Abstract
It has been reported that 30% calorie restriction (CR) for 3 mo results in large increases in mitochondrial biogenesis in heart, brain, liver, and adipose tissue, with concomitant increases in respiration and ATP synthesis. We found these results surprising, and performed this study to determine whether 30% CR does induce an increase in mitochondria in heart, brain, liver, adipose tissue, and/or skeletal muscle. To this end, we measured the levels of a range of mitochondrial proteins, and mRNAs. With the exception of long-chain acyl-CoA dehydrogenase protein level, which was increased ∼60% in adipose tissue, none of the mitochondrial proteins or mRNAs that we measured were increased in rats subjected to 30% CR for 14 wk. There was also no increase in citrate synthase activity. Because it is not possible to have an increase in mitochondria without any increase in key mitochondrial proteins, we conclude that 30% CR does not induce an increase in mitochondria in heart, brain, liver, adipose tissue, or skeletal muscle in laboratory rodents.—Hancock, C. R., Han, D.-H., Higashida, K., Kim, S. H., Holloszy, J. O. Does calorie restriction induce mitochondrial biogenesis? A reevaluation.
Keywords: aging, citrate synthase, cytochrome c, PGC-1α
Nisoli et al. (1) reported that 30% calorie restriction (CR) of mice for 3 mo resulted in large increases in mitochondria in brain, heart, liver, and adipose tissue. This effect was evidenced by increases in mitochondrial DNA, the proteins cytochrome c (cyt c) and cytochrome oxidase subunit IV (COXIV), and the mRNA levels of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and a number of transcription factors that regulate mitochondrial biogenesis. A subsequent study on humans, which has been interpreted to support the concept that CR increases mitochondrial biogenesis, has extended this finding to skeletal muscle (2). The findings of Nisoli et al. (1) appear to be generally accepted, and have led to incorporation of the concept that CR induces mitochondrial biogenesis into hypothetical models of how CR mediates its effects on aging (3).
Adaptations normally occur in response to increases or decreases in metabolic or physiological demands, and are usually tissue specific. For example, exercise that results in an increased need for ATP generation in muscle induces an increase in mitochondrial biogenesis in skeletal muscle (4–6). Cold, which results in an increased need for heat production, induces increases in uncoupling protein 1 and mitochondria in brown adipose tissue (7, 8). On the other hand, a 30% lower food intake results in a decreased need for substrate oxidation as a result of lower substrate availability. It therefore seemed strange to us that CR, which decreases the need for oxidative metabolism, would induce increased biogenesis of mitochondria.
The heart has a very high content of mitochondria, and a further increase in mitochondria resulting from overexpression of PGC-1α in transgenic mice is maladaptive, causing disruption of myofibrillar architecture and heart failure (9, 10). Therefore, the finding of Nisoli et al. (1) that CR resulted in large increases in mitochondria and respiration in heart muscle seemed particularly surprising, as it does not fit with the evidence that CR results in maintenance of good diastolic cardiac function into old age (11). Nisoli et al. (1) also reported that, as a result of the increase in mitochondria, CR results in increased oxygen consumption in several tissues, particularly white adipose tissue (WAT), and that this increase in respiration results in an increase in the steady-state concentration of ATP. This is also a strange finding, because the rate of oxygen consumption is determined by the rate at which cells utilize ATP, not by mitochondrial content. Furthermore, the steady-state concentration of ATP in cells does not increase due to an increase in respiration mediated by an increase in mitochondria. For example, a ∼2-fold adaptive increase in muscle mitochondria induced by exercise training has no effect on resting oxygen consumption or ATP concentration (12). Furthermore, Drew et al. (13) have shown that CR has no effect on ATP concentration in skeletal muscle or heart.
In light of these puzzling findings, the purpose of the present study was to determine whether we could reproduce the finding that 30% CR results in large increases in mitochondria in brain, heart, liver, and adipose tissue. We also evaluated the effect of CR on mitochondrial content of skeletal muscle.
