Abstract
Study Objectives:
To explore the time of day effects of alcohol on sleep, we examined sleep following alcohol administered at four times of day and three homeostatic loads during a 20-hr forced desynchrony (FD) protocol.
Participants:
Twenty-six healthy young adults (21–25 yrs) were studied.
Design:
Participants were dosed at 4 clock times: 0400 (n = 6; 2 females), 1600 (n = 7; 4 females), 1000 (n = 6; 1 female) or 2200 (n = 7; 2 females). Participants slept 2300 to 0800 for at least 12 nights before the in-lab FD study. Double blind placebo and alcohol (vodka tonic targeting 0.05g% concentration) beverages were each administered three times during FD at different homeostatic loads: low (4.25 or 2.24 hrs awake), medium (8.25 or 6.25 hrs awake), high (12.25 or 10.25 hrs awake) in the 0400 and 1600 or 1000 and 2200 groups, respectively. Sleep was staged and subjected to spectral analysis.
Measurements and Results:
Breath Alcohol Concentration (BrAC) confirmed targeted maximal levels. At bedtime, BrAC was 0 in the low and medium homeostatic load conditions; however, at high homeostatic load, BrAC was still measurable. Spectral characteristics of sleep were unaffected with alcohol at any time of day. Few alcohol related changes were seen for sleep stages; however, with alcohol given at 0400 at a high homeostatic load there was an increase in wake.
Conclusions:
These data lend support to the idea that alcohol may be disruptive to sleep; however, our findings are inconsistent with the idea that a low dose of alcohol is a useful sleep aid when attempting to sleep at an adverse circadian phase.
Citation:
Van Reen E; Tarokh L; Rupp TL; Seifer R; Carskadon MA. Does timing of alcohol administration affect sleep? SLEEP 2011;34(2):195-205.
Keywords: Sleep, alcohol, timing, EEG, forced desynchrony
AN ASSOCIATION BETWEEN SLEEP AND ALCOHOL HAS BEEN RECOGNIZED FOR CENTURIES AND STUDIED SCIENTIFICALLY SINCE THE 1930S.1 A CONSISTENT anecdotal belief regarding the association between alcohol and sleep is that alcohol facilitates sleep; as such, alcohol is commonly used as a sleep aid.2 Contradicting this belief are data that show chronic alcohol use and abuse disrupt sleep and that sleep remains disrupted in abstinent alcoholics.3 Several factors complicate our understanding of alcohol's effects on sleep. First, few studies have controlled participants' prior sleep; most studies that have examined alcohol's effects on sleep have done so under “normal” or “usual” sleep schedules without specification or verification. Second, the literature includes a mixture of alcohol doses, times of administration relative to sleep, and times of day of administration. These issues cloud rather than clarify our understanding of the association between alcohol and sleep.
Some inconsistencies exist among previous findings of alcohol's effects on subsequent nighttime sleep, even given similar, single alcohol doses. For example, Yules and colleagues4 examined the effects of a single high dose of alcohol (1 g/kg) given 4 h before bedtime on 3 consecutive nights in 4 healthy males and found no differences for minutes of wake, latency of sleep onset, or NREM sleep compared to control nights, but did find a reduction in REM sleep for the first 2 alcohol nights (though these observed differences were not tested statistically due to the small sample size). Another study examining a single high dose of alcohol (dose = 0.9 g/kg) in 7 healthy males on 3 consecutive nights5 reported increased wakefulness in the second half of the night with alcohol on nights 2 and 3 compared to placebo, and faster sleep onset latency and increased sleep stage 1 in the first half of the night when data from all alcohol nights were examined together for alcohol compared to placebo.5 Van Reen and colleagues6 examined the sleep stage variables in 7 women (mean age = 23.5 y) following alcohol administration (dose = 0.49 g/kg) ending 60 min before bedtime compared to placebo. NREM stage 4 showed an increase in the first 2 h of sleep, and REM was reduced across the entire sleep episode with alcohol compared to placebo. The one consistent finding from these 3 studies is that REM sleep was reduced on the alcohol night. Other studies similarly have shown a decrease in REM sleep with low and moderate alcohol doses,5,7 though not all concur.8 Findings regarding the effects of a single dose of alcohol on subsequent sleep latency and NREM sleep are less consistent.
A greater interval for alcohol administration relative to nocturnal sleep was examined by Landolt and colleagues, who examined the effects of alcohol (0.55 g/kg) on the sleep of 10 healthy men (mean age = 61.6 y) when alcohol was given 6 h before bedtime, at which point breath alcohol was not detectable.9 Thus, any effects of alcohol on sleep were considered “residual” rather than direct. Not only was interval to bedtime longer than in other studies, but the alcohol was given earlier in the day, presumably at a lower homeostatic load (i.e., less pressure to sleep). Observed residual effects of alcohol on sleep included reduced sleep efficiency and total sleep time for the second half of the sleep episode, along with twice as much total wake and reduced NREM sleep stage 1 compared to placebo. REM sleep also showed a residual effect with an overall reduction with alcohol compared to placebo. The extent of these effects is surprising, given that alcohol levels were undetectable at bedtime, thus raising the question of whether the observed effects were due to time of alcohol administration relative to how long the participants were awake or to circadian phase at the time of administration or bedtime.
In summary, the effects of alcohol on sleep stage variables differ as a function of dose and timing relative to bedtime. With alcohol given close to nocturnal sleep time, studies find a reduction of sleep onset latency5,7 and reduced REM sleep.4,5,7 In addition, an alcohol-related increase of stage 4 sleep early in the sleep episode was also reported,6–8 although several studies failed to confirm these findings. Also of note is that the effects of alcohol on sleep appear to linger beyond the time alcohol is metabolized, and these residual effects occurred with alcohol given in the late afternoon.9
The studies described above leave open a number of issues that complicate our understanding of alcohol's effects on sleep. One such issue is the timing of alcohol administration relative to circadian phase and sleep/wake. The issue of how the timing of alcohol affects sleep may be relevant for individuals in judging the relative safety of drinking for waking activities or the effects on sleep. The impact of a relatively low dose of alcohol on performance, for example, is notable when alcohol is given at the start of the “biological night” and with a high homeostatic load.10 Whether alcohol timing may disrupt or enhance a shift workers day sleep is unknown. We apply the 2-process model of sleep/wake regulation to examine these issues. According to this model, sleep and sleep stage distribution are controlled by 2 biological systems: a sleep-dependent homeostatic process and a sleep-independent circadian process.11,12 The homeostatic drive to sleep rises during waking and is dissipated by sleep. On the other hand, gating of sleep and waking are also influenced by an internal daily biological oscillation. This model is at the core of the experimental design described in this paper and provides a framework for interpreting our results.
