Abstract
The clathrin-associated, heterotetrameric adaptor protein (AP) complexes, AP-1, AP-2, and AP-3, recognize signals in the cytosolic domains of transmembrane proteins, leading to their sorting to endosomes, lysosomes, lysosome-related organelles, and/or the basolateral membrane of polarized epithelial cells. One type of signal, referred to as “dileucine-based,” fits the consensus motif (D/E)XXXL(L/I). Previous biochemical analyses showed that (D/E)XXXL(L/I) signals bind to a combination of two subunits of each AP complex, namely the AP-1 γ-σ1, AP-2 α-σ2, and AP-3 δ-σ3 hemicomplexes, and structural studies revealed that an imperfect variant of this motif lacking the (D/E) residue binds to a site straddling the interface of α and σ2. Herein, we report mutational and binding analyses showing that canonical (D/E)XXXL(L/I) signals bind to this same site on AP-2, and to similar sites on AP-1 and AP-3. The strength and amino acid requirements of different interactions depend on the specific signals and AP complexes involved. We also demonstrate the occurrence of diverse AP-1 heterotetramers by combinatorial assembly of various γ and σ1 subunit isoforms encoded by different genes. These AP-1 variants bind (D/E)XXXL(L/I) signals with marked preferences for certain sequences, implying that they are not functionally equivalent. Our results thus demonstrate that different AP complexes share a conserved binding site for (D/E)XXXL(L/I) signals. However, the characteristics of the binding site on each complex vary, providing for the specific recognition of a diverse repertoire of (D/E)XXXL(L/I) signals.
Keywords: Adaptor Proteins, Endocytosis, Intracellular Trafficking, Protein Sorting, Protein-Protein Interactions, Dileucine Signals
Introduction
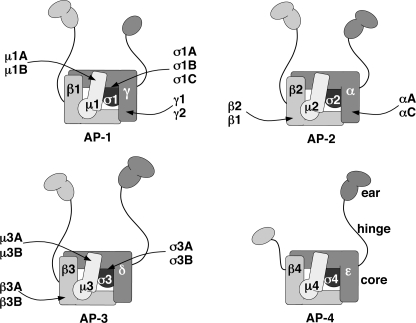
Sorting of transmembrane proteins to endosomes, lysosomes, lysosome-related organelles, and the basolateral plasma membrane of polarized epithelial cells is driven by the recognition of signals in the cytosolic domains of the transmembrane proteins by adaptor proteins that are components of membrane coats (1–4). Key components of this system are the heterotetrameric adaptor protein (AP)4 complexes, AP-1 (γ-β1-μ1-σ1), AP-2 (α-β2-μ2-σ2), AP-3 (δ-β3-μ3-σ3), and AP-4 (ϵ-β4-μ4-σ4) (subunit composition shown in parentheses) (see Fig. 1) (1–4). AP-1, AP-2, and AP-3 associate with clathrin, whereas AP-4 is most likely part of a nonclathrin coat. Another property common to AP-1, AP-2, and AP-3, but not AP-4 is their potential heterogeneity due to the existence of multiple subunit isoforms encoded by different genes, including two γ (γ1 and γ2), two μ1 (μ1A and μ1B), and three σ1 (σ1A, σ1B and σ1C) for AP-1; two α (αA and αC) for AP-2; and two β3 (β3A and β3B), two μ3 (μ3A and μ3B), and two σ3 (σ3A and σ3B) for AP-3 (1). In addition, β1 can substitute for β2 in the AP-2 complex (5–6), the only known case in which a subunit of one AP complex can be incorporated into another. Thus, combinatorial assembly of different subunit isoforms could give rise to twelve AP-1, four AP-2, and eight AP-3 complexes (see Fig. 1). It is not known, however, whether most of these combinations occur in cells and whether particular subunit isoforms endow the complexes with different functional properties.
FIGURE 1.
Subunit heterogeneity of heterotetrameric AP complexes. The schematic shows the subunit composition and isoforms of the four AP complexes (for review, see Ref. 1). Combinatorial assembly of the various subunit isoforms could result in up to twelve AP-1 complexes, four AP-2, eight AP-3, and one AP-4. The inclusion of AP-1 β1 as an AP-2 subunit is based on the observed formation of β1-containing AP-2 complexes upon knockdown of AP-2 β2 (5) or disruption of the corresponding gene (6). The AP complexes have been represented according to the structures of the AP-1 and AP-2 core complexes (47, 50) and of the ear domains of AP-1 γ (51, 52), AP-2 α (53–54) and AP-2 β2 (55). The schematic depicts a core comprising the trunk domains of the large subunits (γ, α, δ, or ϵ and β1-β4 for AP-1, -2, -3, or -4, respectively) together with the corresponding medium (μ) and small subunits (σ). The hinge and ear domains of the large subunits are shown protruding from the core of the complexes (see the AP-4 schematic). The depiction of two subdomains (an N-terminal IgG-like β sandwich and a C-terminal platform) in the ear domains of AP-1 β1, AP-3 δ, AP-3 β3, and AP-4 ϵ is based on alignment with AP-2 α and β2 subunits and secondary structure predictions. The prediction of a single C-terminal platform in AP-4 β4 is based on the lack of conservation of the N-terminal IgG-like β sandwich in this subunit as compared with the corresponding subunits in other AP complexes.
AP-1, AP-2, and AP-3 recognize sorting signals fitting the “tyrosine-based,” YXXØ, and “dileucine-based,” (D/E)XXXL(L/I) consensus motifs (where Ø is an amino acid with a bulky hydrophobic side chain, i.e. leucine, isoleucine, methionine, valine, or phenylalanine) (2–4, 7). Although both types of signals play similar roles in protein sorting, they bind to different sites on the AP complexes. Yeast two-hybrid and other protein interaction assays showed that YXXØ signals bind to the μ subunits of AP-1, AP-2, and AP-3 (8–14). (D/E)XXXL(L/I) signals, on the other hand, do not bind to any single AP subunit but to combinations of γ-σ1, α-σ2, and δ-σ3 subunits, as demonstrated by the use of yeast three-hybrid (Y3H) and in vitro binding assays (15–17).
