Abstract
The proinflammatory cytokine IL-18 was investigated for its role in human myocardial function. An ischemia/reperfusion (I/R) model of suprafused human atrial myocardium was used to assess myocardial contractile force. Addition of IL-18 binding protein (IL-18BP), the constitutive inhibitor of IL-18 activity, to the perifusate during and after I/R resulted in improved contractile function after I/R from 35% of control to 76% with IL-18BP. IL-18BP treatment also preserved intracellular tissue creatine kinase levels (by 420%). Steady-state mRNA levels for IL-18 were elevated after I/R, and the concentration of IL-18 in myocardial homogenates was increased (control, 5.8 pg/mg vs. I/R, 26 pg/mg; P < 0.01). Active IL-18 requires cleavage of its precursor form by the IL-1β-converting enzyme (caspase 1); inhibition of caspase 1 also attenuated the depression in contractile force after I/R (from 35% of control to 75.8% in treated atrial muscle; P < 0.01). Because caspase 1 also cleaves the precursor IL-1β, IL-1 receptor blockade was accomplished by using the IL-1 receptor antagonist. IL-1 receptor antagonist added to the perifusate also resulted in a reduction of ischemia-induced contractile dysfunction. These studies demonstrate that endogenous IL-18 and IL-1β play a significant role in I/R-induced human myocardial injury and that inhibition of caspase 1 reduces the processing of endogenous precursors of IL-18 and IL-1β and thereby prevents ischemia-induced myocardial dysfunction.
During ischemia and reperfusion, numerous endogenous mediators, such as small-molecule second messengers, are produced that affect myocardial function. Within minutes of an ischemic episode, myocardial contractile force diminishes, and the overall recovery of contractile force largely depends on the duration of the ischemic period (1). For example, during an ischemic event, Ca+2 homeostasis is perturbed, oxygen-derived free radicals are generated, and nitric oxide (NO) synthesis and release takes place. In addition, there is also local production of cytokines, particularly tumor necrosis factor α (TNF-α) and IL-1β (2). In the intact heart, these cytokines contribute to ischemia-induced myocardial dysfunction by inducing expression of the genes for inducible NO synthase (1), cyclooxygenase 2, and phospholipase A2, as well as vascular adhesion molecules and several chemokines. As a result, there is immediate depression of myocardial contractile force mediated by small-molecule messengers, followed by cytokine-mediated neutrophil infiltration that further damages heart muscle. Animal hearts studied in the absence of blood or blood products elaborate TNF-α (3) and IL-1β during an ischemic challenge. Cardiomyocytes also lose contractile force because of the action of these endogenous cytokines (4).
Most of the experimental data concerning TNF-α- and IL-1β-mediated myocardial dysfunction are derived from animal studies. However, human myocardial tissues obtained from patients undergoing elective cardiopulmonary bypass procedures have been studied under controlled ex vivo conditions (5, 6). In this experimental model, human atrial trabeculae are suspended in a blood-free physiologically oxygenated buffer bath and then exposed to an episode of simulated ischemia. During this time, contractile force decreases dramatically; when the tissue is reexposed to oxygen, the contractile force returns but is diminished (60%–70% reduction) and evidence of myocardial damage is observed by release of creatine kinase (CK) (5, 6). When TNF bioactivity is specifically neutralized during ischemia/reperfusion (I/R), a greater return of contractile force is observed, suggesting that endogenous myocardial TNF activity contributes to the contractile dysfunction induced by the ischemic event (7).
In the present study, we asked whether the cytokine IL-18 contributes to human ischemia-induced myocardial dysfunction. IL-18 is a proinflammatory cytokine structurally and functionally related to IL-1β (8–10). IL-1β and IL-18 are initially synthesized as inactive precursors requiring the IL-1β-converting enzyme (ICE or caspase 1) for cleavage to mature biologically active molecules (11, 12). Although IL-1β and IL-18 have distinct cell surface receptors, the receptor chains for each cytokine are members of the same receptor superfamily (13, 14), and signal transduction is similar (15). For IL-18, however, there is a third receptor-like chain, the IL-18 binding protein (IL-18BP), that has no transmembrane domain (16). IL-18BP is a constitutively produced, secreted, and potent inhibitor of IL-18 activity (16, 17). To assess a role for endogenous IL-18 in the heart, a specific natural inhibitor of IL-18 activity, IL-18BP, was added to the suprafusing bath during I/R.
Materials and Methods
Reagents.
