Abstract
Excessive sympathetic drive is a hallmark of chronic heart failure (HF). Disease progression can be correlated with plasma norepinephrine concentration. Renal function is also correlated with disease progression and prognosis. Because both the renal nerves and renin-angiotensin II system are activated in chronic HF we hypothesized that excessive renal sympathetic nerve activity decreases renal blood flow in HF and is associated with changes in angiotensin II type 1 receptor (AT1R) and angiotensin II type 2 receptor (AT2R) expression. The present study was carried out in conscious, chronically instrumented rabbits with pacing-induced HF. We found that rabbits with HF showed a decrease in mean renal blood flow (19.8 ± 1.6 in HF vs. 32.0 ± 2.5 ml/min from prepace levels; P < 0.05) and an increase in renal vascular resistance (3.26 ± 0.29 in HF vs. 2.21 ± 0.13 mmHg·ml−1·min in prepace normal rabbits; P < 0.05) while the blood flow and resistance was not changed in HF rabbits with the surgical renal denervation. Renal AT1R expression was increased by ∼67% and AT2R expression was decreased by ∼87% in rabbits with HF; however, kidneys from denervated rabbits with HF showed a near normalization in the expression of these receptors. These results suggest renal sympathetic nerve activity elicits a detrimental effect on renal blood flow and may be associated with alterations in the expression of angiotensin II receptors.
Keywords: cardiorenal syndrome, norepinephrine, renal sympathetic nerve activity, angiotensin II
heart failure (HF) affects over 5 million people in the United States (38). Sympathoexcitation has long been implicated in the progression of HF. Excessive sympathoexcitation, as evidenced by plasma norepinephrine (NE) levels, is associated with lower left ventricular ejection fraction and higher mortality (11). Another indicator of HF severity and clinical outcomes is renal function. Assessing renal function, via serum creatinine, is an important prognostic tool and can be more predictive of outcomes in advanced HF than cardiac measurements (27, 28). A recent clinical study showed that estimated glomerular filtration rate (GFR) was the strongest predictor of outcomes, irrespective of NYHA class and treatment strategy (28). In hospitalized patients, worsening renal function was associated with greater complications, longer stays, more frequent admissions, and poorer survival (50). The renal dysfunction seen in HF patients is not necessarily independent of the excessive sympathetic tone and can feed back via afferent signaling to increase sympathetic drive to the kidney (45, 46).
The kidney generates ANG II generation through the release of renin by the juxtaglomerular cells. In addition, cellular elements within the kidney contain a local renin-angiotensin system (RAS). No matter the origin, ANG II has important consequences in the kidney, in health and disease. In some instances, angiotensin type 1 receptor (AT1R) stimulation results in decreases in GFR and renal blood flow (RBF) that result in net sodium and water reabsorption (6). The response of the vasculature to ANG II is altered in disease states characterized by sympathoexcitation, such as hypertension and HF. For instance, in spontaneously hypertensive rats (SHR), the sensitivity of renal vessels to ANG II is enhanced (32). Additionally, the vasodilator response to ANG II type 2 receptor (AT2R) stimulation is reduced in the afferent arterioles of young SHR (19). These responses could be due to altered receptor expression and/or changes in the signaling pathways activated in disease states.
Both receptor subtypes have been identified in the kidney of most species, including the rabbit (7). Previous studies have focused on the role of the AT1R in mediating the deleterious effects of ANG II, while the AT2R has largely been overlooked, perhaps because of the relatively low level of expression in adult tissue. On the other hand, recent work suggests that the AT2R may be an important additional mediator of ANG II action. Otsuka et al. (43) showed that both the AT1R and AT2R are involved in vascular remodeling seen in the aorta of SHR; AT1R stimulation increased extracellular matrix formation and cellular hypertrophy, whereas stimulation of the AT2R primarily affected vascular smooth muscle cell (VSMC) hypertrophy. Many studies have investigated a dual action of ANG II on VSMC relaxation. Systemic overexpression of AT2R by viral transfection resulted in a greater depressor action of losartan which was blocked by the AT2R antagonist PD123319, implicating AT2R in systemic vasodilation (34).
Renal nerve activation can have profound influences on renal hemodynamics (13). Handa and Johns (24) showed that renal sympathetic nerve stimulation results in a decrease in RBF that is abolished by administering an angiotensin-converting enzyme inhibitor (ACE-I). The influence of the renal nerves on tubular function is related, in part, to the release or production of ANG II. Early work by Mogil et al. (40) hypothesized that intact nerves are required for the normal response to changes in sodium metabolism by activation of the RAS. More recent evidence in support of this hypothesis indicates that both (renal nerves and an intact RAS) are required for acute renal nerve stimulation to influence sodium reabsorption in anesthetized rats (24). Several studies have investigated how these two mechanisms are associated. It has been shown that ANG II acts primarily on the presynaptic nerve terminal AT1R to modulate release of NE (3, 12, 13). Renal nerve stimulation is also related to the RAS through the β1-adrenoreceptors on the juxtaglomerular cells, which results in renin release. HF may exaggerate this response through an increase in renal sympathetic nerve activity and changes in receptor profiles, as well as alter cellular signaling pathways within the kidney. However, the direct effect of long-term renal nerve activation on the expression of ANG II receptors in the HF state has not been investigated.
Therefore, in the present study we hypothesized that increased renal sympathetic nerve activity in HF would contribute to increased renal vascular resistance (RVR). We also hypothesized that there would be associated changes in ANG II receptor expression. To this end, we employed animals with and without chronic renal denervation (DnX) and measured RBF and renal vascular AT1R and AT2R protein expression.
MATERIALS AND METHODS
Animal Model
Experiments were carried out using 30 male New Zealand White rabbits weighing between 3 and 4 kg. All experiments were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and conformed to the guidelines for the care and use of animals of the American Physiological Society and the National Institutes of Health.