X-ray crystallographic analyses have shed light on the structural basis for the interactions of YXXØ and (D/E)XXXL(L/I) signals with the AP-2 complex (18–20). Both binding sites are located on the AP-2 “core,” a domain formed by the amino-terminal regions of α and β2, and the entire μ2 and σ2 subunits (Fig. 1). The YXXØ-binding site comprises two hydrophobic pockets on μ2 that accommodate the Y and Ø residues of the signals (18). The binding site for (D/E)XXXL(L/I) signals likely corresponds to that of a dileucine-containing “Q-peptide” from CD4, (RMpSQIKRLLSE), which was recently identified by Kelly et al. (20). The Q-peptide does not strictly conform to the definition of a (D/E)XXXL(L/I) signal, although the Gln residue at position −4 or the phosphorylated Ser residue at position −5 in the CD4 peptide could behave similar to the Asp or Glu residue at position −4 in the canonical signals (the first critical leucine is considered position 0). This site consists of hydrophobic pockets on σ2 that fit the Leu and (L/I) residues, and a basic patch straddling the boundary of α and σ2 that might bind the Gln, phosphorylated Ser, or (D/E) residues of the signals (20). The AP-2 core occurs in two conformations: an inactive conformation in which both signal-binding sites are occluded by interaction with different parts of β2 and an active conformation in which both binding sites are exposed on a surface that is coplanar with a PtdIns-4,5-P2-binding site on α (21). This conformational change is triggered by phosphorylation of a threonine residue in μ2 (22) and results in increased affinity of AP-2 for PtdIns-4,5-P2-enriched domains of the plasma membrane, thus allowing simultaneous recognition of both types of signal (23). The exact location of the signal-binding sites on AP-1 and AP-3, and the mechanistic details of the interactions have not been determined.
Herein, we report the use of site-directed mutagenesis and Y3H assays to map the binding sites for (D/E)XXXL(L/I) signals on AP-1 and AP-3. We find that these AP complexes have a (D/E)XXXL(L/I)-binding site similar to that of AP-2. Analysis of the fine specificity of interactions of various (D/E)XXXL(L/I) signals with AP-1, AP-2, and AP-3 subunits, however, reveals both signal- and AP-complex-dependent differences. We also investigated the assembly of different AP-1 subunit isoforms in cells and the ability of these isoforms to recognize (D/E)XXXL(L/I) signals. We demonstrate that heterotetramers containing all possible combinations of γ and σ1 isoforms, with the exception of γ2-β1-μ1-σ1C, are assembled in vivo. Finally, we show that the AP-1 γ1-σ1A, γ1-σ1B, and γ1-σ1C hemicomplexes recognize all (D/E)XXXL(L/I) signals tested, whereas γ2-σ1A and γ2-σ1B have a more restricted specificity. These findings indicate that AP-1, AP-2, and AP-3 share a conserved binding site for (D/E)XXXL(L/I) signals, albeit with distinct specificity determined by the exact nature of the signal, as well as the particular AP complex and subunit isoforms involved in the interactions. Based on these observations, we argue that a proper definition of AP complexes must include the specific composition of subunit isoforms.
EXPERIMENTAL PROCEDURES
DNA Constructs
Complementary DNAs encoding HA- or Myc-tagged human σ1A, σ1B, and σ1C (C-terminal tags in all cases) were subcloned in the pXS modification of the pCDL-SRα296 vector (24) or in pCR3.1 (Invitrogen) expression plasmids, respectively.
Antibodies
Mouse monoclonal antibodies to γ1 (clone 100/3), β1/β2 (clone 100/1), and α (clone 100/2) were obtained from Sigma-Aldrich. Mouse monoclonal antibodies to the HA (HA.11) and Myc (clone 9E10) epitopes were purchased from Covance Research Products (Princeton, NJ). Rabbit polyclonal antibodies to μ1 (RY/1) and σ1A (DE/1) were gifts from L. Traub (University of Pittsburgh). The rabbit polyclonal antibody to β3 (β3C1) was described by Dell'Angelica et al. (25) and the rabbit polyclonal anti-ϵ by Boehm et al. (26).
Northern Blot Analysis
Two commercial nylon membranes (Human 12-lane MTNTM Blot and Human MTNTM Blot III, Clontech, Mountain View, CA) with immobilized human poly(A)+ RNA (1 μg) from different tissues were used for Northern blot analysis. Membranes were hybridized with a 32P-labeled human σ1C probe (splice variant 1) prepared using the MegaprimeTM DNA labeling system (GE Healthcare) and [α-32P]dCTP (GE Healthcare).
Y3H Analysis
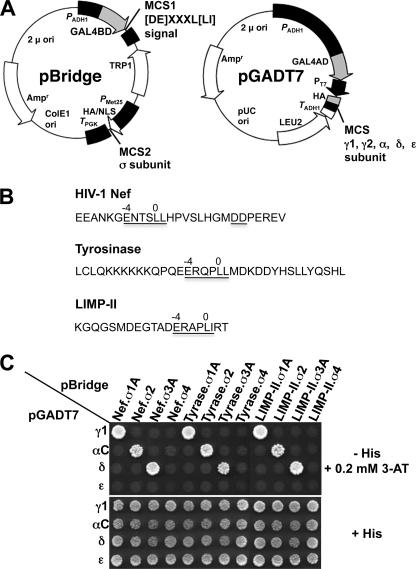
Y3H vector construction and assays were performed as described previously (see Fig. 2A) (15, 27). Double transformants were selected in medium lacking leucine, tryptophan, and methionine but containing histidine (+His) and subsequently plated in +His medium, as well as in the same medium lacking histidine (−His). Interactions between dileucine signals and AP subunits result in activation of the GAL4 promoter and activation of HIS3 gene transcription as evidenced by growth on −His plates. Experiments were also performed on −His plates containing 3-amino-1,2,4-triazole (3-AT), a competitive inhibitor of the His3 protein to minimize background growth due to nonspecific interactions and also to detect differences in the avidity of interactions. Parallel plating on +His plates provided a control for loading and viability of double transformants.
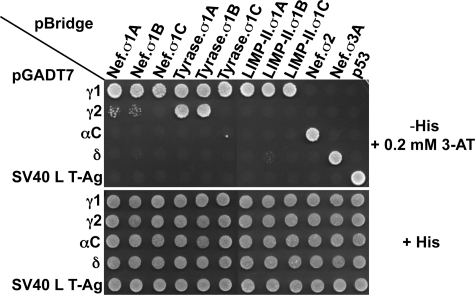
FIGURE 2.
Y3H analysis of the interaction of (D/E)XXXL(L/I) signals with AP hemicomplexes. A, the schematic shows the two vectors used in the Y3H analysis. The cDNAs encoding the (D/E)XXXL(L/I) signals (full-length HIV-1Nef NL4–3 variant or the cytosolic tails of mouse tyrosinase or human LIMP-II) were subcloned into multiple cloning site 1 (MCS1) of the GAL4 binding domain vector pBridge, whereas the small AP subunits (σ1, σ2, σ3, or σ4) were subcloned into MCS2 of the same vector. The sequences encoding full-length mouse γ1, human γ2, rat αC, human δ, or human ϵ were subcloned into the GAL4 activation domain vector pGADT7. ori, origin of replication; Amp, ampicillin resistance gene; NLS, nuclear localization signal. B, sequences of the HIV-1 Nef (NL4–3 variant) flexible loop and mouse tyrosinase and human LIMP-II cytosolic tails with signals conforming to the (D/E)XXXL(L/I) are underlined. The diaspartate motif at +10 and +11 from the HIV-1 Nef ENTSLL dileucine signal that is required for binding to AP-2 (35) is also underlined. The mouse tyrosinase cytosolic tail contains a second (D/E)XXXL(L/I) motif (DYHSLL) C-terminal to the ERQPLL shown in the schematic. Although this second sequence is present in mouse and rat tyrosinase, it is not conserved in other species such as humans, is not involved in lysosomal/melanosomal sorting (56), and is not required for interaction with AP complexes (34). C, all (D/E)XXXL(L/I) signals tested interact with AP-1, AP-2, and AP-3 but not AP-4. Double transformants were plated in medium lacking histidine, leucine, tryptophan, and methionine (−His), to detect interaction among constructs, and in medium lacking only leucine, tryptophan, and methionine (+His) as a control for loading and viability of double transformants. In this experimental set, the −His plates were supplemented with a low concentration (0.2 mm) of 3-AT (a competitive inhibitor of the His3 protein) to minimize background growth due to nonspecific interactions. The lack of interaction of AP-4 with the various (D/E)XXXL(L/I) signals was also observed in −His plates lacking 3-AT. The image shown represents a composite of different plates from the same experiment. Results shown are representative of at least three experiments with similar results. Tyrase, tyrosinase. For details, see “Experimental Procedures.”
