Abstract
In previous work we found that vitamin D-deficient and also calcium-deficient rats developed hypocalcemia and an impairment of bone formation and mineralization. The present study of thyroparathyroidectomized (TPTX) rats was undertaken to determine the effect of hypocalcemia without secondary hyperparathyroidism. TPTX rats fed a normal diet developed hypocalcemia and hyperphosphatemia in association with impairment of osteoblastic bone matrix formation and of mineralization of newly formed matrix. The serum calcium × phosphorus product was not decreased. The decreased formation was largely due to a reduction in matrix apposition indicating decreased synthetic activity of individual ostcoblasts. In contrast to the above results, when TPTX rats were fed a high-calcium diet to prevent hypocalcemia, no impairment of either formation or mineralization was found. From the results of these two experiments, it is reasonably certain that hypocalcemia was responsible for the inhibition of formation and mineralization. Moreover, based on the magnitude of the changes in serum calcium and bone parameters in TPTX rats, hypocalcemia could have accounted for the inhibition of formation and mineralization in calcium-deficient as well as vitamin D-deficient rats.
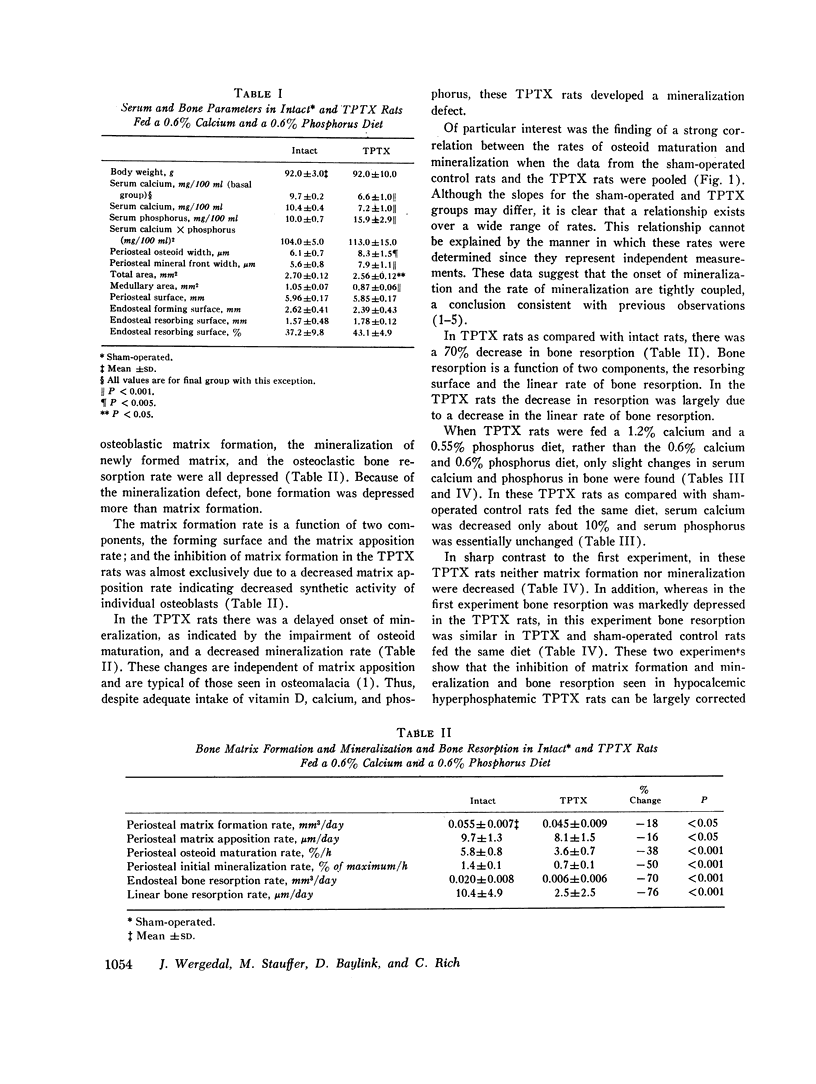
In TPTX rats the mineralization defect was manifested by decreases in both the rate of osteoid maturation (indicating a delayed onset of mineralization) and the rate of mineralization. A strong correlation (r = 0.95, P < 0.001) was observed between these two rates suggesting a tight coupling of these two aspects of mineralization.