Surgical Preparation
All rabbits underwent sterile thoracic instrumentation as described previously (36, 37). In brief, a platinum wire pacing electrode was sutured to the epicardium of the left ventricle. A ground electrode was also secured, usually to the left atrial appendage. All wires were tunneled and exited through the skin at the midscapular region. The chest was closed and evacuated; in the same setting, a radiotelemetry device (model PA-C40, Data Sciences International, St. Paul, MN) was implanted into the right femoral artery, and the tip of the catheter was positioned in the descending aorta for blood pressure and heart rate measurements in the conscious state. Rabbits were allowed to recover for at least 2 wk before the subsequent surgery.
After recovering from the thoracic surgery, all rabbits were subjected to a second surgery. Through a left flank incision, the left renal artery was exposed to allow placement of a flow probe (2-mm S series perivascular probe, Transonic Systems, Ithaca, NY). Before placement of the flow probe, in the DnX animals, all visible nerves adjacent to the renal artery and vein were carefully dissected and excised. The flow probe cable was placed around the vessel, tunneled under the skin, and exteriorized in the midscapular region, dorsal to the cardiac wires. Rabbits were again allowed to recover for 2 wk before pacing was begun.
Induction of HF
After recovery from surgery, baseline hemodynamic measurements were taken for ∼10 min in the conscious rabbit quietly resting in a Plexiglas box in the laboratory on at least 3 separate days. HF was induced by chronic and sustained ventricular pacing as described previously (37). In brief, animals were paced at a rate of 360–380 bpm with the use of a small, lightweight pacing unit of our own design. Rabbits were paced continuously for 3 wk. The progression into HF was monitored by weekly echocardiograms (Accuson Sequoia 512 C; 4-MHz probe) in the conscious state. Cardiac dimensions (left ventricular end-diastolic diameter, left ventricular end-systolic diameter, fractional shortening, and ejection fraction) and other hemodynamic parameters were measured using standard formulas from the parasternal short axis view in the M mode configuration. In addition to left ventricular dimension and contractility changes in the heart, clinical signs of HF such as ascites, pulmonary congestion, and cachexia were evident as symptoms of this HF model. When animals were in stable HF with an ejection fraction of ∼40% (20 ± 2 days, intact, vs. 17 ± 2 days, DnX, not significant, P = 0.41), additional baseline recordings were taken as described above (10 min on 3 separate days) to evaluate renal blood flow (RBF), arterial pressure, heart rate, and RVR. At the conclusion of the experiments, animals were euthanized with an overdose of pentobarbital sodium; kidneys were quickly removed and placed on ice for further biochemical analysis.
Experimental Protocols
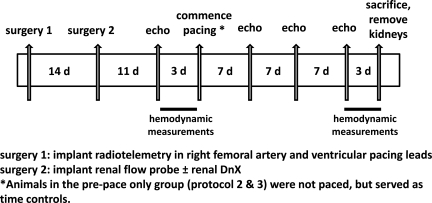
Three protocols were followed in this study. A representative timeline is depicted in Fig. 1. A total of 30 animals were used: 10 animals for protocol 1, 17 for protocol 2 (5 from protocol 1); and 18 for protocol 3 (10 from protocol 1.)
Fig. 1.
Representative timeline for the animals used in this study. Protocol 1 focused on the in vivo measurements (10 animals); protocol 2 focused on the validation of the denervation (DnX) maneuver (17 animals); and protocol 3 focused on the analysis of renal receptor expression (19 animals). Some animals were used in more than one protocol (see the text).
Protocol 1 (10 animals).
This set of experiments focused on in vivo measurement of RBF. Rabbits in this protocol underwent surgical preparation and induction of HF as described above. Five rabbits were subjected to unilateral renal denervation (DnX) during the renal surgery; five rabbits remained intact. In vivo measurements were made in the pre- and postpace states.
Protocol 2 (17 animals).
This protocol was designed to validate the effectiveness of the surgical DnX maneuver. Animals were surgically instrumented as described above and were subjected to the same pacing procedure, 5 DnX (from protocol 1) and 5 additional intact. After the renal surgery, the effectiveness of renal denervation was tested using the response of the renal vasculature to cigarette smoke. The response to oropharyngeal delivery of cigarette smoke (60 ml) is one intervention to near maximally activate the sympathetic nervous system (41). The five intact animals that did not have their nerves severed during the renal surgery served as the pre- and postpace controls to compare the RBF response to smoke administration. Briefly, the heart was paced just above the resting heart rate using an external pacemaker to circumvent the bradycardia evoked by the smoke response. A 60-ml syringe was filled with cigarette smoke. The smoke was then delivered near the external nares of the rabbit. The RBF data are expressed as percent change from baseline in response to smoke (30-s average during the peak response).
A separate group of animals (n = 7) was used to analyze tissue NE content. These animals underwent the same surgical preparation as described above for DnX and were euthanized ∼4 wk after the renal surgery. We measured NE content in both the left (denervated) and right (innervated) kidneys to compare values within the same animal. Samples of kidney cortex were collected after euthanasia. Tissue homogenates were subjected to an ELISA (GenWay Biotech, San Diego, CA) to determine NE content to verify that renal denervation was sustained until the end of the experimental protocol. NE content was normalized to protein concentration, measured using a protein assay kit (Pierce, Rockford, IL) after extraction.
Protocol 3 (18 animals).
This protocol focused on analyzing renal ANG II receptor expression. All 10 animals from protocol 1 were used in this protocol (postpace intact and postpace DnX). Additionally, nine rabbits with the same surgical preparation as described above were not paced but maintained over the same time period as those subjected to chronic pacing. Five of these nine had intact renal nerves; four were DnX.
