barker RJ, Zhu C, Gourdie RG. ZO-1 alters Connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamasawa N, Sik A, Morita M, Yasumura T, Davidson KG, Nagy JI, Rash JE. Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: Implications for ionic homeostasis and potassium siphoning. Neuroscience. 2005;136:65–86. doi: 10.1016/j.neuroscience.2005.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Ransom BR. Electrical coupling between astrocytes and between oligodendrocytes studied in mammalian cell cultures. Glia. 1988;1:64–73. doi: 10.1002/glia.440010108. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Orthmann JL, Enriquez A, Paul DL, Scherer SS. Unique distributions of the gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia. 2004;47:346–357. doi: 10.1002/glia.20043. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Yum SW, Scherer SS. Cellular mechanisms of connexin32 mutations associated with CNS manifestations. J Neurosci Res. 2002;68:522–534. doi: 10.1002/jnr.10255. [DOI] [PubMed] [Google Scholar]
- Kunzelmann P, Schroder W, Traub O, Steinhauser C, Dermietzel R, Willecke K. Late onset and increasing expression of the gap junction protein connexin30 in adult murine brain and long-term cultured astrocytes. Glia. 1999;25:111–119. doi: 10.1002/(sici)1098-1136(19990115)25:2<111::aid-glia2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Li X, Ionescu AV, Lynn BD, Lu S, Kamasawa N, Morita M, Davidson KG, Yasumura T, Rash JE, Nagy JI. Connexin47, connexin29 and connexin32 co-expression in oligodendrocytes and cx47 association with zonula occludens-1 (ZO-1) in mouse brain. Neuroscience. 2004;126:611–630. doi: 10.1016/j.neuroscience.2004.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kamasawa N, Ciolofan C, Olson CO, Lu S, Davidson KG, Yasumura T, Shigemoto R, Rash JE, Nagy JI. Connexin45-containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques, forming bihomotypic gap junctions, with scaffolding contributed by zonula occludens-1. J Neurosci. 2008;28:9769–9789. doi: 10.1523/JNEUROSCI.2137-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci (USA) 2001;98:9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddenkemper T, Grote K, Evers S, Oelerich M, Stogbauer F. Neurological manifestations of the oculodentodigital dysplasia syndrome. J Neurol. 2002;249:584–595. doi: 10.1007/s004150200068. [DOI] [PubMed] [Google Scholar]
- Maglione M, Tress O, Haas B, Karram K, Trotter J, Willecke K, Kettenmann H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia. 2010;58:1104–1117. doi: 10.1002/glia.20991. [DOI] [PubMed] [Google Scholar]
- Manthey D, Banach K, Desplantez T, Lee CG, Kozak CA, Traub O, Weingart R, Willecke K. Intracellular domains of mouse connexin26 and -30 affect diffusional and electrical properties of gap junction channels. J Membr Biol. 2001;181:137–148. doi: 10.1007/s00232-001-0017-1. [DOI] [PubMed] [Google Scholar]
- Massa PT, Mugnaini E. Cell junctions and intramembrane particles of astrocytes and oligodendrocytes: a freeze-fracture study. Neuroscience. 1982;7:523–538. doi: 10.1016/0306-4522(82)90285-8. [DOI] [PubMed] [Google Scholar]
- Masters JR. HeLa cells 50 years on: The good, the bad and the ugly. Nat Rev Cancer. 2002;2:315–319. doi: 10.1038/nrc775. [DOI] [PubMed] [Google Scholar]
- Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J Neurosci. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Majdan M, Awatramani R, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J Neurosci. 2006;26:10984–10991. doi: 10.1523/JNEUROSCI.0304-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: Roles in stem cell proliferation and morphological plasticity? J Comp Neurol. 2001;431:88–104. doi: 10.1002/1096-9861(20010226)431:1<88::aid-cne1057>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Ionescu AV, Lynn BD, Rash JE. Connexin29 and connexin32 at oligodendrocyte and astrocyte gap junctions and in myelin of the mouse central nervous system. J Comp Neurol. 