Abstract
Vitamin D regulates the renin angiotensin system in experimental animals, but corresponding human data are limited. We examined the relation between plasma 25-hydroxyvitamin D and elements of the renin angiotensin system in 184 normotensive individuals in high sodium balance; these included circulating levels of plasma renin activity and angiotensin II, and the renal plasma flow response to infused angiotensin II, which is an indirect measure of the intrinsic renin angiotensin system activity in the kidney. Compared to individuals with sufficient 25-hydroxyvitamin D levels (≥ 30 ng/mL), those with insufficiency (15 - 29.9 ng/mL) and deficiency (<15 ng/mL) had higher circulating angiotensin II levels (p-trend = 0.03). Moreover, those with vitamin D deficiency had significantly blunted renal plasma flow responses to infused angiotensin II (mean decrease of 115 mL/min/1.732 in renal plasma flow vs. 145 ml/min/1.73m2 among those with sufficient vitamin D levels; p-trend = 0.009). Although plasma renin activity was higher among individuals with insufficient levels of vitamin D, the result was not statistically significant. These data suggest that low plasma 25-hydroxyvitamin D levels may result in upregulation of the renin angiotensin system in otherwise healthy humans.
Keywords: vitamin D, renin angiotensin system, hypertension, epidemiology, human
Introduction
Observational studies strongly support an inverse association between plasma 25-hydroxyvitamin D (25[OH]D) concentration and blood pressure as well as hypertension.1-14 A major proposed mechanism linking vitamin D with hypertension involves vitamin D mediated suppression of the renin angiotensin system (RAS), yet data derive mostly from in vitro and animal studies.15-19
Human investigation of the association between vitamin D and the RAS has been scant. Resnick originally reported that plasma renin activity (PRA) and 1,25(OH)2D were inversely correlated (r = −0.65) among 61 individuals on an ambient diet.20 Several years later, Burgess reported a similar association in 10 hypertensives (r = −0.76).21 Interestingly, in a randomized trial that documented a 14 mmHg decrease in SBP with vitamin D supplementation compared with placebo, the authors also noted a trend toward a decrease in circulating Ang II levels (−13.1 pg/mL, p=0.14) relative to placebo.22
To test the hypothesis that there is a mechanistic role for vitamin D in regulation of the RAS in humans, we examined the relation between plasma 25(OH)D concentration with both circulating renin and Ang II levels as well as the renal plasma flow (RPF) response to infused Ang II, which correlates inversely with endogenous intrarenal RAS activity,23-27 among 184 normotensive individuals.
Methods
Study population
Participants in this study included 184 normotensive white and black men and women, recruited as healthy volunteers from the general population, who completed renal plasma flow RPF studies in high sodium balance at one of four General Clinical Research Centers, including Brigham and Women's Hospital in Boston, the University of Utah Medical Center in Salt Lake City, Vanderbilt University Hospital in Nashville, and the Hôpital Européen Georges Pompidou in Paris. We examined normotensive participants because of our prior observations that 25(OH)D levels are inversely associated with the risk of incident hypertension among previously normotensive individuals.3, 4 We analyzed participants during high sodium balance because the range of RPF responsiveness in high sodium balance is greater than in low sodium balance, and thus allows for easier detection of inter-individual differences.24 Additionally, high sodium balance more closely mimics the average ambient diet and thus enhances generalizability of the study findings. The Institutional Review Boards at each of the contributing institutions approved the study, and all participants provided written informed consent.
Participants were classified as normotensive, defined by a seated blood pressure < 140/90 mmHg, not taking anti-hypertensive medications, and furthermore not having a first-degree relative with hypertension onset before the age of 60 years. All participants underwent a screening history and physical and laboratory examination. Other exclusion criteria included diabetes mellitus, chronic kidney disease (defined as a serum creatinine > 1.5 mg/dL), or other significant medical problem including coronary heart disease or active malignancy.
Study protocol
All participants consumed a high salt diet (200 mmol of sodium) for 3 to 7 days prior to the study. High sodium balance was defined by a 24-hour urine sodium excretion ≥ 150 mmol. Participants were admitted to the General Clinical Research Center the night before the RPF study.
