Abstract
Vesicle release from photoreceptor ribbon synapses is regulated by L-type Ca2+ channels, which are in turn regulated by Cl− moving through calcium-activated chloride [Cl(Ca)] channels. We assessed the proximity of Ca2+ channels to release sites and Cl(Ca) channels in synaptic terminals of salamander photoreceptors by comparing fast (BAPTA) and slow (EGTA) intracellular Ca2+ buffers. BAPTA did not fully block synaptic release, indicating some release sites are <100 nm from Ca2+ channels. Comparing Cl(Ca) currents with predicted Ca2+ diffusion profiles suggested that Cl(Ca) and Ca2+ channels average a few hundred nanometers apart, but the inability of BAPTA to block Cl(Ca) currents completely suggested some channels are much closer together. Diffuse immunolabeling of terminals with an antibody to the putative Cl(Ca) channel TMEM16A supports the idea that Cl(Ca) channels are dispersed throughout the presynaptic terminal, in contrast with clustering of Ca2+ channels near ribbons. Cl(Ca) currents evoked by intracellular calcium ion concentration ([Ca2+]i) elevation through flash photolysis of DM-nitrophen exhibited EC50 values of 556 and 377 nM with Hill slopes of 1.8 and 2.4 in rods and cones, respectively. These relationships were used to estimate average submembrane [Ca2+]i in photoreceptor terminals. Consistent with control of exocytosis by [Ca2+] nanodomains near Ca2+ channels, average submembrane [Ca2+]i remained below the vesicle release threshold (∼400 nM) over much of the physiological voltage range for cones. Positioning Ca2+ channels near release sites may improve fidelity in converting voltage changes to synaptic release. A diffuse distribution of Cl(Ca) channels may allow Ca2+ influx at one site to influence relatively distant Ca2+ channels.
INTRODUCTION
Visual responses originating in photoreceptor outer segments are transmitted to the rest of the visual system by altering synaptic release from the terminals of rods and cones. Synaptic vesicles are tethered near the active zone at a platelike structure known as the ribbon (Schmitz 2009). Glutamate release from photoreceptor synapses requires only submicromolar levels of Ca2+, much lower than Ca2+ levels typically required for vesicle release at other synapses (Beutner et al. 2001; Bollmann et al. 2000; Heidelberger et al. 1994; Rieke and Schwartz 1996; Schneggenburger and Neher 2000; Thoreson et al. 2004). Therefore synaptic release from photoreceptors does not necessitate the high levels of Ca2+ that are typically found only in nanodomains immediately adjacent to Ca2+ channels. Nevertheless, L-type Ca2+ channels that mediate vesicle release from photoreceptors are clustered in the terminal (Nachman-Clewner et al. 1999; Morgans 2001; Morgans et al. 2005; Specht et al. 2009; Steele Jr et al. 2005; Xu and Slaughter 2005) beneath synaptic ribbons (tom Dieck et al. 2005), suggesting that release sites are quite close to Ca2+ channels. However, it is also possible that synaptic release from photoreceptors might occur at ectopic sites located some distance from the ribbon, as occurs at bipolar cell ribbon synapses (Midorikawa et al. 2007; Zenisek et al. 2003, 2008).
In addition to stimulating vesicle release, Ca2+ influx stimulates Ca2+-activated chloride [Cl(Ca)] channels localized to photoreceptor terminals (Barnes and Hille 1989; Cia et al. 2004; MacLeish and Nurse 2007). In cones, where the reversal potential of chloride (ECl) is −38 mV (Thoreson and Bryson 2004), activation of Cl(Ca) channels will tend to stabilize the dark membrane potential. In rods, where ECl is −20 mV (Thoreson et al. 2002), activation of Cl(Ca) channels promotes membrane depolarization. Although depolarization enhances Ca2+ channel activity, the dominant effect of this Cl− efflux in rods appears to be an inhibition of Ca2+ channel activity by actions mediated at intracellular anion binding sites on Ca2+ channels (Babai et al. 2010; Thoreson et al. 2000). In this way, Cl− efflux can act as a negative feedback mechanism to limit excessive Ca2+ entry into rods. The strength of feedback interactions between Ca2+ and Cl(Ca) channels will be influenced by the distance between the two types of channels.
This study analyzed the spatial relationships between Ca2+ channels, vesicle release sites, and Cl(Ca) channels in photoreceptors by comparing electrophysiological measurements to profiles of Ca2+ diffusion predicted from a model developed by Ward and Kenyon (2000). The results indicate an extremely tight coupling between Ca2+ channels and ribbon release sites in cones, whereas Cl(Ca) channels are more dispersed throughout the synaptic terminals of rods and cones. Consistent with physiological data, antibodies to the putative Cl(Ca) channel, TMEM16A, produced more diffuse immunostaining in photoreceptor terminals than antibodies to synaptic Ca2+ channels. Similar to the use of Ca2+-activated K+ channels as submembrane Ca2+ sensors (Burrone et al. 2002; Roberts et al. 1990), we also measured the Ca2+ dependence of Cl(Ca) channels and used this relationship to convert Cl(Ca) current measurements into average submembrane Ca2+ levels in rod and cone terminals.
METHODS
Retinal tissue preparation
Experiments were performed using aquatic-phase tiger salamanders (Ambystoma tigrinum, 18–25 cm; Kons Direct, Germantown, WI or Charles Sullivan, Nashville, TN). Care, handling, and experimentation procedures were approved by the University of Nebraska Medical Center Institute for Animal Care and Use Committee or the UCLA Animal Research Committee. Animals were decapitated and pithed and each eye was enucleated. After surgical removal of the cornea, iris, and lens, the resultant eyecup was quartered. Eyecup pieces were placed vitreous side down onto filter paper (2 × 5 mm, type AAWP; 0.8 μm pores; Millipore, Bedford, MA) and the retina was isolated in a cold amphibian saline solution containing (in mM): 111 NaCl, 2.5 KCl, 1.8 CaCl2, 0.5 MgCl2, 10 HEPES, and 5 glucose (pH 7.8). Tissue was sliced into 125-μm-thick sections using a razorblade (#121-6; Ted Pella, Redding, CA) tissue slicer (Stoelting, Wood Dale, IL). Slices were placed in a recording chamber for viewing on an upright fixed-stage microscope (E600FN; Nikon, Tokyo), fitted with a ×60, 1.0 numerical aperture (NA) water-immersion objective (Nikon).
Calculation of free Ca2+ profiles
To model profiles of free Ca2+, we used an Excel-based macro program to calculate Ca2+ diffusion away from a voltage-gated L-type Ca2+ channel in the presence of diffusible buffers (Ward and Kenyon 2000; on-line at //www.medicine.nevada.edu/physio/docs/default.htm). Parameters for the simulation are provided in Table 1.
Table 1.
Parameters to determine free Ca2+ around a Ca2+ channel in the presence of a diffusible chelator
| Parameter | Value | Reference |
|---|---|---|
| DCa | 0.2 μm2/ms | Neher 1986 |
| ICa | 0.25 pA | Church and Stanley 1996 |
| EGTA Kd | 1.58 × 10−7 M | Naraghi 1997 |
| EGTA Kon | 2.7 × 106 M−1 · S−1 | Naraghi 1997 |
| BAPTA Kd | 1.8 × 10−7 M | Naraghi 1997 |
| BAPTA Kon | 4.5 × 108 M−1 · S−1 | Naraghi 1997 |
Electrophysiology
Patch pipettes were pulled on a PP-830 vertical puller (Narishige USA, East Meadow, NY) from borosilicate glass pipettes (OD: 1.2 mm; ID: 0.9 mm, with internal filament; World Precision Instruments, Sarasota, FL) and had tips <2 μm in diameter with resistance values ranging between 12 and 18 MΩ. For Ca2+ buffering experiments, we used a pipette solution containing (in mM): 42 CsCl, 48 Cs-gluconate, 1.9 MgCl2, 32.9 HEPES, 9.4 tetraethylammonium chloride (TEA-Cl), 9.4 magnesium adenosine triphosphate (MgATP), 0.5 guanosine triphosphate (GTP), plus 0.5 ethylene glycol tetraacetic acid (EGTA), 5 EGTA, or 5 1,2-bis(o-aminophenoxy)ethane,N,N,N′,N′-tetraacetic acid (BAPTA) (pH 7.2). For measurements of depolarization-evoked tail current amplitudes illustrated in Figs. 8 and 9, we used a pipette solution with a predicted ECl of −39 mV that consisted of (in mM): 11.3 CsCl, 75 Cs-gluconate, 1.9 MgCl2, 32.9 HEPES, 9.4 TEA-Cl, 9.4 MgATP, 0.5 GTP, and 5 EGTA (pH 7.2). We adjusted membrane potentials with this solution for a measured liquid junction potential of −7 mV.
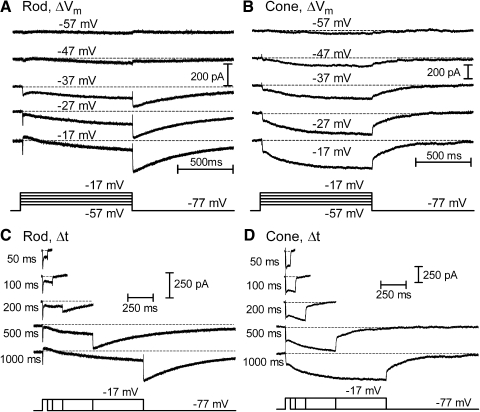
Fig. 8.
Tail currents evoked by depolarizing test steps over a range of voltages (−57 to −17 mV) and durations (50 ms to 1 s). A and B each show the currents evoked in a rod (A) and cone (B) by changes in the amplitude of a 1-s test step from −57 to −17 mV (10-mV increments). Small inward ICa is detectable during steps to −57 mV and above. Cl(Ca) tail currents become visible with steps to −47 mV and above. C and D each illustrate currents evoked by changing the duration of a strong test step (−77 to −17 mV) from 50 ms to 1 s in a rod (C) and cone (D). Inward ICa values were evoked by the step and Cl(Ca) tail currents were observed following termination of the test step with pulses of ≥200 ms in these examples. Passive membrane resistance (Rm) and membrane capacitance (Cm) were subtracted using a P/8 protocol. ECl = −39 mV.