We use the method of forced desynchrony (FD) to isolate alcohol's effects on sleep as a function of these 2 processes.13 FD provides access to the independent contributions of the homeostatic and circadian processes because the timing of sleep and wake vary across circadian phases due to the imposition of a non–24-h day length. A common FD method uses either a 20- or 28-h day length. Conditions under which the FD protocol is performed are controlled to maximize the separation of the homeostatic and circadian processes by timing light exposure, activity levels, and meal size.
No previous studies have systematically varied homeostatic load and/or the time of day to investigate the effects of alcohol on sleep and the sleep EEG; however, data from one study that examined effects of morning or afternoon alcohol (dose = 0.5 g/kg) on sleep onset latency indicated that time of day of consumption had differential effects on sleep onset latency.14 Whether such differential effects may carry over to other aspects of sleep and/or the sleep EEG is not known and is a major gap in this line of research.
The goal of the present study, therefore, was to examine the effects on sleep stages and spectral characteristics of sleep EEG of a moderate dose of alcohol compared to placebo given at 4 different circadian phases and at 3 different homeostatic loads using a 20-h forced desynchrony protocol. We hypothesized that the homeostatic and circadian processes would interact to influence the effects of alcohol on subsequent sleep. Based on previous literature, one would expect to find increased slow wave sleep (SWS) and decreased sleep onset latency (SOL) with alcohol administered at a high homeostatic load and at the start of the circadian night (i.e., 22:00). In addition, our analyses were designed to explore direct and residual effects of alcohol as a function of circadian phase.
METHODS
Participants
Healthy young adult volunteers were recruited using flyers posted in local businesses and colleges, as well as radio and newspaper advertisements. Potential participants were screened initially with brief telephone questions regarding current or past medical/psychiatric conditions, drinking practices, and sleep habits. Our goal was to recruit a sample of healthy young participants free from conditions and/or substances known to affect sleep and/or response to alcohol. Inclusion was based on a report of consuming alcoholic beverages on ≥ 2 occasions per month and ≥ 2 drinks per occasion, but averaging no more than 14 drinks per week. Family history of alcohol abuse or dependence was screened with a Family History Screen15; the Diagnostic and Statistical Manual-fourth edition (DSM-IV) criteria using a computer-driven structured interview with the alcohol abuse/dependence modules from the Diagnostic Interview Schedule (DIS) was used to identify personal history of alcohol abuse or dependence. Volunteers classified with past or current alcohol abuse/dependence and participants with a positive parental history of alcohol abuse/dependence were excluded from the study to reduce variability in our sample.
Positive findings on paper and pencil self-report inventories, including the Revised Symptom Checklist-90 (SCL-90-R)16 and Beck Depression Inventory-II (BDI-II)17 excluded individuals with current major depression or a personal history of psychopathology (e.g., schizophrenia, bipolar disorder). Self-reports on telephone interviews and questionnaires were also used to exclude individuals with chronic medical conditions (e.g., diabetes, cancer), neurological disorders, or a family history of psychopathology. Positive blood tests were used to exclude pregnant women and individuals with abnormal liver function. Further, volunteers with a known sensitivity to alcohol or who were taking medications or drugs that affect the sleep/wake cycle or who smoked were excluded. Additional exclusionary criteria were reports of irregular sleep patterns, travel beyond 3 time zones within 3 months before the scheduled in-lab nights, excessive daytime sleepiness (manifested by ≥ 2 naps per week), and/or a personal or family history of narcolepsy.
Twenty-six healthy young adults aged 21 to 26 years participated in this study. Alcohol was administered centered at 4 different circadian phases based on clock times (04:00, 16:00, 10:00, and 22:00) each participants' group defined by the time: 04:00 (n = 6; 2 females), 16:00 (n = 7; 4 females), 10:00 (n = 6; 1 female), or 22:00 (n = 7; 2 females). Assignment to these alcohol administration groups (04:00, 16:00, 10:00, or 22:00) was determined based on the study schedule when each participant enrolled in the study. These clock times were selected to provide maximum temporal dispersion of alcohol administration (all times 6 h apart) and because previous studies show that alcohol ingested in the morning ˜09:00) increases sedation,14 whereas alcohol taken in the late afternoon (˜17:00) can affect sleep parameters.9 The Lifespan Institutional Review Board for the Protection of Human Subjects approved the protocol for this study, and informed consent was obtained from all participants. Participants received monetary compensation.
PROCEDURES
At-Home Protocol
All participants slept on a fixed 9-h (23:00–08:00) stabilization sleep schedule for ≥ 12 nights at home before coming into the laboratory. Adherence to the schedule was confirmed by actigraphy, sleep diary, and evening and morning phone calls to the lab's timed-stamped answering machine; compliance was examined after one week and on the first in-lab study day. Participants were asked to abstain entirely from alcohol, medications, drugs, and caffeine within 12 h before bedtime for the entire study. Urine toxicology was performed one week before the in-lab study and at the study start to confirm that participants were drug free. Breath alcohol concentration (BrAC) levels were obtained using a hand-held breath analyzer (AlcoSensor IV; Intoximeters, In, St. Louis, MO) when participants arrived at the laboratory to begin the study. In addition, all female participants took a home pregnancy test on the first in-lab night to confirm that they were not pregnant.
Participants stayed in the lab continuously for 13 consecutive nights and the intervening 12 days, beginning with an adaptation night. The 9-h (23:00–08:00) adaptation night was used to screen for sleep disordered breathing and periodic limb movements and to allow participants to adapt to sleeping in the laboratory. Participants arrived approximately 5 h before scheduled bedtime on the first in-lab night for orientation to the laboratory procedures and to be prepared for sleep recordings as described below.