On the day of the study, two intravenous catheters were inserted; one for infusions and the other for blood collection. Participants remained supine during the study. An 8 mg/kg loading dose of para-aminohippuric acid (PAH) was administered to 60 minutes prior the administration of Ang II. This loading dose was immediately followed by a continuous infusion of PAH at 12 mg/min, to achieve plasma PAH concentrations in the middle of the range at which tubular secretion dominates excretion. At this concentration of PAH, clearance is independent of plasma levels and effective RPF (as PAH clearance) was calculated from steady-state plasma PAH concentrations as previously described.28, 29 Effective RPF was normalized to a body surface area of 1.73 m2. Ang II was then infused at 3 ng/kg/min for 55 minutes. Three pre-Ang II measurements of RPF and three post-Ang II measurements of RPF were made.
Day of study measurements
Blood pressure was monitored every 5 minutes during the study using a Dinamap automated device (Critikon, Tampa, FL). All PAH assays were performed by the same technician using an autoanalyzer technique. The intra-assay and inter-assay coefficients of variation (CVs) for the PAH assay were < 5% and < 10%, respectively. Plasma renin activity (PRA) was measured using a radioimmunoassay (DiaSorin, Stillwater, MN). The sensitivity of this assay is 0.01 ng/mL/hour and the CV is <10%. The Ang II radioimmunoassay (ALPCO, Windham, NH) measures Ang II by a double-antibody RIA on plasma samples that are pre-treated with a “home-made” cocktail of enzyme-inhibitors against aminopeptidase, protease and angiotensin converting enzyme. The sensitivity of this method is 0.6 pg/mL (range is 0.6-500 pg/mL) and the CV is <15%. Urine sodium was measured by an ion-selective electrode using automatically diluted specimens (ISE Indirect) and the COBAS Integra 400 (Roche Diagnostics, Indianapolis, IN).
Measurement of 25(OH)D
Measurements of 25(OH)D in plasma were performed using a competitive binding chemiluminescent assay from Nichols Institute Diagnostics (San Juan Capistrano, CA). The sensitivity of this assay is 4 ng/mL, and the coefficient of variation ranges from 4.4-8.4%.
Before measuring 25(OH)D on all study participants, we performed a pilot study to determine whether assaying 25(OH)D levels on stored frozen samples was feasible and reliable. We thawed, aliquoted, and measured 25(OH)D levels on frozen samples from 19 participants who also had 25(OH)D levels measured on fresh samples from the original day of the high sodium study. The correlation coefficient comparing levels from fresh and frozen samples was 0.97.
Statistical analyses
Participants were categorized by vitamin D status based upon 25(OH)D levels: optimal (≥ 30.0 ng/mL), insufficient (15.0-29.9 ng/mL), and deficient (< 15.0 ng/mL). Baseline characteristics according to vitamin D status were analyzed by univariate linear regression (for continuous variables) or the chi-square test of trend (for categorical variables). For the baseline association between vitamin D status and both PRA and Ang II concentration, we also performed multivariable linear regression adjusting for age, race, and BMI.
The primary outcome, specifically RPF response to Ang II infusion, was calculated by subtracting the median post-Ang II PAH clearance from the median baseline pre-Ang II PAH clearance. At the present time, there is no readily available means of directly assessing intrarenal RAS activity in humans. The renal plasma flow response to infused Ang II is an indirect measure of the intrinsic activity of the intrarenal RAS,27 and numerous lines of evidence indicate the RPF response to Ang II in high sodium balance is inversely correlated with endogenous RAS activity.23-26
The association between vitamin D status and the primary outcome of RPF response to Ang II was analyzed using multivariable linear regression, with participants who had optimal vitamin D status defined as the reference group. Unadjusted analyses were first performed to generate data for intuitively interpretable figures, and then multivariable analyses were performed to adjust these estimates for age, race, sex, body mass index (calculated as the weight [in kilograms] divided by the height [in meters] squared), systolic blood pressure, diastolic blood pressure, 24-hour urine sodium, and baseline (pre-Ang II) RPF. The relations between vitamin D category with PRA and Ang II were also analyzed with linear regression.
Because black race was strongly associated with lower 25(OH)D levels, we performed a secondary analysis after excluding black individuals in order to determine whether or not our findings were being driven exclusively by race.
