Abstract
In a study of changes in digestive enzymes after massive intestinal resection and the mechanisms by which such changes occur, rats were sacrified 4 wk after removal of the proximal two-thirds of the small intestine. Alterations in the mucosal levels of sucrase, enterokinase, and dipeptide hydrolase (L-leucyl-L-alanine substrate) were examined in the light of associated changes in protein. DNA and wet mucosal weight, measured in standardized gut segments from various regions of intestine.
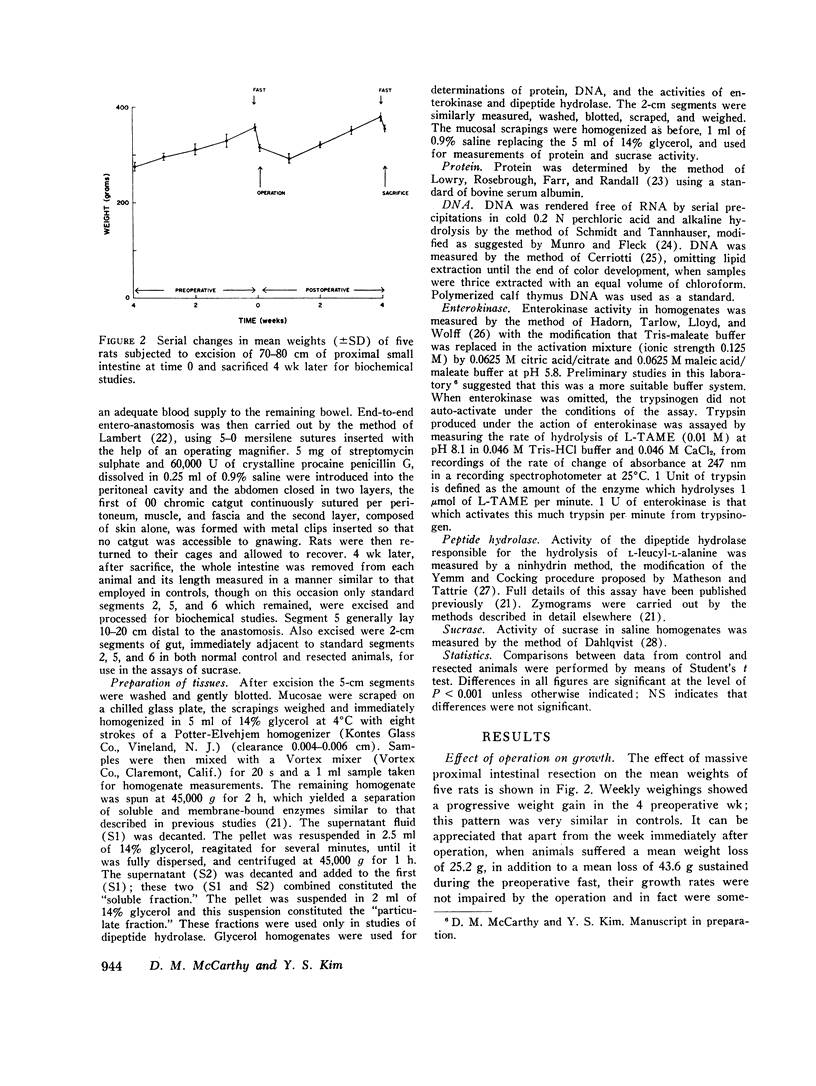
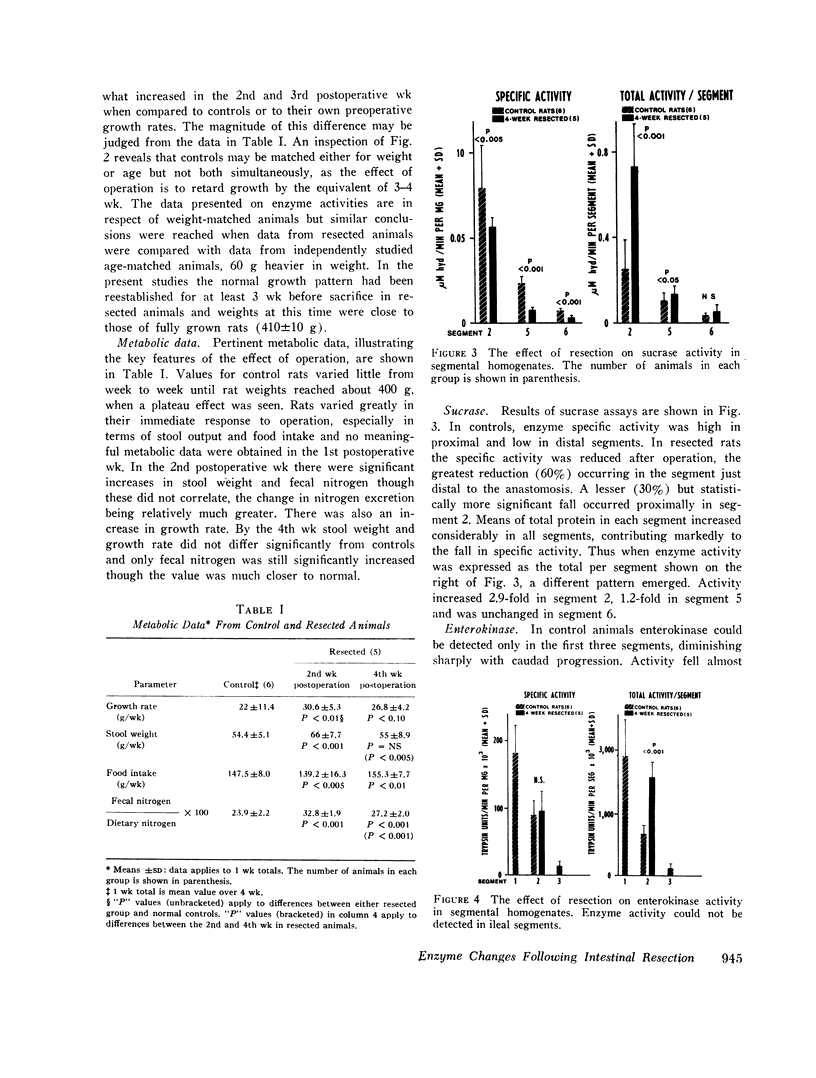
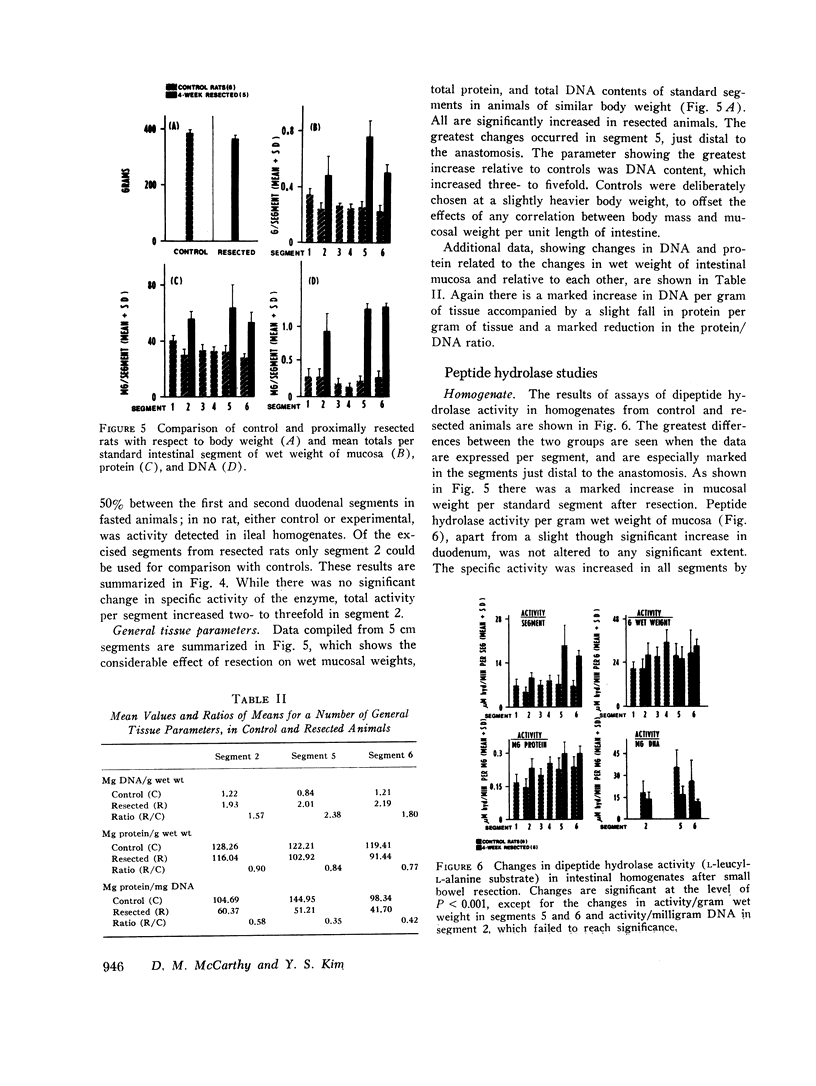
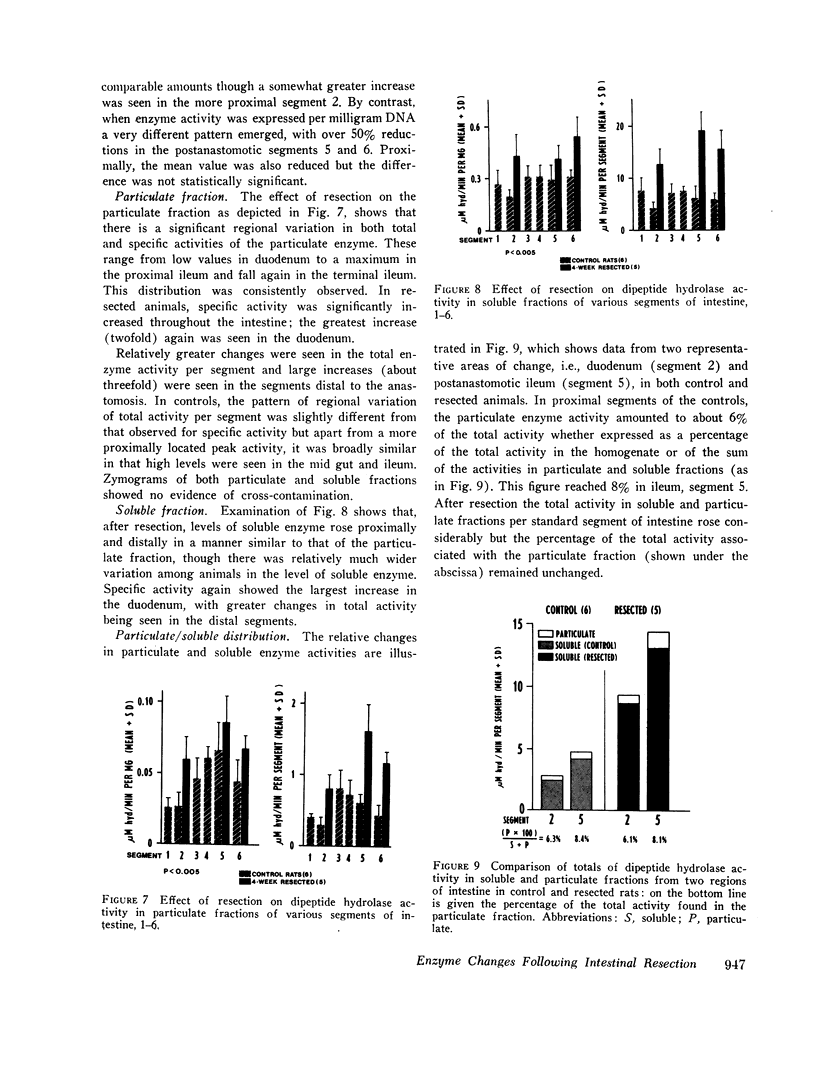
Metabolic studies showed that normal growth patterns were reestablished after the operation but significant elevations in stool weight and fecal nitrogen occurred in the second postoperative week, falling towards normal by the 4th wk. In standard gut segments wet weight of mucosa, protein, and DNA rose, especially in distal segments, DNA increasing disproportionately. Mucosal levels of the proximally distributed and membrane-bound enzymes, sucrase and enterokinase, showed similar patterns of change: when enzyme activity was expressed in terms of the total per segment, proximally there were considerable increases in both enzymes, but, expressed in terms of specific activity, that of sucrase fell and that of enterokinase was unaltered. By contrast, the largely soluble and more distally distributed dipeptide hydrolase increased more in distal segments and the increases in total activity were accompanied by lesser increases in specific activity. However, in spite of increases in total activity, enzyme activity per milligram DNA fell by over 50% in postanastomotic segments. Subcellular distribution studies showed no change in the percentage of the total activity which was membrane-bound and zymograms confirmed that no new dipeptide hydrolase had appeared after resection.
