Abstract
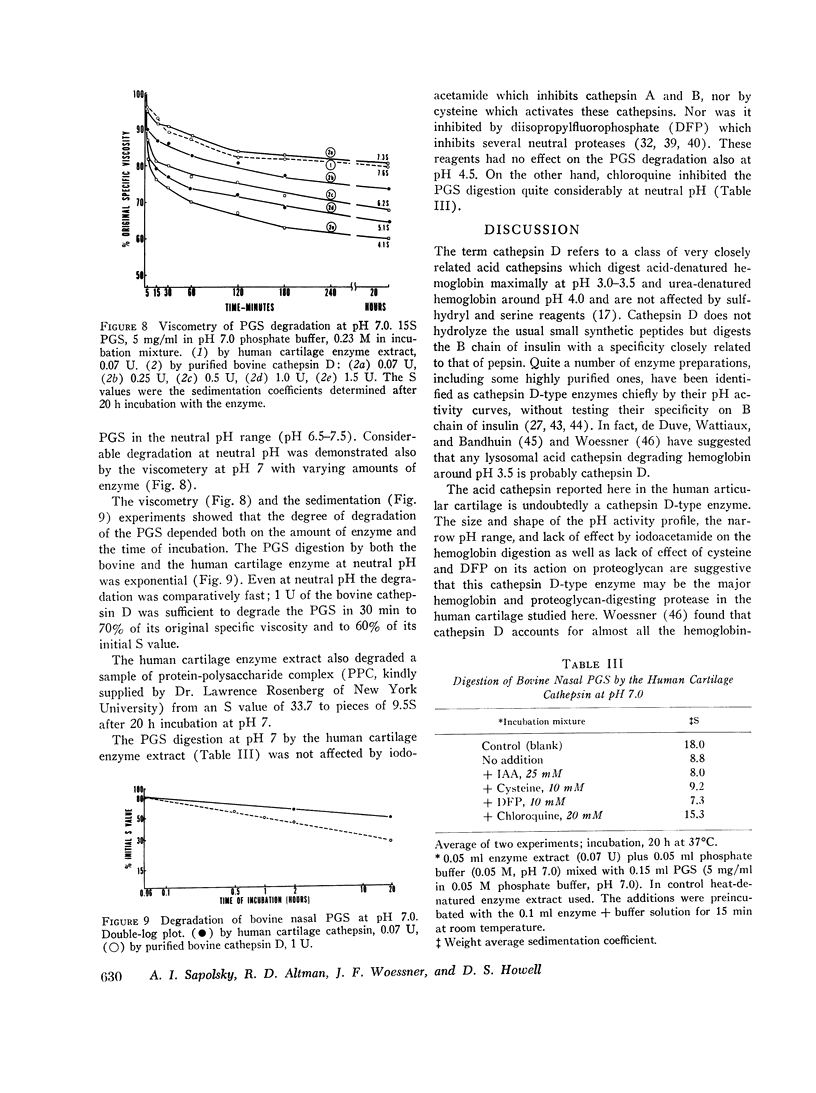
In recent years the lysosomal cathepsins have been implicated as important agents in the physiological degradation of various cartilages. In the present study, the nature of cathepsin present in human articular cartilage was investigated by microtechniques and a possible role for cathepsins in the cartilage degradation observed in osteoarthritis was sought. The results of this study indicated that the hemoglobin and proteoglycan-digesting activity in the human cartilage observed is predominantly that of a cathepsin D-type enzyme. This cathepsin D-type enzyme activity was present in two to three times greater amounts in yellowish or ulcerated articular cartilage from patients with primary osteoarthritis than in control “normal” human cartilages. The human cathepsin D-type enzyme, as well as a highly purified cathepsin D from bovine uterus degraded proteoglycan subunit (PGS) maximally at pH 5. Both enzyme preparations were inactive on hemoglobin at pH 6-8, but degraded PGS considerably at neutral pH. The activity of the human cathepsin extract was not affected by reagents which inhibit or activate cathepsins A and B. Neutral proteases which are active on hemoglobin or are inhibited by diisopropylfluorophosphate (DFP) were not detected in these preparations, but contamination by another type of neutral protease cannot be excluded. Chloroquine inhibited the degradation of PGS at neutral pH by the human cartilage enzyme extract.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y., Evans L., Stainthorpe E., Lack C. H. Characterization of cathepsins in cartilage. Biochem J. 1967 Nov;105(2):549–557. doi: 10.1042/bj1050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y., Evans L. Studies on the cathepsins in elastic cartilage. Biochem J. 1969 May;112(4):427–433. doi: 10.1042/bj1120427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BOLLET A. J., HANDY J. R., STURGILL B. C. Chondroitin sulfate concentration and protein-polysaccharide composition of articular cartilage in osteoarthritis. J Clin Invest. 1963 Jun;42:853–859. doi: 10.1172/JCI104777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barland P., Janis R., Sandson J. Immunofluorescent studies of human articular cartilage. Ann Rheum Dis. 1966 Mar;25(2):156–164. doi: 10.1136/ard.25.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Lysosomal acid proteinase of rabbit liver. Biochem J. 1967 Aug;104(2):601–608. doi: 10.1042/bj1040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollet A. J. Connective tissue polysaccharide metabolism and the pathogenesis of osteoarthritis. Adv Intern Med. 1967;13:33–60. [PubMed] [Google Scholar]
