Abstract
Somatostatin (SST) inhibits growth hormone (GH) secretion and regulates multiple processes by signaling through its receptors sst1–5. Differential expression of SST/ssts may contribute to sex-specific GH pattern and fasting-induced GH rise. To further delineate the tissue-specific roles of SST and sst1–5 in these processes, their expression patterns were evaluated in hypothalamus, pituitary, and stomach of male and female mice under fed/fasted conditions in the presence (wild type) or absence (SST-knockout) of endogenous SST. Under fed conditions, hypothalamic/stomach SST/ssts expression did not differ between sexes, whereas male pituitary expressed more SST and sst2A/2B/3/5A/5TMD2/5TMD1 and less sst1, and male pituitary cell cultures were more responsive to SST inhibitory actions on GH release compared with females. This suggests that local pituitary SST/ssts can contribute to the sexually dimorphic pattern of GH release. Fasting (48 h) reduced stomach sst2A/B and hypothalamic SST/sst2A expression in both sexes, whereas it caused a generalized downregulation of pituitary sst subtypes in male and of sst2A only in females. Thus, fasting can reduce SST sensitivity across tissues and SST input to the pituitary, thereby jointly contributing to enhance GH release. In SST-knockout mice, lack of SST differentially altered sst subtype expression levels in both sexes, supporting an important role for SST in sex-dependent control of GH axis. Evaluation of SST, IGF-I, and glucocorticoid effects on hypothalamic and pituitary cell cultures revealed that these hormones could directly account for alterations in sst2/5 expression in the physiological states examined. Taken together, these results indicate that changes in SST output and sensitivity can contribute critically to precisely define, in a tissue-dependent manner, the sex-specific metabolic regulation of the GH axis.
Keywords: somatostatin receptors, fasting, somatostatin-knockout mice, pituitary, hypothalamus, stomach
somatostatin (SST) is a cyclic tetradecaneuropeptide that was initially isolated from hypothalamus (HPT) based on its capacity to inhibit growth hormone (GH) secretion from pituitary (PIT) somatotropes (6). SST has also been shown to be expressed in peripheral tissues, where the gastrointestinal tract (GIT) is the primary source of circulating SST (13). SST exerts pleiotropic effects, including modulation of neurotransmission, metabolism, and immune function, as well as inhibition of endocrine and exocrine secretion (13, 34, 49). SST exerts these effects through a family of five G protein-coupled receptor subtypes with seven transmembrane domains (TMD), termed sst1–5, which are encoded by separate genes (39, 49, 54). In addition, functional splice variants of ssts have been identified with seven TMD (39, 43, 65) or fewer, including the truncated sst5 variants recently identified by our group in humans (sst5TMD5 and sst5TMD4) (16) and in mice (sst5TMD4, sst5TMD2, and sst5TMD1) (12). Like SST, sst subtypes are expressed throughout the body, including the brain, PIT, and GIT, where the relative abundance of sst subtypes as well as SST is dependent on the species, tissue, sex, and nutritional state analyzed (34, 39, 49).
Experimental studies in humans, rodents, and other mammals have demonstrated that GH is released in a pulsatile manner, which is clearly different between sexes (19, 23, 40, 66). Specifically, under normal (fed) conditions, GH release in males tends to be highly organized, with high amplitude pulses and low baseline values. In contrast, GH release from females is more disorganized, with increased pulse frequency and elevated baseline values. Once secreted into the bloodstream, it is also clear that the sexually dimorphic pattern of GH exerts sex-specific effects on structural growth as well as liver function (68). Based on data generated mainly from rats, it has been hypothesized that one of the mechanisms responsible for elevated baseline GH release in females could be a reduction in SST output from its main tissue sources (HPT and GIT) (1, 10, 34, 40, 41, 44, 50, 62). This is supported by the observation that SST-knockout male mice display a feminized pattern of ultradian GH rhythm with an increase in interpulse GH release (28), which is associated with a more feminine pattern of hepatic gene expression (38). GH is also elevated in male and female SST-knockout mice, and in females, PIT expression of the main stimulatory receptors involved in GH secretion [GH-releasing hormone receptor (GHRH-R) and ghrelin receptor (GHS-R)] (30) is elevated. Interestingly, enhanced GH output in the absence of endogenous SST led to an increase in IGF-I only in females (30). Although SST is clearly important in maintaining the sexually dimorphic pattern of GH release and action, the relative contribution of tissue-specific SST/ssts expression remains to be clarified.
Changes in SST/ssts may also be involved in the fasting-induced rise in GH. However, the effect of fasting on circulating GH levels has been reported to be species dependent. Specifically, whereas fasting suppresses GH pulse release in male rats (34, 64), it enhances GH release in the majority of mammalian species studied to date, including humans and mice (22, 34–36). It has been suggested that the fasting-induced rise in GH could be due in part to downregulation of SST/sst signaling (34–36, 47, 52, 59, 60, 63), since reductions in hypothalamic SST and pituitary and hypothalamic sst subtype expression (mainly sst2 and sst5) have been observed in rats and mice (34–36). The apparent role that SST plays in the sexually dimorphic pattern of GH release, coupled with data suggesting that changes in SST/sst expression contribute to the fasting-induced GH release, led us to conduct the current study with the aim of further delineating the tissue-specific roles of SST/sst in these processes by evaluating the expression pattern of SST and sst subtypes and variants (sst1, -2A, -2B, -3, -4, -5, -5TMD4, -5TMD2, and -5TMD1) in the HPT, PIT, and GIT of male and female mice under fed and fasted conditions in the presence or absence of endogenous SST [wild type, (SST+/+) vs. SST-knockout (SST−/−)]. In addition, a mouse HPT cell line, N6 (3), and primary PIT cell cultures from male and female mice were used to ascertain whether SST can regulate the expression of its own receptors in this specific context.
MATERIALS AND METHODS
In vivo animal models.
All experimental procedures were approved by the Animal Care and Use Committees of the University of Cordoba and University of Illinois at Chicago. Male and female C57Bl/6J mice were purchased from Charles River Laboratories (Barcelona, Spain) or Jackson Laboratory (Bar Harbor, ME) at 8 wk of age. SST-knockout mice in a C57Bl/6 background were generously provided by Dr. Ute Hochgeschwender (Oklahoma Medical Research Foundation, Oklahoma City, OK) (71), and these mice were bred to C57Bl/6J mice to establish an experimental colony, as reported previously (30, 31). Mice were housed under standard conditions of light (12:12-h light-dark cycle) and temperature (22–24°C), with free access to tap water and food (standard rodent chow; LabDiet, St. Louis, MO). Mice were handled daily at least 1 wk prior to euthanasia to acclimate them to personnel and handling procedures and were euthanized by decapitation, without anesthesia, under fed conditions unless otherwise specified. At 9–11 wk of age, male and female mice were weighed, and food was withdrawn (0800–0900) from a subset of mice, whereas the remaining mice received food ad libitum (n = 4–10 mice/genotype/treatment group). Forty-eight hours later, mice were weighed and euthanized by decapitation without anesthesia. Blood and tissues (stomachs, PIT and HPT) were immediately collected, processed, frozen in liquid nitrogen and stored at −80°C for further analysis, as described below.