Cell Transfection
M1 human fibroblasts (a gift from Eric Long, NIAID, National Institutes of Health) were stably transfected with pXS-human σ1A-HA, pXS-human σ1B-HA, pXS-human σ1C-HA, or pcDNA3-HA-human γ2 (a gift from K. Nakayama, Kyoto University). Selection of cells stably transfected with pXS was achieved by co-transfection with pCI-neo (Promega, Madison, WI). M1 cells stably transfected with pcDNA3-HA-human γ2 were subsequently subjected to transient transfection with pCR3.1-human σ1A-myc, human σ1B-myc or human σ1C-myc using the FuGENETM reagent (Roche Applied Science).
Metabolic Labeling and Immunoprecipitation-recapture Analysis
Transfected cells (two 150-mm plates per condition) were subjected to metabolic labeling for 12–15 h using EasyTag ExpressTM 35S protein labeling mix (PerkinElmer Life Science, Waltham, MA). Preparation of Triton X-100 extracts and immunoprecipitation-recapture experiments were performed as described previously (28, 29). The composition of the solubilization and immunoprecipitation buffer was 50 mm Tris-HCl, pH 7.4, 1% Triton X-100, 300 mm NaCl, 5 mm EDTA supplemented before use with 10 mm iodoacetamide, 2 μg/ml leupeptin, and 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride. The immunoprecipitates bound to protein A- or protein G-Sepharose beads were denatured in 100 mm Tris-HCl pH 7.4, 1% SDS, 10 mm DTT, diluted ∼20-fold with immunoprecipitation buffer and subjected to an additional round of immunoprecipitation (recapture step). The recapture beads were dissolved in Laemmli sample buffer and analyzed by SDS-PAGE and phosphorautoradiography (Typhoon 9200 PhophorImager, GE Healthcare).
RESULTS
Y3H Analysis of Interaction of (D/E)XXXL(L/I) Signals with AP Hemicomplexes
We used a Y3H assay (Fig. 2A) (15, 27) to determine the pattern of interaction of different (D/E)XXXL(L/I) signals with AP complexes. The signals tested included ENTSLL (from a flexible loop in the Nef protein of HIV-1) (30–32), ERQPLL (from the mouse tyrosinase cytosolic tail) (33, 34), and ERAPLI (from the human LIMP-II cytosolic tail) (33) (Fig. 2B). In line with our previous studies (15, 35), we found that all of these signals interact with the AP-1 γ1-σ1A, AP-2 αC-σ2, and AP-3 δ-σ3A hemicomplexes but not with the homologous AP-4 ϵ-σ4 hemicomplex (Fig. 2C). These interactions require that both subunits belong to the same AP complex, as mismatched pairs did not show interaction (Fig. 2C).
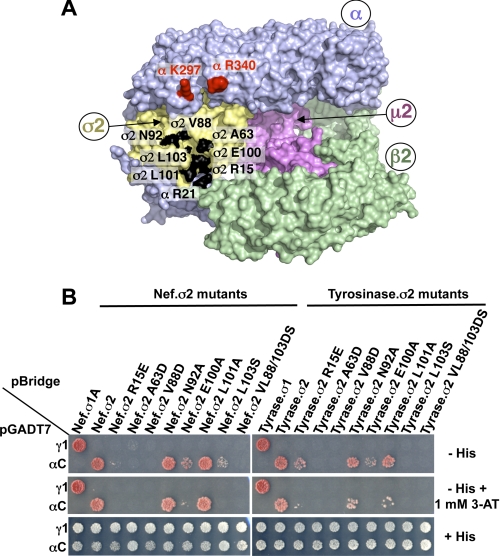
Mutational Mapping of Binding Site for Canonical (D/E)XXXL(L/I) Signals on σ2
We next examined the structural determinants for interaction of AP-2 with the (D/E)XXXL(L/I) signals described above. The crystal structure of the AP-2 core in complex with the dileucine-containing Q-peptide from CD4 showed that most of the residues involved in the interaction were on the σ2 subunit (Fig. 3A) (20). To determine whether these σ2 residues were required for interaction with canonical (D/E)XXXL(L/I) signals, we mutated them and tested for interaction of the mutant proteins with the Nef and tyrosinase signals using the Y3H assay (Fig. 3B). The results showed that several σ2 mutations, including A63D, V88D, E100A, and L103S, largely abolished the interaction of αC-σ2 with both signals (Fig. 3B). Other σ2 mutations affected the interactions with Nef and tyrosinase differently. For instance, the N92A and L101A mutations had no effect on the interaction with the Nef signal but decreased the interaction with the tyrosinase signal, particularly as seen in the presence of 1 mm 3-AT (Fig. 3B). From these results, we concluded that canonical (D/E)XXXL(L/I) signals bind to the same site as the CD4 Q-peptide but exhibit distinct requirements for specific residues within the binding site. The lower sensitivity of the Nef dileucine signal to σ2 substitutions may be due to a stabilizing effect of the previously described diaspartate motif at +10 and +11 from the ENTSLL (Fig. 2B), which is absent in the tyrosinase signal and has been proposed to interact with a basic patch comprising Lys297 and Arg340 on the α subunit (Fig. 3A) (27).
FIGURE 3.
Analysis of AP-2 residues involved in the interaction with Nef and tyrosinase (D/E)XXXL(L/I) signals. A, residues in the α (Arg21) and σ2 (Ala63, Val88, Asn92, Glu100, Leu101, and Leu103) subunits that were subjected to mutagenesis are shown in black on the surface representation of the three-dimensional structure of the AP-2 core complex (Protein Data Bank codes 1GW5 and 2VGL) (50). The α, β2, μ2, and σ2 subunits are depicted in light blue, green, magenta, and gold, respectively. Shown in red on the α subunit are residues Lys297 and Arg340, which are also required for the binding of the AP-2 α-σ2 hemicomplex to HIV-1 Nef (27). B, Y3H assays showing the effect of σ2 substitutions on the interaction of the AP-2 α-σ2 hemicomplex with HIV-1 Nef and tyrosinase (Tyrase) (D/E)XXXL(L/I) signals. Experiments were performed as indicated in the legend to Fig. 2. Positive controls included the interaction of (D/E)XXXL(L/I) signals with the AP-1 γ-σ1 and AP-2 α-σ2 hemicomplexes, whereas double transformants expressing (D/E)XXXL(L/I) signals and discordant γ and σ2 pairs were used as negative controls. Double transformants were plated on −His and −His plus 1 mm 3-AT medium to analyze the interactions at different levels of stringency and on +His medium as a control for loading and viability. The image shown represents a composite of different plates from the same experiment.