Fig. 9.
Conversion of the amplitude of depolarization-evoked Cl(Ca) tail currents into submembrane [Ca2+] for different test step voltages and durations. ICl(Ca) (A, rods; B, cones) amplitude was converted into [Ca2+]i (C, rods; D, cones) attained at Cl(Ca) channels using the sigmoidal curves fit to the data in Fig. 7. Currents exceeding 153 pA in cones or 134 pA in rods were assigned a concentration value of 10 μM. Stimuli that failed to evoke inward tail currents were assigned a concentration value of 31 nM.
Recording electrodes were positioned with Huxley–Wall micromanipulators (Sutter Instrument, Novato, CA) and visualized through the eyepieces or with a video camera (Watec 502H; Rock House Products International, Middletown, NY) mounted on the microscope. Photoreceptors were voltage clamped using a Multiclamp or Axopatch 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA). Currents were acquired using a Digidata 1322 interface and pCLAMP 9.2 software (Molecular Devices). In all experiments, retinal slices were superfused with the amphibian saline solution described earlier bubbled with 100% O2.
Flash photolysis of DM-nitrophen
Flash photolysis of the photolysable Ca2+ chelator, DM-nitrophen (Invitrogen, Carlsbad, CA), produces rapid and spatially homogeneous increases in intracellular calcium ion concentration ([Ca2+]i) throughout the terminal. [Ca2+]i measured at the center of the terminal therefore provides a measure of the change in submembrane [Ca2+]. The pipette solution for photolysis experiments consisted of (in mM): 10 DM-nitrophen, 5 CaCl2, 4 diethylenetriaminepentaacetic acid, 4 MgCl2, 26 Cs-gluconate, 78 HEPES, 6.5 TEA-Cl, 11 Na2ATP, 0.5 GTP (pH 7.2). DM-nitrophen was photolyzed by flashes of UV light derived from a xenon arc flash lamp (Rapp Optoelectronic, Hamburg, Germany). Oregon Green BAPTA-6F (OGB-6F, 0.5 mM, Kd = 3 μM; Invitrogen) was also included in the pipette solution to measure [Ca2+]i. Confocal Ca2+ measurements were made using a laser confocal scanhead (UltraVIEW Live Cell Imaging System; PerkinElmer, Waltham, MA), equipped with a cooled charge-coupled device camera (Orca ER, Hamamatsu, Hamamatsu City, Japan) mounted onto a fixed-stage upright microscope (E600 FN). Images were acquired at 60- to 150-ms intervals with single-frame durations of 48–145 ms and pixel values were binned 2 × 2. To convert OGB-6F fluorescence measurements into Ca2+ levels, we used the following formula (Helmchen 2000)
| (1) |
where ΔF/F represents the fractional change in fluorescence resulting from stimulation. (ΔF/F)max was determined from the maximal fluorescence change produced by application of 500-ms depolarizing steps to −10 mV. We used the Kd of 3 μM for OGB-6F provided by Invitrogen. The resting Ca2+ concentration ([Ca2+]rest) for each solution was determined ratiometrically using 0.2 mM Fura-2 as described previously (Thoreson et al. 2004). As a test of Ca2+ measurements obtained with Eq. 1, we compared OGB-6F measurements with measurements made using a higher-affinity dye, OGB- 1 (Kd = 0.17 μM). We evoked intraterminal calcium changes by applying depolarizing test steps (−70 to −10 mV) of varying duration (50–500 ms). Despite large differences in the Kd, the same depolarizing stimuli yielded similar intraterminal [Ca2+] measurements with the two different dyes (Choi et al. 2008).
The increase in Cl(Ca) current as a function of increasing Ca2+ was fit with a sigmoidal binding curve
| (2) |
where h is the slope factor and Imax is the top of the sigmoidal curve. The bottom value was constrained to zero.
Immunohistochemistry
Salamanders were placed in an induction tank (∼10 L) containing MS-222 (2 g/L). Following induction, the animal was removed and decapitated with large shears, after which the brain and spinal cord were rapidly pithed. After enucleation, each eye was opened along the ora serrata; the cornea, lens, and vitreous body were then removed and the eyecups were immersion-fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4) for 15 min at room temperature. The eyecups were then cryoprotected in 25% sucrose overnight at 4°C. Prior to cutting the tissue with a cryostat the retina was washed with 0.1 M PB, embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and rapidly frozen with dry ice or liquid nitrogen. Cryostat sections of the retina were cut at 12–15 μm, mounted onto gelatin-coated slides, air dried, and stored at −20°C.
All tissue was labeled using the indirect immunofluorescence technique (Stella Jr et al. 2007). Briefly, retinal sections were warmed for 10 min at 37°C. Tissue was preincubated in a 0.1 M PB mixture containing 10% normal goat serum (NGS) or donkey serum (DS; Invitrogen), 1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO), and 0.5% Triton-X 100 (Sigma-Aldrich) for 1 h. The sections were then incubated in primary antibodies overnight at 4°C, which were all diluted in 0.1 M PB (pH 7.4) containing 3% NGS or DS, 1% BSA, and 0.5% Triton-X 100. The primary antibody/antigen complex was detected using secondary antibodies conjugated to either Alexa 488 or Alexa 568 (Invitrogen). Following incubation with the antibody, retinal sections were washed three times for 10 min with 0.1 M PB to remove any unbound primary or secondary antibody. For double-labeling experiments tissue sections were incubated in a mixture of primary antibodies followed by a mixture of secondary antibodies. All slides were allowed to air dry in the dark at room temperature and coverslipped with Prolong Gold anti-fade (Invitrogen).
Images of retinal sections were acquired using a Zeiss Laser Scanning Microscope 510 META (Zeiss, Thornwood, NY) with a C-Apochromat ×40 1.2 NA water objective. To identify fluorescent signals, different lasers were used for excitation: for Alexa 488 the 488-nm argon laser line was used and for Alexa 568 the 543-nm HeNe laser line was used. Little or no staining was observed when using the secondary antibodies alone. During acquisition of signals from double-labeled specimens, scans were collected sequentially to prevent spectral bleed-through. Specific band-pass filters were used to achieve proper separation of signals (for double labeling 488/505–530, 543/560LP). In some scans, fluorescent emissions were further separated using a linear unmixing algorithm (Zeiss LSM ver. 4.2). To validate this process and reduce any potential mismatch or bleed-through of fluorescence among different channels, separation of fluorescent emissions was achieved by processing reference samples that were immunostained with a single label to visualize their entire emission spectra and localization within the retina. Most images were acquired at a 12-bit resolution of 2,048 × 2,048 and, in some cases, 1,024 × 1,024. To increase signal-to-noise ratio, images were averaged on-line (e.g., n = 4) and the scan speed and photo multiplier detector gain were decreased. The digital fluorescent images were single confocal scans taken in the same planes as corresponding differential interference contrast (DIC) images. Most digital images were acquired at an approximate optical thickness of 0.5 μm or 1.0 Airy units. Digital images were saved as Zeiss .LSM files and final publication quality images were exported in .TIFF format at 300 dpi. Images were processed and adjusted for brightness and contrast using Adobe Photoshop CS4 Extended (Adobe Systems, Mountain View, CA).
Antibodies
A rabbit polyclonal antibody to TMEM16A raised against a 620 amino acid peptide was used at a dilution of 1:500 (ab53212; Abcam, Cambridge, MA). This antibody has been shown to react with both human and rodent sequences (manufacturer's data sheet). We also used another rabbit polyclonal antibody raised against a 17 amino acid segment on the N terminus (1:100, SIG5419; Zyagen, San Diego, CA). A mouse monoclonal antibody against glial fibillary acidic protein (GFAP) was used at a dilution of 1:1,000 (catalog no. CH 22102; Neuromics, Edina, MN) to identify Müller cells in the salamander retina (Sassoè Pognetto et al. 1992). A sheep polyclonal antibody raised against amino acids 712 to 730 of the human α1F calcium channel pore-forming subunit (a generous gift from Dr. Catherine Morgans, OHSU, Portland, OR) was used at a dilution of 1:100 to label calcium channels on terminals of photoreceptors (Morgans 2001). Staining in the retina with this antibody was blocked by the peptide used to develop the antibody (Morgans 2001). A monoclonal antibody raised against the synaptic vesicle protein, SV2 (Developmental Studies Hybridoma Bank at the University of Iowa, Iowa City, IA), was used at a dilution of 1:2,000 to label synaptic terminals of photoreceptors in the tiger salamander retina (Mandell et al. 1990; Yang et al. 2002; Zhang and Wu 2009).
Unless otherwise specified, chemicals were obtained from Sigma-Aldrich. The criterion for statistical significance was chosen to be P < 0.05 and evaluated using Student's t-test or one-way ANOVA and GraphPad Prism 4.0. Variability is reported as ±SE.