Assessment of circulating hormones.
Trunk blood was immediately mixed with MiniProtease inhibitor (Roche, Nutley, NJ) and placed on ice and centrifuged, and plasma was stored at −80°C until analysis of GH (mouse/rat ELISA; Diagnostic System Laboratories, Webster, TX), IGF-I (IGF-I 100T RIA kit; Nichols Institute Diagnostic, San Clemente, CA), and corticosterone (Immunodiagnostic Systems, Fountain Hills, AZ) levels.
In vitro cell models.
To investigate whether expression of PIT and HPT sst subtypes could be directly regulated by SST, IGF-I, and glucocorticoids, we used primary PIT cell cultures and the HPT N6 cell line (3). Briefly, PITs of adult male and female C57Bl/6J mice (10 wk of age) were dispersed into single cells and cultured in serum containing α-medium (Invitrogen, Barcelona, Spain), as described previously (12, 29, 31). N6 cells were cultured in monolayer in serum containing α-medium, as described previously (32). After 24 h of culture (200,000 cells/well, 24-well plates, maintained at 37°C in an atmosphere of 5% CO2), medium was removed and cells were preincubated in serum-free medium for 1–2 h, and subsequently the medium was replaced with serum-free medium alone (controls) or containing SST-14 (100 nM), IGF-I (10 nM), or dexamethasone (a synthetic glucocorticoid, 10 nM) for an additional 24 h (3–5 wells/treatment/experiment). Then, total RNA was extracted and reverse-transcribed for determination of mRNA levels by quantitative real-time (qrt)RT-PCR (see below).
RNA isolation, reverse transcription, and gene expression analysis by qrtRT-PCR.
Tissues and pituitary cell cultures were processed for recovery of total RNA using the Absolutely RNA RT-PCR Miniprep Kit (Stratagene, La Jolla, CA) with deoxyribonuclease treatment. The amount of RNA recovered was determined using the Ribogreen RNA Quantification Kit (Molecular Probes, Eugene, OR). Total RNA (1 μg for whole tissues and 0.15 μg for pituitary cell cultures) was reverse-transcribed using random hexamer primers with enzyme and buffers supplied in the cDNA First Strand Synthesis kit (MRI Fermentas, Hanover, MD). cDNA was treated with ribonuclease H, and duplicate aliquots (1 μl) were amplified by qrtRT-PCR, where samples were run against synthetic standards to estimate mRNA copy number. Details regarding the development, validation, and application of a qrtRT-PCR to measure expression levels of mouse transcripts have been reported previously (12, 29, 30). Briefly, thermocycling and fluorescence detection were performed using a Stratagene Mx3000p real-time PCR machine. The final volume of the PCR reaction was 25 μl, including 50 ng of sample, 12.5 μl of brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA), 0.375 μl of each primer (10 μM stock solution), and 10.375 μl of dH2O. Thermal cycling profile consisted of a preincubation step at 95°C for 10 min, followed by 40 cycles of denaturation (95°C, 30 s), annealing (61°C, 1 min), and extension (72°C, 30 s). Final PCR products were subjected to graded temperature-dependent dissociation to verify that only one product was amplified. To determine the starting copy number of cDNA, RT samples were PCR amplified, and the signal was compared with that of standard curve run on the same plate. Standard curves consisted of 1, 101, 102, 103, 104, 105, and 106 copies of synthetic cDNA template for each of the transcripts of interest. Standard curves were generated by the Stratagene Mx3000p Software, and the slope of a standard curve for each template examined was between −3.31 and −3.35 (r2 of the standard curve between 0.997 and 1.002), indicating that the efficiency of amplification was close to 100%, meaning that all templates in each cycle were copied. Specific sets of primers used in this study to measure expression levels of GH, SST, and all sst isoforms/variants (sst1, sst2A, sst2B, sst3, sst4, sst5, sst5TMD4, sst5TMD2, and sst5TMD1) are shown in Supplemental Table S1 (Supplemental Material for this article is available at the AJP-Endocrinology and Metabolism web site). To control for variations in the amount of RNA used in the RT reaction and the efficiency of the RT reaction, mRNA copy number of the transcript of interest was adjusted by the mRNA copy number of cyclophilin A (used as housekeeping gene), where cyclophilin A mRNA levels did not significantly vary between experimental groups, within tissue type (data not shown).
Statistical analysis.
Raw data were evaluated for heterogeneity of variance, and where found, values were log-transformed. Samples from all groups within an experiment were processed at the same time; therefore, the in vivo effects of sex/genotype/fasting and the in vitro effects of SST, IGF-I, and dexamethasone were assessed by one- or two-way ANOVA followed by a Newman-Keuls test for multiple comparisons or by Student's t-test, as appropriate. P < 0.05 was considered significant. All data are expressed as means ± SE. The in vivo effects of sex/genotype/fasting were obtained from a minimum of four animals per group. Results from in vitro studies were obtained from at least three separate independent experiments carried out on different days and with different cell preparation. All statistical analyses were performed using the GB-STAT software package (Dynamic Microsystems, Silver Spring, MD).
RESULTS AND DISCUSSION
Relationship between tissue-specific expression of SST and sst subtypes on pituitary GH synthesis and release in male and female mice.