To try to identify changes within the resistance vasculature, we isolated microvessels. A microvessel-enriched lysate was obtained from the cortex of kidneys by a sieving procedure, slightly modified from that previously described (20). Kidneys were longitudinally sectioned, and the cortex was separated from the medulla. Samples were ground against an 8- in., 180-μm stainless steel mesh. The tissue was rinsed with HBSS buffer and placed into a small flask containing 1 mg/ml collagenase. The flask was placed into a 37°C water bath and gently shaken (100 rpm) for ∼15–20 min. A microscope was used to determine digestion of connective tissue and tubular elements and at least a 60% microvascular yield of vessels ≤100 μm. The mixture was passed over another 150-μm stainless steel mesh and rinsed several times with cold HBSS to increase our microvascular yield to at least 70% vessels.
Once the vessel-enriched sample had been prepared, protein extraction took place and the concentration of protein extracted was measured using a protein assay kit (Pierce). Protein extracts were mixed with 2× 4% SDS sample buffer to obtain equal final amounts of protein in solution. The samples were boiled for 5 min and then loaded onto a 10% SDS-PAGE gel (30 μg protein/well) for electrophoresis using a Bio-Rad minigel apparatus at 40 mA/gel for 45 min. The fractionized protein on the gel was transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA) and subjected to electrophoresis at 300 mA for 90 min. The membrane was probed with primary antibodies (AT1R rabbit polyclonal antibody, 1:500, Santa Cruz Biotechnology; AT2R rabbit polyclonal antibody, 1:500, Santa Cruz Biotechnology; GAPDH mouse monoclonal antibody, 1:1,000, Santa Cruz Biotechnology) and a secondary antibody [goat antirabbit IgG-horseradish peroxidase (HRP), 1:5,000, Santa Cruz Biotechnology; goat antimouse IgG-HRP, 1:5,000, Santa Cruz Biotechnology] and treated with enhanced chemiluminescence substrate (Pierce) for 5 min at room temperature. The bands on the membrane were visualized and analyzed using UVP BioImaging Systems (Epi Chemi II Darkroom, UVP, Upland, CA). GAPDH served as the loading control for the membranes. Protein data are reported as the ratio of receptor to GAPDH signal. The specificity of these antibodies was examined by preabsorption with blocking peptides targeted to the epitopes of the AT1R and AT2R. These data are provided in the online supplementary material for this paper (available on the journal web site).
Data Analysis
Data are expressed as means ± SE. All hemodynamic data were acquired using a 16-channel Powerlab (model 16 SP; ADInstruments, Colorado Springs, CO) with the rabbit resting quietly in a plexiglas box in the laboratory. RVR was calculated online using the Chart5 (ADInstruments) program by dividing mean arterial pressure (MAP) by mean RBF. While central and renal venous pressures were undoubtedly elevated following induction of HF, this was neglected for this calculation. Each rabbit was studied prepacing and after chronic ventricular pacing (postpace) for hemodynamic and smoke response data. A paired t-test was used for in vivo data (comparing the pre- and postpacing data in the same animal) and NE content (comparing the effect of DnX on the left kidney compared with the right intact kidney); differences between groups (the effect of DnX) were determined by a one-way ANOVA followed by the post hoc Bonferroni test to compare individual groups. A probability value of P < 0.05 was considered statistically significant.
RESULTS
Baseline Hemodynamics
Table 1 compares baseline parameters in prepace, postpace, prepace DnX, and postpace DnX groups in the 10 rabbits from protocol 1. There were no significant differences among groups in the prepace state. After induction of HF, there was no significant change in baseline HR; however, in animals with HF, there was a reduction in MAP. In postpace DnX animals, the reduction in MAP did not reach significance (P = 0.49). Left ventricular end systolic diameter increased significantly in the HF state. Left ventricular end diastolic diameter did not significantly increase in either the intact or DnX groups (P = 0.22, intact, and P = 0.11, DnX). Fractional shortening and ejection fraction were significantly reduced after 3 wk of pacing in both groups.
Table 1.
Baseline hemodynamics and echo data from experimental groups before and after continuous ventricular pacing for 3 wk (protocol 1)
| Prepace Intact (n = 5) | Postpace Intact (n = 5) | Prepace DnX (n = 5) | Postpace DnX(n = 5) | |
|---|---|---|---|---|
| Weight, kg | 3.25 ± 0.07 | 3.32 ± 0.05 | 3.50 ± 0.13 | 3.61 ± 0.12 |
| HR, beats/min | 213 ± 8 | 233 ± 11 | 235 ± 5 | 226 ± 13 |
| MAP, mmHg | 69.6 ± 3.7 | 62.5 ± 4.4* | 62.2 ± 4.6 | 59.9 ± 4.9 |
| LVEDD, mm | 15.9 ± 0.7 | 17.0 ± 1.0 | 15.3 ± 0.6 | 17.1 ± 0.5 |
| LVESD, mm | 10.3 ± 0.4 | 14.1 ± 1.0* | 9.6 ± 0.4 | 13.9 ± 0.5* |
| FS, % | 35.6 ± 0.7 | 17.1 ± 1.0* | 37.1 ± 0.7 | 18.6 ± 1.0* |
| EF, % | 68.8 ± 0.9 | 38.6 ± 2.1* | 70.9 ± 0.8 | 41.5 ± 1.9* |
Values are mean ± SE; n = no. of rabbits. HF, heart failure; DnX, (left) unilateral renal denervation; HR, heart rate; MAP, mean arterial pressure; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; FS, fractional shortening; EF, ejection fraction.
P < 0.05 vs. respective sham group.