IL-18BPa isoform was expressed with a N-terminal (His)6 tag in Chinese hamster ovary cells and purified to homogeneity (supplied by Interpharm Laboratories, Nes Ziona, Israel). The ability of IL-18BPa-(His)6 to neutralize IL-18 has been described (17). The IL-1 receptor antagonist (IL-1Ra) was supplied by Amgen Biologicals. The ICE inhibitor (ICEi) Ac-Try-Val-Ala-Asp-chloromethylketone (YVAD) was purchased from Alexis Biochemicals (San Diego) and solubililized in DMSO at 10 mg/ml. The ICEi was diluted in Tyrode's solution before being used. On human peripheral blood mononuclear cells, the ICEi reduces endotoxin-induced secretion of mature IL-1β by 92%, as measured by ELISA (Cistron Biotechnology, Pine Brook, NJ).
Isolated Atrial Trabeculae.
Patients undergoing elective coronary artery bypass surgery with a pump oxygenator require insertion of a canula into the right atrium. At that time, a small segment of the right atrial appendage is routinely excised and discarded. Trabeculae were obtained from this discarded tissue. Human atrial tissue was placed in oxygenated modified Tyrode's buffer solution at 4°C. Modified Tyrode's solution was prepared daily with deionized distilled water and contained d-glucose at 5.0 mmol/liter, CaCl2 at 2.0 mmol/liter, NaCl at 118.0 mmol/liter, KCl at 4.0 mmol/liter, MgSO4⋅7H2O at 1.2 mmol/liter, NaHCO3 at 25.0 mmol/liter, and NaH2PO4 at 1.2 mmol/liter. The substrate-free Tyrode's solution contained choline chloride at 7 mmol/liter to maintain osmolarity. Unless otherwise indicated, chemicals and reagents were obtained from Sigma. Two to four trabeculae (4–7 mm long and <1.0 mm in diameter) were attached to a force transducer and immersed in a heated (37°C) 30-ml bath of modified Tyrode's solution; a 92.5% O2/7.5% CO2 mixture was bubbled during normoxia. This gas mixture provided an O2 partial pressure of >350 mmHg (1 mmHg = 133 Pa), a partial pressure of CO2 of 36–40 mmHg, and a pH of 7.35–7.45. Each parameter was checked routinely with an automated blood gas analyzer. The organ bath temperature was maintained at 37°C throughout the experiment. During simulated ischemia, the gas mixture was switched to 92.5% N2/7.5% CO2. This mixture produced an O2 partial pressure of <50 mmHg. The buffer solution was changed every 20 min except during the 30-min period of simulated ischemia.
Experimental Design.
Trabeculae were equilibrated for 90 min to increase the baseline stretch force to 1,000 mg and to allow stabilization of developed force. Trabeculae that failed to generate more than 250 mg of developed force were excluded from the study. During the 90 min of equilibration, pacing was performed with platinum electrodes (Radnoti Glass, Monrovia, CA) for field stimulation. The electrodes were placed on either side of the trabeculae, stimulated (Grass SD9 stimulator, Warwick, RI) with 6-ms pulses at a voltage 20% above threshold, and paced at 1 Hz during normoxia and at 3 Hz during ischemia. Contractions were monitored by force transducers (Grass FT03) and recorded with a computerized preamplifier and digitizer (MacLab Quad Bridge, MacLab/8e, AD Instruments, Milford, MA) and continuously monitored with a Macintosh computer.
After equilibration, trabeculae from a single patient were studied under three experimental conditions: control conditions consisted of 90 min of normoxic suprafusion; I/R consisted of 30 min of simulated ischemia followed by 45 min of reperfusion; and the third condition consisted of an anticytokine intervention. In the latter case, the anticytokine was added to the suprafusion bath just before the onset of ischemia and was present throughout the 45 min of reperfusion.
Preserved Trabecular CK Activity.
End reperfusion tissue (90 min) CK activity was determined as described (18). Tissues were homogenized in 100 vol of ice-cold isotonic extraction buffer (5, 18). The assay was performed with a CK kit (Sigma) by using an automated spectrophotometer. Results are presented as units of CK activity per mg (wet weight of tissue).
RNA Isolation and Reverse Transcription-Coupled PCR.
Fresh trabeculae were homogenized in Tri-Reagent (Molecular Research Center, Cincinnati), and total RNA was isolated with chloroform extraction and isopropanol precipitation. The RNA was solubilized in diethyl-pyrocarbonate-treated water, DNase-treated, and quantitated by using GeneQuant (Amersham Pharmacia Biotech). cDNA methods have been described (19). For each PCR, the following sequence was used: preheat at 95°C for 15 min, then cycles of 94°C for 40 s, 55°C for 45 s, and 72°C for 1 min, with a final extension phase at 72°C for 10 min. The optimal number of cycles was determined as 35. The primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and human IL-18 (19) and for human IL-18BPa (17) have been reported. The PCR products were separated on a 1.5% agarose gel containing 0.5× TBE (50 mM Tris/45 mM boric acid/0.5 mM EDTA, pH 8.3) with ethidium bromide at 0.5 mg/ml, visualized by UV illumination, and photographed. Densitometry was performed on the negative image (IMAGEQUANT software, Molecular Dynamics), and the relative absorbance of the IL-18 and IL-18BP PCR products was corrected against the absorbance obtained for GAPDH.