- Bollet A. J., Nance J. L. Biochemical Findings in Normal and Osteoarthritic Articular Cartilage. II. Chondroitin Sulfate Concentration and Chain Length, Water, and Ash Content. J Clin Invest. 1966 Jul;45(7):1170–1177. doi: 10.1172/JCI105423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. N., YIELDING K. L. SPECTROPHOTOMETRIC STUDIES OF THE INTERACTION OF CHLOROQUINE WITH DEOXYRIBONUCLEIC ACID. J Biol Chem. 1965 Jul;240:3123–3131. [PubMed] [Google Scholar]
- Chrisman O. D. Biochemical aspects of degenerative joint disease. Clin Orthop Relat Res. 1969 May-Jun;64:77–86. [PubMed] [Google Scholar]
- Cowey F. K., Whitehouse M. W. Biochemical properties of anti-inflammatory drugs. VII. Inhibition of proteolytic enzymes in connective tissue by chloroquine (resochin) and related antimalarial antirheumatic drugs. Biochem Pharmacol. 1966 Aug;15(8):1071–1084. doi: 10.1016/0006-2952(66)90272-3. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- DINGLE J. T., LUCY J. A., FELL H. B. Studies on the mode of action of excess of vitamin A. 1. Effect of excess of vitamin A on the metabolism and composition of embryonic chick-limb cartilage grown in organ culture. Biochem J. 1961 Jun;79:497–500. doi: 10.1042/bj0790497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- FELL H. B., DINGLE J. T. Studies on the mode of action of excess of vitamin A. 6. Lysosomal protease and the degradation of cartilage matrix. Biochem J. 1963 May;87:403–408. doi: 10.1042/bj0870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FESSEL J. M., CHRISMAN O. D. ENZYMATIC DEGRADATION OF CHONDROMUCOPROTEIN BY CELL-FREE EXTRACTS OF HUMAN CARTILAGE. Arthritis Rheum. 1964 Aug;7:398–405. doi: 10.1002/art.1780070406. [DOI] [PubMed] [Google Scholar]
- Granda J. L., Ranawat C. S., Posner A. S. Levels of three hydrolases in rheumatoid and regenerated synovium. Arthritis Rheum. 1971 Mar-Apr;14(2):223–230. doi: 10.1002/art.1780140205. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Janis R., Smith C. Cartilage matrix depletion by rheumatoid synovial cells in tissue culture. J Exp Med. 1967 Dec 1;126(6):1005–1012. doi: 10.1084/jem.126.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Haschen R. J., Krug K. Distribution patterns of proteolytic enzymes in normal and leukaemic human leucocytes. Nature. 1966 Jan 29;209(5022):511–512. doi: 10.1038/209511a0. [DOI] [PubMed] [Google Scholar]
- Janoff A., Zeligs J. D. Vascular injury and lysis of basement membrane in vitro by neutral protease of human leukocytes. Science. 1968 Aug 16;161(3842):702–704. doi: 10.1126/science.161.3842.702. [DOI] [PubMed] [Google Scholar]
- KELLGREN J. H., LAWRENCE J. S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUSCOMBE M. Acid phosphatase and catheptic activity in rheumatoid synovial tissue. Nature. 1963 Mar 9;197:1010–1010. doi: 10.1038/1971010a0. [DOI] [PubMed] [Google Scholar]