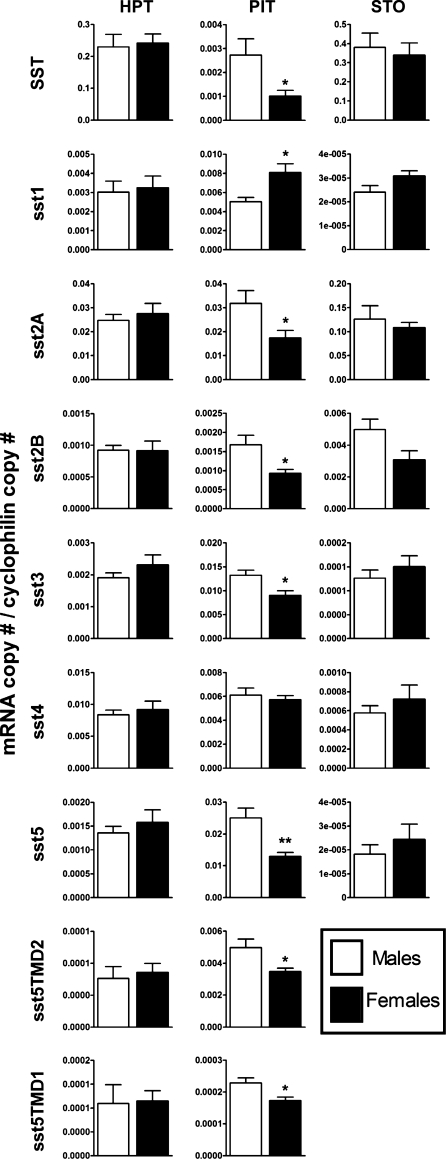
As reviewed previously, one of the factors involved in the sexually dimorphic pattern of GH release could be SST/ssts system (1, 5, 10, 19, 23, 34, 40, 41, 44, 50, 61, 62, 66). To explore the tissue-specific contribution of this system in the sexually dimorphic pattern of GH release, the expression levels of HPT and stomach SST and sst subtypes were compared between male and female mice under fed conditions. The absolute mRNA levels for each transcript are shown in Table 1. As reported previously (29, 51), SST is highly expressed in the stomach and HPT of mice (Table 1), whereas the relative expression of the sst subtypes varied between tissues. Specifically, in the HPT, sst2A > sst4 ≥ sst1 > sst3 > sst5 ≥ sst2B > > sst5TMD1 = sst5TMD2 (mRNA levels of sst5TMD4 were not detected; Table 1), whereas in stomach, sst2A > > sst2B > sst4 > > sst3 > sst1 = sst5 (mRNA levels of all truncated sst5 variants were not detected; Table 1). Although expression patterns/levels varied broadly between HPT and stomach, there were no differences in the expression level of each transcript between sexes (Fig. 1), a finding that lessens the potential role of SST/sst in these tissues in the sexually dimorphic pattern of GH release. Nevertheless, the possibility that our measurement of whole hypothalamic and stomach extracts may have masked changes in SST/sst expression in specific HPT nuclei (periventricular, paraventricular, arcuate) (34, 37, 67) or GIT regions and cell types (13) should not be excluded. In fact, cell-specific regulation of SST expression is supported by the observation of some (11, 20, 28, 41, 42) but not all authors (5, 26) showing that female rats and mice have lower immunodetectable SST in specific hypothalamic regions (i.e., median eminence, periventricular nucleus) compared with males, where these changes are associated with a reduction in SST mRNA levels in the periventricular nucleus but not in the arcuate nucleus. Also, Zhang et al. (72) observed that sst1 mRNA-expressing cells were two- to threefold greater in the arcuate, but not in the ventromedial, nucleus of the HPT in males compared with female rats. Thus, future (neuro)anatomic studies in these animal models may help to unequivocally ascertain this apparent lack of sex-related differences in SST/ssts at the HPT and stomach levels.
Table 1.
Absolute cDNA copy number/0.05 μg total RNA of SST and all sst transcripts in different tissues (hypothalamus, pituitary, and stomach) of male and female mice as determined by quantitative real-time RT-PCR
| Hypothalamus |
Pituitary |
Stomach |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| SST | 133,150 ± 20259 | 113,784 ± 14,472 | 496 ± 132 | 140 ± 43* | 55,687 ± 7,551 | 39,896 ± 5,513 |
| sst1 | 1,965 ± 283 | 2,146 ± 313 | 1,025 ± 102 | 1,831 ± 306* | 5 ± 0 | 6 ± 0 |
| sst2A | 12,931 ± 2156 | 12,097 ± 1,525 | 5,906 ± 1,250 | 2,782 ± 620* | 16,407 ± 289 | 14,160 ± 2,128 |
| sst2B | 334 ± 34 | 347 ± 38 | 249 ± 39 | 116 ± 23* | 1,010 ± 139 | 559 ± 92 |
| sst3 | 1,090 ± 107 | 950 ± 99 | 2,422 ± 234 | 1,384 ± 239* | 26 ± 3 | 30 ± 7 |
| sst4 | 5,392 ± 645 | 3,529 ± 695 | 1,195 ± 103 | 802 ± 133 | 116 ± 14 | 130 ± 21 |
| sst5 | 508 ± 47 | 539 ± 64 | 3,300 ± 508 | 1,732 ± 305* | 4 ± 1 | 4 ± 1 |
| sst5TMD4 | ND | ND | ND | ND | ND | NM |
| sst5TMD2 | 37 ± 1 | 38 ± 2 | 860 ± 58 | 548 ± 41* | ND | NM |
| sst5TMD1 | 45 ± 7 | 51 ± 9 | 40 ± 2 | 27 ± 1* | ND | NM |
Values represent means ± SE of the mRNA copy number of each transcript (n = 4-9 mice/sex/tissue). SST, somatostatin; sst, SST receptor isoform/variant; ND, not detected; NM, not measured.
P < 0.05, values that differ within sex.
Fig. 1.
Somatostatin (SST) and SST receptor isoform/variant (sst) expression in hypothalamus (HPT), pituitary (PIT), and stomach (STO) of fed male (open bars) and female (filled bars) wild-type C57Bl6/J mice as assessed by quantitative real-time (qrt)RT-PCR. Values represent means ± SE (n = 4–9 mice/sex/tissue) of absolute mRNA copy numbers (adjusted by cyclophilin mRNA copy number). Symbols (*P < 0.05, **P < 0.01) indicate values that differ within sex.
However, in addition to SST produced in the HPT and stomach, our laboratory and others have shown that SST mRNA is present in the PIT (14, 28, 30, 51), suggesting that local production of SST could also play a relevant role in controling somatotrope function. In fact, our present results show that PITs of females express less SST compared with male mice (496 ± 132 copies in males vs. 140 ± 43 copies/0.05 μg total RNA in females, P = 0.026; Table 1 and Fig. 1), which is consistent with a previous report showing similar results using nonquantitative RT-PCR methods (28). The relative expression levels of the PIT sst subtypes differed from that observed in the HPT and stomach, with sst2A ≥ sst5 ≥ sst3 = sst1 = sst4 > sst5TMD2 > > sst2B > > > sst5TMD1 (mRNA levels of sst5TMD4 were not detected; Table 1), in both males and females. In this case, comparison of the levels of expression for each transcript between sexes revealed a marked divergence, since female PIT expressed lower levels of mRNA for the primary sst subtypes mediating the actions of SST on GH release, sst2A, and sst5 (34) as well as other sst isoforms/variants (sst2B, sst3, sst5TMD2, and sst5TMD1; Fig. 1). These results are consistent with reports on rats (24, 72) showing that the female PIT expresses less sst2 and sst5 compared with male PIT. Although the majority of sst subtypes were lower in the female PIT, the expression of sst1 was greater compared with males, as reported previously by others (55) but not observed by all (72). It remains to be determined what factors are responsible for these sex-dependent differences in SST/sst expression in the PIT. The limited data available demonstrate that direct application of estrogen to female rat PIT cell cultures increases sst2A/B and sst3 mRNA levels, whereas it decreases sst1 expression (15). It should be noted that these changes are opposite to that observed between males and females in mice (present study) and rats (24, 72), suggesting that either estrogen is not directly responsible for these differences or the timing or dose of estrogen used did not appropriately mimic the actions of estrogens in vivo. There is also a possibility that nonsteroidal gonadal factors may play a role in these differences, as shown previously for sex-dependent difference in hypothalamic expression of SST and GHRH in rats (27).