Baseline RBF and RVR
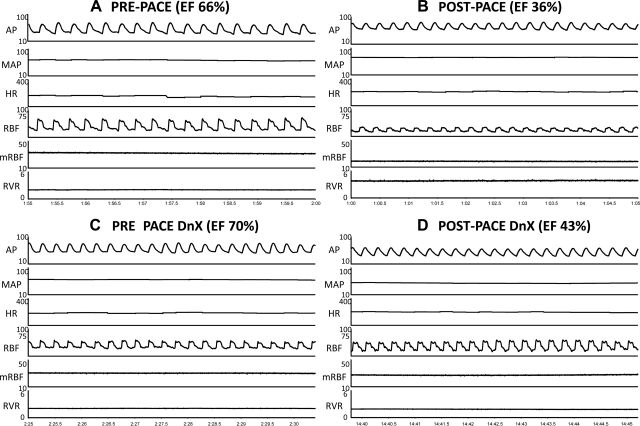
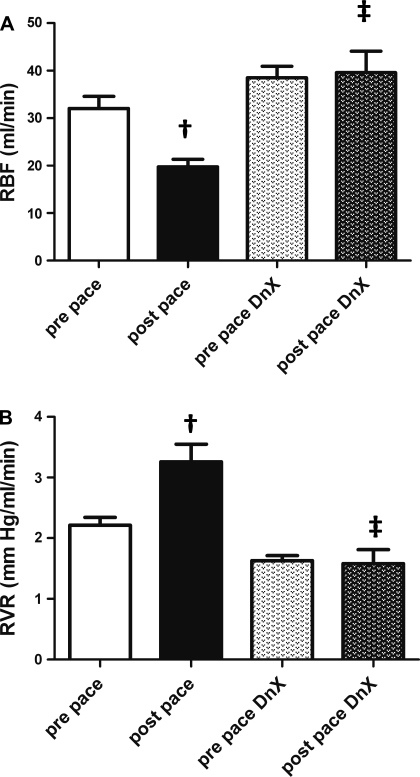
To determine the influence of renal DnX on RVR, we measured RBF and arterial pressure in intact and DnX groups before and after chronic pacing. Figure 2 shows an original hemodynamic recording of arterial pressure, HR, RBF, and RVR in the same animals in the prepace state and after 3 wk of continuous ventricular pacing in an intact [prepace (Fig. 2A), and postpace (Fig. 2B)] and in a renal DnX rabbit [prepace (Fig. 2C) and postpace (Fig. 2D)]. Note the maintenance of RBF in the DnX rabbit after 3 wk of ventricular pacing (Fig. 2, C vs. D) despite a similar reduction in ejection fraction (see Table 1 for group data). Figure 3 shows mean group comparisons of RBF and RVR. Induction of HF significantly reduced baseline RBF to 19.8 ± 1.6 ml/min (vs. prepace, 32.0 ± 2.5 ml/min, P < 0.05), whereas there was no reduction in RBF in the postpace DnX group (38.5 ± 2.4 prepace DnX vs. 39.6 ± 4.5 ml/min postpace DnX). RBF in the postpace DnX group was not different from that in prepace intact animals.
Fig. 2.
Original recordings (protocol 1). All panels represent a 5-s recording of baseline hemodynamics. A: prepace intact animal. B: the same animal as in A after 3 wk of continuous ventricular pacing. C: is a prepace DnX animal. D: the same animal as in C after 3 wk of continuous pacing. AP, arterial pressure; MAP, mean arterial pressure; HR, heart rate; RBF, renal blood flow; mRBF, mean renal blood flow; RVR, renal vascular resistance. EF indicates the ejection fraction of the rabbit on the day of the recording.
Fig. 3.
Renal hemodynamics (protocol 1). A: RBF was measured via flow probe around the left renal artery in 5 intact and 5 DnX animals before and after 3 wk of continuous pacing The value for 1 animal is the average of a 10-min recording on 3 separate days. †P < 0.05 vs. respective prepace group. ‡P < 0.05 vs. postpace; n = 5. B: RVR in 5 intact and 5 DnX animals before and after the pacing protocol. The value for 1 animal is the average of a 10-min recording on 3 separate days. †P < 0.05 vs. respective prepace group. ‡P < 0.05 vs. postpace; n = 5.
RVR showed similar changes to RBF (Fig. 3B). RVR of intact postpace animals was significantly elevated compared with the prepace state (2.21 ± 0.13 prepace vs. 3.26 ± 0.29 mmHg·ml−1·min postpace, P < 0.05). In postpace DnX animals, RVR was not significantly different from the prepace state (1.63 ± 0.08 prepace DnX vs. 1.58 ± 0.23 mmHg·ml−1·min postpace DnX.) In addition, RVR from intact postpace animals was significantly higher than postpace DnX animals (3.26 ± 0.29 HF vs. 1.58 ± 0.23 mmHg·ml−1·min postpace DnX, P < 0.0001). These data confirm a potent role for the renal nerves in altering renal hemodynamics in the setting of chronic HF.
Response to Smoke and Renal NE Content
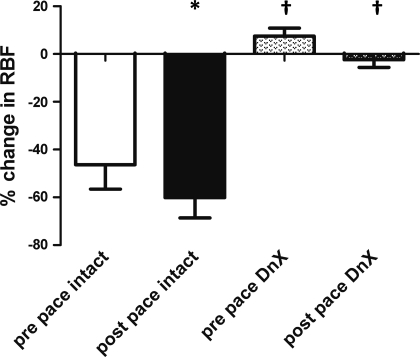
All animals that underwent the surgical denervation maneuver exhibited a blunted reduction in RBF in response to cigarette smoke (Fig. 4) which functionally confirmed adequate renal denervation. The same rabbits were tested after 3 wk of pacing, and intact rabbits still showed a marked reduction in RBF in response to smoke while DnX rabbits showed virtually no reduction. In addition, in a separate group of animals there was a significant reduction in tissue NE content in the DnX kidneys compared with the intact kidneys [20.2 ± 8.8 pg NE/μg protein, right (intact) vs. 0.5 ± 0.2 pg NE/μg protein, left (DnX), P < 0.05]. These data verify the effectiveness of the DnX procedure throughout the duration of the experimental protocols.
Fig. 4.
Validation of DnX (protocol 2). Shown is percent change in RBF in response to oropharyngeal smoke before and after pacing in intact (n = 5) and renal DnX animals (n = 5). *P < 0.05, postpace vs. prepace intact group. †P < 0.05 DnX vs. intact group.