IL-18 Determinations.
Fresh trabeculae were homogenized as described above for CK measurements. IL-18 was analyzed with liquid-phase electrochemiluminescence (ECL, Igen, Gaithersburg, MD). Mouse anti-human IL-18 mAb (R & D Systems) was labeled with ruthenium (Igen). In addition, affinity-purified goat anti-human IL-18 antibody (R & D) was labeled with biotin (Igen). The biotinylated antibody was diluted to a final concentration of 1 μg/ml in PBS (pH 7.4) containing 0.25% BSA, 0.5% Tween-20, and 0.01% azide (ECL buffer). Per assay tube, 25 μl of the biotinylated antibody was preincubated at room temperature with 25 μl of streptavidin-coated paramagnetic beads (Dynal, Great Neck, NY) at 1 μg/μl for 30 min by vigorous shaking. Samples to be tested (25 μl) or standards were added to tubes followed by 25 μl of ruthenylated antibody (final concentration, 1 μg/μl, diluted in ECL buffer). The tubes were then shaken for 24 h. The reaction was quenched by the addition of PBS at 200 μl per tube and the amount of chemiluminescence was determined with an Origen Analyzer (Igen). The limit of detection for IL-18 is 16 pg/ml.
Confocal Microscopy.
Human atrial tissue obtained during insertion of the canula of the pump oxygenator was placed in a plastic holder of 1 cm (3), embedded, and frozen in tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC) on isopentane cooled with dry ice. Frozen sections (5 μm) were cut on a Leica CM 1850 cryostat (Leica, Deerfield, IL). The slides were fixed for 10 min in 4% paraformaldehyde, air-dried, and incubated for 20 min in PBS supplemented with 10% normal goat serum. Sections were incubated in a 1:100 dilution of rabbit anti-human IL-18 antibody (Peprotech, Rocky Hill, NJ) or nonimmune rabbit IgG at 1 μg/ml as negative control. The antibodies were diluted in PBS containing 1% BSA. After an overnight incubation at 4°C, the sections were washed three times with 0.5% BSA in PBS. The sections were then incubated with a secondary goat anti-rabbit antibody conjugated to Alexa488 (Molecular Probes) for 60 min at room temperature in the dark. Nuclei were stained blue with bisbenzimide (Sigma) at 1 μg/100 ml. After staining, sections were washed and examined with the Leica DM RXA (Leica) confocal laser scanning system and analyzed with SLIDEBOOK software for MacIntosh (Intelligent Imaging Innovations, Denver).
Statisical Analysis.
Data are expressed as the mean ± SEM. Mean changes in developed force were calculated relative to the control value at 90 min for each patient's tissue. Statistical significance of differences between groups were determined by factorial ANOVA with Bonferroni–Dunn post hoc analysis. Statistical analyses were performed with STAT-VIEW 4.51 software (Abacus Concepts, Calabasas, CA).
Results
The Effect of Neutralization of Endogenous IL-18 with IL-18BP on Postischemic Developed Force.
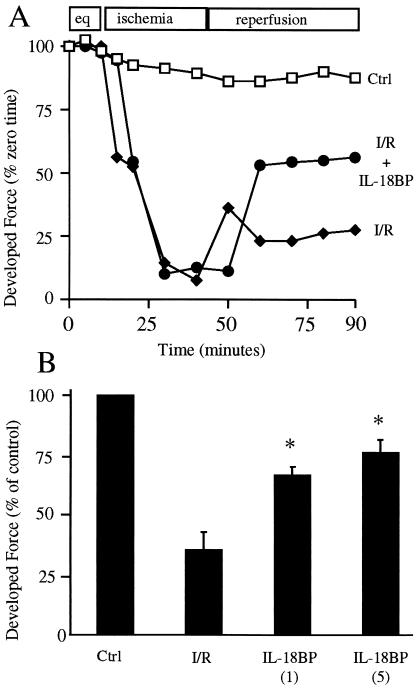
Fig. 1A demonstrates the kinetic response of trabeculae to I/R injury. The final 15 min of equilibration are shown and normalized to 100% at the beginning of the experimental period. Control trabeculae are suprafused under normoxic conditions throughout the experiment. As shown, there is a reduction (10%) in the developed force in the control trabeculae. Trabeculae subjected to ischemia exhibit a rapid decline in contractile function; on reperfusion, contractile force returns to approximately 25% of the control developed force. In contrast, trabeculae exposed to ischemia but in the presence of IL-18BP returned to 55% of the control developed force. To assess the I/R response of heart tissues from several patients, the level of developed force in the control trabeculae at 90 min was set at 100% for each patient's sample, and the relative percent change in developed force for the experimental groups was calculated.
Figure 1.