Forced Desynchrony Schedule
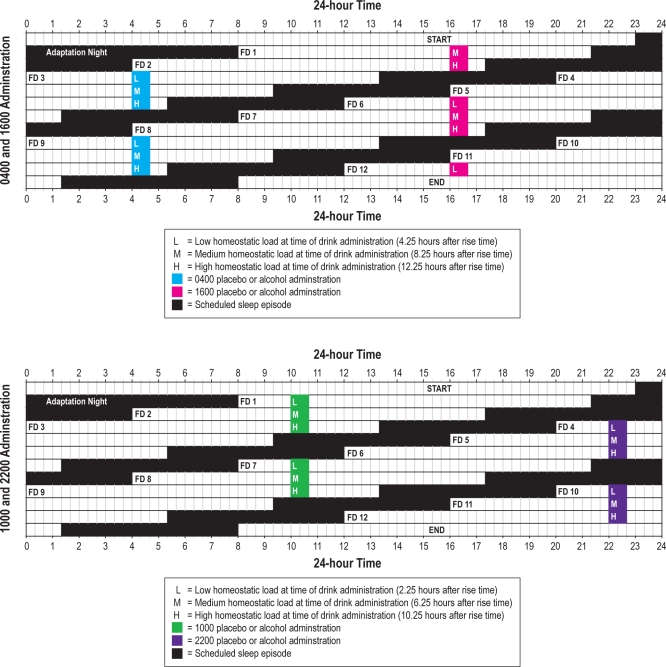
A 20-h forced desynchrony (FD) schedule began immediately upon waking after adaptation night and continued for 12 cycles (Figure 1), with two-thirds (13h 20m) of each cycle awake and one-third (6h 40m) scheduled for sleep. Thus, participants were awakened at 08:00 on FD1, stayed awake until scheduled bedtime at 21:20, awakened to begin FD2 at 04:00, and so forth, precessing 4 h each “day” in real time. Participants were not told the time of day while in the laboratory to minimize expectancies based on knowledge of time. By completing 12 cycles on a 20-h day length, participants completed 2 full cycles in which the timing of the sleep/wake schedule on FD days 1-6 was repeated on FD days 7–12.
Figure 1.
A schematic of the 12 forced desynchrony (FD) cycles demonstrating that “protocol time” of drinking–in terms of length of time awake–was the same for drinks administered 6 cycles apart.
Small meals of equal caloric content were provided at 2-h intervals to limit the influence of meals on measures of interest (including alcohol metabolism). Caloric content of meals was calculated for each individual based upon daily calorie requirements adjusted for height, weight, and sex. Water was provided ad libitum throughout the protocol. The light level (incandescent) in the laboratory was < 15 lux during the waking parts of the protocol and < 1 lux during scheduled hours of sleep to minimize masking effects of light on circadian rhythms and to avoid suppressing melatonin production.
Alcohol Administration Protocol
Participants were not told they would receive a placebo beverage but that they would receive either a low or moderate dose of alcohol to control for alcohol expectancies. Participants also rinsed their mouths with mouthwash (Dawn Mist, Donovan Laboratories, Tampa, FL) before they began drinking to mask the taste of the beverage. The alcohol beverage (“moderate dose”) was vodka (Smirnoff 80 proof) mixed with chilled tonic water in a 1:4 ratio, with a wedge of lime placed in the drink. The placebo beverage (“low dose”) was a chilled tonic and lime drink of the same volume with 3 drops of vodka floated on the surface just before serving. The moderate dose of alcohol was calculated taking into account body weight and sex (men = 0.54 g/kg, women = 0.49 g/kg) and was formulated to achieve a breath alcohol concentration of 0.05g%. The dose of alcohol used in this protocol is considered a moderate dose equivalent to 2 or 3 standard drinks. Beverage administration was double blind; the staff person who prepared the drinks did not interact with participants and did not disclose beverage content to staff members administering the drinks. This individual also read the BrAC from the breathalyzer devices; participants were not told their levels.
During the beverage administration and for approximately 4.5 h or until bedtime, participants sat in individual rooms in bed with their back at a 70-degree angle, had limited social interaction, and were monitored with polysomnography (PSG) and video. Beverages were distributed equally into 3 glasses given at 10-min intervals with instructions to complete each glass by the end of the 10 minutes. BrAC was measured at approximately 30-min intervals in the hours following beverage administration for 4 h 35 min or until bedtime.
Alcohol and placebo administrations across the 12 FD cycles were counterbalanced within subjects (randomly assigned) such that the protocol time of drinking—in terms of length of time awake—was the same for drinks administered 6 cycles apart (see Figure 1). In other words, the alcohol or placebo beverage was administered at the same clock time within each group (04:00, 10:00, 16:00, 22:00); however, as sleep/wake timing occurred 4 h earlier on each cycle of the FD, the length of time relative to waking differed by 4 h on iterative days. For example, when an individual in the 10:00 administration group was given alcohol on FD1, the drink was centered 2.25 h after rise time (i.e., given at a time with low homeostatic load), and the same individual was administered placebo on FD7 also centered 2.25 h after scheduled rise time (also low homeostatic load). As Figure 1 shows, the medium and high homeostatic load (HL) days for the 10:00 group occurred on FD days 2 and 8 and FD days 3 and 9, respectively. Because the protocol was designed to attain maximum temporal dispersion of alcohol administration (6 h), the timing of beverage administration relative to scheduled sleep was unbalanced across groups. The drinking window ended 4.5 h, 8.5 hours, and 12.5 hours after scheduled rise time for the 04:00 and 16:00 administration groups and at 2.5 h, 6.5 h, and 10.5 h after scheduled rise time for the 10:00 and 22:00 administration groups. Thus, the 04:00 and 16:00 groups were examined separately from the 10:00 and 22:00 groups, and results will be presented separately throughout.
Polysomnographic (PSG) Recordings and Analysis
Sleep was continuously recorded by PSG in the laboratory during the 13 scheduled sleep episodes (adaptation night and FD cycles 1–12) and monitored by a trained technician. PSG included central and occipital referential electroencephalogram (EEG) derivations (C3/A2 and C4/A1 and O1/A2 and O2/A1), along with right and left electrooculogram (EOG), electromyogram (EMG; mentalis, submentalis), and electrocardiogram (ECG). EEG electrode placements were measured using the international 10-20 system.18 Respiration (oral/nasal thermocouple) and leg EMG were recorded only on the adaptation night to screen for sleep disordered breathing or periodic limb movement disorder; none was detected.