As exploratory analyses, we also examined the association between vitamin D status and baseline levels of aldosterone and also the blood pressure response to infusion of Ang II. Finally, we explored whether there was an interaction between vitamin D status and gender for our primary endpoint.
Values in the results are means ± standard error, unless otherwise specified. A p-value < 0.05 was considered statistically significant. P-values were two-tailed. All statistical analyses were performed using SAS statistical software, version 9.1 (Cary, NC).
Results
Baseline characteristics
The mean age of participants was 40.1 years (standard deviation [SD], 12.0 years); 52.2% were female, and 14.7% were black. Plasma 25(OH)D levels ranged from 4.8 ng/mL to 52.4 ng/mL (mean, 22.3 ng/mL), and 79.9% of participants had levels that were insufficient or deficient defined as a level < 30 ng/mL. The mean body mass index (BMI, calculated as the weight in kg divided by the height in meters squared) of the group was 25.3 kg/m2 (SD, 3.9 kg/m2), and the mean systolic/diastolic blood pressures were 111/67 mmHg (SD, 12/8 mmHg). Sodium loading was successful as confirmed by 24-hour urine collection (mean sodium, 224 mmol; SD, 80 mmol).
Baseline characteristics according to category of 25(OH)D are displayed in Table 1. Individuals with lower 25(OH)D levels were more likely to be black (p<0.001), and had higher diastolic blood pressures (p=0.006). Other baseline characteristics did not significantly vary according to vitamin D status.
Table 1.
Baseline study characteristics stratified by level of plasma 25(OH)D.
| Characteristic | ≥30 ng/mL (N=37) | 15-29.9 ng/mL (N=108) | <15 ng/mL (N=39) | p-value |
|---|---|---|---|---|
| Age, years | 42.2 (9.5) | 40.0 (12.2) | 38.2 (13.5) | 0.15 |
| Black, % | 0 | 5 | 55 | <0.001 |
| Female, % | 59 | 50 | 51 | 0.49 |
| BMI, kg/m2 | 25.8 (4.2) | 25.3 (3.7) | 25.1 (4.3) | 0.41 |
| Systolic BP, mmHg | 111 (12) | 110 (12) | 114 (10) | 0.21 |
| Diastolic BP, mmHg | 66 (8) | 66 (8) | 71 (6) | 0.006 |
| 24-hr urine sodium, mmol | 241 (87) | 217 (78) | 225 (79) | 0.43 |
| Basal RPF, ml/min/1.73m2 | 575 (125) | 590 (143) | 572 (97) | 0.99 |
Continuous variables are expressed as mean (standard deviation); categorical variables are expressed as %.
P-values were determined by univariate linear regression (for continuous variables), or the chi-square test (for categorical variables).
Vitamin D and baseline PRA and Ang II
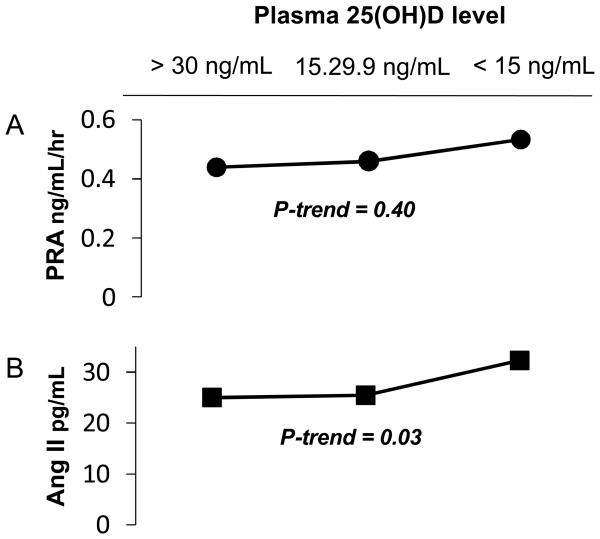
PRA did not significantly differ according to vitamin D status (Figure 1A). PRA levels were low, as anticipated in high sodium balance. Among all participants, mean PRA was 0.09 ± 0.10 ng/mL/hr higher among vitamin D deficient individuals compared to those with sufficient vitamin D levels (p-trend = 0.40). In multivariable models adjusted for age, race, and BMI, PRA was 0.17 ± 0.13 ng/mL/hr higher among those with vitamin D deficiency (p-trend = 0.17). When the analysis was restricted to whites, the mean PRA was 0.15 ± 0.14 ng/mL/hr higher comparing vitamin D deficient to sufficient individuals (p-trend = 0.24). Circulating aldosterone levels also did not significantly differ between categories of 25(OH)D (p-trend = 0.37).