MATERIALS AND METHODS
Animals and CR
This research was approved by the Animal Studies Committee of Washington University School of Medicine. Male Wistar rats weighing ∼90 g were obtained from Charles River Laboratories (Wilmington, MA, USA) and individually housed in a temperature- and light-controlled animal facility. They were fed a rodent laboratory chow diet (Purina, St. Louis, MO, USA) and provided with water ad libitum. The rats were assigned to either a freely eating or a CR group. Food intake of the freely eating rats was measured every other day, and the CR rats were given food equal to 70% of the average amount of food eaten by the freely eating controls. After the experimental group had been fed the CR diet for 14 wk, the rats were anesthetized with an intraperitoneal injection of pentobarbital sodium, and the heart, brain, liver, epididymal fat pads, and triceps muscles were dissected out, frozen in liquid nitrogen, and kept at −80°C until analyzed.
Western Blot Analysis. Frozen tissues were powdered and then homogenized in a 10:1 (v/w) ratio of ice-cold buffer containing: 50 mM Tris-HCl (pH 7.4); 1% Nonidet P-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 0.1 mM 4-(aminoethyl)-benzenesulfonyl fluoride; 1 mM NaF; 1 μg/ml each aprotinin, leupeptin, and pepstatin; 0.1 mM bis-peroxovanadium-1,10-phenanthrolene; and 2 mg/ml β-glycerophosphate. The homogenates were frozen and thawed 3 times to disrupt the mitochondria and rehomogenized. The homogenates were then centrifuged at 1000 g for 15 min at 4°C, and the supernatant was collected. Protein concentration was measured by the method of Lowry et al. (14), and sample volumes were adjusted to give the same protein concentration. Aliquots of supernatant were solubilized in Laemli buffer, and proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes, as described previously (4, 15). Blots were probed with the following antibodies: PGC-1α (Calbiochem, Gibbstown, NJ, USA), citrate synthase (CS; Alpha Diagnostics, San Antonio, TX, USA), COXI and COXIV (Invitrogen, Carlsbad, CA, USA), NADH ubiquinone oxidoreductase (NUO; Invitrogen), cyt c (BD Biosciences, San Jose, CA, USA), ATP synthase subunit α (ATPSα'3b Invitrogen), long-chain acyl CoA dehydrogenase (LCAD; a gift from Daniel P. Kelly, Washington University School of Medicine, St. Louis, MO, USA), actin (Research Diagnostics Fitzgerald Industries International, Concord, MA, USA), β-tubulin (Sigma-Aldrich, St. Louis, MO, USA), acetyl CoA carboxylase (ACC; Cell Signaling Technology, Danvers, MA, USA), or fatty acid synthase (FAS; Abcam, Cambridge, MA, USA). The blots were then incubated with the appropriate horseradish-conjugated secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA). Antibody bound protein was detected by ECL.
CS activity
The activity of CS was measured by the method of Srere (16).
Determination of mRNA
RNA was extracted from triceps muscle as described previously (17). PGC-1α, cyt c, core protein 1 of respiratory chain complex III (Core 1), COX1, and mitochondrial ATPS mRNAs were determined using semiquantitative RT-PCR as described previously (18) using the primers shown in Table 1. Transcript intensity was expressed relative to 18 s (Ambion, Austin, TX, USA).
Table 1.
Primer sequences used for RT-PCR
| Primer | Sequence |
|
|---|---|---|
| Forward | Reverse | |
| Cyt c | 5′-GGAGGCAAGCATAAGACTGG-3′ | 5′-GTCTGCCCTTTCTCCCTTCT-3′ |
| COXIV | 5′-TGGCCCACGTCAAGCTGCTG-3′ | 5′-CATGGCCAGGCCCACCACTG-3′ |
| Core 1 | 5′-GCCATGTTGTCGGTCGCTGC-3′ | 5′-GGCCACCAAAGGACGGGTCG-3′ |
| ATPS | 5′-GCAAGGATGCTGTCCGTGCG-3′ | 5′-TCAGGCCCACTCGTCTGCGA-3′ |
| PGC1α | 5′-GTGCAGCCAAGACTCTGTATGG-3′ | 5′-GTCCAGGTCATTCACATCAAGTTC-3′ |
Plasma insulin
Insulin was measured using a rat insulin ELISA kit from Crystal Chem, Inc., (Downers Grove, IL, USA).