The PSG data acquisition equipment changed over the course of the study; thus, 2 systems were used to record sleep. For the first 9 participants, EEG signals were filtered with Grass Model 8 amplifiers (high-pass EEG filter, 0.3 Hz; low-pass EEG filter, 35 Hz; notch filter 60 Hz) and recorded on the Albert Grass Heritage System (GAMMA (Astromed, Grass, West Warwick, RI). EEG signals were digitized on-line (12 bit AD converter; Butterworth filter, −12 dB/octave; low-pass filter, −6dB at 35 Hz; time constant 1.0 second; collected and stored at a resolution of 128 Hz for the EEG). The GAMMA PSG digital records were saved as European Data Format (EDF) files. The TWin system (Astromed, Grass, West Warwick, RI) was used for the digital PSG recording on 17 participants; signals were collected unfiltered with TWin AS40 bedside amplifiers, and signals were filtered off-line (high-pass EEG filter 0.3 Hz; low-pass filter 35 Hz; notch filter 60 Hz). These signals were collected digitally with a sampling resolution of 400 Hz. In order to verify that the EEG signals obtained from the 2 systems were comparable, a calibration signal was input into both systems simultaneously and the output was recorded. Fast Fourier transform (FFT) of the output revealed that the EEG signals from the 2 systems were in good agreement from 1 to 16 Hz, but small discrepancies emerged at high frequencies. Therefore, frequencies > 16 Hz are not included in the spectral analyses described below.
Sleep stages were scored (blind to alcohol condition) visually from digital records off-line in 30-sec epochs using C3/A2, EOG, and EMG tracings according to the criteria of Rechtschaffen and Kales.19 Inter-scorer reliability was 90%; intra-scorer reliability was 91%. The following variables were analyzed for this report: total sleep time (TST; minutes of sleep scored within the scheduled sleep episode), sleep onset latency (SOL; minutes from lights out to the first of 3 consecutive epochs of sleep), wake after sleep onset (WASO; minutes of wake after falling asleep and before final arousal), total minutes of wake for the entire sleep episode (includes SOL, WASO, and any wake after the final arousal), number of awakenings (number of transitions from sleep to wake), sleep efficiency percent ([TST/(TST+WASO)] × 100), minutes of NREM stages 1, 2, and slow wave sleep (SWS: stages 3 + 4), and minutes of REM sleep.
EEG spectral analysis was performed after affected portions of the 30-sec epochs containing EEG artifacts (e.g., eye blinks, eye movements, movement artifacts, or signal noise) were excluded by careful visual inspection of the EEG signals. The derivation C3/A2 was subjected to spectral analysis off-line using an FFT routine (TMEC, Vitascore, Holland). The FFT routine was applied to artifact-free portions of 30-sec epochs (tapering window, moving averages of 4-sec epochs) of sleep. Thus, the mean spectra for each artifact-free portion of each epoch were used for each 30-sec epoch. This analysis resulted in a frequency resolution of 0.25 Hz. Power spectral data (μV2) for 0.25 Hz bins were then averaged within 30-sec epochs into 1-Hz bins from 1 to 16 Hz. The lowest 0.25 Hz frequency bins (0.25 and 0.5 Hz) were excluded from the initial 1-Hz bin analysis because of possible contamination by slow-frequency artifact. Power spectral data were aligned with sleep stage data, and EEG power spectra were computed separately across sleep for NREM and REM sleep.
Circadian Phase
Saliva samples (2 mL) were collected during FD waking episodes at 30-min intervals for determination of melatonin. Samples were frozen within 4 h of collection and subsequently subjected to radioimmunoassay. Dim light melatonin onset (DLMO) phase was assessed for all participants by linear interpolation between rising values, crossing a threshold value of 4 pg/mL. Melatonin data for 2 individuals could not be used due to low melatonin levels. The intrinsic circadian period for each participant was estimated using dim light melatonin onset, and period was computed by linear regression from these data.
Statistical Analyses
Sleep stage variables
One striking feature of the FD protocol is that large and predictable effects are seen for many sleep stage variables due to the scheduled time for sleep occurring across circadian phases. This feature of the protocol design means that sleep variables differ as a function of FD “night,” since the circadian phase at which sleep is scheduled changes by about 4 h each FD cycle. This phenomenon has been well characterized20 and affects virtually all sleep stage variables, though the effect is minimal for SWS. Thus, to examine alcohol's effects on sleep vs. placebo and across groups, correction factors were derived to minimize this protocol effect.
The first step in the sleep stage analyses, therefore, was to obtain correction values to account for effects attributable to circadian phase of each FD cycle. The sleep data for variables from each FD night were aggregated across participants given placebo or no beverage during the preceding wake episode. The statistical package SPSS (Chicago, IL) was used to compute the mean values across these participants (n = 18 to 21) for all-night sleep stage variables separately for each FD day (1–12) (see supplemental tables for descriptive statistics). These values served as normalized averages and were fitted with a 5th order polynomial using Excel (Microsoft Office 2006) to compute predicted meansleep variables for each FD day (see supplemental figures). The 5th order polynomial fits were good to excellent for all variables, ranging from r2 = 0.58 (number of awakenings) to r2 = 0.89 (minutes of REM sleep). Predicted mean values for each FD day were subtracted from each individual's alcohol and placebo condition values for the corresponding FD day, and these deviations from the predicted mean values as the variables included in the analyses. This normalization process was necessary for analyses comparing effects of alcohol between groups.
Sleep variable deviation scores were assessed with multivariate analysis of variance (MANOVA). The between-group factor was alcohol administration Group (04:00 vs. 16:00 and 10:00 vs. 22:00), and within-group factors were homeostatic load at time of alcohol intake (i.e., length of time awake low, medium, high, 4 h,8 h, or 12 h after scheduled rise time for the 04:00 and 16:00 groups; 2 h, 6 h, or 10 h after scheduled rise time for the 10:00 and 22:00 groups); and Condition (alcohol, placebo). Sources of variance assessed included main effects of Group, homeostatic load (HL), and Condition, as well as all interaction terms.
Analysis of Spectral EEG
EEG spectral power is also influenced by circadian phase.20 In addition, spectral EEG shows pronounced individual differences in the morphology and amplitude of the EEG spectrum21 compared to sleep stages, limiting options for analyzing these data to within-subject comparisons. Therefore, separate analyses of spectra were performed within each administration group (04:00, 10:00, 16:00, and 22:00) and for each HL condition (low, medium, high) to compare alcohol and placebo nights. Bootstrap analyses (with 5,000 iterations) were used to compute statistical significance of the effects of alcohol on EEG spectral data. The application of the bootstrap method to sleep EEG data has previously been described.22 Group differences were not assessed.