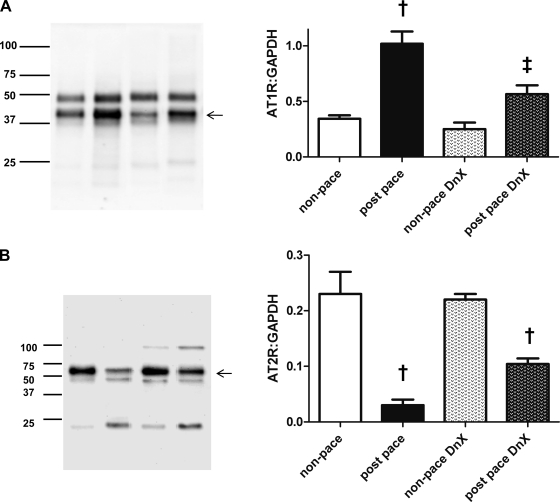
AT1R and AT2R Expression
To examine a possible mechanism by which the renal nerves influence renal hemodynamics in HF, we evaluated changes in ANG II receptor expression in renal cortical vessels from four groups of rabbits (nonpaced, nonpaced DnX, postpace, and postpace DnX; see materials and methods). As shown in Fig. 5, in the presence of intact renal nerves, renal AT1R expression was significantly greater in paced rabbits (1.02 ± 0.11) than those that had not been paced (0.34 ± 0.03, P < 0.05). However, this increase was significantly lower in paced DnX then in nonpaced DnX (0.25 ± 0.06 nonpaced DnX vs. 0.57 ± 0.08 postpace DnX, P > 0.05.) The expression of AT1R in the postpace DnX group was significantly lower than in postpace intact animals. These data suggest that AT1R expression is regulated (directly or indirectly) by renal nerves in animals with chronic HF.
Fig. 5.
Angiotensin receptor expression in kidney microvasculature (protocol 3). A: ANG II type 1 receptor (AT1R) was measured in a microvessel-enriched lysate obtained from 4 separate groups of animals (see materials and methods) and normalized to the loading control GAPDH. †P < 0.05 vs. respective nonpaced group. ‡P < 0.05 vs. postpace; n = 5 except n = 4, nonpaced DnX. B: ANG II type 2 receptor (AT2R) was measured in a microvessel-enriched lysate obtained from 4 separate groups of animals (see materials and methods) and normalized to the loading control GAPDH. †P < 0.05 vs. respective nonpaced group. ‡P < 0.05 vs. postpace; n = 5 except n = 4, nonpaced DnX. Arrows denote bands representing AT1R (A) and AT2R (B). Presence of the doublet at the site of the band of interest is likely detection of a posttranslational modification of the receptor. Fainter bands detected are likely nonspecific binding or protein fragments.
We also examined AT2R protein expression in cortical microvessels from each group of rabbits. Interestingly, we found reciprocal changes in expression of AT2R compared with that of the AT1R. In intact postpace animals, there was a reduction in the expression of AT2R compared with intact nonpaced animals (0.23 ± 0.04 nonpaced vs. 0.03 ± 0.01 in the postpace group, P < 0.05; Fig. 5B). In paced DnX animals, this reduction was diminished (0.22 ± 0.01, nonpaced DnX, vs. 0.10 ± 0.01 in the paced DnX group, P < 0.05).
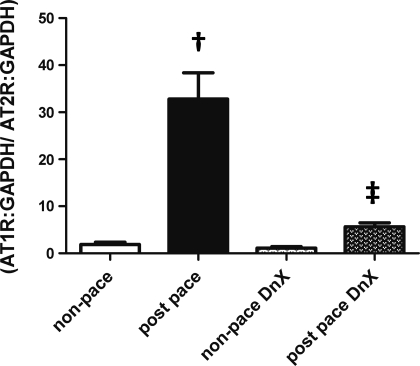
Because the balance between AT1R and AT2R signaling may predict the state of vascular tone, we determined the relative balance of these two receptors in cortical microvessels (Fig. 6). In rabbits with intact renal nerves, the ratio of AT1R to AT2R expression was greatly increased in paced rabbits (1.9 ± 0.5 nonpaced vs. 32.8 ± 5.6 in postpace, P < 0.01). This effect of pacing was greatly blunted by renal DnX (1.12 ± 0.1 nonpaced DnX vs. 5.6 ± 0.8 in the paced DnX group, P > 0.05.)
Fig. 6.
Relative balance of angiotensin receptor expression in kidney microvasculature (protocol 3). Shown is the balance of AT1R to AT2R expression in the 4 groups of rabbits studied. †P < 0.05 vs. respective nonpaced group. ‡P < 0.05 vs. postpace; n = 5 except n = 4, nonpaced DnX.
DISCUSSION
It is well established that the renal sympathetic nerves modulate a variety of renal parameters, including RBF, renin release, GFR, and salt and water reabsorption (14, 15). The current study was designed to determine the influence of the renal sympathetic nerves on renal hemodynamics and ANG II receptor expression in cortical blood vessels of animals with chronic HF. Using chronic renal DnX, we documented in awake, instrumented rabbits with pacing-induced HF the influence of the renal nerves in mediating changes in RBF, RVR, and the expression of both AT1R and AT2R.
Following renal DnX, the increased AT1R and decreased AT2R expression was abrogated in rabbits with chronic HF. In addition, RBF and RVR were similar to prepace intact rabbits ∼5 wk following renal DnX. These data are consistent with the view that the renal nerves are potent modulators of renal function and may be important in ANG II receptor regulation in the setting of chronic HF. However, in our study, there was still an increase in the expression of AT1R and a decrease in AT2R in the kidneys from postpace DnX rabbits. We found this change was not necessarily associated with changes in RBF. This finding could be due to changes in circulating factors that, while not influencing RBF, may influence gene expression in the vasculature.
