Abstract
Methoxyestradiol (2-ME; estradiol metabolite) inhibits vascular smooth muscle cell (VSMC) growth and protects against atherosclerosis and vascular injury; however, the mechanisms by which 2-ME induces these actions remain obscure. To assess the impact of 2-ME on biochemical pathways regulating VSMC biology, we employed high-density oligonucleotide microarrays to identify differentially expressed genes in cultured human female aortic VSMCs treated with 2-ME acutely (4hrs) or long-term (30hrs). Both single gene analysis and Gene Set Enrichment Analysis (GSEA) revealed 2-ME-induced down-regulation of genes involved in mitotic spindle assembly and function in VSMCs. Also GSEA analysis identified effects of 2-ME on genes regulating cell-cycle progression, cell migration/adhesion, vasorelaxation, inflammation and cholesterol metabolism. Transcriptional changes were associated with changes in protein expression including inhibition of cyclin-D1, cyclin-B1, cdk6, cdk4, tubulin polymerization and cholesterol and steroid synthesis and up-regulation of cyclooxygenase-2 (COX-2) and matrix metalloproteinase-1. Microarray data suggested that 2-ME may activate peroxisome proliferator-activated receptors (PPARs) in VSMCs, and 2-ME has structural similarities with rosiglitazone (PPARγ agonist). However, our finding of weak activation and lack of binding of 2-ME to PPARs suggests that 2-ME may modulate PPAR-associated genes via indirect mechanisms, potentially involving COX-2. Indeed the anti-mitogenic effects of 2-ME at concentrations that do not inhibit tubulin polymerization were blocked by the PPAR antagonist GW9662 and COX-2 inhibitor NS398. Finally, we demonstrated that 2-ME inhibited hypoxia-inducible factor-1α. Identification of candidate genes that are positively or negatively regulated by 2-ME provides important leads to investigate and better understand the mechanisms by which 2-ME induces its vasoprotective actions.
Keywords: 2-methoxyestradiol, PPAR, vascular smooth muscle cells, microarray analysis
INTRODUCTION
As with cancer, abnormal cell growth plays a key role in cardiovascular diseases. For example, vascular smooth muscle cells (VSMC) proliferation is involved in vascular remodeling that occurs at sites of atherosclerosis, hypertension-induced vascular changes and injury-induced restenosis1. Hence, drugs capable of inhibiting VSMC growth by targeting key mitogenic mechanisms are effective in protecting against cardiovascular disease2. Thus it is not surprising that various anti-mitotic therapies used in cancer also protect against vascular proliferative disorders3.
Methoxyestradiol (2-ME) is an endogenous metabolite of estradiol with no affinity for ERs, yet exerts anti-carcinogenic effects by inhibiting growth of cancer cells and neovascularization of tumors and is in phase-II clinical trials for cancer4,5. Consistent with the notion that anti-cancer drugs may be useful in cardiovascular diseases, a series of studies by us show that 2-ME is a potent inhibitor of mitogen-induced VSMC proliferation, migration and extracellular matrix synthesis6–8 and that 2-ME attenuates injury-induced neointima formation9 and cholesterol induced atherosclerosis10. Interestingly, sequential metabolism of estradiol to methoxyestradiols, such as 2-ME, plays a major role in mediating the inhibitory effects of estradiol on VSMC growth6–8, suggesting that 2-ME could also be a non-carcinogenic alternative for estrogen therapy in postmenopausal women.
Although 2-ME has therapeutic potential to treat vaso-occlusive disorders, the mechanisms by which it inhibits VSMC growth and neointima formation remain poorly defined. For example, whether 2-ME inhibits VSMC growth by down-regulating growth-inducing pathways or by up-regulating growth-inhibitory pathways remains unclear. Moreover, the receptors via which 2-ME mediates its actions remain elusive11.
Accordingly, the main goal of the present study was to investigate the actions of 2-ME on VSMCs. This was accomplished using three approaches. First, we employed transcriptional profiling in VSMCs using high-density oligonucleotide microarrays to identify 2-ME-induced changes in the expression of transcripts involved in cell-cycle progression, cell-motility, vasorelaxation, cholesterol homeostasis/metabolism and plaque formation/stability that are known to influence vascular remodeling processes and VSMC growth during cardiovascular diseases (atherosclerosis, restenosis, plaque stability) and estrogen therapy12,13. Second, we confirmed the main results of the microarray transcriptional outcomes by investigating the effects of 2-ME on the protein expression or activity of key molecular targets. Third, motivated by the structural similarities of 2-ME with peroxisome proliferator-activated receptor (PPAR)γ ligands (such as rosiglitazone) and by the fact that similar to PPARγ ligands 2-ME protects against metabolic syndrome-induced disorders, improves insulin sensitivity and inhibits VSMC growth1,14, we investigated whether 2-ME may, in part, mediate its actions via PPARγ.
METHODS
Culture of Human Aortic Smooth Muscle Cell (HASMCs)
Female HASMCs in 6th to 8th passage were cultured under standard tissue culture conditions in M231 culture medium containing growth supplement (Cascade Biologics, Inc.)15. The growth medium and serum for VSMC culture was steroid/hormone-free.
Microarray Assay
Subconfluent monolayers of HASMCs grown to sub-confluence were treated with either vehicle (DMSO; 1μl/ml) or 3μM of 2-ME (dissolved in DMSO) in the presence of 5% fetal calf serum for 4-hours (acute phase response/early gene induction) and for 30-hours (late-phase). Following treatment with 2-ME the total RNA was isolated and processed for microarray analysis (for details see supplementary data at http://hyper.ahajournals.org).
Growth Studies
[3H]Thymidine incorporation (index of DNA-synthesis), [3H]proline incorporation (index of collagen-synthesis) and cell-proliferation were conducted as previously described15 (see details in supplemental methods at http://hyper.ahajournals.org).