Effect of IL-18BP on ischemia-induced myocardial contractile dysfunction. (A) Kinetic response to ischemic injury. After equilibration (eq), control (Ctrl) trabeculae were suprafused under normoxic conditions throughout the experiment. Trabeculae were subjected to I/R in the absence or presence of IL-18BP (5 μg/ml) as described in the experimental model. The vertical axis indicates percent of developed force compared with initiation of the experiment (time 0). The data are derived from trabeculae of a single patient and are representative of the methods used to calculate the mean change in developed force at 90 min. (B). Postischemic developed force after neutralization of IL-18 with IL-18BP. Results are expressed as the mean percent change in developed force relative to Ctrl after completion of reperfusion (90 min). Numbers in parentheses indicate IL-18BP in μg/ml (n = 6). *, P < 0.01 compared with I/R.
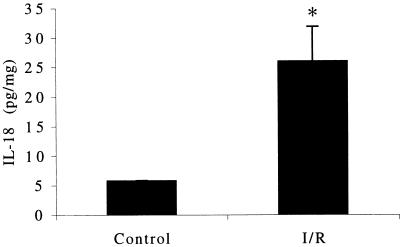
As shown in Fig. 1B, postischemic developed force in untreated trabeculae (I/R) was reduced to a mean of 35% of control. However, in the presence of IL-18BP, this reduction was attenuated to a mean of 66.2% of control at 1 μg/ml and 76% of control at 5 μg/ml, respectively. These results suggest that I/R leads to release of biologically active IL-18 after processing endogenous precursor IL-18 by ICE. Therefore, IL-18 was measured in freshly obtained atrial tissue. As shown in Fig. 2, basal IL-18 was present in trabeculae obtained before the insertion of the of pump-oxygenator canula into the right atrium. After 90 min of equilibration, 30 min of ischemia, and 45 min of reoxygenation, trabeculae were homogenized, and IL-18 levels determined. There was a 4.5-fold increase in IL-18 in the tissue after I/R (Fig. 2).
Figure 2.
Myocardial IL-18 protein content. Trabeculae were homogenized after 90 min of suprafusion under normoxic conditions (Control) or 45 min after 30 min of ischemia. Trabeculae were matched from the same subjects. IL-18 levels are indicated on the vertical axis in pg/ml (n = 4). *, P < 0.01.
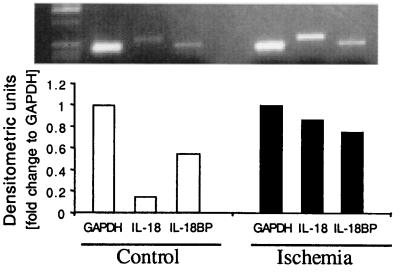
Steady-state mRNA levels for IL-18 and IL-18BP were also determined in these tissues. We observed basal gene expression for IL-18 and IL-18BP in the freshly obtained preischemic atrial homogenates (Fig. 3). Similar to the increase in IL-18 protein, I/R induced a further increase in steady-state IL-18 mRNA levels (4.7-fold increase). IL-18BP gene expression was also observed in freshly obtained atrial tissue and increased only modestly (1.3-fold) after I/R.
Figure 3.
Steady-state IL-18 and IL-18BP mRNA levels in control and ischemic atrial tissue. Tissues obtain after conditions described in Fig. 2 were snap-frozen in liquid nitrogen and kept frozen at −70°C. After homogenization in Tri-Reagent and mRNA isolation, levels of IL-18 and IL-18BP mRNA were determined by reverse transcription-coupled PCR. Data are from one of two subjects evaluated. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Location of IL-18 in Human Myocardium.
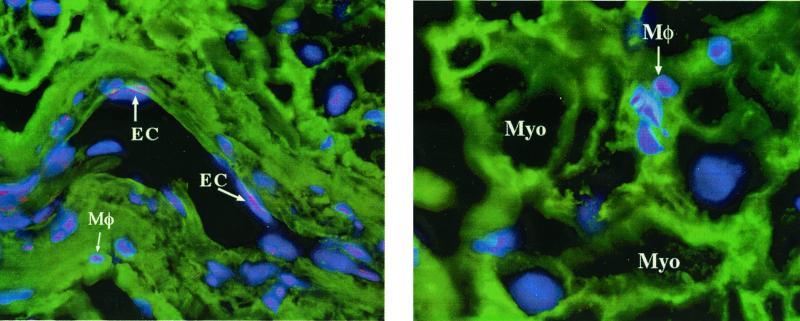
Because IL-18 protein, as measured by ECL, and IL-18 mRNA are present in freshly obtained myocardial homogenates, we used histochemical staining to determine the location of IL-18. Atrial tissues was obtained just before insertion of the pump-oxygenator canula and was immediately snap-frozen. As shown in Fig. 4, IL-18 was observed in resident myocardial macrophages and within the vascular endothelial cells. The IL-18 in macrophages and endothelial cells is present before any operation-related ischemia takes place and is present in the absence of contact with any foreign surfaces. The localization of IL-18 in resident macrophages and endothelial cells is consistent with previous studies of constitutive preformed precursor IL-18 in freshly obtained human peripheral monocytes from healthy subjects (20). Therefore, we conclude that preformed precursor IL-18 exists in the myocardium of patients scheduled for coronary artery bypass for ischemic heart disease.
