Abstract
Background
There is a need to develop new bone anabolic agents because current bone regeneration regimens have limitations. The Wingless-type MMTV integration site (Wnt) pathway has emerged as a crucial regulator of bone formation and regeneration.
Objective
Toreview the molecular basis for Wnt pathway modulation and discuss potential strategies that target it and improve bone mass.
Methods
Data in peer-reviewed reports and meeting abstracts are discussed.
Results/Conclusions
Neutralizing inhibitors of Wnt signaling have emerged as promising and feasible strategies. Small molecule inhibitors of GSK3β increase bone mass, lower adiposity and reduce fracture risk. Neutralizing antibodies to Dickkopf 1, secreted Frizzled-related protein 1 and sclerostin produce similar outcomes in animal models. These drugs are exciting breakthroughs, but they are not without risks. The challenges include tissue-specific targeting and consequently, long-term safety.
Keywords: Osteoporosis, GSK-3, Dkk1, Sclerostin, Sfrp1, SOST
1. Introduction
The clinical need to develop new anabolic agents is high because current bone regeneration options have limitations, anti-resorptive therapies have unknown long-term health consequences, and the demand for therapies is rising as the population ages. It is estimated that more than 200 million people worldwide (1 in 3 women and 1 in 12 men over the age of 50 years) suffer from osteoporosis, with 3 to 4 times as many at risk because of low bone mass [1, 2]. Osteoporosis-related fractures often begin a downward spiral in health and independence for the elderly. The current standard of care is an anti-resorptive treatment such as bisphosphonates, hormone replacement, and selective estrogen receptor modulators. Although these treatments are effective, controversy exists about their long-term effects on general skeletal health, particularly with regards to repair of microfractures, as well as on breast cancer risk and heart health [3, 4]. Teriparatide (Forteo™, parathyroid hormone (PTH) residues 1–34) is the only anabolic drug available at this time. The regulations on Teriparatide use vary by country (e.g., two years of maximum use in the USA) and it is usually only prescribed to patients with established osteoporosis, whom have already suffered a fracture, or in whom bisphosphonates are ineffective or contraindicated. Teriparatide is administered daily via subcutaneous injection and stimulates new bone formation, but has several side effects, including hypercalcemia and hypercalciuria. Moreover, high doses of PTH can cause osteosarcomas in rats [5].
New bone anabolic agents could also be used to treat non-union fractures. Approximately one-third of people fracture a bone within their lifetime and 5–10% of those fractures fail to heal normally and result in a non-union, leading to staggering economic consequences [2, 6, 7]. Bone morphogenic proteins (BMP) 2 and 7 are used respectively to fuse vertebrae and to treat non-union long bone fractures that occur after trauma and for which an allograft is not suitable. BMPs have short half-lives and cannot be delivered systemically, thus, they are not available to treat osteoporosis. New anabolic agents that enhance bone regeneration locally and improve bone density systemically would reduce fracture risk and improve the quality of life for millions of people.
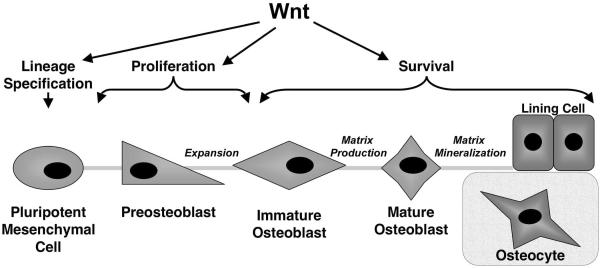
The search for new anabolic therapies is focused on biological pathways that stimulate osteoblast lineage cells to differentiate from a progenitor cell, to proliferate faster and/or to produce more organic matrix proteins. Osteoblasts are derived from mesenchymal progenitor cells in the bone marrow or pericytes. Their maturation process includes consecutive stages of proliferation, matrix production and matrix mineralization (Figure 1). Osteoblasts can ultimately become osteocytes, which are mechanosensory cells within the mineralized matrix, or lining cells that protect the bone surface and form the canopy of a basic multicellular unit (BMU) wherein remodeling occurs [8]. Several signaling pathways, including BMPs, PTH, endothelin, fibroblast growth factors, steroidal hormones, insulin-like growth factors, and prostaglandin agonists, have emerged as positive factors regulating osteoblast maturation, but the Wnt (Wingless-type MMTV integration site) family of ligands has arguably generated the most interest and excitement in recent years. In this report, we review data that highlight the importance Wnt pathways in osteoblast maturation and bone formation. We also discuss the molecular basis, promise and potential limitations of strategies to augment Wnt-dependent bone formation.