Effects of 2-ME on Intracellular Mechanisms
Changes in the expression of cell-cycle regulatory proteins (cyclin-B1, cyclin-D1), COX-2 and hypoxia-inducible factor-1α (HIF-1α) were analyzed by Western-blots. Labelling with radioactive γ-32P-Adenosine-triphosphate was used to assess cyclin-dependent kinase-6 (cdk6) activity. The influence of 2-ME on the dynamics of tubulin polymerization and HIF-1α was assayed by immunofluorescence microscopy and as previously described9.
PPAR Receptor Binding and Activation Experiments
Radioligand based scintillation proximity assays were used to assess binding of 2-ME to PPARs, whereas, PPAR luciferase reporter assay was employed to measure PPAR activity (for details see supplementary methods at http://hyper.ahajournals.org).
Changes in Endogenous Cholesterol, Progesterone and Testosterone Levels
Animal handling and experimentation were in accordance with the Swiss animal protection laws. All of the protocols were approved by institutional animal care and use committee. Male Wistar Kyoto rats (350–400g; RCC; Fullinsdorf, Switzerland) were anesthetized using 100mg/kg ketamin plus 15mg/kg xylazin, and implanted with osmotic pumps for intravenous delivery of the vehicle (n=9) or 2-ME (n=11) at a rate of 350μg/kg/day. A 90% PEG-400 solution in saline (10%) was used as vehicle. After 14-days the animals were sacrificed and blood samples drawn and serum prepared to assess the levels of cholesterol, progesterone and testosterone9.
Statistics
Microarray experiments were conducted in triplicates, whereas all growth experiments were performed in triplicates or quadruplicates with three to four separate cultures. Statistical analysis of microarray data was performed according to standard methods (see supplemental data for details). Statistical analysis was performed using ANOVA, paired Students’t-test or Fisher’s Least Significant Difference test as appropriate. A value of P<0.05 was considered statistically significant.
RESULTS
Effects of 2-ME on Gene Expression in HASMCs
We investigated both a short-term (4-hours) and a prolonged (30-hours) treatment of cultured HASMCs with 3μM 2-ME on modulation of gene expression. Transcriptional changes after short-term treatment (4-hours) were indicative of the beginning of transcriptional responses only (See supplementary table-S1 at http://hyper.ahajournals.org), with no statistical significant results. Hence, the changes following long term treatment (30-hours) were considered more relevant. Indeed, after 30-hours of treatment, significant transcriptional changes were manifest. The top 20 genes that were down-regulated after a 30-hour treatment with 2-ME clearly demonstrated that 2-ME interfered with key processes associated with nuclear division and cell mitosis (See supplementary table-S2 at http://hyper.ahajournals.org). In this regard, 2-ME down-regulated/inhibited genes promoting cell-cycle and cell-growth at multiple levels. Long term treatment with 2-ME also up-regulated several genes, 20 of which were significant (See supplementary table-S3 at http://hyper.ahajournals.org).
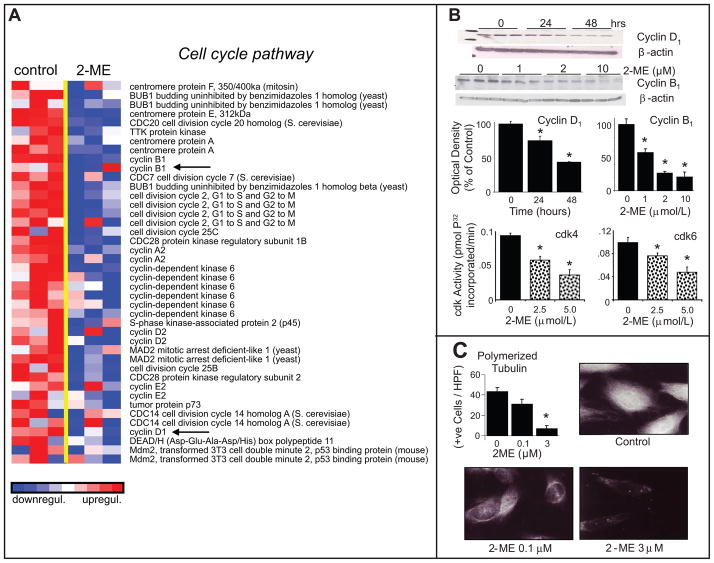
Gene Set Enrichment Analysis (GSEA) identified functionally-related gene sets which respond to 2-ME in a coordinated manner and are relevant for cardiovascular disease. As shown in Figure-1A, treatment with 2-ME significantly down-regulated the expression of genes within the cell-cycle pathway that are essential for cell-growth. Consistent with these observations, treatment of HASMCs with 2-ME down-regulated the expression of cyclin-D1 and cyclin-B1 and inhibited cdk6 and cdk4 activity (Figure-1B). Moreover, consistent with its down-regulatory effects on microtubule processes which are critical for cell division, 2-ME inhibited tubulin polymerization, a key process for cytokinesis (Figure-1C). The cell-cycle distribution in growing cells versus serum starved cells treated with FCS for 30-hours were similar (See supplementary figure-S1 at http://hyper.ahajournals.org). Treatment with 2-ME did not influence HASMC viability (See supplementary figure-S2 at http://hyper.ahajournals.org), nor did it induce apoptosis (See supplementary figure-S2 at http://hyper.ahajournals.org) or aneuploidy (See supplementary figure-S3 at http://hyper.ahajournals.org).
Figure 1.
Inhibitory effects of 2-ME on serum-induced activation of pathways regulating cell cycle. Panel-A depicts the microarray analysis of transcriptional changes in HASMCs treated with 2-ME (3 μmol/L) for 30-hours. Expression levels are depicted as color scale from red (up-regulation) to blue (down-regulation). Gene Set Enrichment Analysis (GSEA) revealed down-regulation of key genes known to induce cytokinesis. Panel-B shows Western-blots and bar graph (changes in optical density; mean±SEM) depicting the significant inhibitory effects of 2-ME on cyclin-D1and cyclin-B1 expression and on cdk4 and cdk6 activity in HASMCs treated with 2-ME. Panel-C illustrates representative photomicrographs and bar graph depicting the inhibitory effects of 2-ME on tubulin polymerization in proliferating HASMCs. The tubulin polymerization experiments were conducted in triplicate. Western blotting and cdk activity data are presented as mean±SEM (n=3). *p<0.05 versus vehicle-treated control. Arrows indicate the genes for which protein expression was confirmed.