Figure 1.
Association between vitamin D status and PRA and Ang II levels in high sodium balance. (A) Plasma renin activity. (B) Angiotensin II concentration. Mean values are shown. Results are from univariate linear regression.
In contrast, Ang II was significantly higher among individuals with lower 25(OH)D levels (Figure 1B). Comparing individuals with vitamin D deficiency to those whose 25(OH)D levels were 30 ng/mL or more, mean Ang II levels were 7.2 ± 3.6 pg/mL higher (p-trend = 0.04) in univariate analyses. After adjustment for age, race, and BMI, Ang II levels were 9.9 ± 4.4 pg/mL higher among vitamin D deficient individuals (p-trend = 0.01). When this analysis was restricted to white individuals, this same comparison documented 10.8 ± 4.6 pg/mL higher mean Ang II levels among vitamin D deficient individuals (p-trend = 0.005).
Vitamin D and RPF response to Ang II infusion
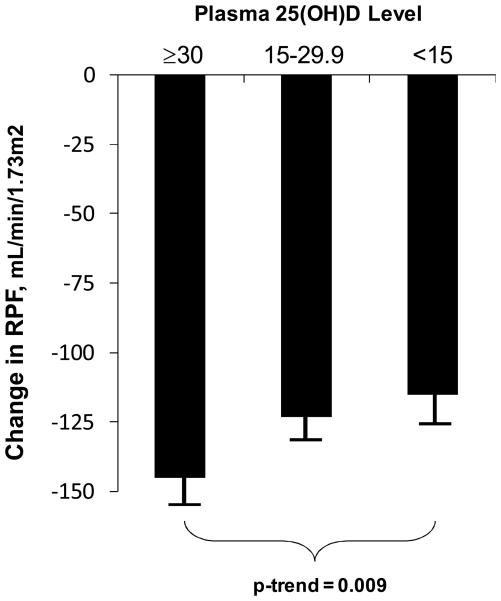
Renal plasma flow responses to Ang II infusion in high sodium balance according to vitamin D status are shown in Figure 2. The mean unadjusted decline in RPF with Ang II infusion in participants whose 25(OH)D level was ≥ 30 ng/mL was 145 ± 13 mL/min/1.73m2. The mean decline in RPF among individuals whose 25(OH)D level was < 15 ng/mL was comparatively blunted (115 ± 10 mL/min/1.73m2; p-trend = 0.001). To account for potential confounding, we used multivariable linear regression adjusting for age, BMI, basal RPF, and race, and the association remained statistically significant; the response to Ang II infusion among vitamin D deficient individuals was 45 ± 13 mL/min/1.73m2 less than among those with optimal 25(OH)D levels (p-trend = 0.009). Further adjustment for the study center had no impact on the results. The change in systolic blood pressure in response to Ang II infusion was also less (by 2 mmHg) among those with vitamin D deficiency compared to those with optimal levels, but this was not statistically significant (p-trend = 0.47).
Figure 2.
Association between vitamin D status and Ang II induced changes in RPF while in high sodium balance. Values are means, and error bars represent standard errors. Results are from univariate linear regression.
To determine whether the association was present in a racially uniform population, we re-analyzed the data after excluding black individuals. The RPF response to Ang II remained significantly blunted among individuals with lower plasma 25(OH)D levels after multivariable adjustment (p-trend = 0.02). Too few blacks were included to analyze them in isolation.
We did not observe any interaction between 25(OH)D levels and gender regarding the RPF response to Ang II infusion (p-interaction = 0.43).
Discussion
Although animal models strongly suggest that vitamin D suppresses the RAS, human data are largely lacking. To our knowledge, this is the first human study to examine the association between plasma 25(OH)D levels and control of the RAS under rigorously controlled dietary conditions. We found that among normotensive individuals, lower 25(OH)D levels were associated with higher circulating Ang II levels and a blunted RPF response to exogenous Ang II infusion, both findings consistent with activation of the RAS in the setting of lower plasma 25(OH)D.