2003a;464:356–370. doi: 10.1002/cne.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Ionescu AV, Lynn BD, Rash JE. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia. 2003b;44:205–218. doi: 10.1002/glia.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE. Connexin26 in adult rodent central nervous system: Demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. J Comp Neurol. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J Neurosci. 2007;27:13949–13957. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DL. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986;103:123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson HL, Garbern JY, Hoban TF, Krajewski KM, Lewis RA, Fischbeck KH, Grossman RI, Lenkinski R, Kamholz JA, Shy ME. Transient central nervous system white matter abnormality in X-linked Charcot-Marie-Tooth disease. Ann Neurol. 2002;52:429–434. doi: 10.1002/ana.10305. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Adhes Commun. 2001;8:315–320. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, De Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Sargiannidou I, Vavlitou N, Aristodemou S, Hadjisavvas A, Kyriacou K, Scherer SS, Kleopa KA. Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS, CNS myelination defects. J Neurosci. 2009;29:4736–4749. doi: 10.1523/JNEUROSCI.0325-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Deschenes SM, Xu YT, Grinspan JB, Fischbeck KH, Paul DL. Connexin32 is a myelin-related protein in the PNS, CNS. J Neurosci. 1995;15:8281–8294. doi: 10.1523/JNEUROSCI.15-12-08281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Xu YT, Nelles E, Fischbeck K, Willecke K, Bone LJ. Connexin32-null mice develop demyelinating peripheral neuropathy. Glia. 1998;24:8–20. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Takashima H, Nakagawa M, Umehara F, Hirata K, Suehara M, Mayumi H, Yoshishige K, Matsuyama W, Saito M, Jonosono M, Arimura K, Osame M. Gap junction protein beta 1 (GJB1) mutations and central nervous system symptoms in X-linked Charcot-Marie-Tooth disease. Acta Neurol Scand. 2003;107:31–37. doi: 10.1034/j.1600-0404.2003.01317.x. [DOI] [PubMed] [Google Scholar]
- Tang W, Zhang Y, Chang Q, Ahmad S, Dahlke I, Yi H, Chen P, Paul DL, Lin X. Connexin29 is highly expressed in cochlear Schwann cells, and it is required for the normal development and function of the auditory nerve of mice. J Neurosci. 2006;26:1991–1999. doi: 10.1523/JNEUROSCI.5055-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RA, Simon EM, Marks HG, Scherer SS. The CNS phenotype of X-linked Charcot-Marie-Tooth disease: more than a peripheral problem. Neurology. 2003;61:1475–1478. doi: 10.1212/01.wnl.0000095960.48964.25. [DOI] [PubMed] [Google Scholar]
- Teubner B, Degen J, Sohl G, Guldenagel M, Bukauskas FF, Trexler EB, Verselis VK, De Zeeuw CI, Lee CG, Kozak CA, Petrasch-Parwez E, Dermietzel R, Willecke K. Functional expression of the murine Connexin 36 gene coding for a neuron-specific gap junctional protein. J Membr Biol. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, Speidel D, Willecke K. Astrocyte cultures from conditional connexin43-deficient mice. Glia. 2004;46:130–141. doi: 10.1002/glia.10350. [DOI] [PubMed] [Google Scholar]
- Uhlenberg B, Schuelke M, Ruschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloglu H, Nurnberg P, Hubner C, Weschke B, Gartner J. Mutations in the gene encoding gap junction protein alpha 12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet. 2004;75:251–260. doi: 10.1086/422763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JJ, Huang SH, Chou KH, Liao PJ, Su CC, Li SY. Identification of mutations in members of the connexin gene family as a cause of nonsyndromic deafness in Taiwan. Audiol Neurootol. 2007;12:198–208. doi: 10.1159/000099024. [DOI] [PubMed] [Google Scholar]
- Zhang JT, Nicholson BJ. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989;109:3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]