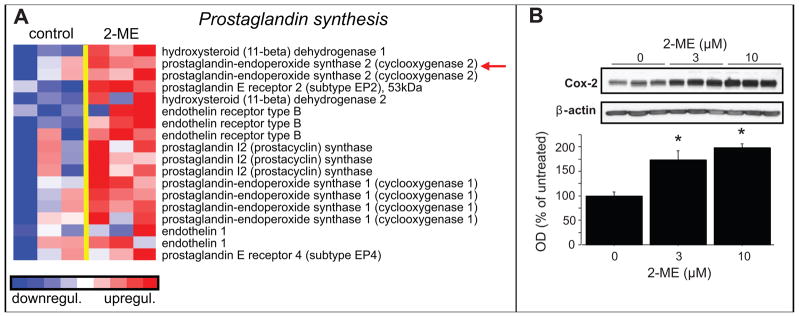
2-ME was also found to regulate genes involved in prostaglandin synthesis, as shown in Figure-2A. Importantly, Western-blots of lysates from HASMCs treated with 2-ME show a significant up-regulation in the expression of COX-2 (Figure-2B), complementary to that observed by microarray analysis.
Figure 2.
Stimulatory effects of 2-ME on prostaglandin synthesis in HASMCs. Panel-A depicts the microarray analysis of transcriptional changes in HASMCs treated with 2-ME (3μmol/L) for 30 hours. Expression levels are depicted as color scale from red (up-regulation) to blue (down-regulation). Gene Set Enrichment Analysis (GSEA) revealed induction of key proteins known to facilitate prostaglandin synthesis. Panel-B shows the significant stimulatory effects of 2-ME on COX-2 protein expression in HASMCs treated with 2-ME. Data are presented as mean±SEM (n=3). *p<0.05 versus vehicle-treated control. Arrows indicate the genes for which protein expression was confirmed.
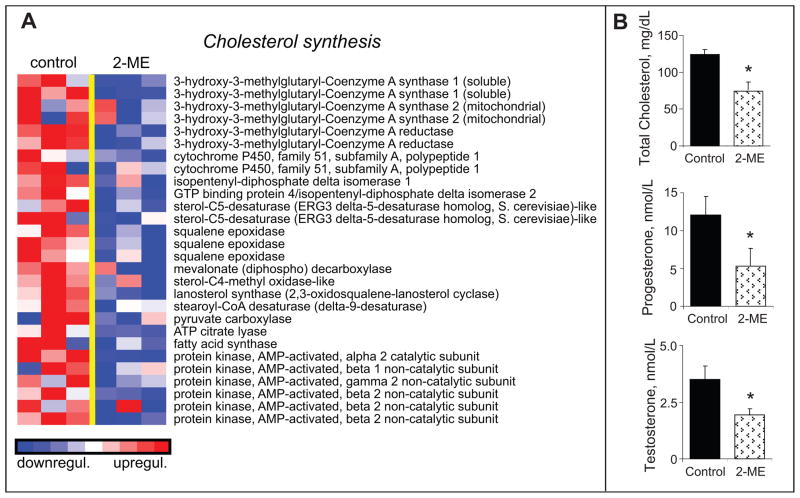
2-ME has been shown to lower cholesterol and protect against cholesterol-induced atherosclerosis10. Consistent with the cholesterol lowering effects of 2-ME, treatment with 2-ME significantly down-regulated genes involved in cholesterol synthesis (Figure-3A). Moreover, treatment of male rats with 2-ME significantly reduced the levels of total cholesterol (Figure-3B) as well as the levels of progesterone and testosterone which require cholesterol as a precursor molecule.
Figure 3.
Inhibitory effects of 2-ME on pathways regulating cholesterol synthesis. Panel-A depicts the microarray analysis of transcriptional changes in HASMCs treated with 2-ME (3μmol/L) for 30-hours. Expression levels are depicted as color scale from red (up-regulation) to blue (down-regulation). Gene Set Enrichment Analysis (GSEA) revealed down-regulation of key genes known to facilitate cholesterol synthesis. Panel-B shows the significant inhibitory effects of 2-ME on circulating levels of cholesterol, progesterone and testosterone in male rats treated for 14-days with 2-ME. Data for cholesterol levels represent mean±SEM (n=7 placebo; and n=7 2-ME treated). *p<0.05 versus vehicle-treated control.
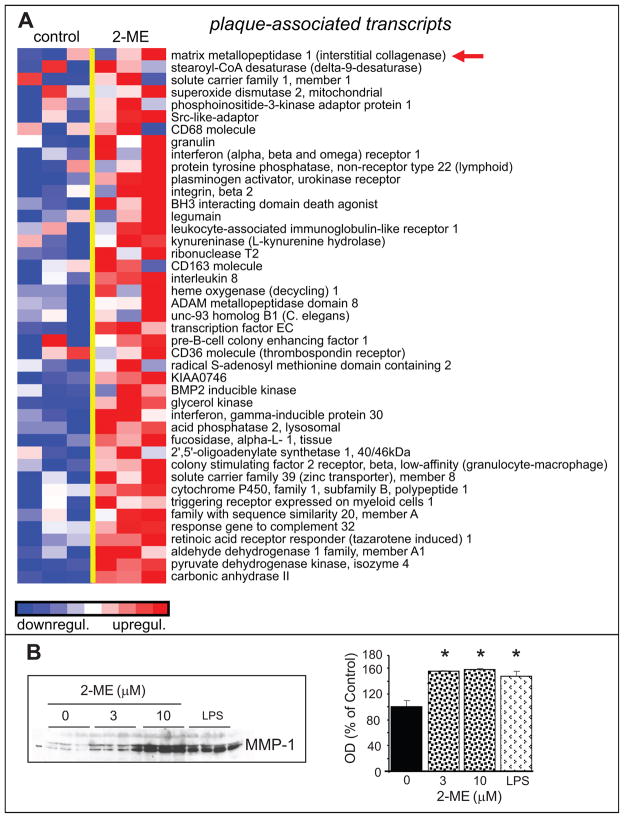
As shown in Figure-4A, GSEA analysis demonstrated that 2-ME up-regulated plaque associated transcripts. Moreover, analysis of matrix metalloproteinase-1 (MMP-1) by Western-blots showed that similar to lipopolysaccharide, 2-ME induced MMP-1 expression in HASMCs (Figure-4B).