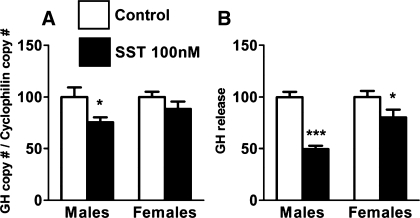
In the current study, the overall reduction in the expression of most sst subtypes, where sst2 and sst5 copy numbers alone account for ∼50% of total sst transcripts (Table 1), supports the hypothesis that the female PIT would be far less responsive to the inhibitory actions of SST. However, it could be argued that these differences are due to the differential contribution of the various PIT cell types in female vs. male PIT (4). To circumvent this problem, we tested the impact of SST on GH synthesis and release from primary PIT cultures prepared from female and male mice using a single dose of SST (100 nM). This dose has previously been demonstrated to exert a maximal inhibitory effect on GH synthesis and/or release in other species [i.e., rat, pigs, primate and human (34, 48, 56)]. Specifically, we found that female mice were indeed significantly less sensitive to the inhibitory actions of SST compared with males (Fig. 2), a response similar to that observed in primary PIT cultures prepared from rats (44, 53). However, future studies using different doses of SST would need to be performed to unequivocally ascertain whether the observed differences between sexes are related to a change in sensitivity (ED50) and/or in the number of receptors (Bmax).
Fig. 2.
Effect of 24-h treatment of SST (100 nM; filled bars) on growth hormone (GH) expression (A) and release (B) in primary PIT cell cultures from male and female mice. GH mRNA copy numbers were determined by qrtRT-PCR, and the values were adjusted by cyclophilin A copy number as an internal control, whereas GH release levels were determined by commercial ELISA. Values represent the mean ± SE of 3 independent experiments (3–5 wells/treatment/experiment) and are expressed as percentage of vehicle-treated controls (set at 100%; open bars) within experiment. Symbols (*P < 0.05, ***P < 0.001) indicate values that differ from vehicle-treated controls.
To our knowledge, this is the first report simultaneously comparing by absolute quantitative methods (qrtRT-PCR) the expression levels (copy number) of SST, as well as all sst isoforms/variants, between male and female mice in key target tissues [central (HPT) and peripheral (PIT and stomach)] for SST actions. The fact that, under normal-fed conditions, 1) no changes in HPT/stomach SST or sst subtype levels were observed between fed male and female mice, 2) male PIT expressed more SST and sst2A/2B/3/5A/5TMD2/5TMD1 and less sst1 compared with PIT of females, and 3) in vitro somatotropes from male mice are more sensitive to the inhibitory actions of SST on GH synthesis and release, compared with female somatotropes, suggests that local PIT alterations of the SST/sst may directly and relevantly contribute to the well-known sexually dimorphic pattern of GH release observed in most mammalian species. However, it should be noted that we do not know whether the changes observed in SST/sst subtype mRNA levels are translated into changes in functional protein levels. This would require species-specific and highly sensitive antibodies for each of the mouse sst subtypes, where such reagents are currently unavailable.
Role of SST and sst subtypes in the fasting-induced rise in circulating GH levels in males and females.
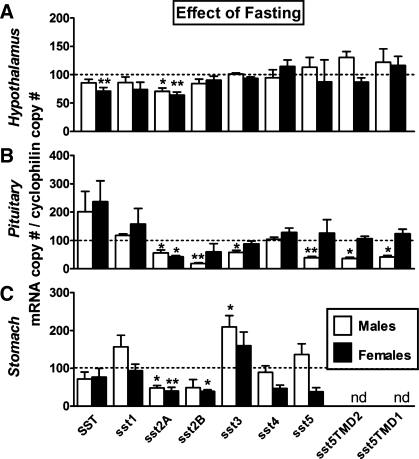
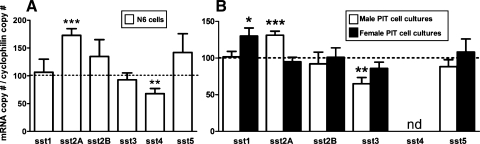
Fasting has been shown to suppress HPT SST mRNA in male mice and ewes (21, 29, 46), consistent with the current observations showing an overall reduction in HPT SST mRNA, which reached significance in females (Fig. 3A). In addition, fasting suppressed HPT sst2A expression in males and females, whereas the expression levels of the other sst subtypes were not significantly altered (Fig. 3A). Since sst2A is the major sst isoform within the HPT (Table 1), these results indicate that the rise in GH levels observed in fasting is associated not only with a reduction in SST expression but also with a downregulation of its central signaling. Evidence supporting an inhibitory role of sst2A signaling in GH-negative feedback has been provided by a study showing that MK-0677 (a GHS-R agonist) stimulated c-fos expression in HPT, and this response could be inhibited by GH pretreatment in intact but not in sst2A-null mice (55). Furthermore, since SST directly stimulated sst2A expression in a HPT cell line (N6; see Fig. 6A), it is not unreasonable to suggest that the specific changes in sst2A expression in response to fasting observed herein could be related, at least in part, to a reduction in local production of SST. In addition, the fall in circulating IGF-I and/or the rise in circulating glucocorticoids that occurs with fasting (33) may contribute to the downregulation of sst2A expression observed in fasting in that IGF-I increased whereas dexamethasone decreased sst2A expression but did not modify sst2B mRNA levels in mouse HPT N6 cell cultures (Supplemental Fig. S1A).
Fig. 3.
Effect of fasting on SST and sst in HPT (A), PIT (B), and STO (C) of male (open bars) and female (filled bars) mice. mRNA copy numbers were assessed by qrtRT-PCR. Values are shown as relative percentage of male or female fed control mice (shown by the dotted line set at 100%) and represent the mean ± SE of 4–9 mice/sex/tissue. Symbols (*P < 0.05, **P < 0.01) indicate values that significantly differ from fed controls within sex.
Fig. 6.
Effect of 24-h treatment of SST (100 nM) on sst subtype expression in mouse hypothalamic N6 cells (A) and in primary PIT cell cultures (B) from male (open bars) and female (filled bars) mice. mRNA copy numbers were determined by qrtRT-PCR, and the values were adjusted by cyclophilin A copy number as an internal control. Values represent the mean ± SE of 3–4 independent experiments (3–5 wells/treatment/experiment) and are expressed as percentage of vehicle-treated controls [shown by the dotted line set at 100% in N6 cells (A) and in male and female mice (B)] within experiment. Symbols (*P < 0.05, **P < 0.01, ***P < 0.001) indicate values that differ from the corresponding vehicle-treated controls.