RESULTS
Breath Alcohol Levels
Breath alcohol concentration (BrAC) taken upon arrival to the sleep lab confirmed a BrAC of 0 for all participants; as expected, all BrAC readings from the placebo nights were also 0. The peak BrAC levels averaged for all alcohol administration occasions within the 4 alcohol administration groups (04:00,16:00, 10:00, and 22:00) were 0.048%, 0.052%, 0.051%, and 0.044g%, respectively. These peak BrAC values were close to the targeted values and did not differ significantly among alcohol administration groups.
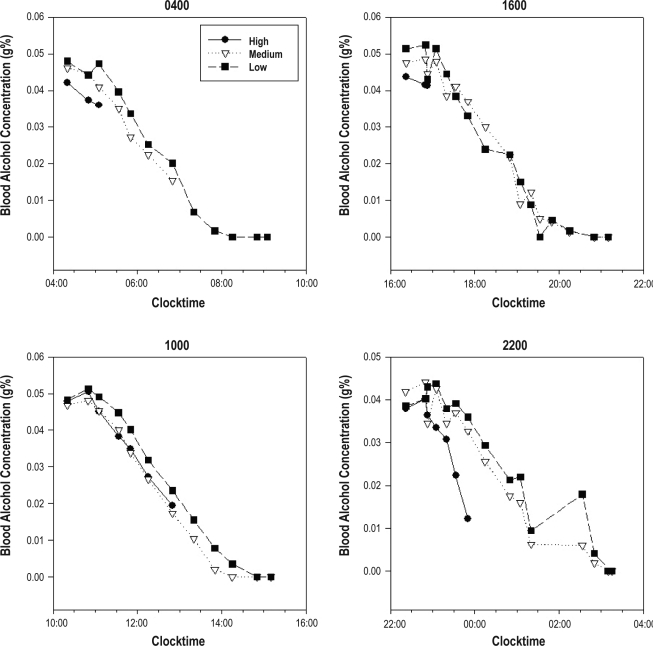
By contrast, the level of BrAC at bedtime showed important differences as a function of the timing of alcohol administration (group), as illustrated in Figure 2: BrAC values at bedtime for the medium and low homeostatic loads were uniformly 0 g%. For the high homeostatic loads, however, BrAC levels were always above 0 g%. The pre-bedtime BrACs for each alcohol administration group in the high HL condition were examined with student t-test (2-tailed significance level P < 0.05). As expected BrAC values at bedtime were higher for the 04:00 and 16:00 administration groups compared to the 10:00 and 22:00 administration groups due to the different intervals of alcohol administration to bedtime.
Figure 2.
The time course of mean breath alcohol concentrations (g%) following alcohol administration for each alcohol administration group (04:00, 16:00, 10:00, and 22:00), at each homeostatic load (HL) condition (high HL condition = black solid line with solid circles; medium HL = grey dotted line with open triangles; low HL = dashed line and solid squares).
Circadian Phase and Period
The circadian period calculations showed that alcohol was administered at distinct circadian phases for the alcohol administration groups being compared. Thus, the circadian phases at the time alcohol was administered for the 04:00 and 16:00 groups never overlapped, and nor for the 10:00 and 22:00 administration groups. Table 1 presents the initial melatonin onset phase, period, and amount of phase shift between alcohol and placebo administration for all participants.
Table 1.
Circadian phase, period, and phase shift
| 04:00 |
16:00 |
||||||
|---|---|---|---|---|---|---|---|
| Participants | Initial DLMO phase | Period | Phase Shift (hours) | Participants | Initial DLMO phase | Period | Phase Shift (hours) |
| 6002 | 20.82 | 24.0 | 0.0 | 3002 | 20.69 | 24.5 | 2.5 |
| 6019 | 21.55 | 24.28 | 1.4 | 3005 | 22.68 | 24.24 | 1.2 |
| 6010 | 23.45 | 24.28 | 1.4 | 4018 | 20.08 | 23.82 | −0.9 |
| 6011 | 21.64 | 24.21 | 1.05 | 4021 | 21.33 | 23.99 | −0.05 |
| 6015 | 22.9 | 24.06 | 0.3 | 5023 | 22.94 | 24.14 | 0.7 |
| 6005* | 5025 | 20.48 | 23.96 | −0.2 | |||
| 6023 | 20.61 | 24.20 | 1.0 | ||||
| 6025* | |||||||
| 10:00 |
22:00 |
||||||
| Participants | Initial DLMO phase | Period | Phase Shift (hours) | Participants | Initial DLMO phase | Period | Phase Shift (hours) |
| 4007 | 22.51 | 24.22 | 1.1 | 4012 | 20.8 | 24.10 | 0.5 |
| 4009 | 22.7 | 24.65 | 3.25 | 4016 | 21.4 | 24.35 | 1.75 |
| 4017 | 22.04 | 24.91 | 4.55 | 4011 | 21.93 | 24.15 | 0.75 |
| 5007 | 21.07 | 24.03 | 0.15 | 4005 | 22.62 | 24.38 | 1.9 |
| 5012 | 21.61 | 24.24 | 1.2 | 5002 | 19.71 | 23.87 | −0.65 |
| 5015 | 19.72 | 23.91 | −0.45 | 5017 | 21.07 | 24.11 | 0.55 |
| 6024 | 21.45 | 23.95 | −0.25 | ||||
Circadian period (h) and phase change (h) from first beverage FD cycle to final beverage FD cycle.
Participants whose melatonin levels were too low to measure phase.
Sleep stage variables
04:00 and 16:00 groups (Table 2):
Table 2.