RESULTS
Distance from Ca2+ channels to release sites
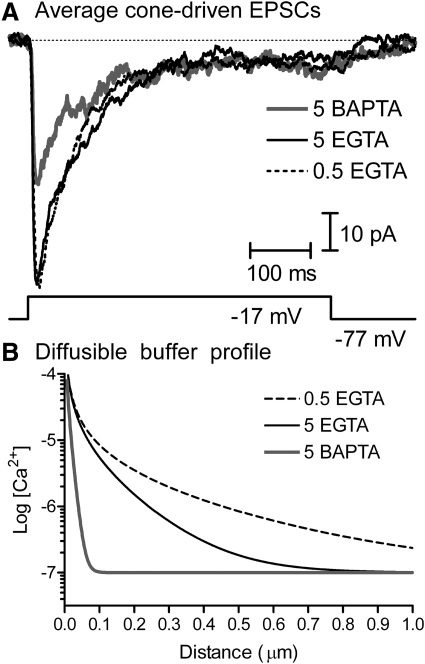
Ca2+ channels are clustered in photoreceptor synaptic terminals (Morgans 2001; Morgans et al. 2005; Nachman-Clewner et al. 1999; Specht et al. 2009; Xu and Slaughter 2005) at the base of the ribbon (tom Dieck et al. 2005), suggesting that release sites are likely to be very close to Ca2+ channels. We analyzed the distance between Ca2+ channels and vesicle release sites by comparing the effects of different diffusible buffers (EGTA or BAPTA) on synaptic release from cones. BAPTA and EGTA have a similar high affinity for Ca2+ but BAPTA has a much faster Kon rate, so it chelates Ca2+ very rapidly near the mouth of a Ca2+ channel. Buffers were introduced into voltage-clamped cones through the patch pipette. To examine effects of the different Ca2+ buffers on synaptic release, cones were depolarized from −77 to −17 mV for 500 ms and postsynaptic currents (PSCs) were recorded simultaneously from voltage-clamped horizontal or off bipolar cells. Recordings were conducted in the presence of cyclothiazide (0.1 mM) to block α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor desensitization. We waited ≥10 min to allow time for diffusion of the Ca2+ buffer to the photoreceptor terminal. Effects of the different buffers on cone-driven PSCs averaged from multiple cell pairs are illustrated in Fig. 1A (0.5 mM EGTA, n = 10; 5 mM EGTA, n = 12; 5 mM BAPTA, n = 7). Cone-driven PSC waveforms were essentially unchanged by elevating the EGTA concentration from 0.5 (dashed line) to 5 mM (black solid line) and diminished, but not fully blocked, by the use of 5 mM BAPTA (gray solid line). Excitatory postsynaptic currents (EPSCs) evoked with shorter 25-ms test pulses were also not blocked by BAPTA (comparison of charge transfer during EPSCs with 0.5 EGTA, 5 EGTA, and 5 BAPTA: P = 0.25, one-way ANOVA), indicating that the persistence of synaptic responses was probably not due to saturation of BAPTA during the 500-ms depolarizing step. Sustained components of the cone-driven PSC were also not blocked by BAPTA, consistent with the hypothesis that both transient and sustained release from cones occurs principally at the ribbon (Jackman et al. 2009).
Fig. 1.
Effects of the diffusible Ca2+ buffers EGTA and BAPTA on EPSCs evoked in horizontal or off bipolar cells by depolarizing steps (−77 to −17 mV for 500 ms) applied to voltage-clamped cones. Records in A show the average postsynaptic current (PSC) from multiple cone/horizontal cell and cone/off bipolar cell pairs obtained after waiting >10 min for diffusion of 5 mM BAPTA (n = 7, solid gray line), 5 mM EGTA (n = 12, solid black line), or 0.5 mM EGTA (n = 10, dashed line) to the cone terminal. Cyclothiazide (0.1 mM) was present in these experiments to prevent AMPA receptor desensitization. EPSCs were diminished but not fully blocked by the addition of 5 mM BAPTA to the patch pipette solution. B: decline in [Ca2+] as a function of distance from an open Ca2+ channel predicted from the “Pore” macro (Ward and Kenyon 2000) with Ca2+ buffering by 5 mM BAPTA (solid gray line), 5 mM EGTA (solid black line), or 0.5 mM EGTA (dashed black line). Model parameters are provided in Table 1. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BAPTA, 1,2-bis(o-aminophenoxy)ethane,N,N,N′,N′-tetraacetic acid; EGTA, ethylene glycol tetraacetic acid; EPSC, excitatory postsynaptic current.
We also tested the effects of these chelators in rods. Like cones, BAPTA did not block PSCs evoked in horizontal cells by test steps applied to voltage-clamped rods (data not shown). However, the persistence of rod-driven PSCs is more difficult to interpret since rod-driven PSCs are at least partly due to synaptic output from neighboring rods coupled to the voltage-clamped cell through gap junctions (Attwell et al. 1984; Cadetti et al. 2005; Zhang and Wu 2004).
We confirmed that Ca2+ buffers could diffuse to the synaptic terminal by comparing intraterminal Ca2+ levels measured with OGB-6F fluorescence in rods 5–10 min after patch rupture (Supplemental Fig. S1).1 The rise in intraterminal Ca2+ evoked by a 100-ms depolarization from −77 to −17 mV declined after the step much more rapidly when the patch pipette contained 5 mM EGTA (τ = 0.22 s, n = 6) or BAPTA (τ = 0.19 s, n = 5) than with 0.5 mM EGTA (τ = 51 s, n = 6). Although the time course of decline is influenced by extrusion, it is dominated by Ca2+ binding to the buffer so these results show that buffers can diffuse through the axon to the rod terminal. Cone pedicles do not possess a thin axon and are thus more accessible to buffers introduced through a patch pipette on the cell body (Mariani 1986; Sherry et al. 1998).
The profiles of [Ca2+] surrounding individual open Ca2+ channels predicted for different buffering conditions were simulated using a model developed by Ward and Kenyon (2000). Parameters for [Ca2+]i simulations under each buffering condition are provided in Table 1 and the predicted Ca2+ profiles are illustrated in Fig. 1B. With 5 mM BAPTA, free Ca2+ surrounding a Ca2+ channel should decline below the release threshold of 400 nM (Duncan et al. 2010; Thoreson et al. 2004) within 50 nm. Even if BAPTA levels attained a concentration at the synapse of only 0.5 mM, 10-fold lower than the pipette concentration, then Ca2+ levels should still fall below release threshold within 100 nm of a Ca2+ channel. Thus conservatively, the persistence of depolarization-evoked PSCs in the presence of BAPTA indicates that Ca2+ channels in cones are <100 nm from synaptic release sites.
Distance from Ca2+ channels to Cl(Ca) channels
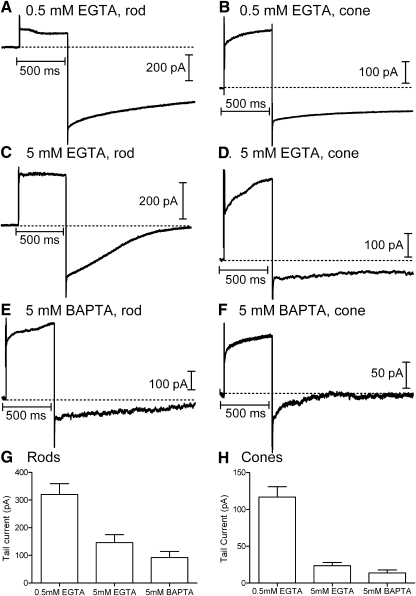
Depolarizing test steps (−77 to −17 mV) applied to rods and cones activate L-type ICa, voltage-dependent K+, Ca2+-activated K+, and Cl(Ca) currents (Attwell and Wilson 1980; Bader et al. 1982; Barnes and Hille 1989). Whole cell currents evoked by depolarizing test steps (−70 to −10 mV, 500 ms) were typically dominated by outward currents (Fig. 2). Using a pipette solution in which ECl = −20 mV, long-lasting inward tail currents were observed after termination of the test step (Fig. 2). The duration of Cl(Ca) tail currents lasts ≤12 s and exhibits considerable variability, declining in direct proportion to the decline in intracellular Ca2+ levels (MacLeish and Nurse 2007). Tail currents could be inhibited by the Cl− channel blocker niflumic acid (0.1 mM) and reversed around ECl (n = 11 for rods and n = 8 for cones; data not shown), consistent with other studies indicating that tail currents reflect calcium-dependent chloride current [ICl(Ca)] activity (Barnes and Deschenes 1992; MacLeish and Nurse 2007; Thoreson et al. 2003). Tail currents were not significantly inhibited by bath application of the vesicular glutamate uptake inhibitor d-threo-β-benzyloxyaspartate (TBOA, 0.1 mM), indicating little contribution from Cl− efflux through excitatory amino acid transporter anion channels (rods: 5 mM BAPTA, P = 0.70, paired t-test, n = 12; 5 mM EGTA, P = 0.70, n = 3; 0.5 mM EGTA, P = 0.64, n = 3; cones: 5 mM BAPTA, P = 0.71, n = 7; 5 mM EGTA, P = 0.97, n = 3; 0.5 mM EGTA, P = 0.54, n = 3).
Fig. 2.
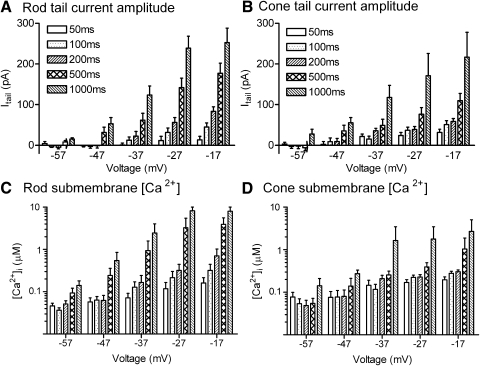
Effects of the diffusible Ca2+ buffers EGTA and BAPTA on depolarization-evoked Ca2+-activated chloride [Cl(Ca)] tail currents in rods and cones. Rod and cone photoreceptors were depolarized by application of a test step from −77 to −17 mV for 500 ms. Peak calcium-dependent chloride current [ICl(Ca)] activation was measured 15 ms after the depolarizing step to avoid recording contamination from the capacitative transient. Depolarization-evoked tail currents arising from the activation of ICl(Ca) persisted in both rods and cones in the presence of 0.5 mM EGTA (A and B), 5 mM EGTA (C and D), and 5 mM BAPTA (E and F). G: mean tail current amplitude measured in rods with Ca2+ buffering provided by 0.5 mM EGTA (316.0 ± 35.0 pA, n = 24), 5 mM EGTA (120.7 ± 21.4 pA, n = 14), and 5 mM BAPTA (78.7 ± 14.6 pA, n = 23). Tail currents were measured 15 ms after the end of the step. Measurements were made ≥10 min after patch rupture. H: tail current amplitudes in cones with Ca2+ buffering provided by 0.5 mM EGTA (123.3 ± 12.4 pA, n = 17), 5 mM EGTA (38.1 ± 8.64 pA, n = 8), and 5 mM BAPTA (27.0 ± 6.70 pA, n = 14).
