Abstract
As a highly reactive substance produced in biological systems by the one-electron reduction of oxygen, superoxide (O2-) seemed a likely candidate as a bactericidal agent in leukocytes. The reduction of cytochrome c, a process in which O2- may serve as an electron donor, was found to occur when the cytochrome was incubated with leukocytes. O2- was identified as the agent responsible for the leukocyte-mediated reduction of cytochrome c by the demonstration that the reaction was abolished by superoxide dismutase, an enzyme that destroys O2-, but not by boiled dismutase, albumin, or catalase.
Leukocyte O2- production doubled in the presence of latex particles. The average rate of formation of O2- in the presence of these particles was 1.03 nmol/107 cells per 15 min. This rate, however, is only a lower limit of the true rate of O2- production, since any O2- which reacted with constituents other than cytochrome c would have gone undetected. Thus. O2- is made by leukocytes under circumstances which suggest that it may be involved in bacterial killing.
Full text
PDF
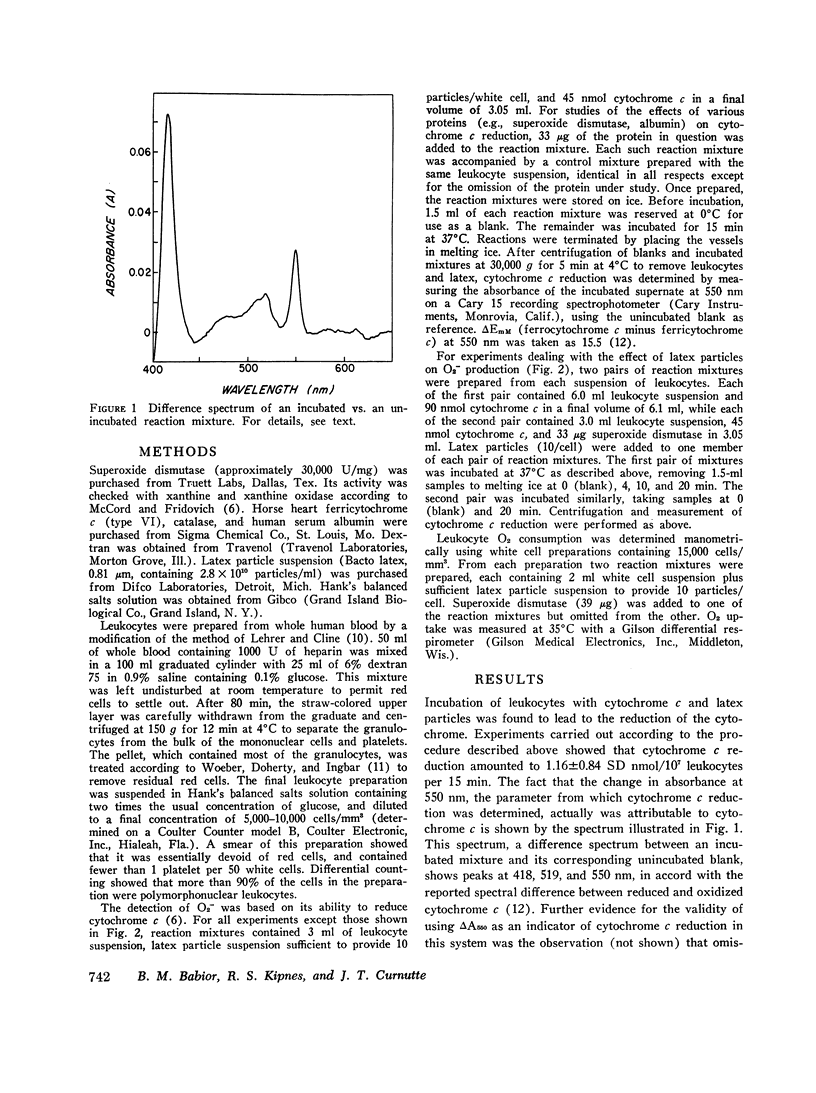
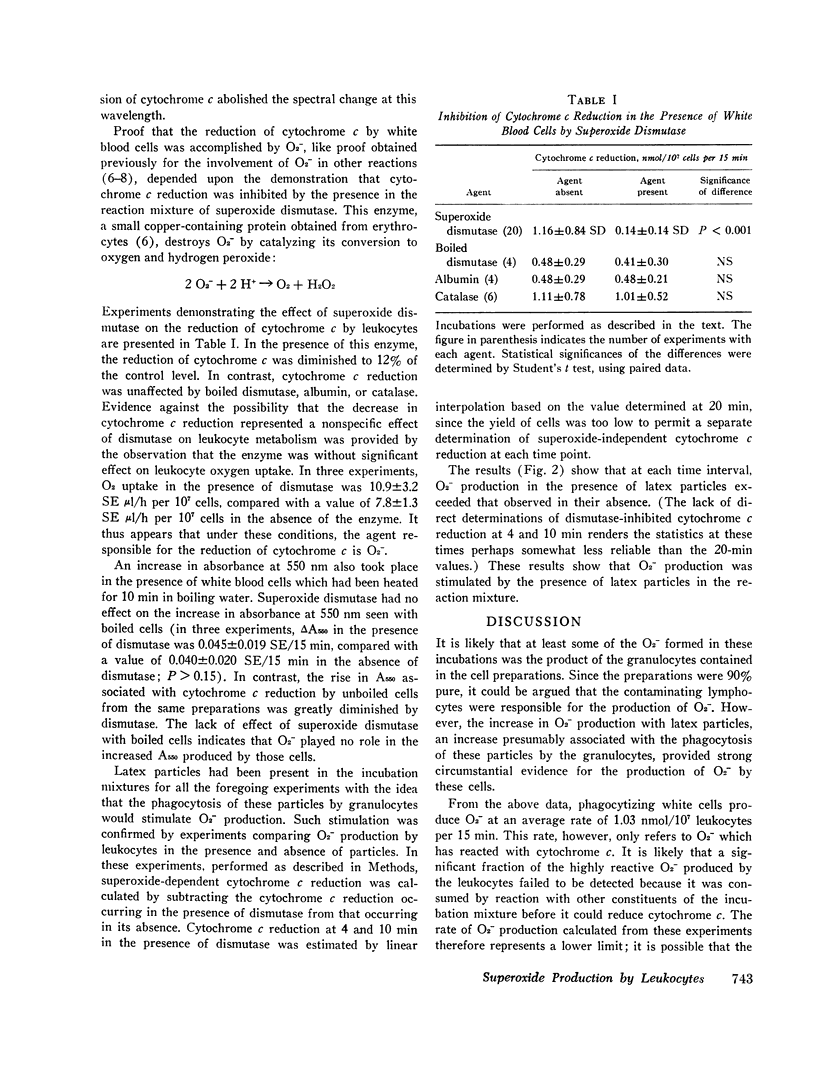
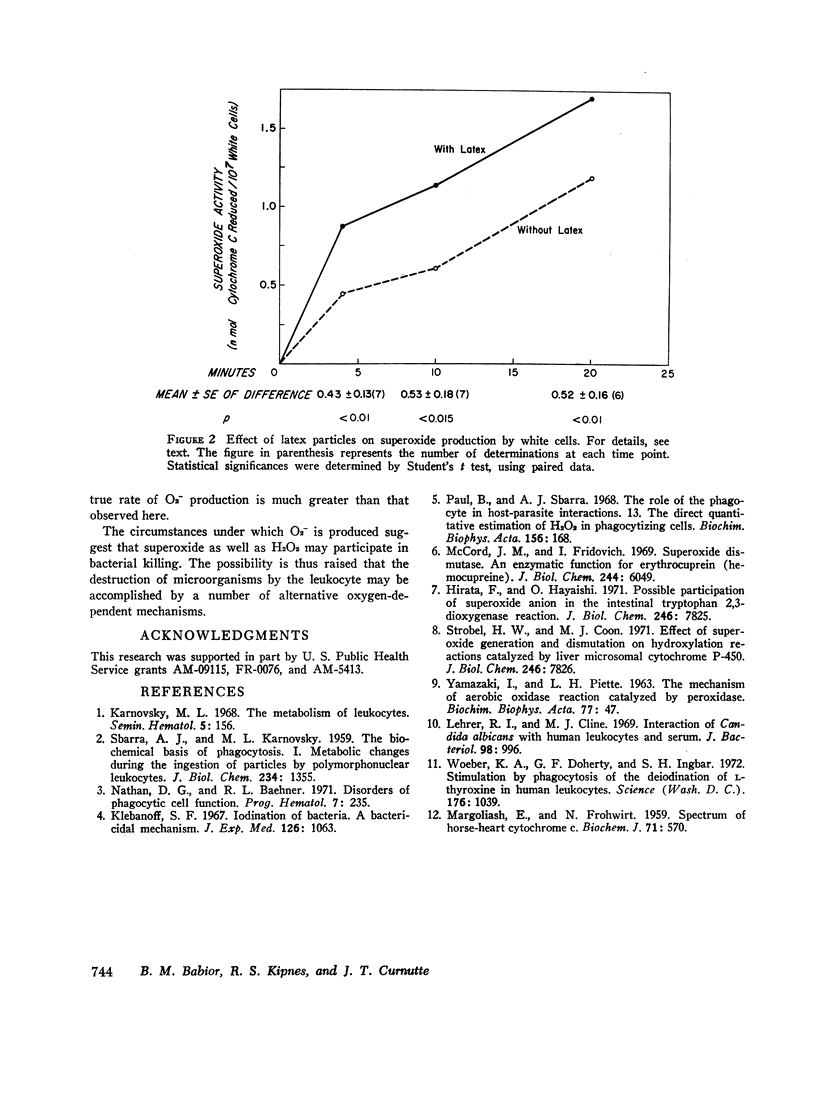