Mean (SD) of deviations from polynomial curve fit values for alcohol administration groups 04:00 and 16:00
| Sleep Variable | Time of Alcohol (Group) | Homeostatic Load & Condition |
|||||
|---|---|---|---|---|---|---|---|
| High Placebo | High Alcohol | Medium Placebo | Medium Alcohol | Low Placebo | Low Alcohol | ||
| SOL1 (minutes) | 04:00 | 0 (5) | −1 (4) | 2 (4) | 8 (14) | 4 (9) | 3 (9) |
| 16:00 | −6 (9) | −10 (7) | 11 (24) | 4 (27) | −11 (8) | −10 (7) | |
| WASO (minutes) | 04:00 | −5 (41) | 6 (44) | −7 (52) | −7 (50) | 8 (69) | 4 (61) |
| 16:00 | −27 (63) | 8 (84) | −3 (22) | −10 (22) | −8 (12) | −3 (24) | |
| Wake2 (minutes) | 04:00 | −1 (10) | 64 (18) | −1 (14) | 38 (12) | 13 (73) | 7 (69) |
| 16:00 | −6 (13) | −8 (16) | 16 (5) | 36 (8) | −19 (8) | −13 (26) | |
| Number of Awakenings | 04:00 | −1 (1) | −4 (2) | −4 (1) | −1 (1) | −3 (5) | −1 (9) |
| 16:00 | 0 (1) | 1 (2) | 1 (2) | −2 (.5) | 1 (10) | 1 (10) | |
| % Sleep Efficiency | 04:00 | 2 (9) | −1 (11) | 2 (13) | 0 (14) | −3 (18) | −1 (18) |
| 16:00 | 8 (16) | 1 (21) | −2 (8) | 2 (9) | 5 (1) | 4 (6) | |
| Stage 1 (minutes) | 04:00 | −11 (9) | −7 (7) | −4 (13) | −3 (9) | −7 (12) | −8 (10) |
| 16:00 | 0 (11) | 0 (14) | 4 (14) | −4 (13) | −2 (10) | −9 (9) | |
| Stage 23 (minutes) | 04:00 | 3 (7) | −38 (10) | 3 (9) | −15 (6) | −2 (41) | −2 (46) |
| 16:00 | 10 (13) | 2 (10) | −4 (6) | −10 (10) | 1 (25) | −3 (22) | |
| SWS (minutes) | 04:00 | 1 (8) | 10 (25) | 9 (19) | 6 (19) | 3 (24) | 10 (9) |
| 16:00 | 2 (17) | −5 (10) | 5 (19) | 2 (25) | 9 (20) | 6 (25) | |
| REM (minutes) | 04:00 | 8 (24) | −4 (26) | −8 (4) | −10 (26) | −6 (26) | −6 (23) |
| 16:00 | 14 (20) | 5 (21) | −12 (10) | −1 (11) | 10 (23) | 20 (20) | |
SOL, Main effect of HL: F2,22 = 3.97, P = 0.003.
Wake, Main effect of Condition: F1,11 = 51.31, P < 0.001; Interaction of Group × Condition: F1,11 = 19.87, P = 0.001; Interaction of Condition × HL: F2,22 = 8.62, P = 0.002; Interaction of Group × Condition × HL: F2,22 = 11.13, P < 0.001.
Stage 2, Main effect of Condition: F1,11 = 59.48, P < 0.001; Interaction of Group × Condition: F1,11 = 16.2, P = 0.002; Interaction of HL × Condition: F1,11 = 7.73, P = 0.003; Interaction of Group × Condition× HL: F2,22 = 5.55, P = 0.011.
As described above, the deviations from mean values predicted by the polynomial curve fits were used for statistical analyses for all of the sleep stage variables. Statistical significance was set at P < 0.006, using a Bonferroni correction factor for multiple comparsions.
The analyses of the 04:00 and 16:00 groups sleep stages showed main effects of condition and interactions that may point to time of day effects. One effect unrelated to the alcohol/placebo condition was that SOL deviation showed a significant main effect of HL, such that when beverage was administered 8.25 h after scheduled rise time, participants took significantly longer to fall asleep compared to beverage administration 12.25 h or 4.25 h after rise time.
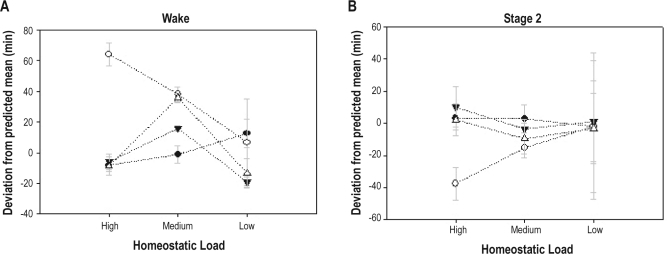
One of the few main effects of alcohol/placebo condition also involved a wake-related variable: Wake deviation in the 04:00 and 16:00 groups was significantly greater for the alcohol condition than the placebo condition (Figure 3A). Wake deviation also manifested significant Group × Condition, HL × Condition, and Group × Condition × HL interactions. These significant interactions were examined with rANOVA within the 04:00 and 16:00 group, showing an interaction (condition × HL) for the 04:00 group; however, the interaction (condition × HL) dropped out for the 16:00 group, indicating 04:00 group was driving the interaction in the MANOVA. A series of post hoc t-tests was performed within the 04:00 group. These post hoc tests showed that wake deviation in the medium (m = −1, SD = 1.2) and low (m = −7, SD = 69) HL conditions did not differ from the placebo average (m = −1.7, SD = 40.3); however, wake deviation was significantly greater when alcohol was given at the high homeostatic load (m = 59.5, SD = 17.4).
Figure 3.
Sleep stage differences: Mean (SE) minutes of (A) wake and (B) stage 2, for alcohol and placebo conditions at each homeostatic load (high, medium, low) for groups 04:00 (solid lines with circles) and 16:00 (dashed lines with triangles). Open symbols indicate placebo condition and solid circles the alcohol condition.
Stage 2 deviation was the only NREM sleep variable that showed statistically significant effects. A main effect of alcohol/placebo condition was found for stage 2 deviation, which was lower for the alcohol condition than the placebo condition (Figure 3B). Stage 2 deviation also showed a Group × Condition and HL × Condition interaction.
Neither main effects nor interactions of alcohol/placebo condition were found for REM sleep deviation.
In summary when alcohol was administered at 04:00 (around the circadian trough) at the highest HL that the protocol allowed for (about 12 h awake), alcohol increased minutes of wake compared to placebo administered at a circadian time 180 degrees out of phase (16:00).
10:00 and 22:00 groups (Table 3):
Table 3.