Fasting did not significantly alter stomach SST expression in male or female mice (Fig. 3C), which is consistent with a previous observation showing that SST mRNA levels were not changed in fasted rats (73). However, fasting downregulated the expression levels of the dominant sst subtypes sst2A and sst2B in both sexes, whereas sst3 mRNA levels were significantly increased only in females. These changes in stomach sst subtype expression may be indirectly involved in the rise in GH observed with fasting by promoting the production of the GH-releasing peptide ghrelin, which is produced primarily by the stomach (9, 25). This hypothesis is supported by 1) the observation that SST and cortistatin (a peptide sharing high structural and functional similarities to SST), as well as octreotide (a sst2A preferring agonist), can downregulate circulating ghrelin levels in fed and fasted humans (2, 7) and rats (57) and 2) our data showing that circulating ghrelin levels are elevated in male (29) and female (data not shown) SST-knockout mice.
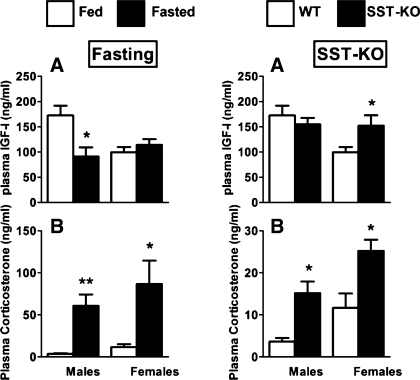
At the level of the PIT, fasting did not significantly alter SST expression in male or female mice (Fig. 3B). However, fasting decreased PIT sst2A/2B/3/5/5TMD2/5TMD1 expression in males, whereas only sst2A mRNA levels were suppressed in females (Fig. 3B), a differential response that may be related to the already reduced PIT expression of sst subtypes in females compared with males (Fig. 1). Nonetheless, given that sst2A is the predominant sst subtype in the PIT and is clearly linked to the inhibitory effect of SST on GH release (34), downregulation of PIT sst2A, coupled with reduced HPT SST input, could represent a primary mechanism by which GH levels increase in response to fasting. The fact that fasting-induced downregulation in PIT sst2/sst5 expression has been observed in male rats (8, 34, 47) and nutrient deprivation has been shown to decrease the GH response to exogenous SST administration in rats, dogs, and humans (33, 52, 59, 60, 63) suggests that the fasting-induced changes in PIT responsiveness to SST is preserved across species. The mechanism by which these changes occur may be directly related to the well-characterized reduction in circulating IGF-I and rise in glucocorticoids observed with fasting (Ref. 36 and Fig. 4, left) since IGF-I can directly upregulate the expression of sst2A (Supplemental Fig. S1B) as well as sst1, sst2B, and sst5 (36) in primary PIT cell cultures from male mice, whereas glucocorticoids directly inhibit sst2A expression (Supplemental Fig. S1B). The role of glucocorticoids is further supported by reports showing that glucocorticoids also decrease PIT sst2 mRNA levels in rats (45, 70) and the presence of consensus glucocorticoid response elements in the promoter of the mouse sst2 gene [as reviewed by Park et al. (45)]. Nevertheless, the actual contribution of systemic IGF-I input in modulating PIT sensitivity to SST in fasted female mice is lessened by the observation that fasting did not result in a significant reduction in total IGF-I levels in females (Fig. 4A, left), although we should not discard the possibility that changes in free (available) IGF-I levels may have occurred, based on studies in humans showing that short-term fasting is characterized by a decrease in bioavailable “free” IGF-I attributed in part to a rise in circulating IGF-binding protein-1 (17).
Fig. 4.
Circulating IGF-I and corticosterone levels of fed (open bars) or fasted (filled bars) (A and B, respectively, left) and SST-intact (open bars) and SST-knockout (SST-KO; filled bars) (A and B, respectively, right) male and female mice. IGF-I and corticosterone values are represented as means ± SE (ng/ml; n = 4–10 mice/treatment-genotype/sex) and were determined by commercial RIA or ELISA kits, respectively. Symbols (*P < 0.05, **P < 0.01) indicate differences between fed and fasted mice or between SST-intact and SST-KO mice within sex.
Role of endogenous SST in regulating its own receptor expression in male and female mice: relationship with circulating GH levels.
Use of SST-knockout mouse models has enabled our laboratory and others to show that SST is required to suppress GH release in males and females and that these actions are critical to preserve the sex-dependent pattern of GH release and its subsequent actions (28, 30). Furthermore, we also discovered that loss of endogenous SST (SST-knockout) is more critical in female than in male mice in suppressing PIT expression of GH, GHRH-R, and GHS-R, resulting in a significant increase in circulating IGF-I levels and a reduction in HPT expression of GHRH, the latter likely due to enhanced negative feedback by GH/IGF-I (19, 36, 40). These sex-dependent differences in the response to SST loss may be related to the fact that in males, but not females, HPT cortistatin is increased, which could in part compensate for SST loss (30). In addition, an upregulation in PIT ghrelin expression as well as GHS-R expression found in female SST-knockout (Supplemental Fig. S2) may also contribute to the enhanced sensitivity of the female SST-knockout GH axis to SST loss, since we have reported recently that locally produced PIT ghrelin could directly participate in the regulation of PIT function independent of circulating ghrelin levels produced by the stomach, thereby acting as a positive ultrashort feedback loop to enhance or facilitate GH release (18). Perhaps some of these or other similar mechanisms are in place in humans in that GH release is more dramatic in women, compared with men, after arginine-mediated SST repression alone or combined with a ghrelin agonist (58, 69).
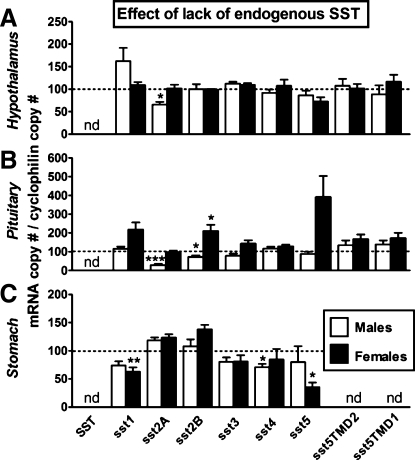
In the current study, we have extended these observations by examining the impact of SST-knockout on tissue-specific expression of sst subtypes in male and female mice. In general, the lack of SST had little impact on HPT sst subtype mRNA levels (Fig. 5), where only the expression of sst2A was significantly downregulated in SST-knockout males. Lack of SST may be directly responsible for the decrease in sst2A expression because, as mentioned above, SST increases sst2A mRNA levels in HPT N6 cells (Fig. 6A); but this alone is unlikely to fully account for the sex differences observed. In fact, using the same in vitro model system, we found that dexamethasone inhibits whereas IGF-I stimulates sst2A mRNA (Supplemental Fig. S1A). Since corticosterone levels are elevated in both male and female SST-knockout mice, whereas IGF-I is enhanced only in females (Fig. 4), we might speculate that the male-specific reduction in PIT sst2A expression could in part be due to the direct inhibitory effect of glucocorticoids unopposed by changes in IGF-I.