Like Ca2+ channels, Cl(Ca) channels are located almost entirely in the synaptic terminals of photoreceptors (MacLeish and Nurse 2007). To analyze the distance between Ca2+ and Cl(Ca) channels in rods and cones, Cl(Ca) tail currents in rods and cones were measured after introducing different concentrations of Ca2+ buffers (0.5 mM EGTA, 5 mM EGTA, and 5 mM BAPTA) into the photoreceptor through the recording pipette. We waited ≥10 min for buffers to diffuse to the synaptic terminal. Figure 2 illustrates recordings obtained from rods and cones using the different buffers. The bar graphs in Fig. 2, G and H show the average amplitude of tail currents measured 15 ms after termination of the test step with the different buffers. In both rods and cones, tail currents were significantly diminished by increasing the EGTA concentration from 0.5 to 5 mM (t-test, rods, P = 0.003; cones P = 0.002, Fig. 2, G–H). BAPTA caused a further reduction in tail currents but did not block them completely. Although the persistence of Cl(Ca) tail currents in the presence of 5 mM BAPTA may be partly due to partial saturation of BAPTA during the lengthy depolarizing step, it also suggests that a small population of Cl(Ca) channels is located within 100 nm of Ca2+ channels.
To estimate the average distance between Ca2+ and Cl(Ca) channels, we compared buffer effects on tail currents with profiles of free Ca2+ predicted for the three buffering conditions (Fig. 1B; Naraghi 1997; Ward and Kenyon 2000). In rods, increasing EGTA from 0.5 to 5 mM reduced tail currents by 82% relative to the greater reduction produced by 5 mM BAPTA. By comparison, the predicted Ca2+ profiles suggest that increasing EGTA from 0.5 to 5 mM should reduce [Ca2+] by 82% relative to the levels predicted for 5 mM BAPTA at a distance of 395 nm from the Ca2+ channel (Fig. 1B). In cones, increasing EGTA from 0.5 to 5 mM reduced tail currents by 89% relative to the reduction produced by 5 mM BAPTA. The same buffer-induced change in Ca2+ levels should occur at a distance of 490 nm from the Ca2+ channel (Fig. 1B). Measurements of the Ca2+ dependence of Cl(Ca) channels described later suggested that their activation is likely to require the binding of two Ca2+ ions. If so, the decline in [Ca2+] should cause a squared decline in Cl(Ca) activation, which would in turn suggest distances of 206 and 263 nm between Ca2+ and Cl(Ca) channels in rods and cones, respectively.
In another approach to assess the average distance between Ca2+ channels and Cl(Ca) channels, we used the calcium dependence of ICl(Ca) to estimate [Ca2+]i from ICl(Ca) amplitude. Sigmoidal concentration–response curves were generated using the half-maximal effective concentration (EC50) and Hill slope values for Cl(Ca) channels, determined from caged Ca2+ experiments described in the following text. Peak current amplitudes were assumed to be slightly larger than the average currents recorded with 0.5 mM EGTA (130 and 330 pA for cones and rods, respectively). Using these curves, we converted tail current measurements into [Ca2+]i and then matched these Ca2+ levels to the predicted decline in Ca2+. For 5 mM EGTA, this yielded estimated distances of 358 and 478 nm in rods and cones, respectively. Estimates with 0.5 mM EGTA are more sensitive to values chosen for the top of the sigmoidal curve, but repeating this procedure with data for 0.5 mM EGTA yielded estimates of 296 and 365 nm for rods and cones, respectively. Distance estimates also depend on parameters used for simulation of the Ca2+ decline. For example, using slightly slower on rates for Ca2+ binding to EGTA from Stern (1992; 1.5 × 106 M−1 · S−1), we obtained distances with 5 mM EGTA of 454 and 596 nm in rods and cones, respectively. Although the precise values differ somewhat, results of these different analyses all agree that the distance between Ca2+ and Cl(Ca) channels averages a few hundred nanometers. The inability of BAPTA to block tail currents completely in rods and cones indicates that there is also a subpopulation of Cl(Ca) channels that are very close to Ca2+ channels. Due to the limited spatial spread of Ca2+ when using BAPTA as a buffer, the amplitude of tail currents measured in the presence of 5 mM BAPTA suggested that Ca2+ and Cl(Ca) channels are separated by only about 60 nm. Together, these results suggest that Cl(Ca) channels are dispersed throughout the synaptic terminal, with some channels quite close to Ca2+ channels and others further away.
Immunohistochemical localization of Cl(Ca) channels
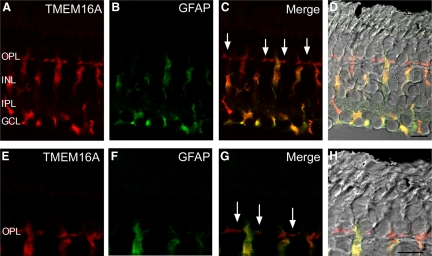
Based on expression cloning and physiological data, TMEM16A has been implicated as a Cl(Ca) channel (Caputo et al. 2008; Hartzell et al. 2009; Rock et al. 2009; Romanenko et al. 2010; Schroeder et al. 2008; Yang et al. 2008). TMEM16A mRNA is present in the outer retina (Gritli-Linde et al. 2009) and the related protein TMEM16B also appears to form Ca2+-activated chloride channels localized to mammalian photoreceptor terminals (Stöhr et al. 2009). We tested whether TMEM16A might contribute to Cl(Ca) channel activity in photoreceptors. Consistent with the presence of TMEM16A protein in photoreceptor terminals, indirect immunofluorescence with an antibody to TMEM16A produced labeling of the outer plexiform layer (OPL) in the tiger salamander retina (Fig. 3A). In addition, TMEM16A labeled cells in the inner nuclear layer (INL) that could also be immunolabeled with antibodies for glial fibrillary acidic protein (GFAP) to label Müller cells (Fig. 3, A–D). This is consistent with electrophysiological evidence for Cl(Ca) channels in salamander Müller cells (Welch et al. 2006). Higher magnification of the OPL suggests that TMEM16A immunolabels photoreceptor terminals (arrows in Fig. 3 indicate rod and cone terminals).
Fig. 3.
TMEM16A is expressed in both the outer plexiform layer (OPL) and Müller cell bodies, which span the entire retina. A: retinal section immunolabeled for TMEM16A (red). B: Müller cells immunolabeled in a retinal section with glial fibillary acidic protein (GFAP, green). C: merged fluorescent image of TMEM16A and GFAP. D: merged TMEM16A (red) and GFAP (green) immunofluorescence with the differential interference contrast (DIC) brightfield image. Arrows in C indicate labeling of TMEM16A in photoreceptor terminals. E: high-magnification zoom region in the OPL illustrating TMEM16A immunolabeling in the synaptic terminals of photoreceptors (red). F: GFAP labeling of the same high-magnification region (green). G: merged fluorescent high-magnification image of TMEM16A and GFAP. H: merged TMEM16A (red) and GFAP (green) with the differential interference contrast (DIC) brightfield image. Arrows in G indicate labeling of TMEM16A in individual photoreceptor terminals. OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars = 10 μm.
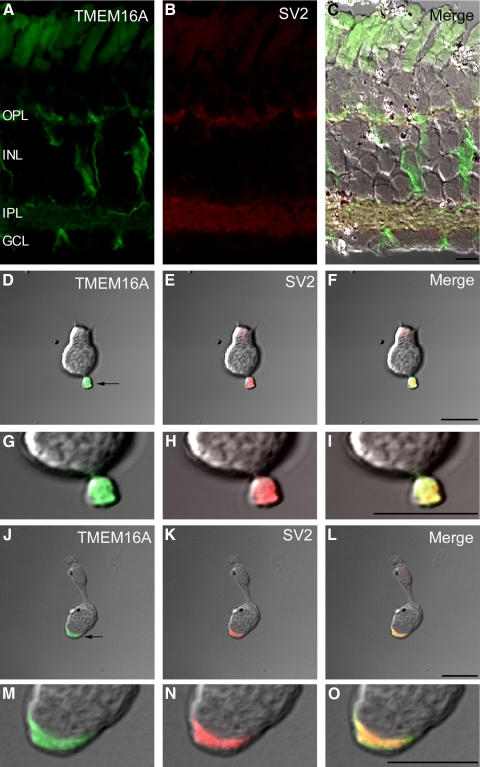
To test whether TMEM16A is expressed in photoreceptor terminals, retinal sections and isolated photoreceptors were double-immunolabeled with the TMEM16A antibody and an antibody to the synaptic vesicle protein, SV2 (Zhang and Wu 2009). TMEM16A expression overlapped with and was limited to SV2 expression in both vertical retinal sections (Fig. 4, A–C) and within the synaptic terminals of isolated photoreceptors (arrows, Fig. 4, D–I), indicating that TMEM16A is localized to the synaptic terminals of photoreceptors. The overlap between TMEM16A and SV2 expression is also consistent with electrophysiological results, suggesting that Cl(Ca) channels are dispersed throughout the presynaptic terminal.
Fig. 4.
TMEM16A and SV2 labeling colocalize at photoreceptor terminals. A: immunolabeling of TMEM16A (green) in a vertical retinal section. B: immunolabeling of the same retinal section with a synaptic vesicle marker, SV2 (red). Autofluorescence in the outer segments was observed without secondary antibodies. C: merged fluorescent and DIC image of TMEM16A and SV2. D: brightfield DIC image of a rod photoreceptor lacking its outer segment immunolabeled with TMEM16 (green). E: brightfield DIC image of the same rod immunolabeled with SV2 (red). F: merged brightfield DIC image with both TMEM16A and SV2. G–I: magnified images of the same rod terminal showing that TMEM16A overlaps completely with SV2. J–L: brightfield DIC images of a small single cone photoreceptor immunolabeled with TMEM16A (green, J), SV2 (red, K), or both (L). M–O: magnified images of the same cone terminal showing that TMEM16A overlaps completely with SV2. OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Synaptic terminal regions are indicated by the arrows in D and J. Scale bar is 10 μm.