The expression of AT1R and AT2R in the kidney has been well documented. Miyata et al. (39) used immunohistochemical and RT-PCR techniques in the rat to identify AT1R in tubular and vascular elements, especially in cortical blood vessels, which corroborates the findings of the current study. Miyata et al. also described AT2R expression in similar locations as the AT1R except no AT2R was detected in the glomeruli or medullary thick ascending limb. Gonzalez et al. (22) showed that AT2R expression was reduced in resistance arteries in response to a high-salt diet.
While the precise mechanism by which renal DnX modulates AT1R and AT2R expression is not clear, the balance between the vasoconstrictor properties of AT1R stimulation and the vasodilator effects of AT2R stimulation was clearly shifted to vasoconstriction in animals with chronic HF. This balance was largely reversed in HF animals following renal DnX. These data support those of Abdulla et al. (2) in which blockade of AT1R reduced RVR in SHR rats. In addition, DiBona and Sawin (16) showed an enhanced vasoconstriction in response to graded renal nerve stimulation in HF rats, and this response was normalized by pretreatment with losartan, an AT1R antagonist. On the other hand, Rajapakse et al. (48) showed a reduction in neurogenic vasoconstriction following AT2R blockade in normal anesthetized rabbits. The differences between this study and the current study are not clear. The pharmacological nature of the study by Rajapakse et al. does not allow for reasonable comparisons to be made.
It is well accepted that activation of the AT2R results in vasodilation by a nitric oxide (NO)-dependant mechanism (30). Interestingly, most studies have pointed to endothelial dysfunction mediated by a decrease in NO release and NO synthase expression in HF (31, 55). Most of these studies have concentrated on vascular beds other than the kidney. We postulate that modulation of the AT2R may be extremely important in the preservation of RBF and renal function in the setting of chronic HF. On the other hand, there are several studies showing the importance of a variety of vasoconstrictor pathways on RBF modulation including the AT1R and the ETA receptor (1, 49). Stimulation of AT1Rs in the vasculature evokes vasoconstriction through direct mobilization of calcium into VSMC and by activation of vascular smooth muscle NADPH oxidase to increase superoxide production (23). This process is accentuated in cardiovascular disease (25), which makes the importance of reducing AT1R expression critical to the maintenance of normal or near-normal renal hemodynamics.
The renal sympathetic nerves terminate on various elements in the kidney (4). These include preglomerular resistance vessels, juxtaglomerular cells, and renal tubular components. There have been no studies evaluating the role of the renal nerves on ANG II receptor expression in the HF state. The changes in receptor expression observed in the present study may be due to a direct effect of the renal nerves on AT1R and AT2R trafficking and/or transcription. Wang et al. (53) suggested that NE increases angiotensinogen gene expression in tubular cells via a cAMP response element upstream of the angiotensinogen gene. This group further implicated the α2- and β1-adrenoreceptors, but not the α-1 adrenoreceptors, in mediating this effect (8). However, Du et al. (18) found in rat aorta and cultured VSMCs that NE induced a downregulation of the AT1R through activation of the α1-adrenoreceptors. Contrary to this finding, in neurons cultured from SHR but not Wistar-Kyoto rats, NE did not alter expression of AT1R (47), indicating genetic differences or disease status may alter regulation of protein expression. On the other hand, activation of the renal sympathetic nerves clearly stimulates juxtaglomerular cell release of renin by a β1-receptor-mediated process (29), which could augment local concentrations of ANG II to initiate upregulation of the AT1R (9). While this mechanism has not been investigated further in the kidney, this laboratory and other have shown a positive feedback system mediated by activation of a variety of transcription factors in response to ANG II to upregulate AT1R message and protein in extrarenal tissues in HF (36, 54). These transcription factors include NF-κB, AP-1, and CREB. It will be important to investigate the downstream signaling pathways that are targeted by increases in renal sympathetic nerve activity.
While our data clearly show a reduction in renal cortical NE content in animals with renal denervation, it is well accepted that circulating catecholamines are elevated in chronic HF states (11). However, in the present study, it does not appear that circulating NE is involved to a major extent in the regulation of renal blood flow in HF since DnX normalized RBF in rabbits with HF.
While we cannot rule out an influence of renal afferents, in the present study we do not believe that interruption of afferent pathways plays a major role in the modulation of either blood flow or ANG II receptor expression. As further evidence for a link between renal efferent nerve activation and AT1R expression, we have previously shown a decrease in RSNA following exercise training in animals with HF (21). Interestingly, in preliminary experiments we have shown that exercise training reduces the upregulated AT1R expression in the renal cortex (10). These data strongly suggest an important contribution of the renal nerves to ANG II receptor expression in hyperadrenergic states and its modulation by both physiological means and by surgical intervention.
The animals in the present study exhibited a significant reduction in cardiac function as a result of chronic pacing (Table 1). However, unilateral renal sympathetic DnX did not appear to influence the severity or course of the disease process. Other studies have shown a palliative effect of reducing global sympathetic nerve activity and/or adrenergic stimulation on cardiac dysfunction in HF (35). There may be several reasons for this discrepancy. First, our model of HF (chronic pacing) results in a dilated cardiomyopathy. Most other studies are performed in the coronary ligation model of HF. Interestingly, in an ischemic model of HF in the rat, bilateral renal denervation before myocardial infarction did improve left ventricular function after 4 wk (42). Second, chronic ventricular pacing is maintained throughout the duration of the study, evoking a progressive and continuous myocardial abnormality. Last, while the kidney contributes to circulating NE (52), we have concentrated on reducing sympathetic outflow only to one kidney in this model, making it unlikely that global sympathetic function has changed.
The so called cardiorenal syndrome is of major importance in the pathophysiology of patients with chronic HF (44). Unfortunately, attempts to block various vasoconstrictor pathways by systemic administration of antagonists (e.g., losartan, bosentan, V1 antagonist) have not been very successful in normalizing renal function in this group of patients (33). Systemic administration of these agents may evoke hypotension, further compromising the ability of the kidney to maintain its blood flow. Recent evidence suggests that intrarenal adenosine receptors may be important in mediating renal vasoconstriction and the cardiorenal syndrome (17). Our data add to the importance of intrarenal receptor modulation in the pathophysiology of the cardiorenal syndrome via the AT1R and AT2R on the renal cortical vasculature.