Statistics
Data are presented as means ± se. Differences between the two groups were evaluated using unpaired t tests.
RESULTS
After 14 wk of CR diet treatment, the average body weight of CR rats was 27% lower than that of the freely eating control rats (426±6 vs. 587±7 g, P<0.001), and average weight gain was 32% lower in the CR group (336±6 vs. 495±9 g, P<0.001). A consistent effect of CR is a reduction in insulin level (19). In the present study, plasma insulin concentration averaged 1.33 ng/ml in the CR group vs. 2.49 ng/ml in the control group (P<0.01).
PGC-1α
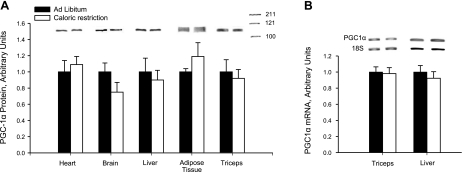
As shown in Fig. 1, the CR diet had no effect on PGC-1α protein expression in the heart, brain, liver, adipose tissue, or skeletal muscles. We also measured PGC-1α mRNA level in liver and muscle, and found no difference between the CR and control groups (Fig. 1). It is well established that PGC-1α is responsible for mediating adaptive increases in mitochondria by coactivating the transcription factors that regulate expression of mitochondrial proteins (20, 21).
Figure 1.
A) PGC-1α protein. Fourteen weeks of 30% CR had no significant effect on PGC-1α protein expression, measured by Western blot analysis. B) PGC-1α mRNA level in triceps muscle and liver were unaffected by CR. Bars represent means ± se for 7–8 rats.
Heart
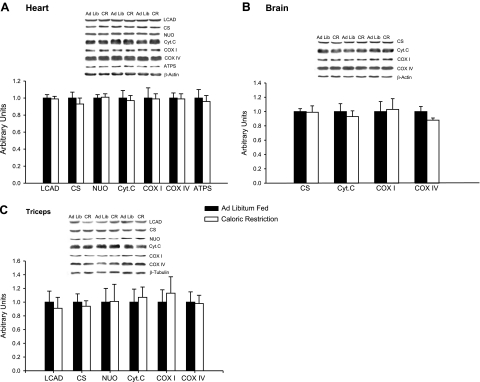
We evaluated the expression of a range of mitochondrial proteins in heart muscle. These included 4 components of the respiratory chain and ATPSα, LCAD as a representative of the fatty acid oxidation pathway, and CS as a representative of the citrate cycle. As shown in Fig. 2A, there was no difference between the levels of any of these proteins in the hearts of the CR and control rats. Because it is not possible to have an increase in mitochondria in the absence of increases in these key mitochondrial proteins, these results show that 14 wk of 30% CR treatment has no effect on mitochondrial biogenesis in the heart.
Figure 2.
A) Heart. Fourteen weeks of 30% CR had no effect on the expression of 7 mitochondrial proteins in the heart. Proteins were measured by Western blot analysis; n = 8 rats. B) Triceps muscle. Fourteen weeks of CR had no effect on the expression of mitochondrial proteins or GLUT4 in skeletal muscle. Proteins were measured by Western blot analysis; n = 8 rats. C) Brain. Fourteen weeks of 30% CR had no effect on the expression of CS, cyt c, or COXI and COXIV proteins in the brain. Proteins were measured by Western blot analysis; n = 7–8 rats. Bars represent means ± se.
Brain
We also determined the effect of the 14 wk of CR treatment on mitochondrial biogenesis in the brain by measurement of a number of key mitochondrial proteins, including CS, cyt c, COXI, and COXIV. The 14 wk of CR did not result in increased mitochondrial biogenesis in the brain, as evidenced by no change in the expression of any of these proteins (Fig. 2C).
