Abstract
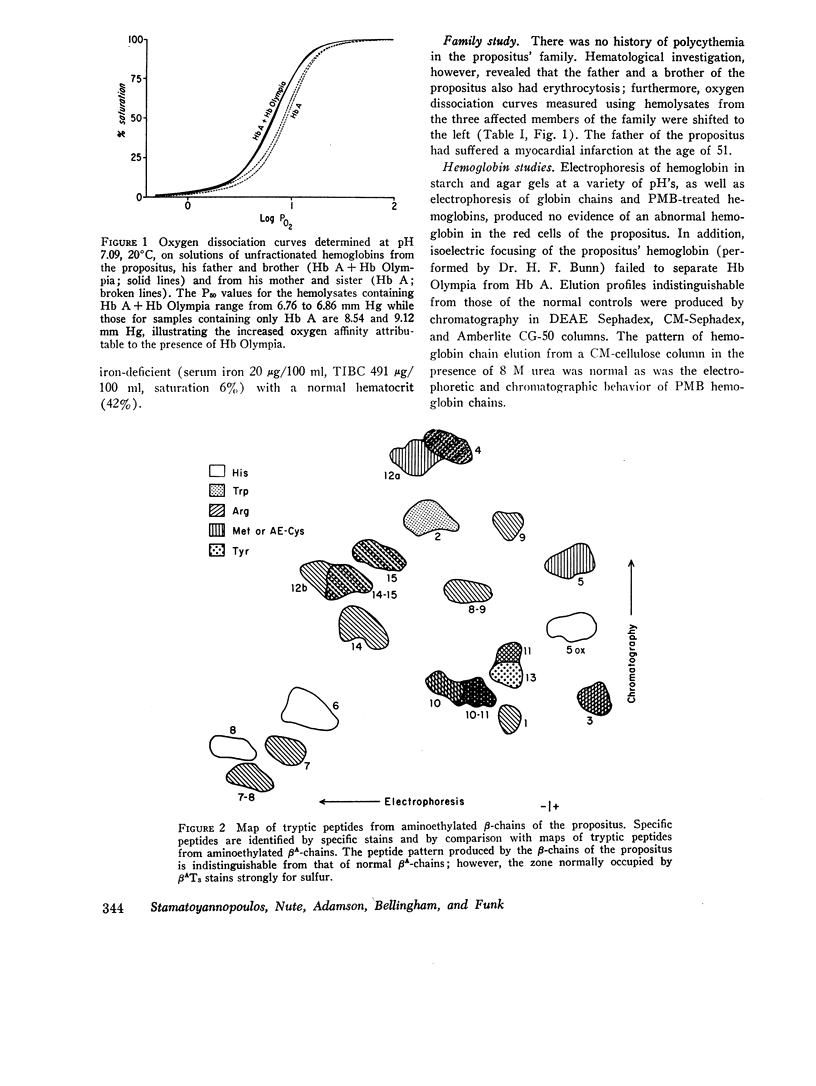
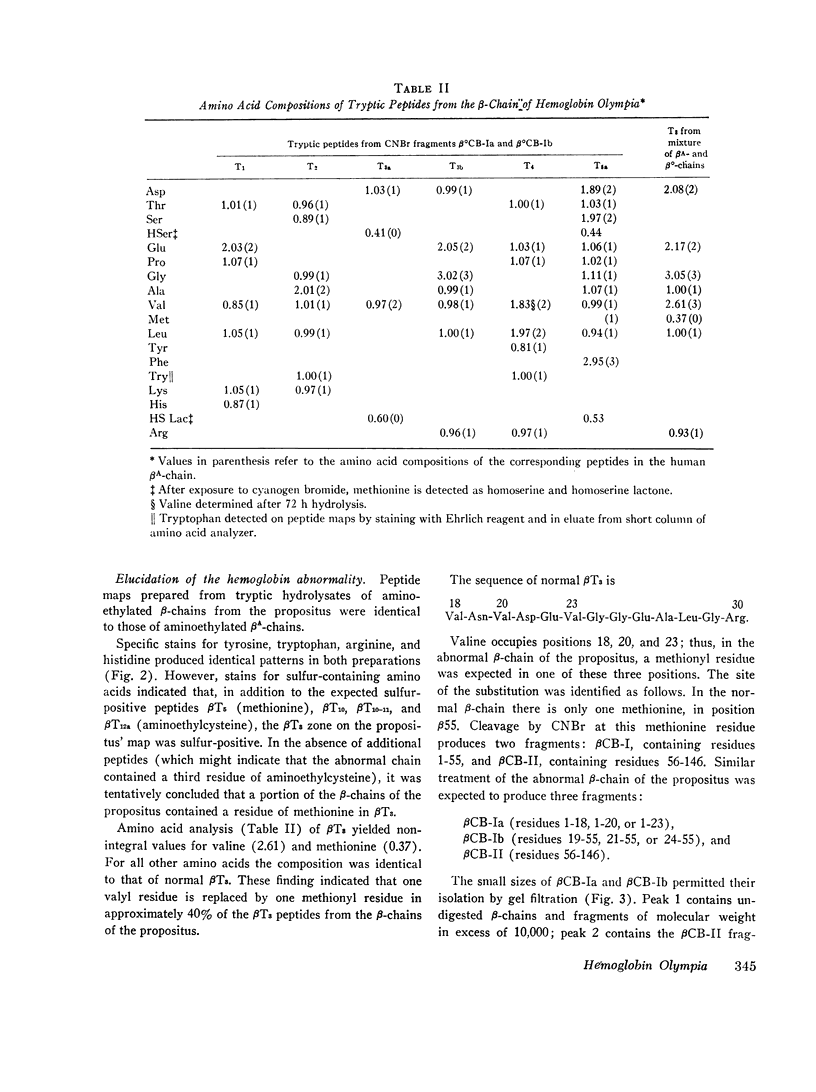
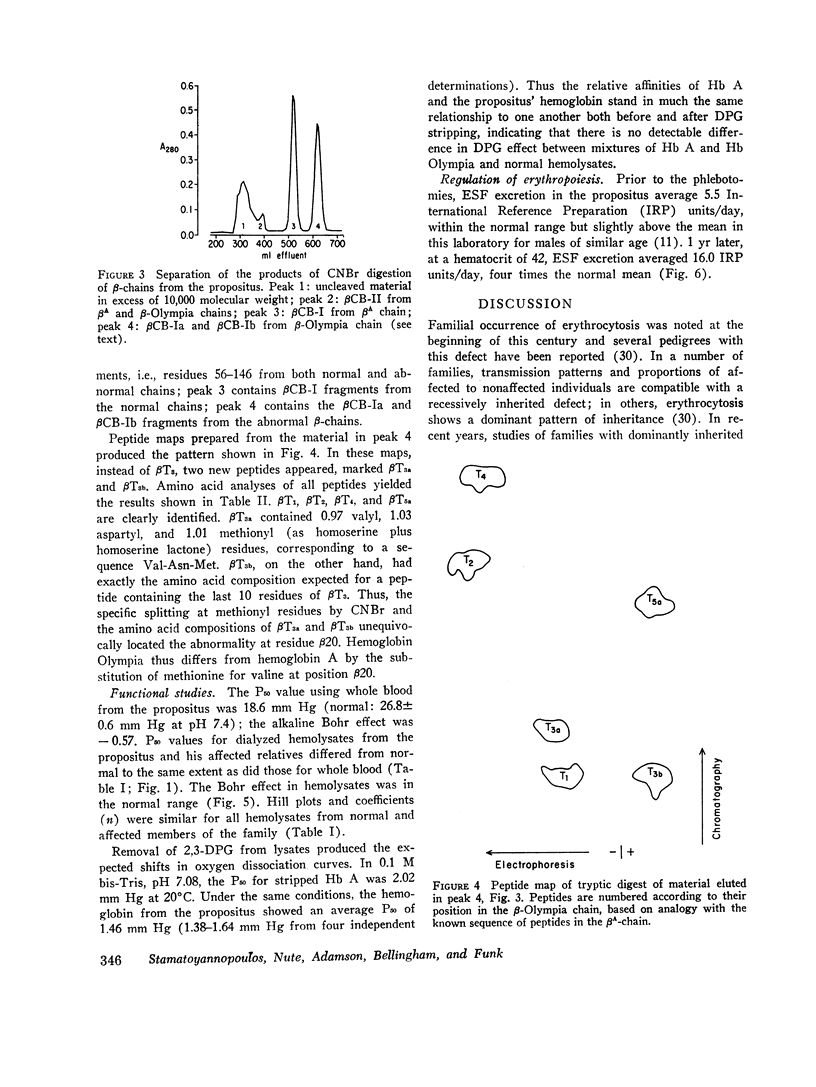
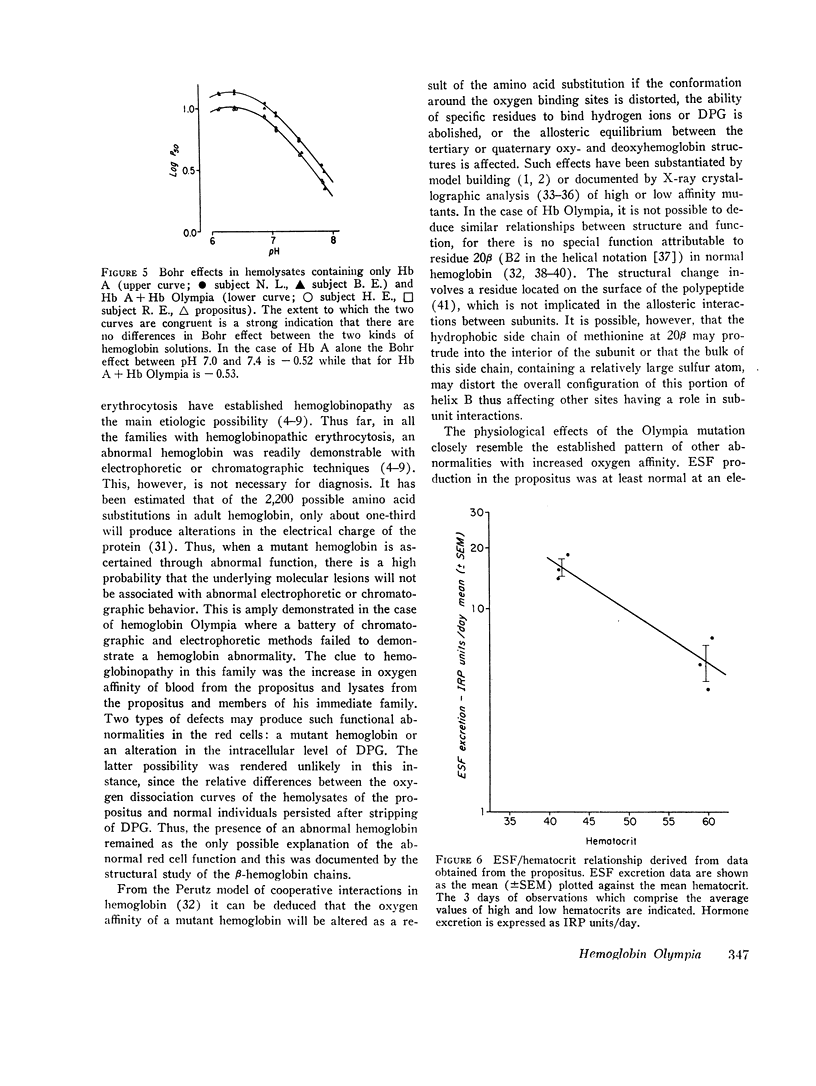
In a family with erythrocytosis, electrophoretic and chromatographic studies failed to demonstrate a hemoglobin variant. However, the oxygen dissociation curves of affected individuals were shifted to the left of normal and this shift persisted when oxygen equilibria were studied in 2.3-diphosphoglycerate-stripped hemolysates. A mutant hemoglobin was evidently present in the red blood cells of the affected persons and was responsible for the increased oxygen affinity and erythrocytosis. Specific staining of tryptic peptide maps of β-chains from the propositus showed that peptide βT3 was positive for a sulfur-containing amino acid. Amino acid analysis yielded a composition identical to that of normal βT3, except that there were 2.6 residues of valine and 0.4 residues of methionine (normal composition: Val = 3.0, Met = 0). This suggested that the β-chains of affected individuals consisted of a mixture of two kinds of chains, 40% of which had a methionyl residue in βT3. Structural studies of isolated cyanogen bromide fragments demonstrated unequivocally that, in the abnormal β-chains, valine in position 20 is replaced by methionine. The new hemoglobin mutant is designated hemoglobin Olympia (β20 (B2) valine → methionine).