Limitations
While the current study clearly shows an influence of the renal nerves on blood flow, vascular resistance, and ANG II receptor expression in HF, we recognize some limitations to this study that should be discussed. First, we did not measure indices of renal function beyond blood flow. We would predict that renal DnX would increase GFR and mobilize salt and water excretion. In fact, classic studies show that inhibition of adrenergic function by either pharmacological or surgical techniques increases salt and water excretion (5, 14). Other studies have shown a natriuretic and diuretic response to chronic renal DnX (51, 56), which we believe would be augmented in the HF state.
Second, the intracellular and downstream pathways for the effects of renal DnX on AT1R and AT2R expression were not evaluated in this study. As indicated above, we and others have shown several signaling molecules that participate in AT1R expression in HF. Little is known about the modulation of the AT2R, especially in the setting of chronic HF.
Third, it is plausible to suggest that one potential mechanism for the upregulation of the AT1R in the kidney of animals with HF is an adrenergic influence on renin release to evoke an ANG II-dependent upregulation of the AT1R (9). Previous studies have reported an increase (26) and no change (25) in the expression of AT1R in response to ANG II infusion. It will be important to carry out studies in which direct intrarenal infusion of AT1R antagonists can be done to evaluate this issue.
In summary, we have shown for the first time a major influence of the renal sympathetic nerves on ANG II receptor expression in resistance vessels in the kidneys of rabbits with chronic, pacing-induced HF. While no cause and effect relationship has been established, it is reasonable to suggest that the imbalance between AT1R and AT2R expression contributes to the reduction in RBF in this disease state. Renal neural influence on membrane receptors should be considered in a description of mechanisms that may participate in the cardiorenal syndrome in chronic HF.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant PO-1 HL-62222. S. C. Clayton was partially supported by a predoctoral fellowship from the American Heart Association, Midwest Affiliate.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Pamela Curry, Michaela Carlson, Johnnie Hackley, and Yu Li for expert technical assistance in these experiments.
REFERENCES
- 1. Abassi Z, Francis B, Wessale J, Ovcharenko E, Winaver J, Hoffman A. Effects of endothelin receptors ET(A) and ET(B) blockade on renal haemodynamics in normal rats and in rats with experimental congestive heart failure. Clin Sci (Lond) 103 Suppl 48: 245S–248S, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Abdulla MH, Sattar MA, Khan MAH, Abdullah NA, Johns EJ. Influence of sympathetic and AT1-receptor blockade on angiotensin II and adrenergic agonist-induced renal vasoconstrictions in spontaneously hypertensive rats. Acta Physiol 195: 397–404, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Balt JC, Mathy MJ, Nap A, Pfaffendorf M, van Zwieten PA. Effect of the AT1-receptor antagonists losartan, irbesartan, and telmisartan on angiotensin II-induced facilitation of sympathetic neurotransmission in the rat mesenteric artery. J Cardiovasc Pharmacol 38: 141–148, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Barajas L. Innervation of the renal cortex. Fed Proc 37: 1192–1201, 1978 [PubMed] [Google Scholar]
- 5. Bencsath P, Fekete MI, Kanyicska B, Szenasi G, Takacs L. Renal excretion of sodium after bilateral renal sympathectomy in the anaesthetized and conscious rat. J Physiol 331: 443–450, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brewster UC, Setaro JF, Perazella MA. The renin-angiotensin-aldosterone system: cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci 326: 15–24, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Burns KD, Homma T, Harris RC. The intrarenal renin-angiotensin system. Semin Nephrol 13: 13–30, 1993 [PubMed] [Google Scholar]
- 8. Chan JS, Wang TT, Zhang SL, Chen X, Carriere S. Catecholamines and angiotensinogen gene expression in kidney proximal tubular cells. Mol Cell Biochem 212: 73–79, 2000 [PubMed] [Google Scholar]
- 9. Cheng HF, Becker BN, Burns KD, Harris RC. Angiotensin II upregulates type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest 95: 2012–2019, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clayton SC, Curry PL, Li Y, Zucker IH. Exercise training and renal denervation attenuate the expression of angiotensin II type 1 and 2 receptors in rabbits with chronic heart failure. FASEB J 22: 159, 2008. 17709607 [Google Scholar]
- 11. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984 [DOI] [PubMed] [Google Scholar]
- 12. DiBona GF. Nervous kidney. Interaction between renal sympathetic nerves and the renin-angiotensin system in the control of renal function. Hypertension 36: 1083–1088, 2000 [DOI] [PubMed] [Google Scholar]
- 13. DiBona GF. Peripheral and central interactions between the renin-angiotensin system and the renal sympathetic nerves in control of renal function. Ann NY Acad Sci 940: 395–406, 2001 [DOI] [PubMed] [Google Scholar]
- 14. DiBona GF. Neurogenic regulation of renal tubular sodium reabsorption. Am J Physiol Renal Fluid Electrolyte Physiol 233: F73–F81, 1977 [DOI] [PubMed] [Google Scholar]
- 15. DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997 [DOI] [PubMed] [Google Scholar]
- 16. DiBona GF, Sawin LL. Losartan corrects abnormal frequency response of renal vasculature in congestive heart failure. Am J Physiol Heart Circ Physiol 285: H1857–H1863, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Dohadwala MM, Givertz MM. Role of adenosine antagonism in the cardiorenal syndrome. Cardiovasc Ther 26: 276–286, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Du Y, Qiu J, Nelson SH, Wang DH. Regulation of type 1 ANG II receptor in vascular tissue: role of alpha1-adrenoreceptor. Am J Physiol Regul Integr Comp Physiol 273: R1224–R1229, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Eiskjaer H, Bagger JP, Mogensen CE, Schmitz A, Pedersen EB. Enhanced urinary excretion of albumin in congestive heart failure: effect of ACE-inhibition. Scand J Clin Lab Invest 52: 193–199, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Endlich N, Endlich K, Taesch N, Helwig JJ. Culture of vascular smooth muscle cells from small arteries of the rat kidney. Kidney Int 57: 