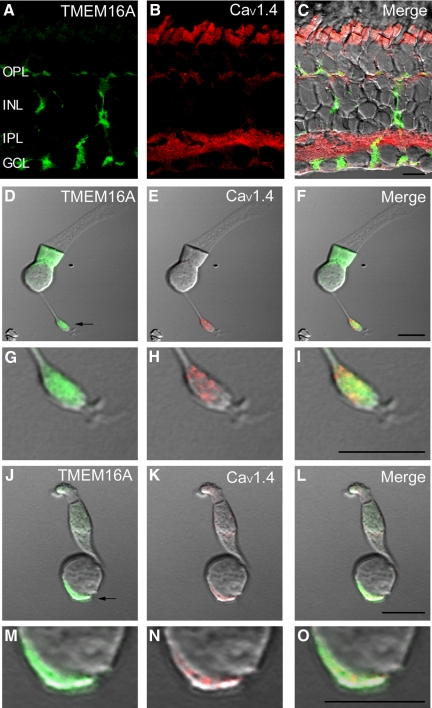
The retina-specific protein CaV1.4 (α1F) has been implicated as the pore-forming subunit of the Ca2+ channel underlying transmitter release from photoreceptors (Bech-Hansen et al. 1998; Firth et al. 2001; Morgans 2001). Similar to the pattern observed in other species (Firth et al. 2001; Morgans 2001), antibodies to CaV1.4 labeled the OPL, photoreceptor inner segments, and IPL of salamander retina (Fig. 5B). CaV1.4 labeling overlapped with TMEM16A in both the OPL and terminals of isolated photoreceptors (Fig. 5). High-magnification images showed punctate labeling of CaV1.4 in rod and cone terminals (Fig. 5, G–I and M–O). The TMEM16A antibody produced more diffuse labeling of photoreceptor terminals.
Fig. 5.
TMEM16A and the Ca2+ channel CaV1.4 are both expressed at photoreceptor terminals. A: TMEM16A labeling (green) in a vertical retinal section. B: CaV1.4 labeling (red) in the same retinal section. C: merged fluorescent and DIC image of TMEM16A and CaV1.4. D–F: brightfield DIC images of a rod photoreceptor immunolabeled with TMEM16A (green, D), CaV1.4 (red, E), or both (F). Autofluorescence in the inner segment region with the green channel was observed without the secondary antibody and is likely due to the presence of NADH and NADPH in the mitochondrial-rich ellipsoid. G–I: magnified images of the same rod terminal illustrate the more punctate expression of CaV1.4 (H) compared with the diffuse labeling of TMEM16A (G) throughout the terminal region. J–L: brightfield DIC images of a large single cone photoreceptor immunolabeled with TMEM16A (green, J), CaV1.4 (red, H), or both (I). M–O: magnified images of the same cone terminal illustrate the more punctate expression of CaV1.4 (N) compared with the diffuse expression of TMEM16A (M) throughout the terminal region. OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Synaptic terminal regions are indicated by the arrows in D and J. Scale bar is 10 μm.
It was not practical to synthesize an antigenic peptide for the Abcam antibody that was raised against a 620 amino acid peptide, so to assess the specificity of labeling in the OPL and photoreceptor terminals, we tested another antibody raised against a different epitope of TMEM16A (Zyagen, San Diego, CA). This antibody showed a similar labeling pattern with immunofluorescence visible in the OPL and IPL, along with diffuse labeling of the terminals of isolated rods and cones (Supplemental Figs. S2 and S3). Immunolabeling was abolished by preincubation with the peptide used to develop the antibody (Supplemental Fig. S2B). We also examined methanol-fixed cells and found that, consistent with results from paraformaldehyde-fixed cells, TMEM16A antibodies produced diffuse staining of the terminal and surrounding regions, whereas CaV1.4 antibodies showed a more concentrated distribution in the synaptic terminals of rods and cones (Supplemental Fig. S3). Together, these anatomical findings are consistent with physiological results, suggesting that Cl(Ca) channels are distributed more diffusely than Ca2+ channels.
Ca2+ dependence of Cl(Ca) channels
We measured the Ca2+ dependence of Cl(Ca) channels in rods and cones by abruptly elevating submembrane Ca2+ levels in photoreceptor terminals via flash photolysis of the caged Ca2+ compound, DM-nitrophen (10 mM). After introduction of DM-nitrophen into the photoreceptor through the patch pipette, flash photolysis produces an instantaneous and homogeneous rise in intraterminal [Ca2+] that can be quantified from the change in fluorescent intensity of OGB-6F (Fig. 6). Rods and cones were voltage-clamped at a membrane potential of −77 mV (illustrated in the left panels of Fig. 6, A and B), below the estimated value of ECl = −39 mV. The activation of Cl(Ca) channels by the increase in [Ca2+]i generated inward currents in both rods and cones (Fig. 6). The amplitude of inward currents evoked in rods and cones by flash photolysis of caged Ca2+ did not differ significantly (P = 0.8, rods; P = 0.54, cones) between trials in which flash photolysis evoked detectable glutamate release (rods, n = 19; cones, n = 14) and trials that failed to evoke a postsynaptic response in simultaneously voltage-clamped horizontal or off bipolar cells (rods, n = 69; cones, n = 20). This is consistent with effects of TBOA described earlier and suggests a minimal contribution from glutamate transporter currents to the inward currents evoked in photoreceptors by flash photolysis of caged Ca2+.
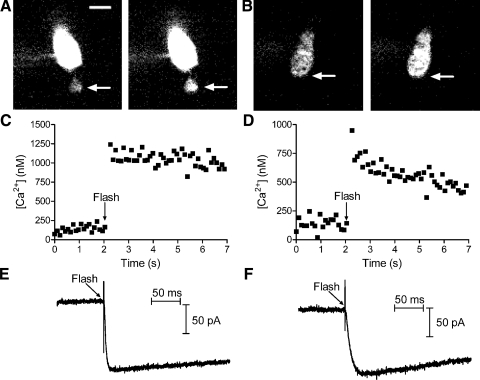
Fig. 6.
Flash photolysis of caged Ca2+ in photoreceptor terminals instantaneously elevated intraterminal [Ca2+] and stimulated inward Cl(Ca) currents. A: grayscale images of Oregon Green BAPTA-6F (OGB-6F) fluorescence in a single confocal section from a rod loaded with the reagent DM-nitrophen. Images were obtained prior to flash photolysis (left) and immediately after flash photolysis (right). Intracellular calcium ion concentration ([Ca2+]i) was measured from a region of interest placed in the synaptic terminal (arrows). Fluorescence is brighter in the soma due to the presence of more dye. B: grayscale images of OGB-6F fluorescence in a single confocal section from a cone before (left) and after (right) flash photolysis of DM-nitrophen. C and D show intraterminal [Ca2+] measured at 60-ms intervals in the rod (C) and cone (D). E and F show inward Cl(Ca) currents evoked in the same rod (E) and cone (F). Cells were voltage-clamped at −77 mV. Ca2+-activated K+ currents were inhibited by Cs+ and tetraethylammonium (TEA) in the pipette solution and reversal potential of chloride (ECl) = −39 mV. Scale bar = 5 μm.
In the examples shown in Fig. 6, ICl(Ca) increased more rapidly in the rod than in the cone. This faster rate is not readily explained by the higher Ca2+ level attained after flash photolysis in the rod since time constants for the rise in ICl(Ca) did not show a clear Ca2+ dependence. This may instead reflect an intrinsic difference between rod and cone currents since ICl(Ca) activated at a significantly faster rate in rods (τ = 3.1 ± 0.25 ms) than that in cones (τ = 4.0 ± 0.30 ms; P = 0.049) when compared over a similar range of Ca2+ levels (rods: 2.62 ± 0.49 μM; cones: 2.60 ± 0.50 μM; P = 0.98).
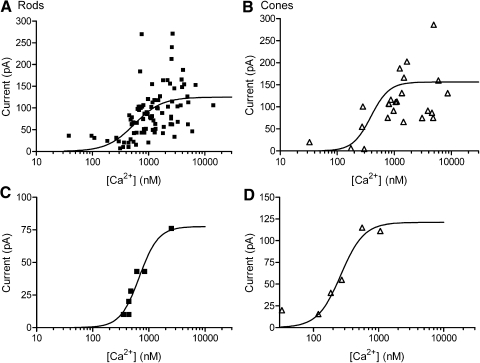
Peak current amplitudes from rods and cones were plotted as a function of intraterminal [Ca2+]i (Fig. 7, A and B). The relationships between amplitude and log [Ca2+]i were assumed to obey standard receptor binding kinetics and therefore fit with sigmoidal binding curves (see methods). In most experiments, we obtained only a single response per cell. Cell-to-cell variability in current amplitude produced scatter in the data. In a few recordings, we held individual cells long enough for [Ca2+]i to recover to basal levels between uncaging flashes, allowing us to make more than one measurement. Figure 7, C and D illustrates recordings from a rod and cone, respectively, in which multiple uncaging flashes were applied. As illustrated by these examples, repeated measurements in individual cells yielded a Ca2+ dependence similar to that in the overall sample. The best-fit sigmoid to the overall sample from rods exhibited an EC50 of 556 nM, with a slope factor of 1.8 (n = 88, Fig. 7A). Data from cones yielded an EC50 of 377 nM, with a slope factor of 2.4 (n = 34, Fig. 7B). Comparisons of the 95% confidence intervals showed no significant differences between the best-fit parameters in rods and cones.
Fig. 7.
Ca2+ dependence of ICl(Ca) in rods and cones. The amplitudes of inward Cl(Ca) currents evoked by flash photolysis of caged Ca2+ were plotted as a function of intraterminal [Ca2+]. Data were fit with sigmoidal concentration–response curves (see methods). The best fit for data from rods (A) was obtained with a half-maximal effective concentration (EC50) of 556 nM and slope factor of 1.76 (n = 88). The best fit for data from cones (B) was obtained with an EC50 of 377 nM and slope factor of 2.44 (n = 34). Repeated measurements in an individual rod (C) and cone (D) yielded a similar sigmoidal relationship. For these 2 cells, the best fits were obtained with EC50 values of 675 and 262 nM and slope factors of 2.56 and 2.44, respectively.