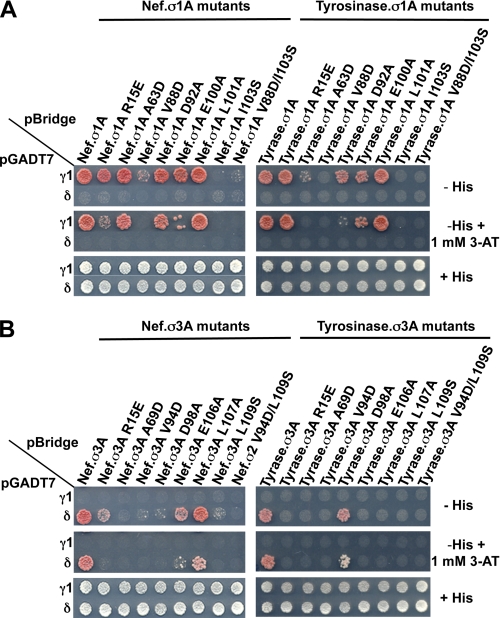
Identification and Characterization of (D/E)XXXL(L/I)-binding Site on σ1 and σ3
We next sought to determine whether AP-1 and AP-3 have a (D/E)XXXL(L/I)-binding site similar to that on AP-2. The σ2 residues that participate in the interaction with (D/E)XXXL(L/I) signals are conserved on σ1A and σ3A (Fig. 4A). We therefore tested the effect of mutating these σ1A and σ3A residues on the interaction with the Nef and tyrosinase signals in Y3H assays. We found that most of these mutations affected the interactions of the corresponding γ1-σ1A and δ-σ3A hemicomplexes with both signals (Fig. 5, A and B). The specific requirements for interaction, however, were signal- and adaptor-dependent. As shown above for αC-σ2, the interactions of γ1-σ1A and δ-σ3A with the Nef signal (Fig. 5, A and B, left panels) were generally less sensitive to substitutions than the corresponding interactions with the tyrosinase signal (Fig. 5, A and B, right panels). In addition, the interaction of both the Nef and tyrosinase signals with γ1-σ1A (Fig. 5A) was stronger and less sensitive to substitutions than the corresponding interactions with δ-σ3A (Fig. 5B). In this context, whereas the interaction with the signals was reduced by most substitutions in σ3A with the exception of L107A (for Nef) and D98A (for tyrosinase) (Fig. 5B), the interaction with σ1A was abolished only by V88D and I103S (for Nef) and also by A63D (for tyrosinase) (Fig. 5A). Among the substitutions that did not abolish binding of the Nef and tyrosinase signals to the γ1-σ1A hemicomplex was σ1A R15E (an effect on Nef was only detected in the presence of 1 mm 3-AT) (Fig. 5A). This substitution involves a basic residue equivalent to σ2 Arg15 which, along with αC Arg21, is part of the positively charged patch that binds to the −4/−5 position of the CD4 Q-peptide (20), a position occupied by Asp or Glu in canonical (D/E)XXXL(L/I) signals. These results indicated that the general features of the AP-2 (D/E)XXXL(L/I)-binding site are conserved on AP-1 and AP-3, although the overall strength of the interactions and the requirement of specific residues on the σ subunits vary for different signals.
FIGURE 4.
Conservation of AP-1 and AP-3 residues potentially involved in interactions with (D/E)XXXL(L/I) signals. A, alignment of human σ1A (RefSeq accession no. NP_001274), σ2 (GenBankTM accession no. AAH06337), and σ3A (GenBankTM accession no. CAG29337). The alignment shows the conservation of σ2 residues (Arg15, Ala63, Val88, Asn92, Glu100, Leu101, and Leu103 shown in black boxes) that interact with residues at different positions of the CD4 Q-peptide (20). Shown in black lettering are the positions on the signal (residue at −4; LL, dileucine pair; or O, other) proposed to bind to the σ residues in the corresponding boxes. B, alignment of N-terminal sequences of human γ1 (GenBankTM accession no. AAH36283), αC (Swiss-Prot accession no. O94973) and δ (GenBankTM accession number AAC51761) showing the conservation of the α subunit Arg residue (αArg15, shown in the black box) proposed to stabilize the −4 position of the CD4 Q-peptide (20). Alignments were generated with CLC Sequence Viewer; decreasing conservation of residues is shown by red to blue rainbow coloring.
FIGURE 5.
Mapping of AP-1 and AP-3 residues involved in interactions with (D/E)XXXL(L/I) signals. The interaction of (D/E)XXXL(L/I) motif-based signals with AP-1 γ-σ1 or AP-3 δ-σ3 mutant hemicomplexes is shown in A and B, respectively. Subcloning of cDNAs sequences encoding (D/E)XXXL(L/I) signals and AP subunits and plating of double transformants were performed as indicated in the legend to Fig. 2. Positive controls included the interaction of (D/E)XXXL(L/I) signals with the AP-1 γ-σ1 or AP-3 δ-σ3 hemicomplexes, whereas double transformants expressing (D/E)XXXL(L/I) signals and discordant γ-σ3 or δ-σ1 pairs were used as negative controls. The image shown represents a composite of different plates from the same experiment. Tyrase, tyrosinase.
Distinct Requirements of Basic Residues on γ-σ1, α-σ2, and δ-σ3 Hemicomplexes for Binding to (D/E)XXXL(L/I) Signals
We also analyzed the role of conserved basic residues on the γ1 and δ subunits at positions equivalent to αC Arg21 (Fig. 4B), given the contribution of this residue to the positively charged patch interacting with the −4/−5 residue of the CD4 Q-peptide (20). To this end, we compared the binding of the Nef, tyrosinase, and LIMP-II signals to the γ1 R15E, αC R21E, and δ R26E mutants and the corresponding wild-type proteins as hemicomplexes with σ1A, σ2, and σ3A, respectively. The results showed that substitution of these basic residues generally decreased interactions with the signals (Fig. 6). The effects were more marked for the γ1 R15E and δ R26E mutations than for the αC R21E mutation and were also more noticeable for the interaction with the Nef and LIMP-II signals than with the tyrosinase signal (Fig. 6). In addition, we observed that the effects were smaller for the αC R21E mutation than for the σ2 R15E mutation (Fig. 6B), whereas similar reductions were observed when comparing the δ R26E and σ3A R15E mutations (Fig. 6C). This suggests that basic residues on both the α and σ2 subunits of AP-2 and the δ and σ3 subunits of AP-3 contribute to the electrostatic interaction with the acidic residue at position −4 of the signals. This is in line with the observation that mutation of both basic residues in the patch (αC Arg21 and σ2 Arg15) is necessary to decrease binding of the AP-2 core to the CD4 Q-peptide (20). In contrast, mutation of γ1 R15E caused a much greater reduction in binding to the (D/E)XXXL(L/I) signals than mutation of σ1A Arg15 (Fig. 6A), indicating that the basic residue contributed by γ1 is the most critical for interaction with the (D/E) residue of the signals. A summary of the results of the mutational analyses is shown in Fig. 7.