Figure 4.
Stimulatory effects of 2-ME on plaque-associated regulatory molecules in HASMCs. Panel-A depicts the transcriptional changes in HASMCs treated with 2-ME (3μmol/L) for 30-hours. Expression levels are depicted as color scale from red (up-regulation) to blue (down-regulation). Gene Set Enrichment Analysis (GSEA) revealed up-regulation of key plaque-associated transcripts. Panel-B shows the significant stimulatory effects of 2-ME on MMP-1 expression in HASMCs treated with 2-ME. HASMCs treated with LPS served as a positive control. Data for microarray analysis and Western-blotting represent mean±SEM (n=3). *p<0.05 versus vehicle-treated control. Arrows indicate the genes for which protein expression was confirmed.
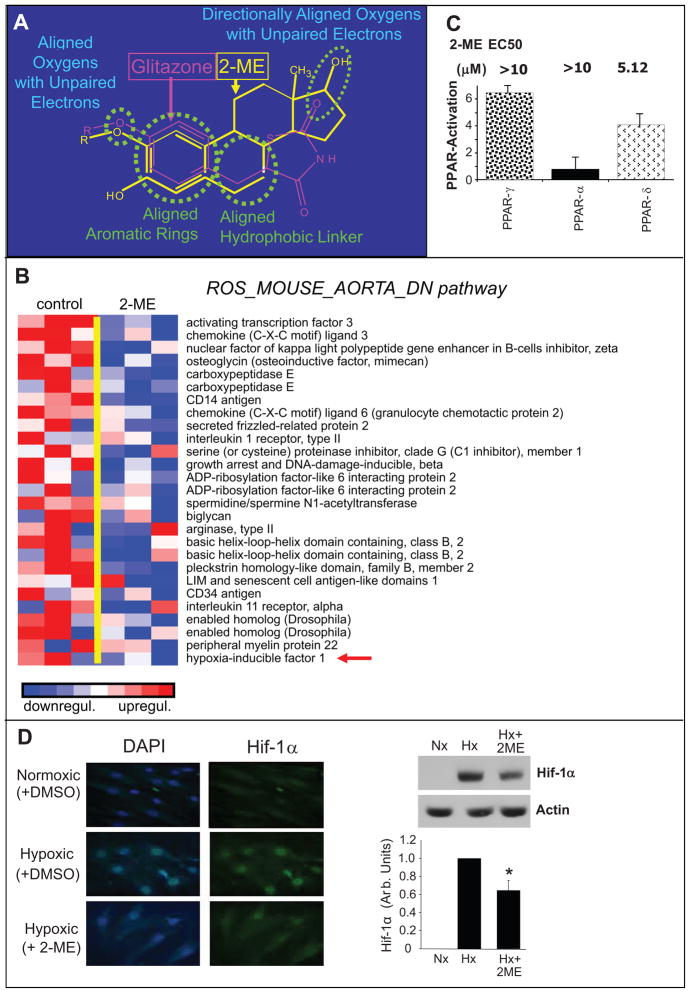
Although 2-ME is known to interact with tubulin5,9, the receptor via which it mediates its actions at lower concentrations remains elusive. 2-ME bears structural similarity with PPAR ligands, for example rosiglitazone (Figure-5A), and indeed we found that 2-ME significantly down-regulated gene transcripts known to be down-regulated by rosiglitazone, as shown in Figure-5B. Moreover, within the drug resistance and metabolism pathway, 2-ME up-regulated the genes for PPARα and PPARγ (See supplementary figure-S4 at http://hyper.ahajournals.org). Hence combining evidence from pharmacological studies and GSEA we hypothesized that 2-ME may potentially be a PPAR ligand. PPAR receptor activation assays using luciferase reporters demonstrated weak activation of PPARγ, PPARα and PPARδ by 2-ME (Figure-5C). Compared to known ligands the PPAR activation by 2-ME was ≥10-fold weaker (See supplementary figure-S5 at http://hyper.ahajournals.org). Moreover, radioligand binding assays showed little affinity of 2-ME for PPARs (See supplementary table-S4 at http://hyper.ahajournals.org); moreover, inhibition of COX-2, known to generate endogenous ligands (prostaglandins/prostacyclins) for PPAR, abrogated 2-ME mediated activation of PPAR (See supplementary figure-S5 at http://hyper.ahajournals.org). Microarray results suggested that, similar to rosiglitazone, 2-ME induced transcripts related to hypoxia and the transcription factor HIF-1α. To further validate this effect we assessed the effects of 2-ME on HIF-1α expression following hypoxia using immunofluorescence microscopy and Western-blots. Exposure of HASMCs to hypoxia induced HIF-1α expression and this stimulatory effect was abrogated in HASMCs treated with 2-ME (Figure-5D).
Figure 5.