Figure 4.
Location of IL-18 in human myocardium. Immunohistochemical staining of human atrial tissue before insertion of atrial canula. (Left) Section through an atrial blood vessel. IL-18 is present within vascular endothelial cells (EC) and in resident myocardial macrophages (Mφ) (Right) Section through atrial muscle. Myocytes (Myo) are identified and IL-18 is observed in the resident macrophages. In this staining technique, IL-18 appears pink.
The Effect of ICE Inhibition on Postischemic Developed Force.
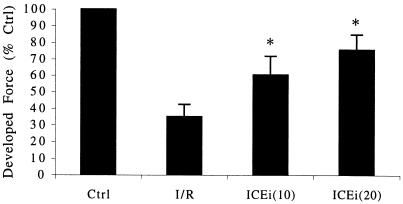
Because IL-18BP effectively attenuated ischemia-induced myocardial dysfunction, we hypothesized that inhibition of the conversion of preformed precursor IL-18 to mature IL-18 would also attenuate ischemia-induced myocardial dysfunction. Therefore, the specific ICE inhibitor YVAD was added to the suprafusion bath before the onset of ischemia. ICE inhibition by the addition of YVAD was continued throughout the ischemic period and during reperfusion. YVAD-mediated inhibition of ICE resulted in attenuation of ischemia-induced myocardial dysfunction, as shown by the improvement in contractile function from 35% of control in I/R to 60% at 10 μg/ml and 75.8% at 20 μg/ml (Fig. 5). These results confirm that biologically active IL-18 in human myocardium is the result of cleavage of preformed precursor IL-18 by ICE. In addition, these results suggest that myocardial ischemia may activate latent ICE.
Figure 5.
Effect of ICE inhibition on postischemic developed force. Results are expressed as the mean percent change in developed force relative to control (Crtl) after I/R. Numbers in parentheses indicate the concentration of ICEi in μg/ml (n = 7). *, P < 0.01 compared with I/R.
The Effect of IL-1Ra on Postischemic Developed Force.
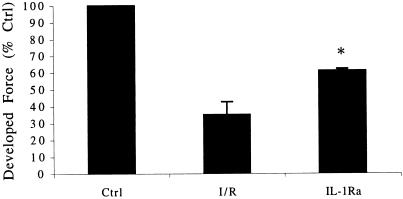
Given that the inhibition of ICE may also reduce processing of endogenous IL-1β, inhibition of IL-1β under saturating concentrations of IL-1Ra will prevent biologically active IL-1β from exerting its effect. As shown in Fig. 6, blockade of IL-1R with IL-1Ra increased the contractile force after I/R from 35% to 61%. Therefore, inhibition of ICE likely exerts its effects via inhibition of the processing of both pro-IL-1β and pro-IL-18.
Figure 6.
Preservation of contractile function after I/R and blockade of IL-1 receptors with IL-1Ra. Results are expressed as the mean percent change in developed force relative to control (Ctrl) after completion of reperfusion. The concentration of IL-1Ra is 20 μg/ml (n = 5). *, P < 0.01 compared with I/R.
Preservation of Cellular Viability.
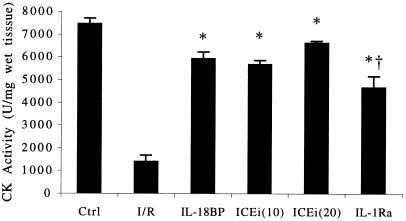
Intracellular levels of CK were used to assess the degree of cellular viability after I/R. In this assay, the higher the CK value, the greater the number of viable cells. Each of the anticytokine interventions resulted in the preservation cellular viability. As demonstrated in Fig. 7, IL-18BP, ICE inhibition (10 and 20 μg/ml), and IL-1Ra increased intracellular CK levels after I/R from 1,399 to, 5,921, 5,675, 6,624, and 4,662 units of CK activity per mg (wet tissue), respectively. These observations suggest that inhibition of I/R-induced activation of IL-18 and IL-1β preserves myocellular viability in this ex vivo model.
Figure 7.
Tissue CK activity after I/R. CK is expressed in units of activity per mg (wet weight of tissue). The experimental conditions are indicated under the horizontal axis. Ctrl and I/R (n = 6); IL-18BP at 5 μg/ml (n = 5); ICEi at 10 and 20 μg/ml (n = 5, each group); IL-1Ra at 20 μg/ml (n = 6). *, P < 0.05 compared with I/R; †, P < 0.05 for ICEi (20) compared with IL-1Ra.