Observational studies strongly support an inverse association between plasma 25(OH)D levels and blood pressure and hypertension.1-14 In addition to numerous cross-sectional analyses, two prospective studies have demonstrated that lower baseline 25(OH)D levels are associated with an increased risk of incident hypertension.3, 4 In the first, which included 613 men and 1,198 women who did not have hypertension at baseline, those with 25(OH)D levels < 15 ng/mL (vitamin D deficiency) compared to ≥ 30 ng/mL had a relative risk (RR) for incident hypertension of 2.7 after adjusting for multiple demographic and lifestyle factors.4 The second prospective study also considered levels of parathyroid hormone (PTH) plus numerous other biomarkers as potential confounders, and found that individuals with vitamin D insufficiency (25[OH]D level < 30 ng/mL) had a 1.5-fold higher risk of developing hypertension compared to those with optimal levels.3
Several putative mechanisms for this relation have been forwarded, including associations between vitamin D deficiency and endothelial dysfunction,22, 30-33 inflammation,34-37 and insulin resistance.38-41 However, a major proposed mechanism was documented by Li et al. in mice lacking the vitamin D receptor gene. Absence of vitamin D signaling in these animals led to an increase in renin gene expression and circulating Ang II levels.15 When placed on a high salt diet, these knockout mice had slight reductions of renin and Ang II, but maintained levels substantially higher than wild type mice controls on a similar diet.15 Furthermore, renin levels remained elevated despite normalization of plasma calcium concentrations, and injection of 1,25(OH)2D reduced renin expression in wild type mice.15, 16 Other animal models support these findings, demonstrating that mice lacking the 1α-hydroxylase gene have a similar phenotype,19 and that 1,25(OH)2D analogs help suppress Ang II mediated kidney injury in diabetic and in 5/6 nephroctomized rats.17, 18
In contrast, few human studies have examined this relationship. The first human study to investigate the association examined 10 normotensive individuals, as well as 51 hypertensive individuals on ambient diets divided into low renin, normal renin, and high renin status.20 The authors reported the highest levels of 1,25(OH)2D in low-renin hypertensives compared to normal and high renin hypertensives (p < 0.01 for both comparisons) as well as normotensives (p < 0.01). Among all 61 individuals, there was an inverse correlation between PRA and 1,25(OH)2D (r = −0.65; p < 0.001).20 The second human study included 10 high-renin hypertensive individuals who were studied initially on a 5-day low sodium diet (10 mmol/d) and then again after a 5-day high sodium diet (100 mmol/d).21 The authors found that changing from a low sodium to high sodium diet led to significant increases in urine calcium excretion and 1,25(OH)2D levels, plus a decrease in PRA. The change in 1,25(OH)2D concentration and PRA were inversely correlated (r = −0.76, p = 0.01).21 The authors hypothesized that sodium loading led to an increase in calcium excretion, which in turn led to an increase in 1,25(OH)2D levels, which then suppressed PRA by increasing juxtaglomerular cell calcium concentrations.21 Levels of 25(OH)D were not measured in either of these studies. One randomized trial of vitamin D supplementation (with ergocalciferol) documented effects on markers of the RAS.22 Sugden et al. randomized 34 individuals with type 2 diabetes and 25(OH)D levels < 20 ng/mL to receive either 100,000 IU of vitamin D2 or placebo. Along with a 6 ng/mL increase in 25(OH)D level at 8 weeks of follow-up with supplementation, systolic blood pressure declined by 14 mmHg compared to placebo. In addition, Ang II levels decreased by 13.1 pg/mL in the vitamin D group relative to the change in the placebo group; conversely, active renin levels (not PRA) were increased in the vitamin D group relative to the change in the placebo group by 2.6 ng/mL.22 Neither of these results was statistically significant.