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson J. W., Parer J. T., Stamatoyannopoulos G. Erythrocytosis associated with hemoglobin Rainier: oxygen equilibria and marrow regulation. J Clin Invest. 1969 Aug;48(8):1376–1386. doi: 10.1172/JCI106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson J. W. The erythropoietin-hematocrit relationship in normal and polycythemic man: implications of marrow regulation. Blood. 1968 Oct;32(4):597–609. [PubMed] [Google Scholar]
- BAGLIONI C. An improved method for the fingerprinting of human hemoglobin. Biochim Biophys Acta. 1961 Apr 1;48:392–396. doi: 10.1016/0006-3002(61)90490-5. [DOI] [PubMed] [Google Scholar]
- BUCCI E., FRONTICELLI C. A NEW METHOD FOR THE PREPARATION OF ALPHA AND BETA SUBUNITS OF HUMAN HEMOGLOBIN. J Biol Chem. 1965 Jan;240:PC551–PC552. [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Charache S., Fairbanks V. F., Maldonado J. E., Noyes A., Gayle E. E. Hemoglobin Malmö Beta-97 (FG-4) histidine--glutamine: a cause of polycythemia. J Clin Invest. 1972 Mar;51(3):666–676. doi: 10.1172/JCI106855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERNOFF A. I., PETTIT N. M., Jr THE AMINO ACID COMPOSITION OF HEMOGLOBIN. 3. A QUALITATIVE METHOD FOR IDENTIFYING ABNORMALITIES OF THE POLYPEPTIDE CHAINS OF HEMOGLOBIN. Blood. 1964 Dec;24:750–756. [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- DONOHUE D. M., MOTULSKY A. G., GIBLETT E. R., PIRZIO-BIROLI G., VIRANUVATTI V., FINCH C. A. The use of chromium as red-cell tag. Br J Haematol. 1955 Jul;1(3):249–263. doi: 10.1111/j.1365-2141.1955.tb05508.x. [DOI] [PubMed] [Google Scholar]