2468–2475, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115: 3095–3102, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez M, Lobos L, Castillo F, Galleguillos L, Lopez NC, Michea L. High-salt diet inhibits expression of angiotensin type 2 receptor in resistance arteries. Hypertension 45: 853–859, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Handa RK, Johns EJ. Interaction of the renin-angiotensin system and the renal nerves in the regulation of rat kidney function. J Physiol 369: 311–321, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst 4: 51–61, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Harrison-Bernard LM, El-Dahr SS, O'Leary DF, Navar LG. Regulation of angiotensin II type 1 receptor mRNA and protein in angiotensin II-induced hypertension. Hypertension 33: 340–346, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102: 203–210, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 113: 671–678, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Holdaas H, DiBona GF, Kiil F. Effect of low-level renal nerve stimulation on renin release from nonfiltering kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 241: F156–F161, 1981 [DOI] [PubMed] [Google Scholar]
- 30. Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther 120: 292–316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katz SD, Schwarz M, Yuen J, LeJemtel TH. Impaired acetylcholine-mediated vasodilation in patients with congestive heart failure. Role of endothelium-derived vasodilating and vasoconstricting factors. Circulation 88: 55–61, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Kost CK, Jr, Jackson EK. Enhanced renal angiotensin II subtype 1 receptor responses in the spontaneously hypertensive rat. Hypertension 21: 420–431, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Krum H, Iyngkaran P, Lekawanvijit S. Pharmacologic management of the cardiorenal syndrome in heart failure. Curr Heart Fail Rep 6: 105–111, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Li H, Gao Y, Grobe JL, Raizada MK, Katovich MJ, Sumners C. Potentiation of the antihypertensive action of losartan by peripheral overexpression of the ANG II type 2 receptor. Am J Physiol Heart Circ Physiol 292: H727–H735, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Lindley TE, Infanger DW, Rishniw M, Zhou Y, Doobay MF, Sharma RV, Davisson RL. Scavenging superoxide selectively in mouse forebrain is associated with improved cardiac function and survival following myocardial infarction. Am J Physiol Regul Integr Comp Physiol 296: R1–R8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ Res 103: 186–193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu JL, Kulakofsky J, Zucker IH. Exercise training enhances baroreflex control of heart rate by a vagal mechanism in rabbits with heart failure. J Appl Physiol 92: 2403–2408, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119: 480–486, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol 277: F437–F446, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Mogil RA, Itskovitz HD, Russell JH, Murphy JJ. Renal innervation and renin activity in salt metabolism and hypertension. Am J Physiol 216: 693–697, 1969 [DOI] [PubMed] [Google Scholar]
- 41. Mousa TM, Gao L, Cornish KG, Zucker IH. Effects of angiotensin II on autonomic components of nasopharyngeal stimulation in male conscious rabbits. J Appl Physiol 98: 1607–1611, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Nozawa T, Igawa A, Fujii N, Kato B, Yoshida N, Asanoi H, Inoue H. Effects of long-term renal sympathetic denervation on heart failure after myocardial infarction in rats. Heart Vessels 16: 51–56, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Otsuka S, Sugano M, Makino N, Sawada S, Hata T, Niho Y. Interaction of mRNAs for angiotensin II type 1 and type 2 receptors to vascular remodeling in spontaneously hypertensive rats. Hypertension 32: 467–472, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Patel J, Heywood JT. Management of the cardiorenal syndrome in heart failure. Curr Cardiol Rep 8: 211–216, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Petersson M, Friberg P, Eisenhofer G, Lambert G, Rundqvist B. Long-term outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur Heart J 26: 906–913, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Phillips JK. Pathogenesis of hypertension in renal failure: role of the sympathetic nervous system and renal afferents. Clin Exp Pharmacol Physiol 32: 415–418, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Raizada MK, Sumners C. Lack of alpha-1-adrenergic receptor-mediated downregulation of angiotensin II receptors in neuronal cultures from spontaneously hypertensive rat brain. Mol Cell Biochem 91: 111–115, 1989 [DOI] [PubMed] [Google Scholar]
- 48. Rajapakse NW, Eppel GA, Widdop RE, Evans RG. ANG II type 2 receptors and neural control of intrarenal blood flow. Am J Physiol Regul Integr Comp Physiol 291: R1669–R1676, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Roux S, Breu V, Ertel SI, Clozel M. Endothelin antagonism with bosentan: a review of potential applications. J Mol Med 77: 364–376, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Shlipak MG, Massie BM. The clinical challenge of cardiorenal syndrome. Circulation 110: 1514–1517, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Szalay L, Colindres RE, Jackson R, Gottschalk CW. Effects of chronic renal denervation in conscious restrained rats. Int Urol Nephrol 18: 3–18, 1986 [DOI] [PubMed] [Google Scholar]
- 52. Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol 491: 881–887, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang TT, Wu XH, Zhang SL, Chan JS. Molecular mechanism(s) of action of norepinephrine on the expression of the angiotensinogen gene in opossum kidney cells. Kidney Int 54: 785–795, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension 52: 679–686, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Westermann D, Riad A, Richter U, Jager S, Savvatis K, Schuchardt M, Bergmann N, Tolle M, Nagorsen D, Gotthardt M, Schultheiss HP, Tschope C. Enhancement of the endothelial NO synthase attenuates experimental diastolic heart failure. Basic Res Cardiol 104: 499–509, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Wilson DR, Honrath U, Sole M. Effect of acute and chronic renal denervation on renal function after release of unilateral ureteral obstruction in the rat. Can J Physiol Pharmacol 57: 731–737, 1979 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.