FIGURE 6.
Substitution of γ, α, δ, and σ residues potentially interacting with the −4 position of the (D/E)XXXL(L/I) motif affects the interaction with HIV-Nef, tyrosinase, and LIMP-II signals. The interaction of the different (D/E)XXXL(L/I) signals with the wild-type or mutant γ-σ1, α-σ2, and δ-σ3 hemicomplexes is shown in A–C, respectively. Subcloning of cDNAs sequences encoding (D/E)XXXL(L/I) signals and AP subunits was performed as indicated in the legend to Fig. 2. Double transformants were plated on −His, −His plus 0.2 mm 3-AT, −His plus 1 mm 3-AT and +His medium (only −His plus 1 mm 3-AT and +His plates shown for simplicity). Similar conclusions were drawn from the analysis of −His and −His plus 0.2 mm 3-AT plates. Tyrase, tyrosinase.
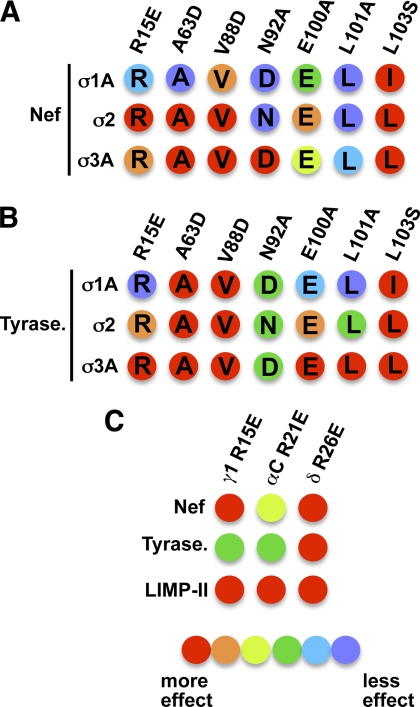
FIGURE 7.
Graphic summary of the effects of substitutions in AP subunits on the interaction of (D/E)XXXL(L/I) motif-based signals. A and B, relative effect of substitutions in σ1A, σ2, and σ3 on the interaction with HIV-1 Nef (A) and tyrosinase signals (B) based on the results shown in Figs. 3 and 5. Numbering of residues indicated on top of schematics correspond to the σ2 sequence (see Figs. 4 and 5 for numbering of corresponding positions in σ1A and σ3A). The effect of substitutions is depicted ranging from red (binding completely abolished) to violet (no effect) in a rainbow gradient (see relative color gradient below C). A “blue shift” (lower sensitivity to mutations) can be observed for σ1A when comparing the effects of substitutions on this subunit with those at equivalent positions in σ2 or σ3A. C, relative effect of γ R15E, αC R21E, and δ R26E substitutions on the interaction with HIV-1 Nef, tyrosinase, and LIMP-II signals based on the results in Fig. 6. The effect of substitutions is depicted as indicated for A and B. Tyrase, tyrosinase.
Assembly of AP-1 Complexes Containing Different γ and σ Subunit Isoforms
Combinatorial assembly of AP subunit isoforms could generate additional diversity in the recognition of sorting signals. This is particularly the case for the AP-1 complex, which could occur in at least 12 varieties (Fig. 1). Of all of the AP-1 subunit isoforms, μ1B is the only one that is restricted to a particular cell type, polarized epithelial cells (36), whereas the other isoforms appear to be widely expressed in mammalian tissues and cells (36–39). Thus, most cells could have up to six different AP-1 complexes containing common β1 and μ1A subunits and variable γ1/γ2 and σ1A/σ1B/σ1C isoforms. This potential heterogeneity begs the questions of whether all of these combinations do assemble in vivo and whether they exhibit any differences in the recognition of (D/E)XXXL(L/I) signals.
We used a biochemical approach to examine the formation of multiple AP-1 variants comprising different γ and σ1 isoforms. To this end, we stably transfected M1 human fibroblasts with HA epitope-tagged σ1A, σ1B, or σ1C constructs. Following metabolic labeling with [35S]methionine, cell lysates were subjected to immunoprecipitation with antibody to the HA epitope and recapture of the solubilized immunoprecipitates with antibodies to other AP subunits. The results of these experiments showed that all three σ1-HA isoforms are incorporated into AP-1 complexes containing γ1, β1, and μ1 subunits but not into complexes including the αC subunit of AP-2, the β3A subunit of AP-3, or the ϵ subunit of AP-4 (Fig. 8, A–C). A similar analysis of M1 cells stably transfected with HA-tagged γ2 showed that this subunit is also specifically incorporated into AP-1 complexes (Fig. 8D). In addition, we subjected these HA-γ2 stable transfectants to transient transfection with Myc-epitope-tagged σ1A, σ1B, or σ1C. The immunoprecipitation-recapture analysis showed that, whereas the γ1 isoform was incorporated into AP-1 complexes containing either σ1A, σ1B, or σ1C, the γ2 isoform was incorporated into AP-1 complexes containing σ1A or σ1B, but not σ1C (Fig. 8E). These experiments thus demonstrated that, of the six possible AP-1 complexes arising from combinations of different γ and σ1 subunits, five can be formed in the transfected M1 cells.
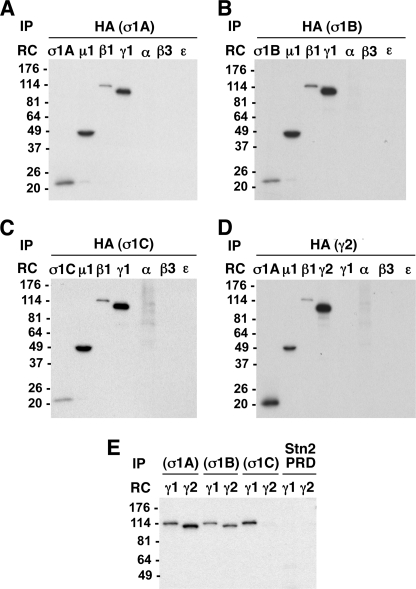
FIGURE 8.