Mean (SD) of deviations from polynomial curve fit values for alcohol administration groups 10:00 and 22:00
| Sleep Variable | Time of Alcohol (Group) | Homeostatic Load & Condition |
|||||
|---|---|---|---|---|---|---|---|
| High Placebo | High Alcohol | Medium Placebo | Medium Alcohol | Low Placebo | Low Alcohol | ||
| Wake (minutes) | 10:00 | −1 (5) | −2 (5) | 10 (20) | 7 (24) | −4 (8) | 1 (27) |
| 22:00 | −4 (5) | −7 (6) | 2 (4) | 3 (4) | 0 (3) | 1 (4) | |
| Stage 1 (minutes) | 10:00 | −1 (3) | 1 (4) | −1 (3) | 0 (4) | 1 (5) | 1 (4) |
| 22:00 | −1 (2) | −1 (2) | 1 (2) | 1 (1) | 1 (3) | 0 (2) | |
| Stage 2 (minutes) | 10:00 | −5 (6) | 3 (9) | −2 (9) | −5 (6) | −1 (7) | −5 (8) |
| 22:00 | 3 (6) | 3 (7) | 2 (6) | 2 (4) | 3 (4) | 3 (3) | |
| SWS (minutes) | 10:00 | 6 (8) | −2 (9) | −8 (9) | −3 (16) | 3 (8) | 1 (17) |
| 22:00 | 1 (7) | 6 (5) | 1 (8) | −2 (7) | −4 (10) | 0 (8) | |
| REM (minutes) | 10:00 | 1 (7) | 0 (5) | 1 (3) | 0 (3) | 0 (0) | 2 (3) |
| 22:00 | 0 (2) | 0 (2) | −5 (2) | 4 (3) | −2 (4) | −5 (2) | |
No main effects or interactions were significant for any NREM or REM sleep stage variables (deviation from the mean) for the 10:00 and 22:00 groups. One wake-related variable showed a significant effect of Group: number of awakenings deviation. The 10:00 group had significantly fewer awakenings deviation compared to the 22:00 group. Although other wake-related variables did not show significant main effects of Group when correcting for multiple comparisons, we note that the 10:00 alcohol administration group showed wide variability and high amounts of wakefulness, which may have influenced the findings, especially given the relatively small sample size.
All-Night Spectral EEG
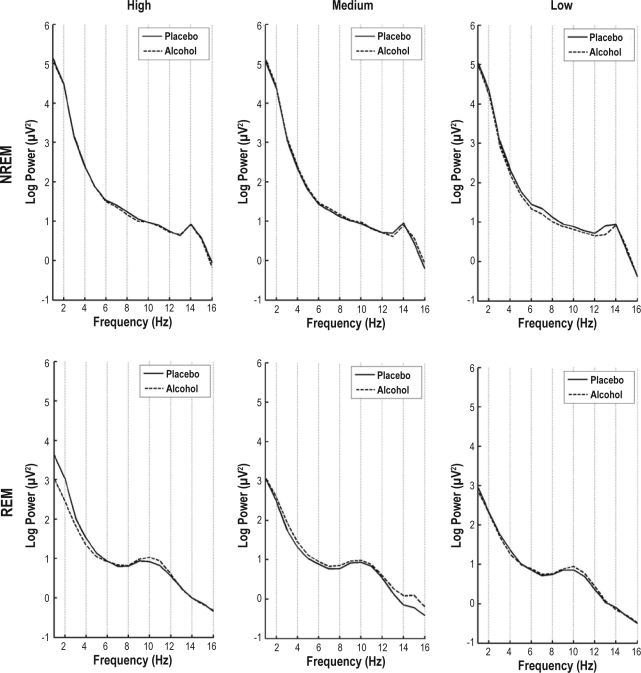
At α set at 0.05, we found no statistically significant differences in the NREM and REM spectra between the alcohol and placebo condition for any Group (04:00, 16:00, 10:00, or 22:00) at any HL condition (low, medium, high). To illustrate, NREM and REM average spectra for the 04:00 group are plotted in Figure 4. Although this group exhibited a significant effect of alcohol on sleep stage variables, the alcohol and placebo spectra are virtually overlapping. Similar overlapping spectra were found for the other groups.
Figure 4.
Spectral EEG power on a log scale for the 04:00 alcohol group for low, medium, and high homeostatic load conditions for NREM and REM sleep. Placebo conditions are in solid lines and alcohol conditions are in dashed lines. There were no statistically significant differences between the alcohol and placebo conditions for any homeostatic load condition.
DISCUSSION
The present study examined the effects of a moderate dose of alcohol compared to placebo given at four different circadian phases and at three different homeostatic loads on sleep stage and spectral EEG characteristics using a 20-hour forced desynchrony protocol. We hypothesized that the homeostatic and circadian processes would interact to influence the effects of alcohol on subsequent sleep. The findings of this study did not support our hypothesis, which was based on previous literature that reported increased SWS and decreased SOL with alcohol administered at a high homeostatic load at the start of the circadian night (i.e., 22:00). On the other hand, we found that when alcohol was administered at 04:00 with the highest homeostatic load, alcohol increased minutes of wake, and there was a trend for reduced minutes of stage 2 sleep compared to alcohol administered at 16:00; the 10:00 and 22:00 alcohol administration timing groups did not show any differential effects of homeostatic load or circadian phase on sleep stage variables.
Findings in the present study of increased wake following alcohol compared to placebo are consistent with one study that examined the effects of two doses (0.5 and 0.75 g%) of alcohol (compared to placebo) given 1 hour before bedtime,23 and also showed a higher percent of wake after sleep onset with alcohol (dose-dependent increase) compared to placebo. Our findings that minutes of wake increased with alcohol consumed at 04:00 (targeting circadian trough) and closest to bedtime (high HL) extends the previous literature by demonstrating an interaction of the homeostatic and circadian systems on alcohol's impact on subsequent sleep. We found a similar trend for homeostatic and circadian interaction effect on NREM stage 2 sleep, such that stage 2 sleep decreased in the groups given alcohol at the circadian trough and highest homeostatic load. This trend may have occurred as a response to increased wake, rather than as a direct effect of alcohol on stage 2 sleep per se.
Our finding that REM sleep (04:00 group) was decreased in the medium HL condition compared to low or high HL conditions indicates possible residual effects of alcohol on sleep that lingered beyond the time that alcohol was metabolized, consistent with previous findings.9 It is unclear, however, why there were residual effects on REM only, and not NREM or wake variables.