Skeletal muscle (triceps)
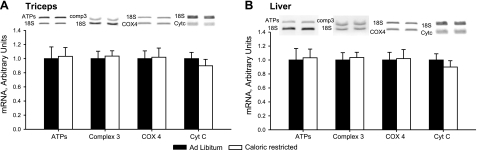
As in the heart, there was no evidence for a CR-induced increase in mitochondrial biogenesis in skeletal muscle, as the levels of 6 key mitochondrial marker proteins did not differ in the CR and ad libitum diet groups (Fig. 2B). Expression of the GLUT4 glucose transporter protein is regulated in parallel with mitochondrial biogenesis by PGC-1α in skeletal muscle (4, 15, 22), so we routinely measure GLUT4 in studies involving mitochondrial adaptive responses in skeletal muscle. CR had no effect on GLUT4 expression in muscle. The mRNA levels of ATPS, Core 1, COXIV, and cyt c were also unaffected by CR in muscle (Fig. 3A).
Figure 3.
Fourteen weeks of 30% CR had no effect on the mRNA levels of cyt c, Core 1 (complex 3), COXIV, or mitochondrial ATPS in triceps muscle (A) or liver (B).
Liver
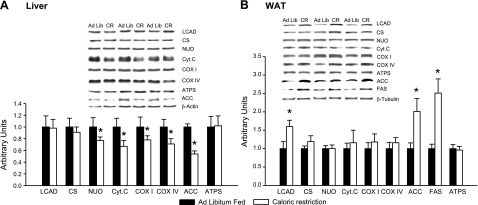
There was also no increase in mitochondria in the liver of the CR animals, as evidenced by the absence of an increase in levels of expression of the mitochondrial proteins shown in Fig. 4A. The four respiratory chain marker proteins measured in this study actually were lower in the livers of the CR animals. Mulligan et al. (23) have reported that 16 wk of CR treatment results in a significant decrease in ACC expression in the liver of mice. This adaptation was also observed in the present study (Fig. 4A). The mRNA levels of a number of mitochondrial inner membrane constituents was unaffected by CR in liver (Fig. 3B).
Figure 4.
A) Liver. Effects of 14 wk of 30% CR on the expression of mitochondrial proteins and also ACC in liver. Proteins were measured by Western blot analysis; n = 8 rats. B) Adipose tissue. Effects of 14 wk of 30% CR on the expression of mitochondrial proteins, as well as ACC and FAS in adipose tissue; n = 7–8 rats. Values are means ± se. *P < 0.05 vs. ad libitum group.
Adipose tissue (epididymal fat pad)
As shown in Fig. 4B, CR had no effect on the level of expression of any of the mitochondrial marker proteins measured in WAT, except for LCAD. Mulligan et al. (23) have reported that 16 wk of CR results in significant increases in the expression of the cytosolic proteins FAS and ACC in adipose tissue. These adaptations also occurred in the present study (Fig. 4B).
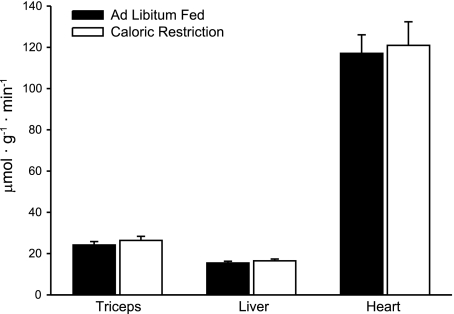
CS activity
As an additional indicator of tissue content of mitochondria, we measured CS activity in liver, heart, and skeletal muscle. As shown in Fig. 5, CR had no effect on CS activity in these tissues.
Figure 5.
CS activity. CR had no effect on CS activity in triceps muscle, liver, or heart.