We found that among 184 normotensive individuals in high sodium balance, lower plasma 25(OH)D levels were associated with significantly higher circulating Ang II concentrations, as well as a blunted RPF response to infused Ang II. Both of these findings support the concept that vitamin D deficiency may be associated with upregulation of the RAS. In contrast, although PRA was higher among individuals with vitamin D insufficiency and deficiency, the association was not statistically significant. This may, in part, be due to insufficient statistical power. Suppression of PRA in the setting of sodium loading may have resulted in levels and ranges that were too low and too narrow to detect a statistical difference given the sample size. It is possible that an association may have been detected had the sample been considerably larger. On the other hand, it is also possible that unlike animals, vitamin D in humans may influence tissue-level production of renin rather than systemic levels. Alternatively, our findings may reflect a renin-independent mechanism for vitamin D suppression of systemic and local Ang II. Indeed, vitamin D signaling may inhibit the expression of angiotensinogen by inhibition of NF-kappa B transcription factors,42, 43 and angiotensinogen may be converted by cathepsins to Ang II in the absence of renin.44, 45
Our study examined plasma 25(OH)D levels; the aforementioned animal and prior human investigations of vitamin D and the RAS, in contrast, focused upon levels of 1,25(OH)2D. Because plasma 25(OH)D is not under homeostatic control while 1,25(OH)2D levels are homeostatically regulated, individuals with low 25(OH)D levels may have normal levels of 1,25(OH)2D; however, the two hormones are generally well correlated.46 In addition, and more importantly, the epidemiologic data supporting a relation between vitamin D and hypertension, as well as vitamin D and cardiovascular disease for that matter, is essentially limited to analyses of 25(OH)D and not 1,25(OH)2D. In order to invoke the RAS as a mechanism to explain the epidemiologic data, therefore, it was critical to analyze levels of 25(OH)D.
Finally, several lines of evidence suggest that 25(OH)D, like 1,25(OH)2D, may be an “active” hormone. The 1α-hydroxylase gene is widely expressed,47 so 25(OH)D may be converted to 1,25(OH)2D locally in various tissues, bypassing the need for conversion in the proximal tubule, and thereby having autocrine and paracrine effects. Furthermore, depending on the conformation of the vitamin D receptor (cis or trans), it may be located either in the cytoplasm or at the plasma membrane, the latter localization associated with its ability to activate second messengers such as protein kinase-C, MAP kinase, and PI3-kinase.48 New data suggest that, while the affinity of cytoplasmic vitamin D receptor is greatest for 1,25(OH)2D, the binding affinity of 25(OH)D for membrane associated vitamin D receptor matches that of 1,25(OH)2D.49 Considering that 25(OH)D concentrations are 1000-fold higher than those of 1,25(OH)2D, it is increasingly apparent that 25(OH)D may be an important active circulating hormone.
Limitations
Our study has limitations. First and foremost, this analysis was cross-sectional, and therefore we cannot demonstrate directionality of the association, nor can we comment on causality. It is possible that up-regulation of the RAS somehow reduced plasma 25(OH)D levels through an unknown mechanism. Even though we adjusted for relevant confounders such as BMI, race, and basal RPF, it is possible that our findings represent residual confounding. Only a randomized controlled intervention could address these possibilities. Second, we did not measure plasma levels of parathyroid hormone or 1,25(OH)2D, and therefore cannot examine whether these hormones mediate or confound our observed association. Third, we had insufficient power to examine these associations in black individuals; this is important because both vitamin D deficiency and hypertension are more prevalent in blacks than in whites. Fourth, we did not gather dietary information, nor did we know whether or not participants were using vitamin D supplements. Nonetheless, this study was cross-sectional, and plasma 25(OH)D levels were measured on blood samples that were collected on the same day that the study was performed. Therefore, dietary or supplemental vitamin D intake was reflected in the plasma 25(OH)D levels analyzed. Because we lacked information about calcium intake, however, we were unable to investigate interactions between vitamin D status and dietary and supplemental calcium. Fifth, we did not have any measurement of renal prostaglandin production in our participants, or whether they used non-steroidal anti-inflammatory drugs. Because renal prostaglandins may regulate the 1α-hydroxylase enzyme, and thus influence conversion of 25(OH)D to 1,25(OH)D,50 it would have been interesting to perform an additional analysis to test an interaction between vitamin D and renal prostaglandins.
Perspectives
Our findings in normotensive individuals are consistent with an association between low plasma 25(OH)D levels upregulation of the RAS. These finding may partly explain the higher risk of developing hypertension observed among individuals with vitamin D insufficiency and deficiency. Randomized trials should be performed to confirm or refute these observations.