In vivo assembly of multiple AP-1 complexes containing different γ or σ1 subunit isoforms. A–D, M1 human fibroblasts were stably transfected with vectors driving the expression of σ1A-HA, σ1B-HA, σ1C-HA, or HA-γ2. Cells were metabolically labeled for 12–15 h with [35S]methionine and [35S]cysteine. Cell lysates were subjected to immunoprecipitation (IP) with anti-HA, and the immunoprecipitates were denatured, diluted, and subjected to additional rounds of immunoprecipitation (RC, recapture) using antibodies against the HA epitope, or μ1, β1, γ1, α, β3, or ϵ subunits (A–C) or σ1A, μ1, β1, HA, γ1, α, β3, or ϵ subunits (D). The recaptured immunoprecipitates were subjected to SDS-PAGE and fluorography. The analysis demonstrates that σ1A, σ1B, and σ1C subunits are all incorporated into AP-1 complexes also containing μ1, β1, and γ1 subunits, but not into AP-2, AP-3, or AP-4 (lack of recapture of σ1A, σ1B, or σ1C by either anti-α, -β3, or -ϵ) (A–C). The γ2 subunit also assembles into AP-1 but not AP-2, AP-3, or AP-4 (D). E, M1 cells stably transfected with HA-γ2 were subjected to transient transfection with vectors driving expression of σ1A-Myc, σ1B-Myc, or σ1C-Myc (a Myc-tagged stonin 2 proline-rich domain (Stn2 PRD) was used as a negative control). Transfected cells were metabolically labeled and lysed, followed by immunoprecipitation with anti-Myc and recapture with either anti-γ1 or anti-HA (for detection of γ2). Note that γ1 is incorporated into AP-1 complexes containing any of the three isoforms of σ1, whereas γ2 can only assemble into AP-1 complexes containing either σ1A or σ1B.
Interaction of (D/E)XXXL(L/I) Signals with AP-1 Hemicomplexes Containing Different γ and σ Isoforms
We next analyzed the ability of hemicomplexes that have different combinations of γ and σ1 subunit isoforms to interact with (D/E)XXXL(L/I) signals using Y3H assays. The results showed that all AP-1 hemicomplexes containing γ1 (γ1-σ1A, γ1-σ1B, and γ1-σ1C) interact with similar avidities with the Nef, tyrosinase, and LIMP-II signals (Fig. 9). In contrast, the γ2-σ1A and γ2-σ1B hemicomplexes displayed signal-dependent interactions; they interacted very weakly with Nef, robustly (similar to the activity of the γ1-σ1A- and γ1-σ1B-containing complexes) with the tyrosinase signal, and not at all with the LIMP-II signal (Fig. 9). The absence of interactions with (D/E)XXXL(L/I) signals observed in yeast transformed with vectors encoding γ2 and σ1C (Fig. 9) is consistent with the biochemical experiments demonstrating the lack of assembly of complexes containing this combination of subunits (Fig. 8E).
FIGURE 9.
γ subunit- and motif-dependent interaction of AP-1 with (D/E)XXXL(L/I) signals. All γ1-based AP-1 hemicomplexes (γ1-σ1A, γ1-σ1B, and γ1-σ1C) display similarly high avidities for (D/E)XXXL(L/I) signals. In contrast, γ2-σ1A and γ2-σ1B interact weakly with HIV-1 Nef, interact strongly with the tyrosinase tail, and do not recognize the LIMP-II signal. The lack of interaction detected for all double transformants expressing γ2 and σ1C is consistent with lack of assembly of AP-1 complexes comprising these two subunits, as evidenced by immunoprecipitation-recapture analysis of metabolically labeled cells (Fig. 8). This lack of interaction cannot be explained by lack of expression of γ2 and σ1C subunits in yeast given the binding of the tyrosinase signal to γ2-σ1A or γ2-σ1B and to γ1-σ1C, respectively. The image shown represents a composite of different plates from the same experiment. SV40 L T-Ag, SV40 large T-antigen (negative control for interactions with pBridge constructs and positive control for interaction with p53).
DISCUSSION
The results of our analyses demonstrate that canonical (D/E)XXXL(L/I) signals bind to the same site on AP-2 as the noncanonical CD4 Q-peptide (20) and that AP-1 and AP-3 have a similar binding site. This is evidenced by the loss of signal binding by the σ2 V88D or L103S substitutions and the homologous σ1A V88D and I103S and σ3A V94D and L109S substitutions. Nonetheless, there are also differences in the interactions that are dependent on both the signals and adaptors (see Fig. 7). The approach that we used to assess differences in (D/E)XXXL(L/I) signal recognition is the substitution of residues on AP-1 and AP-3 that are equivalent to those important for the interaction of AP-2 with the CD4 Q-peptide. Our results show that the binding of these signals to AP-1 is less sensitive to substitution of single residues in its putative binding site than the corresponding interactions with AP-2 and AP-3. Two residues that exemplify these differences are σ1A Arg15 and Leu101, which can be substituted with relatively little impact on the ability of γ1-σ1A to recognize (D/E)XXXL(L/I) signals. In contrast, substitution of the corresponding residues in σ3A (Arg15 and Leu107) decreased the interaction of (D/E)XXXL(L/I) signals with δ-σ3A (especially in the case of tyrosinase). A mixed outcome, inhibition by σ2 R15E and (D/E)XXXL(L/I) signal-dependent effects with σ2 L101A, was observed when analyzing the homologous substitutions in σ2.
The differences in the interaction of (D/E)XXXL(L/I) signals with AP complexes also extend to the interaction of the (D/E) residue with basic residues on both subunits of the hemicomplexes. We found that interaction with γ1-σ1A depends mainly on γ1 Arg15, whereas interactions with the other hemicomplexes involve basic residues on both subunits, namely α Arg21 and σ2 Arg15 in α-σ2 and δ Arg26 and σ3A Arg15 in δ-σ3A (Fig. 6). The requirement of residues in two subunits of each complex (γ and σ1 for AP-1, α and σ2 for AP-2, and δ and σ3 for AP-3) explains why it was necessary to use Y3H assays to detect interactions of (D/E)XXXL(L/I) signals with AP subunits (15). From these experiments, we conclude that although AP-1, AP-2, and AP-3 share a similar binding site, residues in both the signals and the adaptors make different contributions to the interactions, thereby defining the fine specificity of signal recognition events.
Genetic studies have begun to address the physiologic significance of the existence of AP subunit isoforms. AP-1 exhibits the greatest diversification of subunit isoforms because vertebrates express two γ (γ1 and γ2; both ubiquitous), two μ1 (μ1A and μ1B; the first ubiquitous, the second epithelial-specific), and three σ (σ1A, σ1B, and σ1C) paralogs (1). Targeted disruption of the μ1A gene in mouse causes embryonic lethality at day 13.5 (during mid-organogenesis) and only polarized epithelial cells from early embryos exhibit membrane binding of AP-1, likely due to the expression of μ1B (40). Inactivation of the γ1 gene is also lethal and results in death of mouse embryos prior to implantation (41), indicating that γ2 is unable to compensate for the lack of γ1 expression. Recent findings also support specific requirements for the three σ1 isoforms (supplemental Fig. 1A). A mutation in the human σ1A gene leading to premature translation termination causes the neurocutaneous MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma) (42). On the other hand, human σ1B deficiency causes an X-linked mental retardation syndrome characterized by basal ganglia calcifications and elevated protein levels in cerebrospinal fluid (43–45). Moreover, mice deficient in σ1B are viable and fertile but display altered synaptic vesicle recycling in hippocampal synapses, reduced motor coordination and long-term spatial learning and memory deficiencies (39). The σ1C isoform was first identified in a bioinformatics analysis (1) and is also expressed in a variety of mouse (39) and human tissues (supplemental Fig. 1C). Interestingly, three splice variants of σ1C exhibiting differences at their C termini have been identified (supplemental Fig. 1B), but there is no information on the alterations brought about by their lack of expression.