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hirata F., Hayaishi O. Possible participation of superoxide anion in the intestinal tryptophan 2,3-dioxygenase reaction. J Biol Chem. 1971 Dec 25;246(24):7825–7826. [PubMed] [Google Scholar]
- Karnovsky M. L. The metabolism of leukocytes. Semin Hematol. 1968 Apr;5(2):156–165. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Nathan D. G., Baehner R. L. Disorders of phagocytic cell function. Prog Hematol. 1971;7(0):235–254. [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Strobel H. W., Coon M. J. Effect of superoxide generation and dismutation on hydroxylation reactions catalyzed by liver microsomal cytochrome P-450. J Biol Chem. 1971 Dec 25;246(24):7826–7829. [PubMed] [Google Scholar]
- Woeber K. A., Doherty G. F., Ingbar S. H. Stimulation by phagocytosis of the deiodination of L-thyroxine in human leukocytes. Science. 1972 Jun 2;176(4038):1039–1041. doi: 10.1126/science.176.4038.1039. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI I., PIETTE L. H. THE MECHANISM OF AEROBIC OXIDASE REACTION CATALYZED BY PEROXIDASE. Biochim Biophys Acta. 1963 Sep 3;77:47–64. doi: 10.1016/0006-3002(63)90468-2. [DOI] [PubMed] [Google Scholar]