Inhibitory effects of 2-ME on pathways down-regulated by rosiglitazone in HASMCs. Panel-A depicts the structural similarity between 2-ME and PPARγ ligand rosiglitazone. Panel-B depicts the microarray analysis of transcriptional changes in HASMCs treated with 2-ME (3μmol/L) for 30-hours. Expression levels are depicted as color scale from red (up-regulation) to blue (down-regulation). Gene Set Enrichment Analysis (GSEA) revealed down-regulation of key genes known to be down-regulated by rosiglitazone. Panel-C demonstrates the stimulatory effects of 2-ME on PPARδ, PPARγ and PPARα using a transactivation assay. Panel-D depicts representative photomicrographs and Western-blots demonstrating the inhibitory effects of 1μmol/L 2-ME on hypoxia (Hx) induced HIF-1α expression in HASMCs. The bar graph represents the densitometeric analysis of Western-blots (mean±SEM) from three separate experiments. Data for microarray analysis and Western-blotting represent mean±SEM (n=3). The immunostaining experiments were conducted in quadruplicate. *p<0.05 versus HASMCs treated with hypoxia (Hx). Normoxia (Nx). Arrows indicate the genes for which protein expression was confirmed.
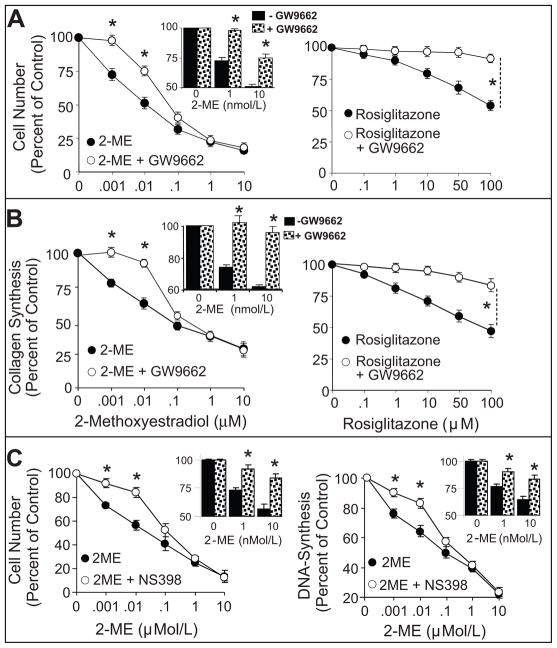
To further investigate the possibility that 2-ME exerts effects mediated by PPAR, we tested whether the growth inhibitory effects of 2-ME are blocked by the PPARγ antagonist GW9662. Serum increased several-fold all indices of cellular growth including DNA-synthesis, collagen-synthesis and cell-proliferation. In serum-treated cells, 2-ME concentration-dependently attenuated cell-proliferation (Figure-6A left-panel), collagen-synthesis (Figure-6B left-panel) and DNA-synthesis (See supplementary figure-S6 at http://hyper.ahajournals.org). Similar to 2-ME, rosiglitazone, a well established PPARγ ligand, concentration-dependently inhibited FCS-induced cell-proliferation (Figure-6A right-panel), collagen-synthesis (Figure-6B right-panel) and DNA-synthesis (See supplementary figure-S6 at http://hyper.ahajournals.org). As compared to rosiglitazone, 2-ME was more potent in inhibiting serum-induced HASMC growth. The lowest concentrations of 2-ME and rosiglitazone that significantly inhibited HASMC growth (DNA-synthesis, cell-proliferation, collagen-synthesis) were 1nmol/L and 10μmol/L, respectively. With 2-ME, a 50% inhibition of DNA-synthesis, cell-proliferation and collagen-synthesis was observed at 10nmol/L, 100nmol/L, and 100nmol/L, respectively; whereas, ≥10μmol/L of rosiglitazone was required to inhibit HASMC growth by 50%. Co-treatment with the PPARγ antagonist GW9662 blocked the inhibitory effects of rosiglitazone. Moreover, GW9662 abrogated the inhibitory effects of low (1–10nmol/L), but not high (>10nmol/L), concentrations of 2-ME on cell-number (Figure-6A left-panel and insert bar graph), collagen-production (Figure-6B left-panel and insert bar graph) and DNA-synthesis (See supplementary figure-S6 at http://hyper.ahajournals.org). Similar to GW9662, NS398 (10-μmol/L; a COX-2 inhibitor) abrogated the inhibitory effects of low (1–10nmol/L), but not high (>10nmol/L) concentrations of 2-ME on cell-proliferation and DNA-synthesis (Figure-6C).
Figure 6.
Depicts the attenuation by PPARγ antagonist GW9662 of the concentration-dependent inhibitory effects of 2-ME (0.001–10μmol/L; left-panels) or rosiglitazone (0.1–100μmol/L; right-panels) on 2.5%FCS-induced cell-number (A) and collagen ([3H]proline-incorporation) (B) in HASMCs. Also shown are the effects of NS398 on 2-ME-induced inhibition of cell number and DNA-synthesis (C). Data represent mean±SEM from 3 separate experiments conducted in triplicates. *p<0.05 significant reversal of the inhibitory effects of 2-ME or rosiglitazone by GW9662.
DISCUSSION
2-ME is an investigational drug in phase-II clinical trials for cancer and has promise as a drug to inhibit neointima formation by inhibiting VSMC growth4,5,9,16. Here, using high-density oligonucleotide microarrays, we provide evidence that 2-ME’s anti-vasoocclusive actions are mediated in part by regulation of specific genes. In this regard, the microarray data provide evidence that 2-ME down-regulates genes that are critically involved in mitotic spindle assembly, cell-cycle progression and cytokinesis and show that 2-ME increases the expression of genes involved in vasorelaxation (e.g. COX-2/prostaglandins) and inhibits genes involved in cholesterol biosynthesis. 2-ME also causes major changes in genes regulating the respiratory chain and redox pathways and plaque stability. Finally, leads from the microarray data coupled with biochemical assays provide evidence that 2-ME mimics the effects of rosiglitazone on HASMC growth, and these effects may in part be PPARγ mediated. Moreover, 2-ME significantly down-regulates gene transcripts and proteins known to be similarly affected by rosiglitazone. These data support the concept that 2-ME may be an endogenous ligand for PPARs or activate PPAR-associated genes downstream form the receptor or via stimulation of an endogenous ligand such as COX-2-derived prostaglandins. Identification of candidate genes that are positively or negatively regulated by 2-ME provides important leads to investigate the mechanisms by which 2-ME induces its vasoprotective and anti-vasoocclusive actions.