Fig. 5.
SST and sst expression in HPT (A), PIT (B), and STO (C) of male and female wild-type (SST-intact control mice shown by the dotted line set at 100%) compared with male (open bars) and female (filled bars) SST-KO mice, as assessed by qrtRT-PCR. Values represent the mean ± SE of 4–9 mice/sex/tissue. Symbols (*P < 0.05, **P < 0.01, ***P < 0.001) indicate values that significantly differ from SST-intact within sex. nd, Not detected.
In contrast to the HPT, lack of endogenous SST was associated with a decrease in stomach sst4 in males and a decrease in sst1 and sst5 mRNA levels in females (Fig. 5C). Although the expression levels of sst1, sst4, and sst5 in the stomach are low compared with sst2A (Table 1), we cannot discount the possibility that fewer sst subtypes would minimize the inhibitory impact of cortistatin (which is expressed in the GIT and is intact in SST-knockout mice) on ghrelin release (7).
At the level of the PIT, we observed that sst2A and sst2B were downregulated in males, whereas sst1, sst2B, and sst5 were upregulated in female SST-knockout mice. These results, taken together with previous reports (28, 30), reinforce the notion that SST is an important player in the sex-dependent differences in GH axis function (34). Downregulation of sst2 isoforms in male PIT may be directly mediated by loss of SST, reduction in IGF-I, and elevation of glucocorticoids, similar to that discussed for HPT sst2 regulation, in that sst2A expression is stimulated by SST and IGF-I (with sst2B expression also being stimulated by IGF-I) and suppressed by dexamethasone in primary PIT cell cultures (Supplemental Fig. S1B). We might speculate that the lack of change in sst2A expression and elevation of sst1, sst2B, and sst5 in PIT of female SST-knockout mice may be related to elevations in IGF-I, since our laboratory has shown that IGF-I can increase these receptor isoforms in mouse primary PIT cell cultures (36).
It should be mentioned that the tissue-specific and relative changes in the expression levels of sst subtypes in SST-knockout mice originally developed in the laboratory of Dr. Ute Hochgeschwender (71) are partially different from those generated using SST-knockout mice developed in the laboratory of Dr. Malcolm Low (28), as reviewed in detail elsewhere (34). It is possible that such differences are due to different strategies for gene deletion, background strain, time of day tested, age, light cycle, diet, stress, and/or analytical techniques used. Therefore, the assessment of the effect of somatostatin replacement in both somatostatin-knockout mouse models should be undertaken in future studies to unequivocally elucidate the precise contribution of endogenous somatostatin on the observed changes in the regulation of sst subtypes and GH axis function in both animal models.
Summary.
The present results strongly support the hypothesis that the sexually dimorphic pattern of GH release is influenced largely by SST/ssts system, where the PIT appears to be a key component in this process, since female PITs express lower levels of SST and ssts and are relatively unresponsive to the inhibitory action of in vitro SST treatment compared with males. Our data also substantiate and extend the work of others implicating a reduction in SST tone in the fasting-induced rise in GH, which may involve a reduction in HPT SST expression as well as an overall downregulation in ssts within the HPT, PIT, and stomach; however, the specific changes in ssts were tissue and sex dependent. Studies examining the direct effects of SST, IGF-I, and dexamethasone on expression of these sst subtypes in a HPT cell line and in primary PIT cell cultures, coupled with the sex-dependent impact of fasting on these end points, may explain in part the sex-dependent impact of fasting on HPT and PIT sst expression. Finally, our data suggest that the GH axis of female mice is more sensitive to the loss of endogenous SST, particularly at the level of the PIT, showing upregulation of GH-stimulatory receptors and a general downregulation of ssts, likely leading to an increase in IGF-I. These changes were not observed in male SST-knockout perhaps because of a compensatory rise in HPT cortistatin. When viewed as a whole, these observations suggest that changes in SST output, as well as sensitivity, at multiple levels of the GH axis play a role in sex difference and metabolic regulation of GH release.
GRANTS
This work is supported in part by grants RYC-2007-00186 and BFU2008-01136/BFI (to R. M. Luque); FI06/00804 (to J. Córdoba-Chacón); FPU-AP20052473 (to M. D. Gahete); BIO-0139, CTS-01705, and BFU2007-60180/BFI (to J. P. Castaño); and NIDDK 30677 and a Veterans Affairs Merit Award (to R. D. Kineman).
DISCLOSURES
The authors have nothing to disclose.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Ute Hochgeschwender (Oklahoma Medical Research Foundation) for the SST-knockout mice.