- Dozy A. M., Huisman T. H. Studies on the heterogeneity of hemoglobin. XIV. Chromatography of normal and abnormal human hemoglobin types on CM-Sephadex. J Chromatogr. 1969 Mar 11;40(1):62–70. doi: 10.1016/s0021-9673(01)96618-x. [DOI] [PubMed] [Google Scholar]
- Easley C. W. Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta. 1965 Sep 13;107(2):386–388. doi: 10.1016/0304-4165(65)90147-9. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Greer J., Perutz M. F. Three dimensional structure of haemoglobin Rainier. Nat New Biol. 1971 Apr 28;230(17):261–264. doi: 10.1038/newbio230261a0. [DOI] [PubMed] [Google Scholar]
- Greer J. Three-dimensional structure of abnormal human haemoglobins Chesapeake and J Capetown. J Mol Biol. 1971 Nov 28;62(1):241–249. doi: 10.1016/0022-2836(71)90143-4. [DOI] [PubMed] [Google Scholar]
- Greer J. Three-dimensional structure of abnormal human haemoglobins Kansas and Richmond. J Mol Biol. 1971 Jul 14;59(1):99–105. doi: 10.1016/0022-2836(71)90415-3. [DOI] [PubMed] [Google Scholar]
- Hamilton H. B., Iuchi I., Miyaji T., Shibata S. Hemoglobin Hiroshima (beta-143 histidine--aspartic acid): a newly identified fast moving beta chain variant associated with increased oxygen affinity and compensatory erythremia. J Clin Invest. 