Abnormal growth of VSMCs plays a key role in the etiology of neointima thickening which contributes to vaso-occlusive disorders and cardiovascular disease1,2. Because 2-ME inhibits injury induced neointima formation as well as cholesterol induced atherosclerosis9,10, screening of genes that are influenced by 2-ME would provide important leads to further investigate the potential role of various pathways in mediating the antiproliferative and anti-vasoocclusive actions of 2-ME. Our previous studies show that as with VSMCs, 2-ME also inhibits growth of glomerular mesangial cells and cardiac fibroblasts17,18. It is likely that our results in HASMCs may provide important leads to the mechanisms via which 2-ME is protective against renal disease associated with glomerulosclerosis as well as against cardiac hypertrophy16,19.
Although multiple mechanisms are known to regulate VSMC growth, cell-cycle regulatory proteins and tubulin play a major role in promoting VSMC proliferation, migration and cytokinesis2. Screening of the genes down-regulated by 2-ME provides strong evidence that 2-ME is a potent inhibitor of factors involved in cell-cycle progression. Moreover, 2-ME down-regulates genes involved in tubulin polymerization, an essential step in nuclear division/cytokinesis as well as cell migration. These conclusions are further supported by the fact that the effects of 2-ME on the expression of genes regulating cell-cycle are also reflected at the protein level for some selected proteins tested. In this context, treatment of VSMCs both down-regulated genes and reduced the activity of proteins involved in G1 to G2 transition. Moreover, mimicking the effects of tubulin-associated genes, 2-ME inhibited tubulin polymerization in VSMCs. Finally, the fact that 2-ME down-regulates ki67 gene expression is also supported by our previous finding that 2-ME inhibits neointima formation9 and this effect is accompanied with inhibition of ki67 positive VSMCs9.
Previous studies from our group provide evidence that in animals with the metabolic syndrome 2-ME protects against cardiorenal disease progression and improves insulin sensitivity14. Our finding that in HASMCs 2-ME mimics the effects of rosiglitazone with respect to down-regulating the expression of multiple transcripts suggests that the protective effects of 2-ME may, in part, be mediated via a PPARγ-linked mechanism. This contention is also supported by the observations that 2-ME has structural similarity to rosiglitazone and that the antimitogenic effects of 2-ME, at concentrations that do not interfere with tubulin polymerization, are blocked by the PPARγ antagonist GW9662. Our finding that 2-ME inhibits hypoxia-induced expression of HIF-1α, a transcription factor known to induce VSMC growth20, suggests that 2-ME may induce its vasoprotective actions by inhibiting HIF-1α. Recent findings that PPAR-γ activation inhibits HIF-1α expression in allergic airway disease in mice21 suggests that the inhibitory effects of 2-ME on HIF-1α in VSMCs may be PPAR-γ mediated. However, it is possible that the effects of 2-ME on PPAR-γ activation and HIF-1α inhibition are independent actions of 2-ME which synergistically inhibit VSMC growth; additional studies are required to evaluate this possibility.
Although the above observations suggest that 2-ME may mediate its effects via PPARs, radioligand-displacement studies detect no direct binding of 2-ME to PPARα, PPARγ or PPARδ at concentrations up to 10μmol/L. However, in transcriptional trans-activation studies, 2-ME activates PPARγ, but with lower potency than that at which it mediates its anti-proliferative effects on VSMC proliferation that are reversible by PPARγ antagonist. These data suggest that 2-ME may mediate its effects via modulation of PPARγ-dependent gene regulation but that this occurs by downstream/upstream or other regulatory events, which are independent of ligand binding. In this context it is interesting to note that in microarray assays treatment of VSMCs with 2-ME up-regulated the genes for PPAR receptors (See supplementary Figure-S4 at http://hyper.ahajournals.org), suggesting that 2-ME’s effects may in part be mediated via modulation of PPAR expression. However the facts that 2-ME induces the expression of COX-2, that COX-2 generates endogenous ligands for PPAR (e.g., prostaglandin-D2/15d-prostaglandin-J2) and that inhibition of COX-2 with NS398 blocks 2-ME-mediated activation of PPAR and inhibition of VSMC growth suggest that 2ME may indirectly influence PPAR associated genes via generation of these endogenous ligands or via targets downstream from PPARs. Since GW9662 has been shown to inhibit COX-2 activity in VSMCs, it is possible that it abrogates the antimitogenic effects of 2-ME by inhibiting COX-2 activity22, similar to NS398. Indeed, the stimulatory effects of 2-ME on COX-2 were attenuated by GW9662 (see supplementary figure-S7 at http://hyper.ahajournals.org). However, more in-depth studies are needed to investigate this aspect.
The microarray data provide evidence that in HASMCs, 2-ME down-regulates genes involved in cholesterol synthesis, suggesting that it may protect against atherosclerosis. Indeed, recent animal studies provide evidence that treatment with 2-ME protects against cholesterol-induced atherosclerosis in ApoE knockout mice10; moreover, 2-ME lowers cholesterol levels in normal as well as Zucker obese rats with the metabolic syndrome14. Because the mechanisms via which 2-ME lowers cholesterol levels remain unclear, the data from microarray analysis of cholesterol synthesis genes regulated by 2-ME provides invaluable leads to further investigate the mechanisms involved. Indeed, our data provide evidence that 2-ME down-regulates the expression of multiple genes which play an important role in cholesterol biosynthesis. In this context, 2-ME inhibits HMG CoA, a target for statins to lower cholesterol23; inhibits squalene epoxidase, which acts downstream from HMG CoA to sequentially convert squalene to 2,3-oxidosqualene and cholesterol24; inhibits mevalonate (diphospho) decarboxylase which participates in cholesterol synthesis downstream from acetyl-CoA by converting mevalonic acid to isopentenyl-PP and squalene25. Because cholesterol is a precursor for steroid synthesis we investigated the levels of progesterone and testosterone in male rats treated with 2-ME. Consistent with our gene array data, along with total cholesterol, 2-ME decreased the circulating levels of both testosterone and progesterone. Since CYP450 is responsible for the conversion of cholesterol to steroids, it is feasible that inhibitory effects of 2-ME also contributes to its inhibitory effects on testosterone and progesterone levels. The effects on estradiol levels could not be assessed as the levels in male animals were below detection limit. Taken together, our findings provide evidence for the potential gene targets via which 2-ME can reduce cholesterol synthesis and subsequently atherosclerosis and cardiovascular disease. This contention is also supported by the recent findings that 2-ME reduces cholesterol and inhibits atherosclerosis in female mice10. Since the gene expression studies were conducted in human female cells whereas the changes in cholesterol and steroid levels measured in male rats treated with 2-ME, additional studies in females are required to further confirm these findings.