Figure 1. Wnts affect multiple stages of osteoblast-linage maturation.
2. Wnt Signaling Pathways
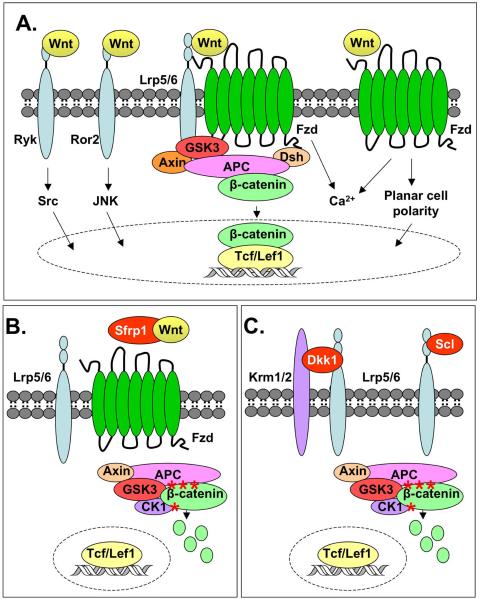
Wnts are cysteine-rich, secreted glycoproteins that activate cell surface receptor-mediated signaling pathways to control of gene expression, cell fate determination, proliferation, and migration. Wnts are required for embryogenesis, organogenesis, postnatal development, and regeneration of adult tissues including lymphocytes, skin, colon, hair follicles, and bone [9]. In humans and mice, there are 19 Wnts that bind to receptor complexes containing one of 10 Frizzled (Fzd) receptors and sometimes one of two low-density lipoprotein receptor-related protein (LRP) 5 or 6 co-receptors, theoretically creating at least 380 potential receptor combinations. Complicating the issue further, Wnts also bind to receptor-like tyrosine kinase (Ryk) and receptor tyrosine kinase-like orphan receptor 2 (Ror2) receptors. Wnt ligands can thus stimulate multiple signaling cascades, including the “canonical” β-catenin pathway, the planar cell polarity (PCP) pathway, calcium (Ca2+), protein kinase A, Src, and c-Jun N-terminal kinase (JNK) pathways [10] (Figure 2A). Wnts have historically been classified as “canonical” (e.g., Wnt 1, 3a, 8, 10b) on the basis of their ability to inhibit glycogen synthase kinase (GSK)3 phosphorylation of β-catenin and its subsequent degradation or as “non-canonical” (e.g., Wnt 4, 5a, 11) if they do not affect β-catenin levels. These simplistic classifications are now being challenged by studies demonstrating that some Wnts (including Wnt1, 5a and 11) stimulate several of the above-mentioned signaling pathways in context-dependent manners that can depend on which receptor is available and active [10, 11]. Filling our gap in understanding of how Wnts bind to specific receptors will be an important step in understanding tissue-specific responses and will be crucial for the identification new targets for intervention.
Figure 2. Activation and inhibition of Wnt signaling pathways.