1969 Mar;48(3):525–535. doi: 10.1172/JCI106010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Imai K., Morimoto H., Kotani M., Watari H., Hirata W. Studies on the function of abnormal hemoglobins. I. An improved method for automatic measurement of the oxygen equilibrium curve of hemoglobin. Biochim Biophys Acta. 1970 Feb 17;200(2):189–196. doi: 10.1016/0005-2795(70)90163-7. [DOI] [PubMed] [Google Scholar]
- JONES R. T. STRUCTURAL STUDIES OF AMINOETHYLATED HEMOGLOBINS BY AUTOMATIC PEPTIDE CHROMATOGRAPHY. Cold Spring Harb Symp Quant Biol. 1964;29:297–308. doi: 10.1101/sqb.1964.029.01.032. [DOI] [PubMed] [Google Scholar]
- Jones R. T., Osgood E. E., Brimhall B., Koler R. D. Hemoglobin Yakina. I. Clinical and biochemical studies. J Clin Invest. 1967 Nov;46(11):1840–1847. doi: 10.1172/JCI105674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H., Carrell R. W. Variations in the structure of human haemoglobin. With particular reference to the unstable haemoglobins. Br Med Bull. 1969 Jan;25(1):14–23. doi: 10.1093/oxfordjournals.bmb.a070664. [DOI] [PubMed] [Google Scholar]
- Morimoto H., Lehmann H., Perutz M. F. Moleuclar pathology of human haemoglobin: stereochemical interpretation of abnormal oxygen affinities. Nature. 1971 Aug 6;232(5310):408–413. doi: 10.1038/232408a0. [DOI] [PubMed] [Google Scholar]
- Muirhead H., Greer J. Three-dimensional Fourier synthesis of human deoxyhaemoglobin at 3.5 Angstrom units. Nature. 1970 Nov 7;228(5271):516–519. doi: 10.1038/228516a0. [DOI] [PubMed] [Google Scholar]