REFERENCES
- 1. Argente J, Chowen JA, Zeitler P, Clifton DK, Steiner RA. Sexual dimorphism of growth hormone-releasing hormone and somatostatin gene expression in the hypothalamus of the rat during development. Endocrinology 128: 2369–2375, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Barkan AL, Dimaraki EV, Jessup SK, Symons KV, Ermolenko M, Jaffe CA. Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. J Clin Endocrinol Metab 88: 2180–2184, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, Shkreta L. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology 145: 393–400, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Ben-Shlomo A, Melmed S. Pituitary somatostatin receptor signaling. Trends Endocrinol Metab 21: 123–133, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouyer K, Loudes C, Robinson IC, Epelbaum J, Faivre-Bauman A. Sexually dimorphic distribution of sst2A somatostatin receptors on growth hormone-releasing hormone neurons in mice. Endocrinology 147: 2670–2674, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179: 77–79, 1973 [DOI] [PubMed] [Google Scholar]
- 7. Broglio F, Koetsveld Pv P, Benso A, Gottero C, Prodam F, Papotti M, Muccioli G, Gauna C, Hofland L, Deghenghi R, Arvat E, Van Der Lely AJ, Ghigo E. Ghrelin secretion is inhibited by either somatostatin or cortistatin in humans. J Clin Endocrinol Metab 87: 4829–4832, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Bruno JF, Xu Y, Song J, Berelowitz M. Pituitary and hypothalamic somatostatin receptor subtype messenger ribonucleic acid expression in the food-deprived and diabetic rat. Endocrinology 135: 1787–1792, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Camiña JP, Carreira MC, Micic D, Pombo M, Kelestimur F, Dieguez C, Casanueva FF. Regulation of ghrelin secretion and action. Endocrine 22: 5–12, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Chowen-Breed JA, Steiner RA, Clifton DK. Sexual dimorphism and testosterone-dependent regulation of somatostatin gene expression in the periventricular nucleus of the rat brain. Endocrinology 125: 357–362, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Chowen JA, Frago LM, Argente J. The regulation of GH secretion by sex steroids. Eur J Endocrinol 151, Suppl 3: U95–U100, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Córdoba-Chacón J, Gahete MD, Duran-Prado M, Pozo-Salas AI, Malagón MM, Gracia-Navarro F, Kineman RD, Luque RM, Castaño JP. Identification and characterization of new functional truncated variants of somatostatin receptor subtype 5 in rodents. Cell Mol Life Sci 67: 1147–1163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corleto VD. Somatostatin and the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 17: 63–68, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Dalm VA, Van Hagen PM, de Krijger RR, Kros JM, Van Koetsveld PM, Van DerLely AJ, Lamberts SW, Hofland LJ. Distribution pattern of somatostatin and cortistatin mRNA in human central and peripheral tissues. Clin Endocrinol (Oxf) 60: 625–629, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Djordjijevic D, Zhang J, Priam M, Viollet C, Gourdji D, Kordon C, Epelbaum J. Effect of 17beta-estradiol on somatostatin receptor expression and inhibitory effects on growth hormone and prolactin release in rat pituitary cell cultures. Endocrinology 139: 2272–2277, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Durán-Prado M, Gahete MD, Martínez-Fuentes AJ, Luque RM, Quintero A, Webb SM, Benito-López P, Leal A, Schulz S, Gracia-Navarro F, Malagón MM, Castaño JP. Identification and characterization of two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J Clin Endocrinol Metab 94: 2634–2643, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Frystyk J. Free insulin-like growth factors—measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm IGF Res 14: 337–375, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Gahete MD, Córdoba-Chacón J, Salvatori R, Castaño JP, Kineman RD, Luque RM. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol Cell Endocrinol 317: 154–160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hartman ML, Veldhuis JD, Thorner MO. Normal control of growth hormone secretion. Horm Res 40: 37–47, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Hasegawa O, Sugihara H, Minami S, Wakabayashi I. Masculinization of growth hormone (GH) secretory pattern by dihydrotestosterone is associated with augmentation of hypothalamic somatostatin and GH-releasing hormone mRNA levels in ovariectomized adult rats. Peptides 13: 475–481, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Henry BA, Rao A, Tilbrook AJ, Clarke IJ. Chronic food-restriction alters the expression of somatostatin and growth hormone-releasing hormone in the ovariectomised ewe. J Endocrinol 170: R1–R5, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, Thorner MO. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest 81: 968–975, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jansson JO, Eden S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6: 128–150, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Kimura N, Tomizawa S, Arai KN, Kimura N. Chronic treatment with estrogen up-regulates expression of sst2 messenger ribonucleic acid (mRNA) but down-regulates expression of sst5 mRNA in rat pituitaries. Endocrinology 139: 1573–1580, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Kineman RD, Luque RM. Evidence that ghrelin is as potent as growth hormone (GH)-releasing hormone (GHRH) in releasing GH from primary pituitary cell cultures of a nonhuman primate (Papio anubis), acting through intracellular signaling pathways distinct from GHRH. Endocrinology 148: 4440–4449, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Kuwahara S, Kesuma Sari D, Tsukamoto Y, Tanaka S, Sasaki F. Age-related changes in growth hormone (GH)-releasing hormone and somatostatin neurons in the hypothalamus and in GH cells in the anterior pituitary of female mice. Brain Res 1025: 113–122, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Lago F, Senaris RM, Emson PC, Dominguez F, Dieguez C. Evidence for the involvement of non-androgenic testicular factors in the regulation of hypothalamic somatostatin and GHRH mRNA levels. Brain Res 35: 220–226, 1996 [PubMed] [Google Scholar]
- 28. Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest 107: 1571–1580, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luque RM, Gahete MD, Hochgeschwender U, Kineman RD. Evidence that endogenous SST inhibits ACTH and ghrelin expression by independent pathways. Am J Physiol Endocrinol Metab 291: E395–E403, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Luque RM, Kineman RD. Gender-dependent role of endogenous somatostatin in regulating growth hormone-axis function in mice. Endocrinology 148: 5998–6006, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology 147: 2754–2763, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology 148: 4601–4611, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Luque RM, Lin Q, Kineman RD. Understanding the interrelationship between metabolism and the GH-axis. In: Hypothalamic-Pituitary Disease and Obesity, 11th International HypoCCS Meeting, edited by Clemmons DR, Attanasio AF. Bristol, UK: BioScientifica, 2009, p. 33–56 [Google Scholar]