Discussion
Generation of oxygen-derived free radicals, NO, calcium overload, or decreased responsiveness of the myofilaments to calcium may contribute to contractile dysfunction after I/R (1). In addition to these immediate-acting mediators, the relationship of cytokines to myocellular dysfunction after I/R remains unclear. Data from the present study suggest that IL-18 and IL-1β are processed and released from their endogenous precursor forms in human heart tissue during ischemic injury and function to suppress contractile force. Furthermore, the processing of the precursors appears to be ICE-dependent, and latent ICE is likely activated by ischemia. Previously, neutralization of endogenous TNF-α was shown to protect human trabeculae from ischemia-induced dysfunction (6). At present, it is likely that the combination of IL-18, IL-1β, and TNF-α accounts for the ischemia-induced dysfunction.
Oxygen metabolites present after ischemia depress myocardial contractile function in several animal models in vitro and in vivo (1). The source of the oxygen radicals is unclear, although xanthine oxidase may be an important mediator of oxyradical production (21). Oxyradicals may interact with cellular proteins, lipids, calcium, and myofilaments to induce contractile depression. In addition to xanthine oxidase, TNF-α is an inducer of oxygen metabolites. In addition, recent data indicate that IL-18 primes human neutrophils for superanion production (C. Silliman, personal communication).
Ischemia is a direct stress signal to the myocyte and, as a result, gene expression of stress-related molecules is elevated. For example, after 15 min of ischemia in rodent hearts perfused with Kreb's buffer, TNF-α gene expression is up-regulated (2). However, the sudden and marked reduction in atrial trabecular function in the present study is apparent within minutes and it is unlikely that cytokines account for the early dysfunction. During reperfusion, however, the failure to return completely to functionality appears to be cytokine-mediated because specific cytokine blockade or neutralization restores functionality to a greater degree than ischemic controls. Depressed function during reperfusion may be caused by oxygen radical-induced loss of myocyte integrity, increased production of NO, or altered calcium flux. Therefore, do IL-1β and/or IL-18 trigger the above changes? The addition of IL-1β to oxygenated human trabeculae suppresses function (22), and it is known that IL-1β induces NOS in cardiac myocytes (23). However, it is not known whether IL-18 acts similarly.
NO is a myocardial depressant. However, the effect of NO after ischemia is controversial. This controversy stems from the different tissue levels NO present depending on which pathway of NO synthesis is activated. Lower levels of NO resulting from synthesis via the constitutive NO synthase pathway appears to protect the myocardium (24), whereas the NO produced from inducible NO synthase, which is significantly higher, leads to myocardial injury (25). After a moderate ischemic insult, induction of inducible NO synthase occurs in the rat myocardium followed by increased NO production (26). This NO subsequently leads to myocardial contractile depression. Using the same trabeculae model as the present study, Cain et al. (22) demonstrated that specific inhibition of NO synthase attenuated TNF-α- and IL-1β-induced human myocardial dysfunction. As discussed, endogenous TNF-α accounts for some of the postischemic myocardial dysfunction. There are numerous hypotheses on how TNF-α mediates ischemia induced myocardial dysfunction. Finkel et al. (25) demonstrated TNF-α induced contractile dysfunction in isolated hamster papillary muscle. This effect was abolished with inhibition of NO synthase. NO has been demonstrated to play a role in TNF-α-induced myocardial dysfunction via desensitization of the myofilaments to calcium (23). In addition, TNF-α may also lead to phosphorylation of troponin, which further desensitizes the myofilaments to calcium.
Calcium is a vital mediator of myocardial contractile function. Changes in intracellular Ca2+, cellular calcium overload, and modulation of the myofilaments response to Ca2+ affect contractile force. The majority of investigations has focused on the role of calcium as the effector of myocardial contractile dysfunction. The relationship between myocardial calcium changes and myocellular contractile dysfunction has been well described (1). After an I/R injury, the myofilaments responsiveness to calcium decreases and is thought to account for most of the decrease in contractile function after ischemia. In addition to calcium overload, an ischemic insult leads to the production and activation of intracellular calcium-dependent proteases. Upon activation, these proteases begin intracellular myofilament proteolysis leading to postischemic contractile dysfunction. Given the protection afforded by the anticytokine interventions in the present study, it is likely that IL-1β and/or IL-18 alter intracellular calcium homeostasis during and after ischemia.
Although mature IL-1β has been shown to directly suppress function when added to human atrial trabeculae (22), it has not been shown whether endogenous IL-1β in the heart participates in ischemia-induced dysfunction. In the present study, inhibition of IL-1β activity by IL-1 receptor blockade indicates that biologically active endogenous IL-1β is present in the heart after ischemia. Furthermore, the formation of active IL-1β in the ischemic heart is ICE-dependent. The data are consistent with the concept that synthesis of the precursor for IL-1β and activation of ICE takes place during I/R.