A) The “canonical pathway” is stimulated when Wnts bind to Frizzled (Fzd) receptors and low-density lipoprotein receptor-related protein (Lrp)5/6 co-receptors (center). This activates Disheveled (Dsh), which inhibits a cytoplasmic complex composed of glycogen synthase kinase (GSK)3β, Axin, and adenomatous polyposis coli (APC). Cytoplasmic β-catenin levels rise and some β-catenin translocates to the nucleus where it associates with T-cell factor (Tcf)/lymphoid enhancer-binding factor (Lef) transcription factors to regulate gene expression. During non-canonical Wnt signaling (right side of the figure), Wnts bind a Fzd receptor, but the downstream signaling events do not involve GSK3β or β-catenin. Two non-canonical Wnt signaling cascades that have been identified are: 1) Wnt/calcium signaling increases intracellular Ca2+ levels and activates protein kinase C and calcium/calmodulin-dependent kinase; and 2) The Wnt/planar cell polarity pathway that signals through Rho/Rac GTPases and c-Jun N-terminal kinase (JNK) to modulate cytoskeletal elements and gene expression. Wnts also bind to receptor tyrosine kinases receptor-like tyrosine (Ryk) and receptor tyrosine kinase-like orphan receptor 2 (Ror2) to activate oncogene (Src) and JNK signaling, respectively (left side of figure). B) Secreted frizzled-related proteins (Sfrps) antagonize canonical Wnt signaling by binding the ligands and preventing their association with Fzd receptors. In the absence of Wnt signaling, GSK3β phosphorylates (asterisks) β-catenin, which marks it for ubiquitination and degradation by the proteosome. C) Dickkoph (Dkk1) suppresses Wnt signaling by forming a ternary complex with Lrp5/6 and Kremen (Krm)1/2. Sclerostin (Scl) also binds to Lrp5/6, but not Krm1/2, to antagonize canonical Wnt signaling.
3. Wnt Signaling Stimulates Bone Formation
The role of Wnt signaling in bone formation gained significant recognition in 2001 when Gong and colleagues reported loss-of-function mutations in the LRP5 co-receptor cause the autosomal recessive disorder osteoporosis-pseudoglioma syndrome (OPPG), which is characterized by low bone mass, ocular defects, and a predisposition to fractures [12]. These findings were recapitulated in germline Lrp5 knockout mice, which developed a low bone mass phenotype similar to patients with OPPG due to decreased osteoblast proliferation [13]. Lrp6 hypomorphic mice are also osteopenic and mice lacking Lrp5 and one copy of Lrp6 have additive reductions in bone mass [14]. Other groups identified a mutation in LRP5 at amino acid G171 in individuals with high bone mass and remarkable skeletal strength [15, 16]. Transgenic mice overexpressing the G171V mutation in preosteoblasts using the Col1a1(3.6) promoter recapitulated the high bone mass phenotype and had significantly stronger bones than wildtype animals [17, 18]. It was recently demonstrated that expression of the gain-of-function G171V mutation in more mature osteoblasts using the Col1a1(2.3) promoter did not affect bone density [19]. The latter study also demonstrated that Lrp5-deficiency in the duodenum, rather than in osteoblasts, decreased osteoblast proliferation indirectly via gut-derived serotonin binding its osteoblast receptor, 5-hydroxytryptamine (Htr1b), and activating the cAMP responsive element binding protein (CREB) transcription factor [19]. These data suggest that Lrp5 deficiency causes bone loss in a Wnt-independent manner, but does not rule out a crucial role for Wnt signaling in osteoblasts. Lrp6 or another co-receptor might be more crucial for Wnt signaling in osteoblast lineage cells, particularly in immature osteoblasts and in progenitor cells.
Since the LRP5 discoveries earlier this decade, many studies have documented a role for Wnt pathway components in bone formation, regeneration and repair. Table 1 summarizes the bone phenotypes associated with genetic altered expression of Wnt signaling pathway components. The overarching conclusion derived from these studies is that activation of the Wnt pathways facilitates osteoblast specification from mesenchymal progenitors and enhances bone mass and strength, while suppression causes bone loss. The interesting and perplexing caveat is that several mechanisms are responsible for altered bone mass. For example, LRP5 appears to regulate osteoblast numbers and proliferation [13], perhaps in a Wnt-independent fashion [19], while β-catenin regulates osteoprotegerin (OPG) production in mature osteoblasts and affects bone resorption without affecting osteoblast numbers [20]. In progenitor cells, β-catenin activation facilitates osteoblast differentiation at the expense of chondrocyte development [21–24], while Wnt5a and Wnt10b increase bone volume by suppressing PPARγ2 activity to block adipogenesis and promote osteoblast lineage maturation [25–27]. These genetic studies, as well as ones showing that Wnt pathway activation enhances osteoblast and osteocyte survival in vitro [18, 23, 28] and that Wnt pathways are active in bone regeneration sites (reviewed in [29]), strongly support crucial roles for Wnts pathways in bone mass accrual. However, recent data suggest that more needs to be done to understand how cells at different stages of maturity respond to Wnts.
Table 1.