Although these genetic studies have provided information on the requirement of specific AP-1 subunit isoforms at the organismal level, the molecular and cellular bases for these requirements are not known. Moreover, except for μ1A and μ1B (46), the assembly of different subunit isoforms into AP-1 had not been demonstrated prior to our study. Our observations provide the first assessment of the in vivo assembly of AP-1 heterotetramers formed by different combinations of γ and σ subunits. We found that σ1A, σ1B, and σ1C can all assemble into AP-1 heterotetramers. In addition, we observed that γ1 is incorporated into complexes containing any σ1 isoform, whereas γ2 can assemble into complexes containing σ1A or σ1B but not σ1C. The structural basis for the incompatibility of γ2 with σ1C is unknown. Although the crystal structure of an AP-1 core complex (trunk of γ1 and β1 subunits along with μ1A and σ1A) has been solved (47), the contacts between the γ1 trunk and σ1A are too extensive to make inferences based on primary structure differences between γ1/γ2 and between σ1A/σ1C.
Our experiments also provide the first indication that certain combinations of subunit isoforms have intrinsically different recognition specificities, as exemplified by the differences in (D/E)XXXL(L/I) signal recognition by AP-1 hemicomplexes containing γ1 or γ2 (Fig. 9). The inability of γ2-containing complexes to interact with signals that are recognized by γ1-containing complexes might explain why γ1-deficient mice are inviable despite the ubiquitous expression of γ2. What might then be the specific role of γ2, given the ubiquitous expression of γ1 and the strong interaction of γ1-based hemicomplexes with dileucine signals? The strong avidity of γ2-σ1A and γ2-σ1B hemicomplexes for the tyrosinase signal (as opposed to other (D/E)XXXL(L/I)-based signals) (Fig. 9) suggests that γ2-containing AP-1 complexes might sort specific cargo to specialized cellular compartments such as melanosomes, a process in which AP-1 has been implicated (48).
AP-1 complex variants containing μ1A or μ1B have been referred to as AP-1A or AP-1B, respectively (49). In light of the broader repertoire of AP subunit combinations that can be assembled and the differences in the activity of some of these combinations, we submit that, whenever relevant, the proper definition of an AP complex must include information on the specific subunits that constitute it (e.g. AP-1 (γ1-μ1A-β1-σ1B) or AP-1 (γ2-μ1A-β1-σ1A)).
Supplementary Material
Acknowledgments
We thank H. Tsai for expert technical assistance and L. Traub and E. Long for kind gifts of reagents.
This work was supported by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- AP
- adaptor protein
- Y3H
- yeast three-hybrid
- 3-AT
- 3-amino-1,2,4-triazole
- HIV-1
- human immunodeficiency virus, type 1.
REFERENCES
- 1. Boehm M., Bonifacino J. S. (2001) Mol. Biol. Cell 12, 2907–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonifacino J. S., Traub L. M. (2003) Annu. Rev. Biochem. 72, 395–447 [DOI] [PubMed] [Google Scholar]
- 3. Robinson M. S. (2004) Trends Cell Biol. 14, 167–174 [DOI] [PubMed] [Google Scholar]
- 4. Traub L. M. (2009) Nat. Rev. Mol. Cell Biol. 10, 583–596 [DOI] [PubMed] [Google Scholar]
- 5. Keyel P. A., Thieman J. R., Roth R., Erkan E., Everett E. T., Watkins S. C., Heuser J. E., Traub L. M. (2008) Mol. Biol. Cell 19, 5309–5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li W., Puertollano R., Bonifacino J. S., Overbeek P. A., Everett E. T. (2010) Cleft Palate Craniofac. J. 47, 566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braulke T., Bonifacino J. S. (2009) Biochim. Biophys. Acta 1793, 605–614 [DOI] [PubMed] [Google Scholar]
- 8. Ohno H., Stewart J., Fournier M. C., Bosshart H., Rhee I., Miyatake S., Saito T., Gallusser A., Kirchhausen T., Bonifacino J. S. (1995) Science 269, 1872–1875 [DOI] [PubMed] [Google Scholar]
- 9. Ohno H., Fournier M. C., Poy G., Bonifacino J. S. (1996) J. Biol. Chem. 271, 29009–29015 [DOI] [PubMed] [Google Scholar]
- 10. Boll W., Ohno H., Songyang Z., Rapoport I., Cantley L. C., Bonifacino J. S., Kirchhausen T. (1996) EMBO J. 15, 5789–5795 [PMC free article] [PubMed] [Google Scholar]
- 11. Rapoport I., Miyazaki M., Boll W., Duckworth B., Cantley L. C., Shoelson S., Kirchhausen T. (1997) EMBO J. 16, 2240–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chuang E., Alegre M. L., Duckett C. S., Noel P. J., Vander Heiden M. G., Thompson C. B. (1997) J. Immunol. 159, 144–151 [PubMed] [Google Scholar]
- 13. Stephens D. J., Crump C. M., Clarke A. R., Banting G. (1997) J. Biol. Chem. 272, 14104–14109 [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y., Allison J. P. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 9273–9278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janvier K., Kato Y., Boehm M., Rose J. R., Martina J. A., Kim B. Y., Venkatesan S., Bonifacino J. S. (2003) J. Cell Biol. 163, 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaudhuri R., Lindwasser O. W., Smith W. J., Hurley J. H., Bonifacino J. S. (2007) J. Virol. 81, 3877–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doray B., Lee I., Knisely J., Bu G., Kornfeld S. (2007) Mol. Biol. Cell. 18, 1887–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Owen D. J., Evans P. R. (1998) Science 282, 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Owen D. J., Setiadi H., Evans P. R., McEver R. P., Green S. A. (2001) Traffic 2, 105–110 [DOI] [PubMed] [Google Scholar]
- 20. Kelly B. T., McCoy A. J., Späte K., Miller S. E., Evans P. R., Höning S., Owen D. J. (2008) Nature 456, 976–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson L. P., Kelly B. T., McCoy A. J., Gaffry T., James L. C., Collins B. M., Höning S., Evans P. R., Owen D. J. (2010) Cell 141, 1220–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ricotta D., Conner S. D., Schmid S. L., von Figura K., Honing S. (2002) J. Cell Biol. 156, 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Höning S., Ricotta D., Krauss M., Späte K., Spolaore B., Motley A., Robinson M., Robinson C., Haucke V., Owen D. J. (2005) Mol. Cell. 18, 519–531 [DOI] [PubMed] [Google Scholar]