Acknowledgements
None
Sources of Funding
This work was supported by NIH grants HL079929 and HL084236.
Footnotes
Conflicts of Interest/Disclosures
John P. Forman declares no competing interests.
Jonathan S. Williams declares no competing interests.
Naomi D.L. Fisher declares no competing interests.
References
- 1.Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003;16:72–75. doi: 10.1016/s0895-7061(02)03197-7. [DOI] [PubMed] [Google Scholar]
- 2.Duprez D, de Buyzere M, de Backer T, Clement D. Relationship between vitamin D3 and the peripheral circulation in moderate arterial primary hypertension. Blood Press. 1994;3:389–393. doi: 10.3109/08037059409102292. [DOI] [PubMed] [Google Scholar]
- 3.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin d levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 5.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes. 2008;57:2619–2625. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gannage-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol. 2009;160:965–971. doi: 10.1530/EJE-08-0952. [DOI] [PubMed] [Google Scholar]
- 7.Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 8.Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 9.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87:136–141. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 10.Kulah E, Dursun A, Aktunc E, Acikgoz S, Aydin M, Can M. Effects of angiotensin-converting enzyme gene polymorphism and serum vitamin D levels on ambulatory blood pressure measurement and left ventricular mass in Turkish hypertensive population. Blood Press Monit. 2007;12:207–213. doi: 10.1097/MBP.0b013e32813fa371. [DOI] [PubMed] [Google Scholar]
- 11.Landin-Wilhelmsen K, Wilhelmsen L, Wilske J, Lappas G, Rosen T, Lindstedt G, Lundberg PA, Bengtsson BA. Sunlight increases serum 25(OH) vitamin D concentration whereas 1,25(OH)2D3 is unaffected. Results from a general population study in Goteborg, Sweden (The WHO MONICA Project) Eur J Clin Nutr. 1995;49:400–407. [PubMed] [Google Scholar]
- 12.Pasco JA, Henry MJ, Nicholson GC, Brennan SL, Kotowicz MA. Behavioural and physical characteristics associated with vitamin D status in women. Bone. 2009;44:1085–1091. doi: 10.1016/j.bone.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Smotkin-Tangorra M, Purushothaman R, Gupta A, Nejati G, Anhalt H, Ten S. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab. 2007;20:817–823. doi: 10.1515/jpem.2007.20.7.817. [DOI] [PubMed] [Google Scholar]
- 15.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol. 2007;18:1796–1806. doi: 10.1681/ASN.2006091028. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, Li YC. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008;73:163–171. doi: 10.1038/sj.ki.5002572. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 20.Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 21.Burgess ED, Hawkins RG, Watanabe M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am J Hypertens. 1990;3:903–905. doi: 10.1093/ajh/3.12.903. [DOI] [PubMed] [Google Scholar]
- 22.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 23.Fisher ND, Price DA, Litchfield WR, Williams GH, Hollenberg NK. Renal response to captopril reflects state of local renin system in healthy humans. Kidney Int. 1999;56:635–641. doi: 10.1046/j.1523-1755.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 24.Hollenberg NK, Chenitz WR, Adams DF, Williams GH. Reciprocal influence of salt intake on adrenal glomerulosa and renal vascular responses to angiotensin II in normal man. J Clin Invest. 1974;54:34–42. doi: 10.1172/JCI107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollenberg NK, Williams GH, Burger B, Chenitz W, Hoosmand I, Adams DF. Renal blood flow and its response to angiotensin II. An interaction between oral contraceptive agents, sodium intake, and the renin-angiotensin system in healthy young women. Circ Res. 1976;38:35–40. doi: 10.1161/01.res.38.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Hollenberg NK, Williams GH, Taub KJ, Ishikawa I, Brown C, Adams DF. Renal vascular response to interruption of the renin-angiotensin system in normal man. Kidney Int. 1977;12:285–293. doi: 10.1038/ki.1977.113. [DOI] [PubMed] [Google Scholar]