- 34. Luque RM, Park S, Kineman RD. Role of endogenous somatostatin in regulating GH output under basal conditions and in response to metabolic extremes. Mol Cell Endocrinol 286: 155–168, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Luque RM, Park S, Kineman RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology 148: 300–309, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Luque RM, Soares BS, Peng XD, Krishnan S, Cordoba-Chacon J, Frohman LA, Kineman RD. Use of the metallothionein promoter-human growth hormone-releasing hormone (GHRH) mouse to identify regulatory pathways that suppress pituitary somatotrope hyperplasia and adenoma formation due to GHRH-receptor hyperactivation. Endocrinology 150: 3177–3185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McMahon CD, Radcliff RP, Lookingland KJ, Tucker HA. Neuroregulation of growth hormone secretion in domestic animals. Domest Anim Endocrinol 20: 65–87, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Meyer RD, Laz EV, Su T, Waxman DJ. Male-specific hepatic Bcl6: growth hormone-induced block of transcription elongation in females and binding to target genes inversely coordinated with STAT5. Mol Endocrinol 23: 1914–1926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Møller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta 1616: 1–84, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Muller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev 79: 511–607, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Murray HE, Simonian SX, Herbison AE, Gillies GE. Correlation of hypothalamic somatostatin mRNA expression and peptide content with secretion: sexual dimorphism and differential regulation by gonadal factors. J Neuroendocrinol 11: 27–33, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Nurhidayat Tsukamoto Y, Sigit K, Sasaki F. Sex differentiation of growth hormone-releasing hormone and somatostatin neurons in the mouse hypothalamus: an immunohistochemical and morphological study. Brain Res 821: 309–321, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J Neurochem 89: 1057–1091, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Painson JC, Tannenbaum GS. Sexual dimorphism of somatostatin and growth hormone-releasing factor signaling in the control of pulsatile growth hormone secretion in the rat. Endocrinology 128: 2858–2866, 1991 [DOI] [PubMed] [Google Scholar]
- 45. Park S, Kamegai J, Kineman RD. Role of glucocorticoids in the regulation of pituitary somatostatin receptor subtype (sst1-sst5) mRNA levels: evidence for direct and somatostatin-mediated effects. Neuroendocrinology 78: 163–175, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Park S, Peng XD, Frohman LA, Kineman RD. Expression analysis of hypothalamic and pituitary components of the growth hormone axis in fasted and streptozotocin-treated neuropeptide Y (NPY)-intact (NPY+/+) and NPY-knockout (NPY−/−) mice. Neuroendocrinology 81: 360–371, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Park S, Sohn S, Kineman RD. Fasting-induced changes in the hypothalamic-pituitary-GH axis in the absence of GH expression: lessons from the spontaneous dwarf rat. J Endocrinol 180: 369–378, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Parmar RM, Chan WW, Dashkevicz M, Hayes EC, Rohrer SP, Smith RG, Schaeffer JM, Blake AD. Nonpeptidyl somatostatin agonists demonstrate that sst2 and sst5 inhibit stimulated growth hormone secretion from rat anterior pituitary cells. Biochem Biophys Res Commun 263: 276–280, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 20: 157–198, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Plotsky PM, Vale W. Patterns of growth hormone-releasing factor and somatostatin secretion into the hypophysial-portal circulation of the rat. Science 230: 461–463, 1985 [DOI] [PubMed] [Google Scholar]
- 51. Ramírez JL, Mouchantaf R, Kumar U, Otero Corchon V, Rubinstein M, Low MJ, Patel YC. Brain somatostatin receptors are up-regulated in somatostatin-deficient mice. Mol Endocrinol 16: 1951–1963, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Rigamonti AE, Marazzi N, Cella SG, Cattaneo L, Müller EE. Growth hormone responses to growth hormone-releasing hormone and hexarelin in fed and fasted dogs: effect of somatostatin infusion or pretreatment with pirenzepine. J Endocrinol 156: 341–348, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Schettini G, Florio T, Meucci O, Landolfi E, Lombardi G, Marino A. Somatostatin inhibition of anterior pituitary adenylate cyclase activity: different sensitivity between male and female rats. Brain Res 439: 322–329, 1988 [DOI] [PubMed] [Google Scholar]
- 54. Schonbrunn A, Gu YZ, Dournard P, Beaudet A, Tannenbaum GS, Brown PJ. Somatostatin receptor subtypes: specific expression and signaling properties. Metabolism 45: 8–11, 1996 [DOI] [PubMed] [Google Scholar]
- 55. Señarís RM, Lago F, Diéguez C. Gonadal regulation of somatostatin receptor 1, 2 and 3 mRNA levels in the rat anterior pituitary. Brain Res Mol Brain Res 38: 171–175, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Siehler S, Nunn C, Hannon J, Feuerbach D, Hoyer D. Pharmacological profile of somatostatin and cortistatin receptors. Mol Cell Endocrinol 286: 26–34, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Silva AP, Bethmann K, Raulf F, Schmid HA. Regulation of ghrelin secretion by somatostatin analogs in rats. Eur J Endocrinol 152: 887–894, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Soares-Welch C, Farhy L, Mielke KL, Mahmud FH, Miles JM, Bowers CY, Veldhuis JD. Complementary secretagogue pairs unmask prominent gender-related contrasts in mechanisms of growth hormone pulse renewal in young adults. J Clin Endocrinol Metab 90: 2225–2232, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Støving RK, Andersen M, Flyvbjerg A, Frystyk J, Hangaard J, Vinten J, Koldkjaer OG, Hagen C. Indirect evidence for decreased hypothalamic somatostatinergic tone in anorexia nervosa. Clin Endocrinol (Oxf) 56: 391–396, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Sugihara H, Emoto N, Shibasaki T, Minami S, Wakabayashi I. Increased pituitary growth hormone-releasing factor (GRF) receptor messenger ribonucleic acid expression in food-deprived rats. Brain Res 742: 355–358, 1996 [DOI] [PubMed] [Google Scholar]
- 61. Tannenbaum GS, Epelbaum J. Somatostatin . In: The Endocrine System: Hormonal Control of Growth, edited by Kostyo JL. New York: Oxford University Press, 1999, p. 221–265 [Google Scholar]
- 62. Tannenbaum GS, Ling N. The interrelationship of growth hormone (GH)-releasing factor and somatostatin in generation of the ultradian rhythm of GH secretion. Endocrinology 115: 1952–1957, 1984 [DOI] [PubMed] [Google Scholar]
- 63. Tannenbaum GS, Painson JC, Lengyel AM, Brazeau P. Paradoxical enhancement of pituitary growth hormone (GH) responsiveness to GH-releasing factor in the face of high somatostatin tone. Endocrinology 124: 1380–1388, 1989 [DOI] [PubMed] [Google Scholar]
- 64. Tannenbaum GS, Rorstad O, Brazeau P. Effects of prolonged food deprivation on the ultradian growth hormone rhythm and immunoreactive somatostatin tissue levels in the rat. Endocrinology 104: 1733–1738, 1979 [DOI] [PubMed] [Google Scholar]
- 65. Vanetti M, Vogt G, Hollt V. The two isoforms of the mouse somatostatin receptor (mSSTR2A and mSSTR2B) differ in coupling efficiency to adenylate cyclase and in agonist-induced receptor desensitization. FEBS Lett 331: 260–266, 1993 [DOI] [PubMed] [Google Scholar]
- 66. Veldhuis JD. Neuroendocrine control of pulsatile growth hormone release in the human: relationship with gender. Growth Horm IGF Res 8, Suppl B: 49–59, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol 286: 75–87, 2008 [DOI] [PubMed] [Google Scholar]
- 68. Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20: 2613–2629, 2006 [DOI] [PubMed] [Google Scholar]
- 69. Wideman L, Weltman JY, Patrie JT, Bowers CY, Shah N, Story S, Veldhuis JD, Weltman A. Synergy of l-arginine and GHRP-2 stimulation of growth hormone in men and women: modulation by exercise. Am J Physiol Regul Integr Comp Physiol 279: R1467–R1477, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Xu Y, Berelowitz M, Bruno JF. Dexamethasone regulates somatostatin receptor subtype messenger ribonucleic acid expression in rat pituitary GH4C1 cells. Endocrinology 136: 5070–5075, 1995 [DOI] [PubMed] [Google Scholar]
- 71. Zeyda T, Diehl N, Paylor R, Brennan MB, Hochgeschwender U. Impairment in motor learning of somatostatin null mutant mice. Brain Res 906: 107–114, 2001 [DOI] [PubMed] [Google Scholar]
- 72. Zhang WH, Beaudet A, Tannenbaum GS. Sexually dimorphic expression of sst1 and sst2 somatostatin receptor subtypes in the arcuate nucleus and anterior pituitary of adult rats. J Neuroendocrinol 11: 129–136, 1999 [DOI] [PubMed] [Google Scholar]
- 73. Zhao Z, Sakata I, Okubo Y, Koike K, Kangawa K, Sakai T. Gastric leptin, but not estrogen and somatostatin, contributes to the elevation of ghrelin mRNA expression level in fasted rats. J Endocrinol 196: 529–538, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.