Consistent with our microarray data, recent gene expression studies on the effects of 2-ME on tumor/cancer cells provide evidence that, similar to VSMCs, 2-ME down-regulates cell cycle, and tubulin associated genes involved in the mitotic spindle assembly checkpoint and inhibits mitogenesis and migration/metastasis25,26. Interestingly, unlike VSMCs, 2-ME induces apoptosis via up-regulation of apoptosis associated genes in cancer cells27.
The beneficial effects of 2-ME on the cardiovascular system, the fact that 2-ME is non-feminizing and the fact that 2-ME is anti-carcinogenic render 2-ME an attractive therapeutic alternative for estrogen therapy. However, because estrogen therapy induces deleterious effects in selective groups of women with pre-established plaques4, it is important to assess whether 2-ME may be a safer substitute to treat this group of postmenopausal women. The present study shows that 2-ME up-regulates several plaque associated transcripts. These effects are also confirmed by Western blots where 2-ME up-regulates the expression of MMP-1, which is implicated in plaque destabilization and rupture28. Taken together these findings suggest that the therapeutic use of 2-ME may be deleterious in subjects with pre-established plaques; however further in-depth studies are required to investigate this possibility. Finally, because recent findings indicate that some PPAR ligands are associated with significant increases in cardiovascular events among high risk patients with type-2 diabetes29, it would be important to investigate whether 2-ME induces deleterious or beneficial actions as an activator of PPAR associated genes.
PERSPECTIVE
2-ME is an investigational drug known to protect against multiple proliferative disorders including vascular and renal diseases and hypertension. Microarray-based screening and identification of candidate genes modulated by 2-ME provides leads for better understanding the mechanisms via which 2-ME induces its vasoprotective and anti-vasoocclusive actions and helps better define 2-MEs potential use as a therapeutic agent.
Supplementary Material
Acknowledgments
Sources of Funding
This work was financially supported by the Swiss National Science Foundation Grants: 32-64040.00, 320000-117998 (RKD), and 3200B0-116110/1 (MZ); the Olten Heart Foundation (EL and RKD); Oncosuisse-Grant OCS-01551-08-2004 (RKD); EMDO-stiftung (RKD); and NIH-grants: HL69846, DK068575 and DK079307 (EKJ).
Footnotes
Disclosures: none
References
- 1.Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer: Common molecular pathways of disease development and progression. Ann NY Acad Sci. 2001;947:271–292. [PubMed] [Google Scholar]
- 2.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression. New therapeutic target for vascular proliferative disease. Circulation. 1998;98:82–89. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 3.Sollott SJ, Cheng L, Pauly RR, Jenkins GM, Monticone RE, Kuzuya M, Froehlich JP, Crow MT, Lakatta EG, Rowinsky EK, Kinsella JL. Taxol inhibits neointimal smooth muscle accumulation after angioplasty in the rat. J Clin Invest. 1995;95:1869–1876. doi: 10.1172/JCI117867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey RK, Imthurn B, Jackson EK. 2-Methoxyestradiol: a potential treatment for multiple proliferative disorders. Endocrinology. 2007;148:4125–4127. doi: 10.1210/en.2007-0514. [DOI] [PubMed] [Google Scholar]
- 5.Mooberry SL. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Current Opinion in Oncology. 2003;15:425–430. doi: 10.1097/00001622-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Dubey RK, Gillespie DG, Zacharia LC, Rosselli M, Korzekwa KR, Fingerle J, Jackson EK. Methoxyestradiols mediate the antimitogenic effects of estradiol on vascular smooth muscle cells via estrogen receptor-independent mechanisms. Biochem Biophys Res Commun. 2000;278:27–33. doi: 10.1006/bbrc.2000.3755. [DOI] [PubMed] [Google Scholar]
- 7.Barchiesi F, Jackson EK, Gillespie DG, Zacharia LC, Fingerle J, Dubey RK. Methoxyestradiols mediate estradiol-induced antimitogenesis in human aortic SMCs. Hypertension. 2002;39:874–879. doi: 10.1161/01.hyp.0000013863.25970.ba. [DOI] [PubMed] [Google Scholar]
- 8.Dubey RK, Gillespie DG, Zacharia LC, Barchiesi F, Imthurn B, Jackson EK. CYP450- and COMT-derived estradiol metabolites inhibit activity of human coronary artery SMCs. Hypertension. 2003;41:807–813. doi: 10.1161/01.HYP.0000048862.28501.72. [DOI] [PubMed] [Google Scholar]
- 9.Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res. 2006;99:266–274. doi: 10.1161/01.RES.0000233318.85181.2e. [DOI] [PubMed] [Google Scholar]
- 10.Bourghardt J, Bergstrom G, Krettek A, Sjöberg S, Borèn J, Tivesten A. The endogenous estradiol metabolite 2-methoxyestradiol reduces atherosclerotic lesion formation in female apolipoprotein E-deficient mice. Endocrinology. 2007;148:4128–4132. doi: 10.1210/en.2007-0259. [DOI] [PubMed] [Google Scholar]