Average submembrane [Ca2+]
Similar to the use of Ca2+-activated K+ channels as submembrane Ca2+ sensors (Burrone et al. 2002; Roberts et al. 1990), we used the empirically determined Ca2+ dependence of ICl(Ca) to assess levels of submembrane Ca2+ attained at Cl(Ca) channels in the presynaptic terminal. We chose 5 mM EGTA as the Ca2+ buffer for these experiments because tail currents and postsynaptic responses exhibited smaller changes over time following patch rupture when using 5 mM EGTA in the pipette solution compared with experiments with 0.5 mM EGTA or 5 mM BAPTA. This suggests that 5 mM EGTA is closer to the endogenous buffering capacity. ECl in the pipette solution (−39 mV) was matched to the ECl in the DM-nitrophen pipette solution used to measure Ca2+ dependence. We stimulated rods and cones with a series of voltage steps from −57 to −17 mV, ranging in duration from 50 ms to 1 s. We subtracted capacitative and leak curents using a P/8 protocol. Figure 8A shows a series of traces illustrating the increase in tail currents evoked in a rod with increasing depolarization from −57 to −17 mV using test steps of 1-s duration. Figure 8B shows the same measurements performed in a cone. Inward currents due to activation of L-type ICa were observed during the test step and inward ICl(Ca) tail currents were observed after termination of the test step. Consistent with ICl(Ca) being driven by Ca2+ influx through L-type Ca2+ channels (Barnes and Hille 1989; MacLeish and Nurse 2007), both ICa and tail currents increased with depolarization above −57 mV. Figure 8, C and D illustrates the effects of increasing test step duration when using a step to −17 mV in a rod and cone, respectively. Tail currents were accentuated by the use of longer test steps, consistent with an increase in [Ca2+]i exceeding the local buffering capacity. The large increase in ICl(Ca) observed in the rod with test steps of 500 ms and 1 s may reflect contributions of Ca2+-induced release of Ca2+ from intracellular stores (Cadetti et al. 2006).
Using the sigmoidal curves fit to rod and cone data in Fig. 7, we converted ICl(Ca) amplitude into [Ca2+]i attained at Cl(Ca) channels. Experiments on Cl(Ca) channel localization suggested that Cl(Ca) channels are dispersed throughout the synaptic terminal. This, in turn, suggests that Ca2+ levels attained at Cl(Ca) channels should provide an estimate of the average submembrane [Ca2+]. Inward Cl(Ca) tail currents exceeding limits of the sigmoidal curve (>153 pA in cones and >125 pA in rods) were assigned a concentration value of 10 μM. Stimuli that failed to evoke inward tail currents were assigned a concentration value of 31 nM. The results of this conversion are shown in Fig. 9, C and D for rods and cones. Consistent with Fura2 measurements (Choi et al. 2008; Steele Jr et al. 2005; Szikra and Krizaj 2006), tail current measurements suggest basal Ca2+ levels (assessed from 50- to 200-ms steps to −57 mV) slightly below 100 nM. Ca2+ levels rose steeply as the membrane potential approached the dark resting potential of −40 mV. Application of a 1-s depolarization to −37 mV, slightly above the average dark potential, caused submembrane [Ca2+] to rise to 2 μM in both rods and cones, consistent with estimates obtained using Ca2+-sensitive dyes (Choi et al. 2008; Steele Jr et al. 2005; Szikra and Krizaj 2006). [Ca2+] attained during 1-s steps to −27 and −17 mV may exceed estimated levels since tail currents evoked by these stimuli often exceeded 125 pA in rods or 153 pA in cones.
DISCUSSION
Release sites are <100 nm from Ca2+ channels in photoreceptor terminals
The persistence of cone-driven PSCs in the presence of BAPTA indicates that a number of release sites are <100 nm from L-type Ca2+ channels in cone terminals. These dimensions are consistent with ultrastructural observations that ribbon-associated synaptic vesicles contact the plasma membrane along the flanks of the synaptic ridge at a distance of about 50 nm from the edge of the ribbon (Lasansky 1973; Raviola and Gilula 1975). This close proximity between Ca2+ channels and release sites is similar to the squid giant synapse (Adler et al. 1991), GABAergic basket cells in the hippocampus (Bucurenciu et al. 2008), mature calyx of Held neurons (Fedchyshyn and Wang 2005), and retinal bipolar cells (Jarsky et al. 2010). However, it differs from many other CNS synapses, where EGTA can significantly depress release, indicating that Ca2+ channels are much further away from release sites. These include immature calyx of Held (Borst and Sakmann 1996; Meinrenken et al. 2002), cortical pyramidal cells (Ohana and Sakmann 1998; Rozov et al. 2001), and hair cells (Moser and Beutner 2000). In capacitance recordings from isolated goldfish bipolar cell terminals, 5 mM EGTA diminished vesicle release (Mennerick and Matthews 1996), whereas introduction of 10 mM EGTA into mouse bipolar cell somas did not diminish synaptic release (Singer and Diamond 2003). The more pronounced effects of EGTA observed in the former study may be due to differences in the preparation and cellular access: Mennerick and Matthews (1996) recorded directly from large goldfish bipolar cell terminals, whereas Singer and Diamond (2003) recorded from rat bipolar cell somas that are separated from the terminal by a long, thin axon. The finding that release sites are situated extremely close to Ca2+ channels in photoreceptors is particularly surprising given the high affinity for Ca2+ exhibited by the release mechanism, which requires only submicromolar levels to stimulate exocytosis (Duncan et al. 2010; Rieke and Schwartz 1996; Sheng et al. 2007; Thoreson et al. 2004). The presence of highly localized Ca2+ nanodomains suggests strong endogenous Ca2+ buffering in photoreceptor terminals.
One consequence of a tight coupling between Ca2+ channels and release sites may be a lower likelihood of ectopic release (i.e., release at sites other than the active zone). In addition to Ca2+ channels in the synaptic terminals of photoreceptors, immunohistochemical studies show faint labeling of the soma and inner segments, suggesting some channels may be located at nonsynaptic sites (Morgans 2001; Morgans et al. 2005; Nachman-Clewner et al. 1999; tom Dieck et al. 2005). However, electrophysiological measurements suggest that 95% of the Ca2+ channels are localized to the synaptic terminal in rods (Xu and Slaughter 2005) and high-resolution confocal microscopy and immunoelectron micrographs show that Ca2+ channels in the terminal are mainly located beneath the arciform density at the base of the ribbon (Specht et al. 2009; tom Dieck et al. 2005). The finding that both transient and sustained components of synaptic release involve release sites <100 nm from Ca2+ channels is thus consistent with the hypothesis that release occurs predominantly at the ribbon in cone photoreceptors (Jackman et al. 2009). However, our data are also consistent with the possibility of nonribbon release sites located very close to nonribbon Ca2+ channels. Like cones, rod-driven EPSCs were not blocked by BAPTA in the patch pipette, but interpretation of these responses is complicated by the presence of synaptic output from neighboring, gap-junctionally coupled rods (Cadetti et al. 2005).
Ca2+ levels rise to micromolar levels beneath the ribbon when photoreceptors are maintained in darkness (Choi et al. 2009; Jackman et al. 2009; Steele Jr et al. 2005; Szikra and Krizaj 2006; present study, Fig. 9). Micromolar Ca2+ levels are sufficient to release many of the vesicles in the readily releasable pool at the base of the synaptic ribbon (Jackman et al. 2009). When maintained in darkness, the sustained release rate is thus determined by the rate at which vesicles replenish the readily releasable pool, not the frequency of individual Ca2+ channel openings (Jackman et al. 2009). Linking the rate of release to replenishment rather than individual channel openings may reduce the synaptic noise that can result from stochastic channel openings. By contrast, when photoreceptors are strongly hyperpolarized in bright light, Ca2+ channel openings occur principally when the cell depolarizes in response to a light decrement. In this situation, positioning Ca2+ channels close to release sites may allow fusion to be closely synchronized with Ca2+ channel opening, thus enhancing fidelity and temporal precision in the conversion of membrane potential changes to synaptic release. The combination of high Ca2+ affinity in the release mechanism with a close proximity to Ca2+ channels may allow the synapse to switch between different output modes to improve release precision under light-adapted conditions when the occurrence of Ca2+ channel openings is closely synchronized to the appearance of a stimulus but reduce synaptic noise in darkness when Ca2+ channel openings occur more randomly.
The distance between Ca2+ and Cl(Ca) channels averages a few hundred nanometers
In addition to stimulating synaptic release, Ca2+ influx through Ca2+ channels activates Cl(Ca) channels. The finding that 5 mM BAPTA did not fully eliminate ICl(Ca) in rods or cones suggests that some Cl(Ca) channels are quite close (<100 nm) to Ca2+ channels. However, other Cl(Ca) channels are further away since the distance between Ca2+ channels and Cl(Ca) channels averaged 300–600 nm in cones and 200–450 nm in rods. By comparison, the distance between individual ribbons in published electron micrographs of salamander retina is often a micron or more (Lasansky 1978; Pang et al. 2008; Townes-Anderson et al. 1985), consistent with the hypothesis that Cl(Ca) channels are dispersed between ribbons.
Until the identity of the channel(s) contributing to Cl(Ca) currents is fully characterized, measurements of these spatial relationships are necessarily limited to physiological approaches. The putative Cl(Ca) channel, TMEM16B, has been shown to be localized to photoreceptor terminals (Schroeder et al. 2008; Stöhr et al. 2009). The present immunohistochemical results indicate that the related isoform, TMEM16A, is also present at photoreceptor synaptic terminals, consistent with the presence of mRNA for the TMEM16A gene in outer retinal layers (Gritli-Linde et al. 2009; Fig. 3). TMEM16A may therefore participate with TMEM16B in generating ICl(Ca) in photoreceptors. Staining for TMEM16A antibodies produced labeling of rod and cone terminals that overlapped extensively with labeling for the synaptic vesicle protein SV2, but was more diffuse than the punctate staining observed with antibodies to CaV1.4 (α1F) Ca2+ channels. Punctate labeling of rod terminals by L-type Ca2+ channel antibodies was also observed by Nachman-Clewner et al. (1999). Although uncertainties remain about the molecular identity of the Cl(Ca) channel, these results are consistent with electrophysiological results, suggesting that Cl(Ca) channels are not clustered as tightly near synaptic ribbons as Ca2+ channels, but more dispersed throughout the synaptic terminals of rods and cones.