- Marks N., Lajtha A. Separation of acid and neutral proteinases of brain. Biochem J. 1965 Oct;97(1):74–83. doi: 10.1042/bj0970074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESS E. M., PORTER R. R., CEBRA J. The isolation and properties of a proteolytic enzyme, cathepsin D, from bovine spleen. Biochem J. 1960 Mar;74:501–514. doi: 10.1042/bj0740501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pita J. C., Müller F. J. Ultracentrifugal studies in capillary cells. I. Determination of sedimentation coefficients. Anal Biochem. 1972 Jun;47(2):395–407. doi: 10.1016/0003-2697(72)90133-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Pal S., Beale R., Schubert M. A comparison of proteinpolysaccharides of bovine nasal cartilage isolated and fractionated by different methods. J Biol Chem. 1970 Aug 25;245(16):4112–4122. [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Sapolsky A. I., Woessner J. F., Jr Multiple forms of cathepsin D from bovine uterus. J Biol Chem. 1972 Apr 10;247(7):2069–2076. [PubMed] [Google Scholar]
- THOMAS L., McCLUSKEY R. T., POTTER J. L., WEISSMANN G. Comparison of the effects of papain n vitamin A on cartilage. I. The effects in rabbits. J Exp Med. 1960 May 1;111:705–718. doi: 10.1084/jem.111.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasi S., Murray R. K., Macmorine D. R., Movat H. Z. The role of PMN-leucocyte lysosomes in tissue injury, inflammation and hypersensitivity. II. Studies on the proteolytic activity of PMN-leucocyte lysosomes of the rabbit. Br J Exp Pathol. 1966 Aug;47(4):411–423. [PMC free article] [PubMed] [Google Scholar]
- Wegelius O., Klockars M., Vainio K. Content of fibrocartilagenolytic enzymes and viscosity of homogenates of joint menisci in rheumatoid arthritis. Scand J Clin Lab Invest. 1970 Jan;25(1):41–45. doi: 10.3109/00365517009046188. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Lysosomes. N Engl J Med. 1965 Nov 18;273(21):1143–concl. doi: 10.1056/NEJM196511182732107. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Spilberg I. Breakdown of cartilage proteinpolysaccharide by lysosomes. Arthritis Rheum. 1968 Apr;11(2):162–169. doi: 10.1002/art.1780110206. [DOI] [PubMed] [Google Scholar]
- Weston P. D. A specific antiserum to lysosomal cathepsin D. Immunology. 1969 Sep;17(3):421–428. [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Acid hydrolases of connective tissue. Int Rev Connect Tissue Res. 1965;3:201–260. doi: 10.1016/b978-1-4831-6753-4.50011-9. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr, Shamberger R. J., Jr Purification and properties of cathepsin D from bovine utrus. J Biol Chem. 1971 Apr 10;246(7):1951–1960. [PubMed] [Google Scholar]
- Wojtowicz M. B., Odense P. The effect of urea upon the activity measurement of cod muscle cathepsin with hemoglobin substrate. Can J Biochem. 1970 Sep;48(9):1050–1053. doi: 10.1139/o70-165. [DOI] [PubMed] [Google Scholar]