TPTX rats also had lower bone resorption rates and higher serum phosphorus levels than sham-operated animals when the normal calcium diet was fed but not when the high-calcium diet was fed. Thus the inhibition of bone resorption in TPTX rats was at least partially prevented by correction of hyperphosphatemia. This is consistent with previous work showing an inverse relationship between serum phosphorus and bone resorption. Accordingly, the depression of bone resorption in TPTX rats was probably due to hyperphosphatemia as well as to hypoparathyroidism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylink D. J., Wergedal J. E. Effets du fluor su la minéralisation des os chez le rat. Med Hyg (Geneve) 1971 Oct 13;29(980):1554–1556. [PubMed] [Google Scholar]
- Baylink D., Morey E., Rich C. Effect of calcitonin on the rates of bone formation and resorption in the rat. Endocrinology. 1969 Feb;84(2):261–269. doi: 10.1210/endo-84-2-261. [DOI] [PubMed] [Google Scholar]
- Baylink D., Stauffer M., Wergedal J., Rich C. Formation, mineralization, and resorption of bone in vitamin D-deficient rats. J Clin Invest. 1970 Jun;49(6):1122–1134. doi: 10.1172/JCI106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylink D., Wergedal J., Stauffer M. Formation, mineralization, and resorption of bone in hypophosphatemic rats. J Clin Invest. 1971 Dec;50(12):2519–2530. doi: 10.1172/JCI106752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylink D., Wergedal J., Thompson E. Loss of proteinpolysaccharides at sites where bone mineralization is initiated. J Histochem Cytochem. 1972 Apr;20(4):279–292. doi: 10.1177/20.4.279. [DOI] [PubMed] [Google Scholar]
- Burkhart J. M., Jowsey J. Morphologic evidence of osteomalacia in the parathyroidectomized dog. Mayo Clin Proc. 1966 Oct;41(10):663–667. [PubMed] [Google Scholar]
- Canas F., Terepka A. R., Neuman W. F. Potassium and milieu intérieur of bone. Am J Physiol. 1969 Jul;217(1):117–120. doi: 10.1152/ajplegacy.1969.217.1.117. [DOI] [PubMed] [Google Scholar]
- Flanagan B., Nichols G., Jr Bone matrix turnover and balance in vitro. I. The effects of parathyroid hormone and thyrocalcitonin. J Clin Invest. 1969 Apr;48(4):595–606. doi: 10.1172/JCI106018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. H., Heaney R. P. Skeletal renewal and metabolic bone disease. N Engl J Med. 1969 Feb 6;280(6):303–concl. doi: 10.1056/NEJM196902062800605. [DOI] [PubMed] [Google Scholar]
- JOWSEY J., ROWLAND R. E., MARSHALL J. H., McLEAN F. C. The effect of parathyroidectomy on haversian remodeling of bone. Endocrinology. 1958 Dec;63(6):903–908. doi: 10.1210/endo-63-6-903. [DOI] [PubMed] [Google Scholar]
- Kalu D. N., Doyle F. H., Pennock J., Foster G. V. Parathyroid hormone and experimental osteosclerosis. Lancet. 1970 Jun 27;1(7661):1363–1366. doi: 10.1016/s0140-6736(70)91271-7. [DOI] [PubMed] [Google Scholar]
- Kelly P. J. Effects of thyroid and parathyroid deficiency on bone remodelling distal to a venous tourniquet. J Anat. 1971 Dec;110(Pt 3):349–361. [PMC free article] [PubMed] [Google Scholar]
- Moore E. W. Ionized calcium in normal serum, ultrafiltrates, and whole blood determined by ion-exchange electrodes. J Clin Invest. 1970 Feb;49(2):318–334. doi: 10.1172/JCI106241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Niemann I. Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology. 1969 Sep;85(3):446–452. doi: 10.1210/endo-85-3-446. [DOI] [PubMed] [Google Scholar]
- Raisz L. G. The pharmacology of bone. Introduction. Fed Proc. 1970 May-Jun;29(3):1176–1178. [PubMed] [Google Scholar]
- Raisz L. G., Trummel C. L., Holick M. F., DeLuca H. F. 1,25-dihydroxycholecalciferol: a potent stimulator of bone resorption in tissue culture. Science. 1972 Feb 18;175(4023):768–769. doi: 10.1126/science.175.4023.768. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Anast C., Arnaud C. Thyrocalcitonin, EGTA, and urinary electrolyte excretion. J Clin Invest. 1967 May;46(5):746–752. doi: 10.1172/JCI105575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Feinblatt J., Nagata N., Pechet M. Effect of ions upon bone cell function. Fed Proc. 1970 May-Jun;29(3):1190–1197. [PubMed] [Google Scholar]
- Rasmussen H. Ionic and hormonal control of calcium homeostasis. Am J Med. 1971 May;50(5):567–588. doi: 10.1016/0002-9343(71)90113-6. [DOI] [PubMed] [Google Scholar]
- Sherrard D., Baylink D., Wergedal J. Bone disease in uremia. Trans Am Soc Artif Intern Organs. 1972;18(0):412-5, 421. doi: 10.1097/00002480-197201000-00102. [DOI] [PubMed] [Google Scholar]
- Waron M., Rich C. Rate of recovery from acute hypocalcemia as a measure of calcium homeostatic efficiency in the dog. Endocrinology. 1969 Dec;85(6):1018–1027. doi: 10.1210/endo-85-6-1018. [DOI] [PubMed] [Google Scholar]
- Wergedal J. E., Baylink D. J. Distribution of acid and alkaline phosphatase activity in undemineralized sections of the rat tibial diaphysis. J Histochem Cytochem. 1969 Dec;17(12):799–806. doi: 10.1177/17.12.799. [DOI] [PubMed] [Google Scholar]
- Wergedal J. E. Enzymes of protein and phosphate catabolism in rat bone. I. Enzyme properties in normal rats. Calcif Tissue Res. 1969;3(1):55–66. doi: 10.1007/BF02058645. [DOI] [PubMed] [Google Scholar]