It is concluded that increases in the segmental totals of various enzymes seen after resection are achieved by disproportinate increases in the number of mucosal cells per segment and that the greatest change in a particular enzyme occurs in the region where the enzyme is normally found in highest concentration.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTHAUSEN T. L., DOIG R. K., UYEYAMA K., WEIDEN S. Digestion and absorption after massive resection of the small intestine. II. Recovery of the absorptive function as shown by intestinal absorption tests in two patients and a consideration of compensatory mechanisms. Gastroenterology. 1950 Sep;16(1):126–139. [PubMed] [Google Scholar]
- Altmann G. G. Influence of bile and pancreatic secretions on the size of the intestinal villi in the rat. Am J Anat. 1971 Oct;132(2):167–177. doi: 10.1002/aja.1001320204. [DOI] [PubMed] [Google Scholar]
- BOOTH C. C., EVANS K. T., MENZIES T., STREET D. F. Intestinal hypertrophy following partial resection of the small bowel in the rat. Br J Surg. 1959 Jan;46(198):403–410. doi: 10.1002/bjs.18004619821. [DOI] [PubMed] [Google Scholar]
- Bury K. D. Disaccharidase activity and carbohydrate absorption after intestinal resection. Surg Forum. 1971;22:367–369. [PubMed] [Google Scholar]
- CERIOTTI G. A microchemical determination of desoxyribonucleic acid. J Biol Chem. 1952 Sep;198(1):297–303. [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Functional compensation after small-bowel resection in man. Demonstration by direct measurement. Lancet. 1966 Jul 16;2(7455):146–147. doi: 10.1016/s0140-6736(66)92426-3. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Eichholz A., Crane R. K. STUDIES ON THE ORGANIZATION OF THE BRUSH BORDER IN INTESTINAL EPITHELIAL CELLS : I. Tris Disruption of Isolated Hamster Brush Borders and Density Gradient Separation of Fractions. J Cell Biol. 1965 Sep 1;26(3):687–691. doi: 10.1083/jcb.26.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn B., Tarlow M. J., Lloyd J. K., Wolff O. H. Intestinal enterokinase deficiency. Lancet. 1969 Apr 19;1(7599):812–813. doi: 10.1016/s0140-6736(69)92071-6. [DOI] [PubMed] [Google Scholar]
- Hanson W. R., Osborne J. W. Epithelial cell kinetics in the small intestine of the rat 60 days after resection of 70 per cent of the ileum and jejunum. Gastroenterology. 1971 Jun;60(6):1087–1097. [PubMed] [Google Scholar]
- Kim Y. S., Birtwhistle W., Kim Y. W. Peptide hydrolases in the bruch border and soluble fractions of small intestinal mucosa of rat and man. J Clin Invest. 1972 Jun;51(6):1419–1430. doi: 10.1172/JCI106938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORAN M. R., ALTHAUSEN T. L. Cellular proliferation of intestinal epithelia in the rat two months after partial resection of the ileum. J Biophys Biochem Cytol. 1960 Jul;7:667–672. doi: 10.1083/jcb.7.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORAN M. R., ALTHAUSEN T. L. Transport of vitamin A in vitro across normal isolated rat intestine and intestine subjected to 'partial' resection. Am J Physiol. 1959 Dec;197:1333–1336. doi: 10.1152/ajplegacy.1959.197.6.1333. [DOI] [PubMed] [Google Scholar]