- 27.Shoback DM, Williams GH, Moore TJ, Dluhy RG, Podolsky S, Hollenberg NK. Defect in the sodium-modulated tissue responsiveness to angiotensin II in essential hypertension. J Clin Invest. 1983;72:2115–2124. doi: 10.1172/JCI111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins PN, Lifton RP, Hollenberg NK, Jeunemaitre X, Hallouin MC, Skuppin J, Williams CS, Dluhy RG, Lalouel JM, Williams RR, Williams GH. Blunted renal vascular response to angiotensin II is associated with a common variant of the angiotensinogen gene and obesity. J Hypertens. 1996;14:199–207. doi: 10.1097/00004872-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Raji A, Williams GH, Jeunemaitre X, Hopkins PN, Hunt SC, Hollenberg NK, Seely EW. Insulin resistance in hypertensives: effect of salt sensitivity, renin status and sodium intake. J Hypertens. 2001;19:99–105. doi: 10.1097/00004872-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Kahonen M, Nappi S, Jolma P, Hutri-Kahonen N, Tolvanen JP, Saha H, Koivisto P, Krogerus L, Kalliovalkama J, Porsti I. Vascular influences of calcium supplementation and vitamin D-induced hypercalcemia in NaCl-hypertensive rats. J Cardiovasc Pharmacol. 2003;42:319–328. doi: 10.1097/00005344-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 31.London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Metivier F. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 32.Wakasugi M, Noguchi T, Inoue M, Kazama Y, Tawata M, Kanemaru Y, Onaya T. Vitamin D3 stimulates the production of prostacyclin by vascular smooth muscle cells. Prostaglandins. 1991;42:127–136. doi: 10.1016/0090-6980(91)90072-n. [DOI] [PubMed] [Google Scholar]
- 33.Wong MS, Delansorne R, Man RY, Vanhoutte PM. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2008;295:H289–296. doi: 10.1152/ajpheart.00116.2008. [DOI] [PubMed] [Google Scholar]
- 34.Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol. 1995;15:5789–5799. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyakoshi H, Aoki T, Hirasawa Y. Immunological effects of 1alpha-hydroxycholecalciferol (1alpha-OH-D3) and its metabolites. Clin Nephrol. 1981;16:119–125. [PubMed] [Google Scholar]
- 37.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Baynes KC, Boucher BJ, Feskens EJ, Kromhout D. Vitamin D, glucose tolerance and insulinaemia in elderly men. Diabetologia. 1997;40:344–347. doi: 10.1007/s001250050685. [DOI] [PubMed] [Google Scholar]
- 39.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 40.Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 41.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract. 1995;27:181–188. doi: 10.1016/0168-8227(95)01040-k. [DOI] [PubMed] [Google Scholar]
- 42.Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto FL, Wong KE, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-{kappa}B pathway. Am J Physiol Renal Physiol. 2009;296:F1212–1218. doi: 10.1152/ajprenal.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Brasier AR. Angiotensinogen gene activation by angiotensin II is mediated by the rel A (nuclear factor-kappaB p65) transcription factor: one mechanism for the renin angiotensin system positive feedback loop in hepatocytes. Mol Endocrinol. 1996;10:252–264. doi: 10.1210/mend.10.3.8833654. [DOI] [PubMed] [Google Scholar]
- 44.Graciano ML, Cavaglieri Rde C, Delle H, Dominguez WV, Casarini DE, Malheiros DM, Noronha IL. Intrarenal Renin-Angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol. 2004;15:1805–1815. doi: 10.1097/01.asn.0000131528.00773.a9. [DOI] [PubMed] [Google Scholar]
- 45.Karlsson C, Lindell K, Ottosson M, Sjostrom L, Carlsson B, Carlsson LM. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab. 1998;83:3925–3929. doi: 10.1210/jcem.83.11.5276. [DOI] [PubMed] [Google Scholar]
- 46.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 47.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 48.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 49.Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal. 2009;2:re4. doi: 10.1126/scisignal.275re4. [DOI] [PubMed] [Google Scholar]
- 50.Wark JD, Larkins RG, Eisman JA, Wilson KR. Regulation of 25-hydroxy-vitamin D-1 alpha-hydroxylase in chick isolated renal tubules: effects of prostaglandin E2, frusemide and acetylsalicylic acid. Clin Sci (Lond) 1981;61:53–59. doi: 10.1042/cs0610053. [DOI] [PubMed] [Google Scholar]