The present studies support the concepts that human atrial myocardium is highly sensitive to IL-18 and IL-1β and that the combination of these two cytokines appear to synergistically depress myocardial function. We have demonstrated (22) that the presence of exogenous IL-1β or TNF-α decreases contractile force in human trabeculae in the absence of ischemia. In addition, the combination of these two cytokines have a synergistic effect on the depression of myocardial contractility. Furthermore, we have preliminary data to suggest that exogenous IL-18 under normoxic conditions also depresses myocardial contractile function.
The ability of ICE inhibition to reduce postischemic dysfunction suggests that the processing of precursor IL-1β and IL-18 are necessary for cytokine-mediated myocardial suppression. The immunohistochemical studies revealed that IL-18 is preformed in the resident macrophages and endothelial cells of atrial tissues from patients with ischemic heart disease but it is not clear whether the precursor IL-1β is also preformed. However, IL-1β mRNA is rapidly increased in rat hearts within 15 min after an ischemic insult (2), and therefore it is likely that there is also increased precursor IL-1β synthesis in atrial trabeculae during ischemia. Ischemia itself may be an activator of latent ICE activity in heart tissue. Several investigators have reported that ICE inhibition during myocardial I/R injury in animals reduces apoptotic cell death. The criteria used for determining cell death was DNA fragmentation and cleavage of poly(ADP)-ribose polymerase (27–29). Importantly, the present studies expand these observations by demonstration that ICE inhibition preserves functionality within the injured tissue immediately after I/R. ICE inhibition also preserves cell viability because CK levels remained high in postischemic tissues treated with an ICE inhibitor.
IL-1β and TNF-α have also been implicated in the pathogenesis of human myocardial suppression in sepsis (30, 31). The mechanism(s) by which IL-1β and TNF-α induce contractile dysfunction has also been linked to NO and changes in cellular calcium handling (31). In addition, inhibition of the sphingomyelin signaling pathway abrogated TNF-α/IL-1β-induced myocardial contractile dysfunction (22). Although the present study does not address the role of NO in IL-18-mediated ischemia-induced dysfunction, TNF-α depresses the myocardium in a NO-dependant pathway (6). Blockade of IL-1 receptors revealed a role for endogenous IL-1β in I/R injury, a finding that was not unanticipated given the large amount of animal data. That endogenous IL-18 also plays a role in the injury was unanticipated but based on the fact that IL-18BP only neutralizes mature IL-18 (16, 17). Because ICE inhibition prevents the cleavage of both precursor IL-1β and IL-18, it would not be surprising that IL-18 and IL-1β act synergistically in suppressing myocardial function. In fact, the specific blockade of TNF-α, IL-1β, or IL-18 attenuates, but does not entirely reverse, I/R-induced myocardial dysfunction, suggesting that the three cytokines act together to suppress myocardial function.
The ability to modulate or interrupt cytokine signaling has been demonstrated in numerous disease states in animal models and clinically in humans (e.g., rheumatoid arthritis and Crohn's disease). IL-1β and TNF-α have been associated with acute myocardial dysfunction and hence are logical targets in patients. However, IL-18, a member of the IL-1 superfamily, has to date not been associated with myocardial dysfunction but should also be considered a target for ischemic therapy based on the present studies. IL-1β and IL-18 share numerous properties including the cleavage of the inactive precursor forms to the active forms by ICE (caspase 1). Given the myocardium's response to proinflammatory cytokines and the ability to interrupt the inflammatory process, we hypothesize that the inhibition of caspase 1 protects ischemia-induced human myocardial dysfunction via inhibition of IL-1β and IL-18 processing.
Acknowledgments
We thank Fabia Gamboni-Robertson for the IL-18 immunohistochemical studies, Dr. Ron Pincus (Interpharm, Nes Ziona, Israel) for the recombinant human IL-18BP, and Amgen for IL-1Ra. These studies are supported by National Institutes of Health Grants AI-15614 amd GM-4922.