Ca2+ dependence of Cl(Ca) channels in photoreceptors
Using flash photolysis of DM-nitrophen to elevate submembrane [Ca2+] instantaneously, we found that ICl(Ca) could be activated by submicromolar [Ca2+] levels in photoreceptors. The slopes of sigmoidal fits to Ca2+ dependence suggest that binding of two or more Ca2+ ions is required for activation. This is consistent with TMEM16 expression studies and studies on Cl(Ca) channels in other tissues that show Hill coefficients ≥2 (Arreola et al. 1996; Evans and Marty 1986; Giovannucci et al. 2002; Kuruma and Hartzell 2000; Pifferi et al. 2009; Reisert et al. 2003; Yang et al. 2008). The EC50 values of 556 nM in rods and 377 nM in cones are similar to the Kd of ICl(Ca) in acinar cells (Arreola et al. 1996; Giovannucci et al. 2002) but lower than Kd values obtained from excised patches containing TMEM16A channels, TMEM16B channels, Cl(Ca) channels from olfactory neurons, or Xenopus laevis oocyte channels (Kuruma and Hartzell 2000; Pifferi et al. 2009; Reisert et al. 2003; Yang et al. 2008). The Ca2+ dependence of Cl(Ca) channels can be influenced by membrane potential, with lower Kd values observed at more positive potentials (Hartzell et al. 2005). For example, TMEM16A exhibits a Kd for Ca2+ of 2.6 μM at −60 mV but only 400 nM at +60 mV (Yang et al. 2008). Thus differences in the Kd could mean that a protein other than TMEM16 is responsible for Cl(Ca) currents in photoreceptors, but can also be explained by the presence of protein partners in situ that modify the local charge environment to produce a Kd similar to that observed at positive potentials.
Using Cl(Ca) channels as Ca2+ sensors, we found that submembrane Ca2+ levels in photoreceptors range from basal levels slightly below 100 nM to 1–2 μM at the dark resting potential, consistent with results obtained using Ca2+-sensitive dyes (Choi et al. 2008; Steele Jr et al. 2005; Szikra and Krizaj 2006). Ca2+ levels rose steeply as the membrane potential approached the dark potential, suggesting that small variations in resting potential can have large effects on resting Ca2+ levels. Over much of the normal operating voltage range in cones (−60 to −40 mV), average submembrane [Ca2+] remained below the threshold of 400 nM needed to stimulate synaptic vesicle exocytosis (Duncan et al. 2010; Thoreson et al. 2004). This is consistent with Ca2+ chelator results indicating that synaptic release is controlled by high Ca2+ levels attained in highly localized nanodomains near Ca2+ channels.
Significance of Ca2+/Cl(Ca) channel relationships
Ca2+ influx through voltage-gated Ca2+ channels activates Cl(Ca) channels and activation of Cl(Ca) channels can in turn influence Ca2+ channel activity (Thoreson et al. 1997, 2000). In rods, ECl averages −20 mV, so activation of ICl(Ca) results in a Cl− efflux. Although the membrane depolarization that results from Cl− efflux stimulates ICa, this stimulatory effect is countered by a simultaneous strong inhibitory influence of Cl− efflux whereby reductions in intracellular Cl− act at anion binding sites on individual Ca2+ channels to directly inhibit channel open probability (Babai et al. 2010; Thoreson et al. 2000). CaV1.4 Ca2+ channels, which are the principal subtype in rods, exhibit slow calcium-dependent inactivation (Baumann et al. 2004; Koschak et al. 2003; McRory et al. 2004). Inhibition of ICa by negative feedback from ICl(Ca) activity may provide an alternative mechanism to limit excessive Ca2+ entry.
ECl in cones averages −38 mV, close to the dark resting potential (Thoreson and Bryson 2004). ICl(Ca) evoked by strong depolarizing steps was smaller in cones than that in rods, suggesting a lower Cl(Ca) current density. Thus Cl(Ca) channel activation is likely to produce only small changes in intracellular Cl− levels that will have only a small effect on Ca2+ channel open probability. The activation of Cl(Ca) channels in cones may instead be more important for stabilizing the membrane potential near its dark resting value (Maricq and Korenbrot 1988).
The present results suggest that Ca2+ influx through an individual Ca2+ channel could potentially influence the activity of Cl(Ca) channels hundreds of nanometers away and thereby indirectly influence the activity of relatively distant Ca2+ channels. Such long-range interactions may be boosted by calcium-induced calcium release in rod terminals (Babai et al. 2010; Cadetti et al. 2006; Krizaj et al. 1999, 2003; Suryanarayan and Slaughter 2006). Regulation of relatively distant Ca2+ channels by the activation of Cl(Ca) channels may help to ensure similarity in the behavior of Ca2+ channels located at different positions along the nearly 1 μm long synaptic ridge in rods. In amphibian rods with multiple ribbons, long-range interactions might also help to reduce functional heterogeneity between channels at neighboring synaptic ribbons (Frank et al. 2009; Meyer et al. 2009).
GRANTS
This work was supported by Research to Prevent Blindness, National Eye Institute Grants EY-10542 to W. B. Thoreson and EY-15573 to N. C. Brecha, National Institutes of Health American Recovery and Reinvestment Act award to support G. E. Riccardi (to N. C. Brecha), a Senior Career Scientist Award from the Department of Veterans Affairs to N. C. Brecha, Fight for Sight grant to K. Rabl, and a University of Nebraska Medical Center Graduate Student Fellowship to A. Mercer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci 11: 1496–1507, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola J, Melvin J, Begenisich T. Activation of calcium-dependent chloride channels in rat paroid acinar cells. J Gen Physiol 108: 35–47, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol 309: 287–315, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Wilson M, Wu SM. A quantitative analysis of interactions between photoreceptors in the salamander (Ambystoma) retina. J Physiol 352: 703–737, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babai N, Kanevsky N, Dascal N, Rozanski GJ, Singh DP, Fatma N, Thoreson WB. Anion-sensitive regions of L-type CaV1.2 calcium channels expressed in HEK293 cells. PLoS One 5: e8602, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babai N, Morgans CW, Thoreson WB. Calcium-induced calcium release contributes to synaptic release from mouse rod photoreceptors. Neuroscience 165: 1447–1456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader CR, Bertrand D, Schwartz EA. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol 331: 253–284, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Deschênes MC. Contribution of Ca and Ca-activated Cl channels to regenerative depolarization and membrane bistability of cone photoreceptors. J Neurophysiol 68: 745–755, 1992 [DOI] [PubMed] [Google Scholar]
- Barnes S, Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol 94: 719–743, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoletti TM, Babai N, Thoreson WB. Vesicle pool size at the salamander cone ribbon synapse. J Neurophysiol 103: 419–423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann L, Gerstner A, Zong X, Biel M, Wahl-Schott C. Functional characterization of the L-type Ca2+ channel Cav1.4α1 from mouse retina. Invest Ophthalmol Vis Sci 45: 708–713, 2004 [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet 19: 264–267, 1998 [DOI] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron 29: 681–690, 2001 [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science 289: 953–957, 2000 [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature 383: 431–434, 1996 [DOI] [PubMed] [Google Scholar]
- Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron 57: 536–545, 2008 [DOI] [PubMed] [Google Scholar]
- Burrone J, Neves G, Gomis A, Cooke A, Lagnado L. Endogenous calcium buffers regulate fast exocytosis in the synaptic terminal of retinal bipolar cells. Neuron 33: 101–112, 2002 [DOI] [PubMed] [Google Scholar]
- Cadetti L, Bryson EJ, Ciccone CA, Rabl K, Thoreson WB. Calcium-induced calcium release in rod photoreceptor terminals boosts synaptic transmission during maintained depolarization. Eur J Neurosci 23: 2983–2990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadetti L, Tranchina D, Thoreson WB. A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics in the salamander retina. J Physiol 569: 773–788, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008 [DOI] [PubMed] [Google Scholar]
- Choi SY, Jackman S, Thoreson WB, Kramer RH. Light regulation of Ca2+ in the cone photoreceptor synaptic terminal. Vis Neurosci 25: 693–700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church PJ, Stanley EF. Single L-type calcium channel conductance with physiological levels of calcium in chick ciliary ganglion neurons. J Physiol 496: 59–68, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cia D, Bordais A, Varela C, Forster V, Sahel JA, Rendon A, Picaud S. Voltage-gated channels and calcium homeostasis in mammalian rod photoreceptors. J Neurophysiol 93: 1468–1475, 2004 [DOI] [PubMed] [Google Scholar]
- Duncan G, Rabl K, Gemp I, Heidelberger R, Thoreson WB. Quantitative analysis of synaptic release at the photoreceptor synapse. Biophys J 98: 2102–2110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MG, Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol 378: 437–460, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of Held synapse. J Neurosci 25: 4131–4140, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth SI, Morgan IG, Boelen MK, Morgans CW. Localization of voltage-sensitive L-type calcium channels in the chicken retina. Clin Exp Ophthalmol 29: 183–187, 2001 [DOI] [PubMed] [Google Scholar]
- Frank T, Khimich D, Neef A, Moser T. Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells. Proc Natl Acad Sci USA 106: 4483–4488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci DR, Bruce JI, Straub SV, Arreola J, Sneyd J, Shuttleworth TJ, Yule DI. Cytosolic Ca(2+) and Ca(2+)-activated Cl(−) current dynamics: insights from two functionally distinct mouse exocrine cells. J Physiol 15: 469–484, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Vaziri Sani F, Rock JR, Hallberg K, Iribarne D, Harfe BD, Linde A. Expression patterns of the Tmem16 gene family during cephalic development in the mouse. Gene Expr Patterns 9: 178–191, 2009 [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol 67: 719–758, 2005 [DOI] [PubMed] [Google Scholar]
- Hartzell HC, Yu K, Xiao Q, Chien L-T, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol 587: 2127–2139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 371: 513–515, 1994 [DOI] [PubMed] [Google Scholar]
- Helmchen F. Calibration of fluorescent calcium indicators. In: Imaging Neurons: A Laboratory Manual, edited by Yuste R, Lanni F, Konnerth A. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2000, p. 32 [Google Scholar]