With regards to the spectral analysis results, we did not find power differences in any frequency bins with alcohol compared to placebo. Few previous studies have looked at the effects of moderate doses of alcohol on the sleep EEG. The three studies that have examined the effects of alcohol on the spectral EEG8,9,6 have reported minimal changes in the sleep EEG with alcohol compared to placebo. Many methodological differences exist across these studies, and the inconsistent findings were difficult to interpret. In addition, controls for homeostatic load and circadian phase were not as explicit as in the current study. We know that cognitive tasks show a combined effect of inadequate sleep and alcohol intake, and this effect is more than additive.24 Therefore, if alcohol and inadequate sleep have a similar (more than additive) effect on sleep, this may help to explain the inconsistent findings in the previous literature in studies where sleep was not assessed prior to in-lab examination.
Our attempts to vary homeostatic load and circadian phase systematically have provided findings largely inconsistent with the notion of differential effects of time of day of alcohol consumption on sleep. Thus, with homeostatic load and circadian phase taken into account, a moderate dose of alcohol did not produce major changes to sleep architecture.
The current study has several limitations that complicate interpretation. In the first place, a small sample size limits power to detect subtle changes in sleep architecture with alcohol. In addition, the alcohol dose was moderate and BrAC was low at bedtime even in the condition with highest homeostatic load. Further, this study only included healthy young adults, which limits generalization to patient populations (e.g., alcoholics or patients with sleep disorders). A final limitation is that the 20-h FD limited the homeostatic load even below the normal waking day. Thus, our “high” homeostatic load condition is only “high” relative to our other conditions.
In summary, our findings lend support to the idea that alcohol may disrupt sleep. We note that our findings—regardless of administration phase or length of time awake (up to 12 h) at dosing—do not support the notion that a moderate alcohol dose is a useful sleep aid, since sleep stage parameters associated with improved sleep (increased SWS, decreased SOL, etc.) did not occur. On the other hand, modest changes may have been missed due to our small sample size. Our strongest finding was that alcohol disrupts sleep when taken before a sleep episode that starts at the phase of peak circadian sleep propensity, i.e., the 04:00 group. Sleep onset is rapid at this phase without alcohol, and sleep pressure wanes as circadian dependent alerting increases.20 If alcohol's disruptive capacity is strongest taken before sleep at this phase, alcohol use may be a pitfall for night shift workers or individuals with jet lag who drink to improve “day sleep.” Our data indicate that alcohol consumption near one's circadian trough and when one has been awake for at least 13.33 hours (i.e., relatively high homeostatic load) is the worst time to consume alcohol in regards to disrupting sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (AA 13252) to Mary A. Carskadon, PhD. The authors would like to thank our consultants on the project, Dr. Tim Roehrs, Dr. J. Todd Arnedt, Dr. Robert Swift, and Dr. Peter Monti. Thanks also go to Christine Acebo for statistical advice. Also, we would like to thank Denise Maceroni for saliva assays and Jennifer Maxwell for assistance with data analysis and compilation. The assistance of the staff at the E.P. Bradley Sleep Lab, and Brown University students in data collection is gratefully acknowledged.
Supplemental Data Tables
Supplemental Data Tables
REFERENCES
- 1.Kleitman N. Sleep and wakefulness. Chicago: University of Chicago Press; 1939. [Google Scholar]
- 2.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. Sleep. 1999;22:S347–S353. [PubMed] [Google Scholar]
- 3.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–39. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- 4.Yules RB, Lippman ME, Freedman DX. Alcohol administration prior to sleep. The effect on EEG sleep stages. Arch Gen Psychiatry. 1967;16:94–7. doi: 10.1001/archpsyc.1967.01730190096012. [DOI] [PubMed] [Google Scholar]
- 5.Rundell OH, Lester BK, Griffiths WJ, Williams HL. Alcohol and sleep in young adults. Psychopharmacologia. 1972;26:201–18. doi: 10.1007/BF00422697. [DOI] [PubMed] [Google Scholar]
- 6.Van Reen E, Jenni OG, Carskadon MA. Effects of alcohol on sleep and the sleep electroencephalogram in healthy young women. Alcohol Clin Exp Res. 2006;30:974–81. doi: 10.1111/j.1530-0277.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- 7.MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young men. J Stud Alcohol. 1982;43:434–44. doi: 10.15288/jsa.1982.43.434. [DOI] [PubMed] [Google Scholar]
- 8.Dijk DJ, Brunner DP, Aeschbach D, Tobler I, Borbély AA. The effects of ethanol on human sleep EEG power spectra differ from those of benzodiazepine receptor agonists. Neuropsychopharmacology. 1992;7:225–32. [PubMed] [Google Scholar]
- 9.Landolt HP, Roth C, Dijk DJ, Borbély AA. Late-afternoon ethanol intake affects nocturnal sleep and the sleep EEG in middle-aged men. J Clin Psychopharmacol. 1996;16:428–36. doi: 10.1097/00004714-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Rupp TL, Acebo C, Seifer R, Carskadon MA. Effects of a moderate evening alcohol dose. II: performance. Alcohol Clin.Exp Res. 2007;31:1365–71. doi: 10.1111/j.1530-0277.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 11.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 12.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–95. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 13.Czeisler CA, Allan JS, Kronauer RE. A method of assaying the effects of therapeutic agents on the period of the endogenous circadian pacemaker in man. In: Montplaisir J, Godbout R, editors. Sleep and biological rhythms. New York: Oxford University Press; 1990. pp. 87–98. [Google Scholar]
- 14.Roehrs T, Zwyghuizen-Doorenbos A, Knox M, Moskowitz H, Roth T. Sedating effects of ethanol and time of drinking. Alcohol Clin Exp Res. 1992;16:553–7. doi: 10.1111/j.1530-0277.1992.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 15.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–82. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis LR. SCL-90-R, Symptom Checklist-90-R. 1975.
- 17.Beck AT. Screening depressed patients in family practice. Postgrad Med. 1972;52:81–5. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- 18.Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–5. [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 20.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 21.Buckelmʊller J, Landolt H, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–6. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33:801–9. doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams DL, MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young women. J Stud Alcohol. 1983;44:515–23. doi: 10.15288/jsa.1983.44.515. [DOI] [PubMed] [Google Scholar]
- 24.Rupp TL, Acebo C, Van Reen E, Carskadon MA. Effects of a moderate evening alcohol dose. I: sleepiness. Alcohol Clin Exp Res. 2007;31:1358–64. doi: 10.1111/j.1530-0277.2007.00433.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data Tables
Supplemental Data Tables