- LORAN M. R., CROCKER T. T. POPULATION DYNAMICS OF INTESTINAL EPITHELIA IN THE RAT TWO MONTHS AFTER PARTIAL RESECTION OF THE ILEUM. J Cell Biol. 1963 Nov;19:285–291. doi: 10.1083/jcb.19.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A., Josefsson L. Quantitative determination of enzymes in different parts of the villi and crypts of rat small intestine. Comparison of alkaline phosphatase, disaccharidases and dipepeptidases. J Histochem Cytochem. 1967 Dec;15(12):713–721. doi: 10.1177/15.12.713. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A. Rat enterokinase: the effect of ions and the localization in the intestine. Biochim Biophys Acta. 1971 Jul 21;242(1):209–225. doi: 10.1016/0005-2744(71)90101-x. [DOI] [PubMed] [Google Scholar]
- Nygaard K. Resection of the small intestine in rats. 3. Morphological changes in the intestinal tract. Acta Chir Scand. 1967;133(3):233–248. [PubMed] [Google Scholar]
- Osborne M. P., Frederick P. L., Sizer J. S., Blair D., Cole P., Thum W. Mechanism of gastric hypersecretion following massive intestinal resection. Clinical and experimental observations. Ann Surg. 1966 Oct;164(4):622–634. doi: 10.1097/00000658-196610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J. The subcellular localization of di- and tri-peptide hydrolase activity in guinea-pig small intestine. Biochem J. 1970 Nov;120(1):195–203. doi: 10.1042/bj1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNELL P. C., SPRAY G. H. Small intestinal function in the rat after massive resections. Gastroenterology. 1956 Oct;31(4):361–368. [PubMed] [Google Scholar]
- Rodgers J. B., Jr, Bochenek W. Localization of lipid reesterifying enzymes of the rat small intestine. Effects of jejunal removal on ileal enzyme activities. Biochim Biophys Acta. 1970 May 5;202(3):426–435. doi: 10.1016/0005-2760(70)90113-x. [DOI] [PubMed] [Google Scholar]
- Rosensweig N. S., Herman R. H. Control of jejunal sucrase and maltase activity by dietary sucrose or fructose in man. A model for the study of enzyme regulation in man. J Clin Invest. 1968 Oct;47(10):2253–2262. doi: 10.1172/JCI105910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn N. An Experimental Contribution to Intestinal Surgery, with Special Reference to the Treatment of Intestinal Obstruction (Continued). Ann Surg. 1888 Feb;7(2):99–115. doi: 10.1097/00000658-188801000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skála I., Konrádová V. Hypertrophy of the small intestine after its partial resection in the rat. Ultrastructure of the intestinal epithelium. Am J Dig Dis. 1969 Mar;14(3):182–188. doi: 10.1007/BF02235879. [DOI] [PubMed] [Google Scholar]
- Tilson M. D., Wright H. K. The effect of resection of the small intestine upon the fine structure of the intestinal epithelium. Surg Gynecol Obstet. 1972 Jun;134(6):992–994. [PubMed] [Google Scholar]
- Touloukian R. J., Spencer R. P. Ileal blood flow preceding compensatory intestinal hypertrophy. Ann Surg. 1972 Mar;175(3):320–325. doi: 10.1097/00000658-197203000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall A. J., Peters T. J. Changes in structure and peptidase activity of rat small intestine induced by prednisolone. Gut. 1971 Jun;12(6):445–448. doi: 10.1136/gut.12.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]
- Wilmore D. W., Holtzapple P. G., Dudrick S. J., Cerda J. J. Transport studies, morphological and histochemical findings in intestinal epithelial cells following massive bowel resection. Surg Forum. 1971;22:361–363. [PubMed] [Google Scholar]