- Jackman SL, Choi SY, Thoreson WB, Rabl K, Bartoletti TM, Kramer RH. Role of the synaptic ribbon in transmitting the cone light response. Nat Neurosci 12: 303–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci 30: 11885–11895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Walter D, Hoda J-C, Heinzle T, Grabner M, Striessnig J. Cav1.4α1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent inactivation. J Neurosci 23: 6041–6049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Bao JX, Schmitz Y, Witkovsky P, Copenhagen DR. Caffeine-sensitive calcium stores regulate synaptic transmission from retinal rod photoreceptors. J Neurosci 19: 7249–7261, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Lai FA, Copenhagen DR. Ryanodine stores and calcium regulation in the inner segments of salamander rods and cones. J Physiol 547: 761–774, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruma A, Hartzell HC. Bimodal control of a Ca2+-activated Cl− channel by different Ca2+ signals. J Gen Physiol 115: 59–80, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond B Biol Sci 265: 471–489, 1973 [DOI] [PubMed] [Google Scholar]
- Lasansky A. Contacts between receptors and electrophysiologically identified neurones in the retina of the larval tiger salamander. J Physiol 285: 531–542, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeish PR, Nurse CA. Ion channel compartments in photoreceptors: evidence from salamander rods with intact and ablated terminals. J Neurophysiol 98: 86–95, 2007 [DOI] [PubMed] [Google Scholar]
- Mandell JW, Townes-Anderson E, Czernik AJ, Cameron R, Greengard P, De Camilli P. Synapsins in the vertebrate retina: absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron 5: 19–33, 1990 [DOI] [PubMed] [Google Scholar]
- Mariani AP. Photoreceptors of the larval tiger salamander retina. Proc R Soc Lond B Biol Sci 227: 483–492, 1986 [DOI] [PubMed] [Google Scholar]
- Maricq AV, Korenbrot JI. Calcium and calcium-dependent chloride currents generate action potentials in solitary cone photoreceptors. Neuron 1: 503–515, 1988 [DOI] [PubMed] [Google Scholar]
- McRory JE, Hamid J, Doering CJ, Garcia E, Parker R, Hamming K, Chen L, Hildebrand M, Beedle AM, Feldcamp L, Zamponi GW, Snutch TP. The CACNA1F gene encodes an L-type calcium channel with unique biophysical properties and tissue distribution. J Neurosci 24: 1707–1718, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinrenken CJ, Borst JG, Sakmann B. Calcium secretion coupling at calyx of held governed by nonuniform channel-vesicle topography. J Neurosci 22: 1648–1667, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron 17: 1241–1249, 1996 [DOI] [PubMed] [Google Scholar]
- Meyer AC, Frank T, Khimich D, Hoch G, Riedel D, Chapochnikov NM, Yarin YM, Harke B, Hell SW, Egner A, Moser T. Tuning of synapse number, structure and function in the cochlea. Nat Neurosci 12: 444–453, 2009 [DOI] [PubMed] [Google Scholar]
- Midorikawa M, Tsukamoto Y, Berglund K, Ishii M, Tachibana M. Different roles of ribbon-associated and ribbon-free active zones in retinal bipolar cells. Nat Neurosci 10: 1268–1276, 2007 [DOI] [PubMed] [Google Scholar]
- Morgans CW. Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci 42: 2414–2418, 2001 [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, Taylor WR. Photoreceptor calcium channels: insight from night blindness. Vis Neurosci 22: 561–568, 2005 [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci USA 97: 883–888, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman-Clewner M, St. Jules R, Townes-Anderson E. L-type calcium channels in the photoreceptor ribbon synapse: localization and role in plasticity. J Comp Neurol 415: 1–16, 1999 [PubMed] [Google Scholar]
- Naraghi T. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium 22: 255–268, 1997 [DOI] [PubMed] [Google Scholar]
- Neher E. Concentration profiles of intracellular calcium in the presence of a diffusible chelator. In: Calcium Electrogenesis and Neuronal Functioning, edited by Heinemann U, Klee M, Neher E, Singer W. Berlin: Springer-Verlag, 1986, p. 80–96 [Google Scholar]
- Ohana O, Sakmann B. Transmitter release modulation in nerve terminals of rat neocortical pyramidal cells by intracellular calcium buffers. J Physiol 513: 135–148, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Barrow A, Jacoby RA, Wu SM. How do tonic glutamatergic synapses evade receptor desensitization? J Physiol 586: 2889–2902, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifferi S, Dibattista M, Menini A. TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflügers Arch 458: 1023–1038, 2009 [DOI] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J Cell Biol 65: 192–222, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Bauer PJ, Yau K-W, Frings S. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J Gen Physiol 122: 349–364, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Schwartz EA. Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J Physiol 493: 1–8, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci 10: 3664–3684, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, O'Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl− secretory channel in mouse airways. J Biol Chem 284: 14875–14880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanenko VG, Catalan MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE. Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285: 12990–13001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol 531: 807–826, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoè Pognetto M, Panzanelli P, Artero C, Fasolo A, Cantino D. Comparative study of glial fibrillary acidic protein (GFAP)-like immunoreactivity in the retina of some representative vertebrates. Eur J Histochem 36: 467–477, 1992 [PubMed] [Google Scholar]
- Schmitz F. The making of synaptic ribbons: how they are built and what they do. Neuroscientist 15: 611–624, 2009 [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature 406: 889–893, 2000 [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z, Choi SY, Dharia A, Li J, Sterling P, Kramer RH. Synaptic Ca2+ in darkness is lower in rods than cones, causing slower tonic release of vesicles. J Neurosci 27: 5033–5042, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Bui DD, Degrip WJ. Identification and distribution of photoreceptor subtypes in the neotenic tiger salamander retina. Vis Neurosci 15: 1175–1187, 1998 [DOI] [PubMed] [Google Scholar]
- Specht D, Wu SB, Turner P, Dearden P, Koentgen F, Wolfrum U, Maw M, Brandstätter JH, tom Dieck S. Effects of presynaptic mutations on a postsynaptic Cacna1s calcium channel colocalized with mGluR6 at mouse photoreceptor ribbon synapses. Invest Ophthalmol Vis Sci 50: 505–415, 2009 [DOI] [PubMed] [Google Scholar]
- Steele EC, Jr, Chen X, Iuvone PM, MacLeish PR. Imaging of Ca2+ dynamics within the presynaptic terminals of salamander rod photoreceptors. J Neurophysiol 94: 4544–4553, 2005 [DOI] [PubMed] [Google Scholar]
- Stella SL, Jr, Hu WD, Vila A, Brecha NC. Adenosine inhibits voltage-dependent Ca2+ influx in cone photoreceptor terminals of the tiger salamander retina. J Neurosci Res. 85: 1126–1137, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MD. Buffering of calcium in the vicinity of a channel pore. Cell Calcium 13: 183–192, 1992 [DOI] [PubMed] [Google Scholar]
- Stöhr H, Heisig JB, Benz PM, Schöberl S, Milenkovic VM, Strauss O, Aartsen WM, Wijnholds J, Weber BH, Schulz HL. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci 29: 6809–6818, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayanan A, Slaughter MM. Synaptic transmission mediated by internal calcium stores in rod photoreceptors. J Neurosci 26: 1759–1766, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikra T, Krizaj D. The dynamic range and domain-specific signals of intracellular calcium in photoreceptors. Neuroscience 141: 143–155, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Bryson EJ. Chloride equilibrium potential in salamander cones. BMC Neurosci 5: 53–60, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Reducing extracellular chloride suppresses dihydropyridine-sensitive calcium currents and synaptic transmission in amphibian photoreceptors. J Neurophysiol 77: 2175–2190, 1997 [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Chloride efflux inhibits single calcium channel open probability in vertebrate photoreceptors: chloride imaging and cell-attached patch-clamp recordings. Vis Neurosci 17: 197–206, 2000 [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Rabl K, Townes-Anderson E, Heidelberger R. A highly Ca2+-sensitive pool of vesicles contributes to linearity at the rod photoreceptor ribbon synapse. Neuron 42: 595–605, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson et al., 2002. Thoreson WB, Stella SL, Jr, Bryson EI, Clements J, Witkovsky P. D2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Vis Neurosci 19: 235–247, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Tranchina D, Witkovsky P. Kinetics of synaptic transfer from rods and cones to horizontal cells in the salamander retina. Neuroscience 122: 785–798, 2003 [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, FejtovÁ A, Bracko O, Gundelfinger ED, Brandstätter JH. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol 168: 825–836, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes-Anderson E, Macleish PR, Raviola E. Rod cells dissociated from mature salamander retina: ultrastructure and uptake of horseradish peroxidase. J Cell Biol 100: 175–188, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Kenyon JL. The spatial relationship between Ca2+ channels and Ca2+-activated channels and the function of Ca2+-buffering in avian sensory neurons. Cell Calcium 28: 233–246, 2000 [DOI] [PubMed] [Google Scholar]
- Welch NC, Lalonde MR, Barnes S, Kelly ME. Calcium-activated chloride channels in Müller cells acutely isolated from tiger salamander retina. Glia 53: 74–80, 2006 [DOI] [PubMed] [Google Scholar]
- Xu JW, Slaughter MM. Large-conductance calcium-activated potassium channels facilitate transmitter release in salamander rod synapse. J Neurosci 25: 7660–7668, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Standifer KM, Sherry DM. Synaptic protein expression by regenerating adult photoreceptors. J Comp Neurol 443: 275–278, 2002 [DOI] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008 [DOI] [PubMed] [Google Scholar]
- Zenisek D. Vesicle association and exocytosis at ribbon and extraribbon sites in retinal bipolar cell presynaptic terminals. Proc Natl Acad Sci USA 105: 4922–4927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci 23: 2538–2548, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Immunocytochemical analysis of photoreceptors in the tiger salamander retina. Vision Res 49: 64–73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang Z, Wu SM. Immunocytochemical analysis of spatial organization of photoreceptors and amacrine and ganglion cells in the tiger salamander retina. Vis Neurosci 21: 157–166, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.