DISCUSSION
The mitochondrial content of tissues can undergo adaptive increases or decreases in response to changes in energy demand and substrate supply. Our laboratory has been studying the effect of various adaptive stimuli and interventions, including exercise, thyrotoxicosis, increases in fatty acids and iron deficiency, primarily in muscle but also in other tissues, for >40 yr (4, 6, 24–26). Our general approach has been to measure the levels of key mitochondrial constituents, such as CS and cyt c, which normally change in parallel with the tissue's maximal capacity for substrate oxidation. The absence of a change in the levels of such mitochondrial markers, indicates that there has been no change in the tissue's content of mitochondria. Although it is not possible for an increase in functional mitochondria to occur without increases in key mitochondrial proteins such as CS, it is possible for increases in the levels of key mitochondrial proteins to occur without an increase in the capacity for substrate oxidation. This occurs under abnormal conditions in which there is no parallel increase or a decrease in another key mitochondrial protein or group of proteins. An example is severe iron deficiency which results in decreases in the cytochromes and other mitochondrial iron-containing proteins (24). Therefore, if we detect an increase in a number of mitochondrial constituents, we also measure the maximal capacity of tissue homogenates and/or mitochondrial preparations for substrate oxidation under conditions in which availability of ADP, inorganic phosphate, and substrate are not limiting to determine whether the increase in mitochondrial enzymes/proteins reflects an increase in functional mitochondria. It is sometimes also of interest, when there is evidence for an increase in mitochondria, to determine whether there is an increase in mitochondrial number, as reflected in an increase in mitochondrial DNA.
The purpose of the present study was to determine whether the finding of Nisoli et al. (1) that CR induces large increases in mitochondrial biogenesis in heart, brain, liver and adipose tissue is reproducible. We found that, except for LCAD in adipose tissue, there was no increase in the level of any of the mitochondrial marker proteins used as indicators of tissue mitochondrial content in heart, brain, liver, adipose tissue, or skeletal muscle of rats subjected to 14 wk of 30% CR. These results show that, contrary to the report of Nisoli et al. (1), 30% CR does not induce an increase in mitochondrial biogenesis in laboratory rodents.
There is extensive evidence that mitochondrial dysfunction and loss of mitochondria, due in large part to mutations in mitochondrial DNA, occur with and play important roles in the deterioration in structure and function of tissues and loss of cells with aging (13, 27–33). Although CR does not induce an increase in mitochondrial biogenesis, it does have a powerful protective effect against the development of deleted mitochondrial genes, mitochondrial enzyme abnormalities, and loss of mitochondria (34–38). It is interesting in this context that Baker et al. (39) found that mitochondrial enzyme levels in skeletal muscle were slightly lower in 8- to 10-mo-old CR vs. ad libitum diet rats; however, as a result of protection against the decrease in mitochondrial proteins, old CR diet animals had higher mitochondrial marker enzyme levels in their muscles than the ad libitum diet controls. This protective effect of CR against a decrease in muscle mitochondria with aging could, in a cross-sectional study on old animals, result in the erroneous conclusion that CR stimulates mitochondrial biogenesis. Both the present study and the study by Nisoli et al. (1) were performed on animals that were too young for an age-related decrease in mitochondria to be a factor.
CR animals usually eat their daily food allotment rapidly, and are then without food until their next feeding. A possible explanation for the increase in LCAD expression in adipose tissue of the CR animals could be increased activation of peroxisome proliferator activated receptor α (PPARα) by increases in intracellular fatty acids due to triglyceride lipolysis during the periods of food deprivation between feedings. Fatty acids are ligands that activate PPARα, which is a transcription factor that regulates transcription of LCAD and other fatty acid oxidation enzymes (40, 41).
Civitarese et al. (2) studied the effects of 6 mo of 25% CR in overweight humans that resulted in a decrease in body fat from 31 to 26.6%, and evaluated the effect of this weight loss program on mitochondrial content of skeletal muscle obtained by biopsy of vastus lateralis muscle. They found that muscle mitochondrial DNA was increased by ∼35%, and interpreted this finding to indicate that CR increases muscle mitochondrial biogenesis in healthy humans (2). This study has been cited as further evidence that CR increases mitochondrial biogenesis. However, this interpretation is open to question, because Civitarese et al. (2) did not observe any change in CS protein content or in the levels of activity of CS, COXII, or β-hydroxacyl-CoA dehydrogenase in muscle of the CR group. In our opinion, it is not possible to have an increase in functional mitochondria without increases in these mitochondrial enzymes. In a similar recent study, Rabol et al. (42) induced a 11% weight loss in healthy overweight women by means of a low-calorie diet and measured the effect of the decrease in calorie intake on CS activity, mitochondrial DNA copy number, and mitochondrial respiration in muscle biopsies. They found that mitochondrial respiration per milligram of tissue decreased by ∼25%, while CS activity and mitochondrial DNA copy number were unchanged in the weight-loss group. In the present study on rats, 30% CR also had no effect on mitochondrial biogenesis in skeletal muscle. Our findings confirm the results of Sreekumar et al. (43), who found that 36 wk of 40% CR had no effect on skeletal muscle content of mitochondria in rats.