Abbreviations
- I/R
ischemia/reperfusion
- CK
creatine kinase
- ICE
IL-1β-converting enzyme
- ICEi
ICE inhibitor
- IL-18BP
IL-18 binding protein
- IL-1Ra
IL-1 receptor antagonist
- YVAD
Ac-Try-Val-Ala-Asp-chloromethylketone
- TNF
tumor necrosis factor
References
- 1.Bolli R. Circulation. 1990;82:723–738. doi: 10.1161/01.cir.82.3.723. [DOI] [PubMed] [Google Scholar]
- 2.Herskowitz A, Choi S, Ansari A A, Wesselingh S. Am J Pathol. 1995;146:419–428. [PMC free article] [PubMed] [Google Scholar]
- 3.Meldrum D R, Cleveland J C, Jr, Cain B S, Meng X, Harken A H. Ann Thorac Surg. 1998;65:439–443. doi: 10.1016/s0003-4975(97)01297-6. [DOI] [PubMed] [Google Scholar]
- 4.Gurevitch J, Frolkis I, Yuhas Y, Paz Y, Matsa M, Mohr R, Yakirevich V. J Am Coll Cardiol. 1996;28:247–252. doi: 10.1016/0735-1097(96)00105-2. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland J C J, Meldrum D R, Cain B S, Banerjee A, Harken A H. Circulation. 1997;96:29–32. doi: 10.1161/01.cir.96.1.29. [DOI] [PubMed] [Google Scholar]
- 6.Cain B S, Meldrum D R, Dinarello C A, Meng X, Banerjee A, Harken A H. J Surg Res. 1998;76:117–123. doi: 10.1006/jsre.1998.5304. [DOI] [PubMed] [Google Scholar]
- 7.Cain B S, Meldrum D R, Meng X, Dinarello C A, Shames B D, Banerjee A, Harken A H. J Surg Res. 1999;83:7–12. doi: 10.1006/jsre.1998.5548. [DOI] [PubMed] [Google Scholar]
- 8.Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita K, Torigoe K, Okura T, Fukuda S, et al. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazan J F, Timans J C, Kaselein R A. Nature (London) 1996;379:591. doi: 10.1038/379591a0. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello C A. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 12.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Nature (London) 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 13.Born T L, Thomassen E, Bird T A, Sims J E. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 14.Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikate T, Murakami T, Sanou O, Kojima H, Fuji M, et al. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 15.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, et al. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 16.Novick D, Kim S-H, Fantuzzi G, Reznikov L, Dinarello C A, Rubinstein M. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim S-H, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello C A. Proc Natl Acad Sci USA. 2000;97:1190–1195. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan L J, Blum H, Banerjee A, Whitman G J. J Surg Res. 1993;54:311–315. doi: 10.1006/jsre.1993.1049. [DOI] [PubMed] [Google Scholar]
- 19.Reznikov L L, Kim S H, Westcott J Y, Frishman J, Fantuzzi G, Novick D, Rubinstein M, Dinarello C A. Proc Natl Acad Sci USA. 2000;97:2174–2179. doi: 10.1073/pnas.040582597. . (First Published February 11, 2000; 10.1073/pnas.040582597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puren A J, Fantuzzi G, Dinarello C A. Proc Natl Acad Sci USA. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlat M I, O'Neill P G, Egan J M, Abernethy D R, Michael L H, Myers M L, Roberts R, Bolli R. Am J Physiol. 1987;252:H566–H577. doi: 10.1152/ajpheart.1987.252.3.H566. [DOI] [PubMed] [Google Scholar]
- 22.Cain B S, Meldrum D R, Dinarello C A, Meng X, Joo K S, Banerjee A, Harken A H. Crit Care Med. 1999;27:1309–1318. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Kelly R A, Smith T W. Circulation. 1997;95:778–781. doi: 10.1161/01.cir.95.4.778. [DOI] [PubMed] [Google Scholar]
- 24.Schluter K D, Weber M, Schraven E, Piper H M. Am J Physiol. 1994;267:H1461–H1466. doi: 10.1152/ajpheart.1994.267.4.H1461. [DOI] [PubMed] [Google Scholar]
- 25.Finkel M S, Oddis C V, Jacob T D, Watkins S C, Hattler B G, Simmons R L. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 26.Chandrasekar B, Streitman J E, Colston J T, Freeman G L. Biochim Biophys Acta. 1998;1406:91–106. doi: 10.1016/s0925-4439(97)00062-8. [DOI] [PubMed] [Google Scholar]
- 27.Holly T A, Drincic A, Byun Y, Nakamura S, Harris K, Klocke F J, Cryns V L. J Mol Cell Cardiol. 1999;31:1709–1715. doi: 10.1006/jmcc.1999.1006. [DOI] [PubMed] [Google Scholar]
- 28.Okamura T, Miura T, Takemura G, Fujiwara H, Iwamoto H, Kawamura S, Kimura M, Ikeda Y, Iwatate M, Matsuzaki M. Cardiovasc Res. 2000;45:642–650. doi: 10.1016/s0008-6363(99)00271-0. [DOI] [PubMed] [Google Scholar]
- 29.Piot C A, Martini J F, Bui S K, Wolfe C L. Cardiovasc Res. 1999;44:536–542. doi: 10.1016/s0008-6363(99)00227-8. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo J E. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz R, Panas D L, Catena R, Moncada S, Olley P M, Lopaschuk G D. Br J Pharmacol. 1995;114:27–34. doi: 10.1111/j.1476-5381.1995.tb14901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]