- 24. Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. (1988) Mol. Cell. Biol. 8, 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dell'Angelica E. C., Ooi C. E., Bonifacino J. S. (1997) J. Biol. Chem. 272, 15078–15084 [DOI] [PubMed] [Google Scholar]
- 26. Boehm M., Aguilar R. C., Bonifacino J. S. (2001) EMBO J. 20, 6265–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaudhuri R., Mattera R., Lindwasser O. W., Robinson M. S., Bonifacino J. S. (2009) J. Virol. 83, 2518–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dell'Angelica E. C., Ohno H., Ooi C. E., Rabinovich E., Roche K. W., Bonifacino J. S. (1997) EMBO J. 16, 917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonifacino J. S., Dell'Angelica E. C. (2001) Curr. Protoc. Cell Biol. 1, 7.2.1–7.2.21 [DOI] [PubMed] [Google Scholar]
- 30. Bresnahan P. A., Yonemoto W., Ferrell S., Williams-Herman D., Geleziunas R., Greene W. C. (1998) Curr. Biol. 8, 1235–1238 [DOI] [PubMed] [Google Scholar]
- 31. Craig H. M., Pandori M. W., Guatelli J. C. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 11229–11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greenberg M., DeTulleo L., Rapoport I., Skowronski J., Kirchhausen T. (1998) Curr. Biol. 8, 1239–1242 [DOI] [PubMed] [Google Scholar]
- 33. Höning S., Sandoval I. V., von Figura K. (1998) EMBO J. 17, 1304–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Theos A. C., Tenza D., Martina J. A., Hurbain I., Peden A. A., Sviderskaya E. V., Stewart A., Robinson M. S., Bennett D. C., Cutler D. F., Bonifacino J. S., Marks M. S., Raposo G. (2005) Mol. Biol. Cell 16, 5356–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindwasser O. W., Smith W. J., Chaudhuri R., Yang P., Hurley J. H., Bonifacino J. S. (2008) J. Virol. 82, 1166–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohno H., Tomemori T., Nakatsu F., Okazaki Y., Aguilar R. C., Foelsch H., Mellman I., Saito T., Shirasawa T., Bonifacino J. S. (1999) FEBS Lett. 449, 215–220 [DOI] [PubMed] [Google Scholar]
- 37. Lewin D. A., Sheff D., Ooi C. E., Whitney J. A., Yamamoto E., Chicione L. M., Webster P., Bonifacino J. S., Mellman I. (1998) FEBS Lett. 435, 263–268 [DOI] [PubMed] [Google Scholar]
- 38. Takatsu H., Sakurai M., Shin H. W., Murakami K., Nakayama K. (1998) J. Biol. Chem. 273, 24693–24700 [DOI] [PubMed] [Google Scholar]
- 39. Glyvuk N., Tsytsyura Y., Geumann C., D'Hooge R., Hüve J., Kratzke M., Baltes J., Boening D., Klingauf J., Schu P. (2010) EMBO J. 29, 1318–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meyer C., Zizioli D., Lausmann S., Eskelinen E. L., Hamann J., Saftig P., von Figura K., Schu P. (2000) EMBO J. 19, 2193–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zizioli D., Meyer C., Guhde G., Saftig P., von Figura K., Schu P. (1999) J. Biol. Chem. 274, 5385–5390 [DOI] [PubMed] [Google Scholar]
- 42. Montpetit A., Côté S., Brustein E., Drouin C. A., Lapointe L., Boudreau M., Meloche C., Drouin R., Hudson T. J., Drapeau P., Cossette P. (2008) PLoS Genet. 4, e1000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tarpey P. S., Stevens C., Teague J., Edkins S., O'Meara S., Avis T., Barthorpe S., Buck G., Butler A., Cole J., Dicks E., Gray K., Halliday K., Harrison R., Hills K., Hinton J., Jones D., Menzies A., Mironenko T., Perry J., Raine K., Richardson D., Shepherd R., Small A., Tofts C., Varian J., West S., Widaa S., Yates A., Catford R., Butler J., Mallya U., Moon J., Luo Y., Dorkins H., Thompson D., Easton D. F., Wooster R., Bobrow M., Carpenter N., Simensen R. J., Schwartz C. E., Stevenson R. E., Turner G., Partington M., Gecz J., Stratton M. R., Futreal P. A., Raymond F. L. (2006) Am. J. Hum. Genet. 79, 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saillour Y., Zanni G., Des Portes V., Heron D., Guibaud L., Iba-Zizen M. T., Pedespan J. L., Poirier K., Castelnau L., Julien C., Franconnet C., Bonthron D., Porteous M. E., Chelly J., Bienvenu T. (2007) J. Med. Genet. 44, 739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Borck G., Mollà-Herman A., Boddaert N., Encha-Razavi F., Philippe A., Robel L., Desguerre I., Brunelle F., Benmerah A., Munnich A., Colleaux L. (2008) Hum. Mutat. 29, 966–974 [DOI] [PubMed] [Google Scholar]
- 46. Fölsch H., Pypaert M., Schu P., Mellman I. (2001) J. Cell Biol. 152, 595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heldwein E. E., Macia E., Wang J., Yin H. L., Kirchhausen T., Harrison S. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 14108–14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Delevoye C., Hurbain I., Tenza D., Sibarita J. B., Uzan-Gafsou S., Ohno H., Geerts W. J., Verkleij A. J., Salamero J., Marks M. S., Raposo G. (2009) J. Cell Biol. 187, 247–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fölsch H., Ohno H., Bonifacino J. S., Mellman I. (1999) Cell 99, 189–198 [DOI] [PubMed] [Google Scholar]
- 50. Collins B. M., McCoy A. J., Kent H. M., Evans P. R., Owen D. J. (2002) Cell 109, 523–535 [DOI] [PubMed] [Google Scholar]
- 51. Nogi T., Shiba Y., Kawasaki M., Shiba T., Matsugaki N., Igarashi N., Suzuki M., Kato R., Takatsu H., Nakayama K., Wakatsuki S. (2002) Nat. Struct. Biol. 9, 527–531 [DOI] [PubMed] [Google Scholar]
- 52. Kent H. M., McMahon H. T., Evans P. R., Benmerah A., Owen D. J. (2002) Structure 10, 1139–1148 [DOI] [PubMed] [Google Scholar]
- 53. Owen D. J., Vallis Y., Noble M. E., Hunter J. B., Dafforn T. R., Evans P. R., McMahon H. T. (1999) Cell 97, 805–815 [DOI] [PubMed] [Google Scholar]
- 54. Traub L. M., Downs M. A., Westrich J. L., Fremont D. H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 8907–8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Owen D. J., Vallis Y., Pearse B. M., McMahon H. T., Evans P. R. (2000) EMBO J. 19, 4216–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Simmen T., Schmidt A., Hunziker W., Beermann F. (1999) J. Cell Sci. 112, 45–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.