In summary, the results of this study show that 30% CR in rats does not induce an increase in mitochondria in heart, brain, liver, WAT, or skeletal muscle. This finding disagrees with the report by Nisoli et al. (1) stating that 30% CR induces large increases in mitochondrial biogenesis in laboratory rodents. The concept that CR induces an increase in mitochondria (1, 2) appears to be widely accepted and is being used to explain some of the beneficial effects of CR on aging and disease prevention (3, 44, 45). Our negative finding is, therefore, important, because it should help to correct this misconception.
Acknowledgments
The authors thank Iheoma Nwaogu and Jamie Votava for their technical assistance.
This research was supported by U.S. National Institutes of Health grant AG00425. C.R.H. was supported by an American Diabetes Association-based postdoctoral fellowship initially and then by Individual National Research Service Award DK076410. K.H. was supported by a Research Fellow of the Japan Society for the Promotion of Science grant.
REFERENCES
- 1. Nisoli E., Tonello C., Cardile A., Cozzi V., Bracale R., Tedesco L., Falcone S., Valerio A., Cantoni O., Clementi E., Moncada S., Carruba M. O. (2005) Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317 [DOI] [PubMed] [Google Scholar]
- 2. Civitarese A. E., Carling S., Heilbronn L. K., Hulver M. H., Ukropcova B., Deutsch W. A., Smith S. R., Ravussin E. CALERIE Pennington Team (2007) Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PloS Med. 4, e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guarente L. (2008) Mitochondria - a nexus for aging, calorie restriction, and sirtuins? Cell 132, 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baar K., Wende A. R., Jones T. E., Marison M., Nolte L. A., Chen M., Kelly D. P., Holloszy J. O. (2002) Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 16, 1879–1886 [DOI] [PubMed] [Google Scholar]
- 5. Booth F. W., Baldwin K. M. (1997) Muscle plasticity: energy demanding and supply processes. In Handbook of Physiology, Sect. 12: Exercise Regulation and Integration of Multiple Systems (Rowell L. B., Shephard J. T., eds) pp. 1075–1123, Oxford University Press, New York [Google Scholar]
- 6. Holloszy J. O., Coyle E. F. (1984) Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. 56, 831–839 [DOI] [PubMed] [Google Scholar]
- 7. Puigserver P., Wu Z., Park C. W., Graves R., Wright M., Spiegelman B. M. (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 [DOI] [PubMed] [Google Scholar]
- 8. Cao W., Daniel K. W., Robidoux J., Puigserver P., Medvedev A. V., Bai X., Floering L. M., Spiegelman B. M., Collins S. (2004) p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 24, 3057–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Russell L. K., Mansfield C. M., Lehman J. J., Kovacs A., Courtois M., Saffitz J. E., Medeiros D. M., Valencik M. L., McDonald J. A., Kelly D. P. (2004) Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ. Res. 94, 525–533 [DOI] [PubMed] [Google Scholar]
- 10. Lehman J. J., Barger P. M., Kovacs A., Saffitz J., Medeiros D. M., Kelly D. P. (2000) Peroxisome proliferator-activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 106, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taffet G. E., Pham T. T., Hartley C. J. (1997) The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J. Gerontol. Biol. Sci. 52, B285–B290 [DOI] [PubMed] [Google Scholar]
- 12. Constable S. H., Favier R. J., McLane J. A., Fell R. D., Chen M., Holloszy J. O. (1987) Energy metabolism in contracting rat skeletal muscle: adaptation to exercise-training. Am. J. Physiol. 253, C316–C322 [DOI] [PubMed] [Google Scholar]