- 11.Dubey RK, Jackson EK. Invited Review: Cardiovascular protective effects of 17β-estradiol metabolites. J Appl Physiol. 2001;91:1868–1883. doi: 10.1152/jappl.2001.91.4.1868. [DOI] [PubMed] [Google Scholar]
- 12.Libby P, Theroux P. Pathophysiology of Coronary Artery Disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 13.Dubey RK, Jackson EK. Invited Review: Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol. 2001;280:F365–F388. doi: 10.1152/ajprenal.2001.280.3.F365. [DOI] [PubMed] [Google Scholar]
- 14.Tofovic SP, Dubey RK, Jackson EK. 2-Hydroxyestradiol attenuates the development of obesity, the metabolic syndrome, and vascular and renal dysfunction in obese ZSF1 rats. J Pharmacol Exp Ther. 2001;299:973–977. [PubMed] [Google Scholar]
- 15.Dubey RK, Gillespie DG, Zacharia LC, Imthurn B, Jackson EK, Keller PJ. Clinically used estrogens differentially inhibit human aortic smooth muscle cell growth and MAP kinase activity. Arterioscleros Thromb Vas Biol. 2000;20:964–972. doi: 10.1161/01.atv.20.4.964. [DOI] [PubMed] [Google Scholar]
- 16.Dubey RK, Jackson EK. Invited Review: Cardiovascular protective effects of 17β-estradiol metabolites. J Appl Physiol. 2001;91:1868–1883. doi: 10.1152/jappl.2001.91.4.1868. [DOI] [PubMed] [Google Scholar]
- 17.Dubey RK, Gillespie DG, Zacharia LC, Keller PJ, Jackson EK. Role of methoxyestradiols in the growth inhibitory effects of estradiol on human glomerular mesangial cells. Hypertension. 2002;39:418–424. doi: 10.1161/hy0202.103297. [DOI] [PubMed] [Google Scholar]
- 18.Dubey RK, Gillespie DG, Zacharia LC, Rosselli M, Jackson EK. Methoxyestradiols mediate the antimitogenic effects of locally applied estradiol on cardiac fibroblast (CF) growth. Hypertension. 2002;39:412–417. doi: 10.1161/hy0202.102837. [DOI] [PubMed] [Google Scholar]
- 19.Dubey RK, Jackson EK. Potential vascular actions of 2-methoxyestradiol. Trends Endocrinol Metab. 2009;20:374–379. doi: 10.1016/j.tem.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz K, Fanburg BL, Beasley D. Hypoxia and hypoxia-inducible factor-1alpha promote growth factor-induced proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;290:H2528–H2534. doi: 10.1152/ajpheart.01077.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lee KS, Kim SR, Park SJ, Park HS, Min KH, Jin SM, Lee MK, Kim UH, Lee YC. Peroxisome proliferator activated receptor-γ modulates reactive oxygen species generation and activation of nuclear factor-κB and hypoxia-inducible factor 1α in allergic airway disease. J Allergy Clin Immunol. 2006;118:120–127. doi: 10.1016/j.jaci.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Bishop-bailey D, Warner TD. PPARγ induce prostaglandin production in vascular smooth muscle cells: indomethacin acts as a peroxisome proliferator-activated receptor -γ antagonist. FASEB J. 2003;17:1925–1927. doi: 10.1096/fj.02-1075fje. [DOI] [PubMed] [Google Scholar]
- 23.Ray KK, Cannon CP. Pathological changes in acute coronary syndromes: The role of statin therapy in the modulation of inflammation, endothelial function and coagulation. J Thrombosis and Thrombolysis. 2004;18:89–101. doi: 10.1007/s11239-004-0205-9. [DOI] [PubMed] [Google Scholar]
- 24.Matzno S, Yamauchi T, Gohda M, Ishida N, Katsuura K, Hanasaki Y, Tokunaga T, Itoh H, Nakamura N. Inhibition of cholesterol biosynthesis by squalene epoxidase inhibitor avoids apoptotic cell death in L6 myoblasts. J Lipid Res. 1997;38:1639–1648. [PubMed] [Google Scholar]
- 25.Sawamura M, Nara Y, Yamori Y. Liver mevalonate 5-pyrophosphate decarboxylase is responsible for reduced serum cholesterol in stroke-prone spontaneously hypertensive rat. J Biol Chem. 1992;267:6051–6055. [PubMed] [Google Scholar]
- 26.Bhati R, Gökmen-Polar Y, Sledge Jr GW, Fan C, Nakshatri H, Ketelsen D, Borchers CH, Dial MJ, Patterson C, Klauber-DeMore N. 2-Methoxyestradiol inhibits the anaphase-promoting complex and protein translation in human breast cancer cells. Cancer Res. 2007;67:702–708. doi: 10.1158/0008-5472.CAN-06-3406. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan D, Li G, Auclair D, Hideshima T, Richardson P, Podar K, Mitsiades N, Mitsiades C, Li C, Kim RS, Munshi N, Chen LB, Wong W, Anderson KC. Identification of genes regulated by 2-methoxyestradiol (2ME2) in multiple myeloma cells using oligonucleotide arrays. Blood. 2003;101:3606–3614. doi: 10.1182/blood-2002-10-3146. [DOI] [PubMed] [Google Scholar]
- 28.Nikkari ST, O’Brien KD, Ferguson M, Hatsukami T, Welgus HG, Alpers CE, Clowes AW. Interstitial Collagenase (MMP-1) Expression in Human Carotid Atherosclerosis. Circulation. 1995;92:1393–1398. doi: 10.1161/01.cir.92.6.1393. [DOI] [PubMed] [Google Scholar]
- 29.Shaya FT, Lu Z, Sohn K, Weir Thiazolidinediones and cardiovascular events in high-risk patients with type-2 diabetes mellitus. A comparison with other oral antidiabetic agents. P&T. 2009;34:490–501. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.