- Nute P. E., Sullivan B. Primate hemoglobins: their structure, function and evolution. I. Amino acid compositions of the tryptic peptides from the beta chain of Cebus albifrons. Comp Biochem Physiol B. 1971 Aug 15;39(4):797–814. doi: 10.1016/0305-0491(71)90104-0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Pulsinelli P., Eyck L. T., Kilmartin J. V., Shibata S., Iuchi I., Miyaji T., Hamilton H. B. Haemoglobin Hiroshima and the mechanism of the alkaline Bohr effect. Nat New Biol. 1971 Aug 4;232(31):147–149. doi: 10.1038/newbio232147a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Reed C. S., Hampson R., Gordon S., Jones R. T., Novy M. J., Brimhall B., Edwards M. J., Koler R. D. Erythrocytosis secondary to increased oxygen affinity of a mutant hemoglobin, hemoglobin Kempsey. Blood. 1968 May;31(5):623–632. [PubMed] [Google Scholar]
- Rosemeyer M. A., Huehns E. R. On the mechanism of the dissociation of haemoglobin. J Mol Biol. 1967 Apr 28;25(2):253–273. doi: 10.1016/0022-2836(67)90141-6. [DOI] [PubMed] [Google Scholar]
- SANGER F., TUPPY H. The amino-acid sequence in the phenylalanyl chain of insulin. I. The identification of lower peptides from partial hydrolysates. Biochem J. 1951 Sep;49(4):463–481. doi: 10.1042/bj0490463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Bellingham A. J., Lenfant C., Finch C. A. Abnormal hemoglobins with high and low oxygen affinity. Annu Rev Med. 1971;22:221–234. doi: 10.1146/annurev.me.22.020171.001253. [DOI] [PubMed] [Google Scholar]
- Yamaoka K. Hemoglobin Hirose: 2 237(C3) tryptophan yielding serine. Blood. 1971 Dec;38(6):730–738. [PubMed] [Google Scholar]