- 13. Drew B., Phaneuf S., Dirks A., Selman C., Gredilla R., Lezza A., Barja G., Leeuwenburgh C. (2003) Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am. J. Physiol. 284, R474–R480 [DOI] [PubMed] [Google Scholar]
- 14. Lowry O. H., Rosenbrough N. J., Farr A. L., Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 15. Baar K., Song Z., Semenkovich C. F., Jones T. E., Han D.-H., Nolte L. A., Ojuka E. O., Chen M., Holloszy J. O. (2003) Skeletal muscle overexpression of nuclear respiratory factor 1 increases glucose transport capacity. FASEB J. 17, 1666–1673 [DOI] [PubMed] [Google Scholar]
- 16. Srere P. A. (1969) Citrate synthase. Methods Enzymol. 13, 3–5 [Google Scholar]
- 17. Otani K., Han D.-H., Ford E. L., Garcia-Roves P. M., Ye H., Horikawa Y., Bell G. I., Holloszy J. O., Polonsky K. S. (2004) Calpain system regulates muscle mass and glucose transporter GLUT4 turnover. J. Biol. Chem. 279, 20915–20920 [DOI] [PubMed] [Google Scholar]
- 18. Terada S., Wicke S., Holloszy J. O., Han D.-H. (2006) The PPARδ activator GW505516 has no acute effect on glucose transport in skeletal muscle. Am. J. Physiol. Endocrin. Metab. 290, E607–E611 [DOI] [PubMed] [Google Scholar]
- 19. Masoro E. J. (2005) Overview of caloric restriction and ageing. Mech. Ageing. Dev. 126, 913–922 [DOI] [PubMed] [Google Scholar]
- 20. Lin J., Handschin C., Spiegelman B. M. (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1, 361–370 [DOI] [PubMed] [Google Scholar]
- 21. Kelly D. P., Scarpulla R. C. (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18, 357–368 [DOI] [PubMed] [Google Scholar]
- 22. Michael L. F., Wu Z., Cheatham R. B., Puigserver P., Adelmant G., Lehman J. J., Kelly D. P., Spiegelman B. M. (2001) Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. U. S. A. 98, 3820–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mulligan J. D., Stewart A. M., Saupe K. W. (2008) Downregulation of plasma insulin levels and hepatic PPARγ expression during the first week of caloric restriction in mice. Exp. Gerontol. 43, 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cartier L.-J., Ohira Y., Chen M., Cuddihee R. W., Holloszy J. O. (1986) Perturbation of mitochondrial composition in muscle by iron deficiency. Implications regarding mitochondrial biogenesis. J. Biol. Chem. 261, 13827–13832 [PubMed] [Google Scholar]
- 25. Garcia-Roves P. M., Huss J. M., Han D.-H., Hancock C. R., Iglesias-Gutierrez E., Chen M., Holloszy J. O. (2007) Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 104, 10709–10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Winder W. W. (1979) Time-course of the T3 and T4-induced increase in rat soleus muscle mitochondria. Am. J. Physiol. 236, C132–C138 [DOI] [PubMed] [Google Scholar]
- 27. Lass A., Sohal B. H., Weindruch R., Forster M. J., Sohal R. S. (1998) Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic. Biol. Med. 25, 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barazzoni R., Short K. R., Nair K. S. (2000) Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J. Biol. Chem. 275, 3343–3347 [DOI] [PubMed] [Google Scholar]
- 29. Wanagat J., Cao Z., Pathare P., Aiken J. M. (2001) Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 15, 322–332 [DOI] [PubMed] [Google Scholar]
- 30. Short K. R., Bigelow M. L., Kahl J., Singh R., Coenen-Schimke J., Raghavakaimal S., Nair K. S. (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U. S. A. 102, 5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kujoth G. C., Hiona A., Pugh T. D., Someya S., Panzer K., Wohlgemuth S. E., Hofer T., Seo A. Y., Sullivan R., Jobling W. A., Morrow J. D., Van Remmen H., Sedivy J. M., Yamasoba T., Tanokura M., Weindruch R., Leeuwenburgh C., Prolla T. A. (2005) Mitochondrial DNA mutations, oxidative strress, and apoptosis in mammalian aging. Science 309, 481–484 [DOI] [PubMed] [Google Scholar]
- 32. Kraytsberg Y., Kudryavtseva E., McKee A. C., Geula C., Kowall N. W., Khrapko K. (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 38, 518–520 [DOI] [PubMed] [Google Scholar]
- 33. Marzetti E., Hwang J. C., Lees H. A., Wohlgemuth S. E., Supont-Versteegden E. E., Carter C. S., Bernabei R., Leeuwenburgh C. (2010) Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim. Biophys. Acta 1800, 235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamilton M. L., Van Remmen H., Drake J. A., Yang H., Guo Z. M., Kewitt K., Walter C. A., Richardson A. (2001) Does oxidative damage to DNA increase with age? Proc. Natl. Acad. Sci. U. S. A. 98, 10469–10474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKiernan S. H., Tuen V. C., Baldwin K., Wanagat J., Djamali A., Aiken J. M. (2007) Adult-onset calorie restriction delays the accumulation of mitochondrial enzyme abnormalities in aging rat kidney tubular epithelial cells. Am. J. Physiol. Renal Physiol. 292, F1751–F1760 [DOI] [PubMed] [Google Scholar]
- 36. Lee C. M., Aspnes L. E., Chung S. S., Weindruch R., Aiken J. M. (1998) Influence of caloric restriction on age-associated skeletal muscle fiber characteristics and mitochondrial changes in rats and mice. Ann. N. Y. Acad. Sci. 854, 182–191 [DOI] [PubMed] [Google Scholar]
- 37. Ward W. F., Qi W., Van Remmen H., Zackert W. E., Roberts L. J., 2nd, Richardson A. (2005) Effects of age and caloric restriction on lipid peroxidation: measurement of oxidative stress by F2-isoprostane levels. J. Gerontol. A Biol. Sci. Med. Sci. 60, 847–851 [DOI] [PubMed] [Google Scholar]
- 38. Judge S., Leeuwenburgh C. (2007) Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am. J. Physiol. Cell Physiol. 292, C1983–C1992 [DOI] [PubMed] [Google Scholar]
- 39. Baker D. J., Betik A. C., Krause D. J., Hepple R. T. (2006) No decline in skeletal muscle oxidative capacity with aging in long-term calorically restricted rats: effects are independent of mitochondrial DNA integrity. J. Gerontol. Biol. Sci. 61, 675–684 [DOI] [PubMed] [Google Scholar]
- 40. Wang Y.-X., Lee C.-H., Tiep S., Yu R. T., Ham J., Kang H., Evans R. M. (2003) Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113, 159–170 [DOI] [PubMed] [Google Scholar]
- 41. Barish G. D., Narkar V. A., Evans R. M. (2006) PPARδ: a dagger in the heart of the metabolic syndrome. J. Clin. Invest. 116, 590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rabol R., Svendsen P. F., Skovbro M., Boushel R., Haugaard S. B., Schjerling P., Schrauwen P., Hesselink M. K., Nilas L., Madsbad S., Dela F. (2009) Reduced skeletal muscle mitochondrial respiration and improved glucose metabolism in nondiabetic obese women during a very low calorie dietary intervention leading to rapid weight loss. Metabolism 58, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 43. Sreekumar R., Unnikrishnan J., Fu A., Nygren J., Short K. R., Schimke J., Barazzoni R., Nair K. S. (2002) Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. Am. J. Physiol. 283, E38–E43 [DOI] [PubMed] [Google Scholar]
- 44. Marzetti E., Anne Lees H., Eva Wohlgemuch S., Leeuwenburgh C. (2009) Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. Biofactors 35, 28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang J. H., Hood D. A. (2009) Age-associated mitochondrial dysfunction in skeletal muscle: Contributing factors and suggestions for long-term interventions. IUBMB Life 61, 201–214 [DOI] [PubMed] [Google Scholar]