Summary of Bone Phenotypes in Genetic Models of Altered Wnt Signaling
| Gene | Role in Wnt pathway(s) | Bone Phenotype(s)* | References |
|---|---|---|---|
| APC | Downregulates of β-catenin signaling | CKO: Increased BMD | [91, 92] |
|
| |||
| Axin2 | Downregulates of β-catenin signaling | CKO: Craniosynostosis; Increased BMD | [93, 94] |
|
| |||
| β-Catenin | Interacts with Lef1/Tcfs to regulate gene expression | CKO: Ectopic formation of chondrocytes | [21–23] |
| Tg: Increased ossification, suppression of chondrocytes | [20, 24] | ||
|
| |||
| Dkk1 | Inhibits β-catenin signaling | Het: High bone mass | [47] |
| Tg: Low bone mass | [46] | ||
|
| |||
| Dkk2 | Inhibits β-catenin signaling | KO: Low bone mass | [95] |
| Het: Normal | |||
|
| |||
| Fzd9 | Receptor for Wnts | KO: Decreased bone formation; Osteopenic | [96] |
|
| |||
| GSK3β | Inhibits β-catenin signaling by targeting β-catenin for degradation | Het: Increased bone formation | [97] |
| Pharmacological inhibition: Increased BMD | [31] | ||
|
| |||
| Krm1/2 | Potentiate capacity of Dkks to inhibit β-catenin signaling | CKO: Increases BMD | [49] |
|
| |||
| Lrp5 | Co-receptor for Wnts | KO: Decreased BMD | [13] |
| CKO (Osteoblasts): Normal | [19] | ||
| CKO (Duodenum): Low BMD | |||
| GOF: Increased BMD | [15, 16] | ||
|
| |||
| Lrp6 | Co-receptor for Wnts | CKO: Decreased BMD | [98] |
|
| |||
| Sfrp1 | Secreted inhibitor of β-catenin signaling | KO: Increased BMD | [76] |
|
| |||
| SOST | Inhibits β-catenin signaling | KO: Increased BMD | [61] |
| Tg: Osteopenic | [62] | ||
|
| |||
| Tcf7 (Tcf1) | Transcription factor activated through interaction with β-catenin | DKO (Tcf7−/−Lef1−/−): Similar to Wnt3a−/− phenotype | [99] |
| KO: Low bone mass, increase in bone resorption | [20] | ||
|
| |||
| Wnt3a | Activates β-catenin pathway | Het: No change in BMD | [25] |
|
| |||
| Wnt5a | Activates β-catenin and noncanonical pathways | Het: Reduced BMD, increased adipogenesis | [25] |
|
| |||
| Wnt10b | Activates β-catenin | KO: Reduced BMD, increased adipogenesis | [26, 27] |
| Tg: Increased BMD | |||
BMD: bone mineral density; CKO: conditional knockout mouse; Het: heterozygous knockout mouse; KO: global knockout; DKO: double global knockout; Tg: transgenic; GOF: gain of function
4. Therapeutic Strategies Targeting the Wnt Pathways
Given the plethora of data showing that Wnt pathway activation promotes bone formation, it has become an attractive target in the search for therapies that increase systemic (e.g., osteoporosis) and focal (e.g., critical size defects and non-union fractures) bone formation. Two basic therapeutic strategies for enhancing bone regeneration through the Wnt signaling pathways exist: adding agonists or blocking naturally occurring antagonists. Recombinants Wnts are difficult and expensive to purify because they are glycoproteins and only palmitoylated forms are active [30]; thus, the former approach is cost-prohibitive. The alternative strategy of inhibiting natural antagonists is a more feasible approach. This is currently being explored by neutralizing secreted inhibitors of Wnt pathways with antibodies or by inactivating intracellular enzymes (e.g., GSK3β) that reduce β-catenin activity with small molecules (Figure 2).
4.1. GSK3β Inhibitors
GSK3β is a crucial regulator of the Wnt-β-catenin pathway. It is a serine-threonine kinase that phosphorylates the amino-terminus of β-catenin, as well as adenomatous polyposis coli (APC) and Axin, members of β-catenin destruction complex, in the absence of a Wnt signal to initiate the degradation of β-catenin (Figure 2B) (reviewed in [9]). Because GSK3β is a kinase, modulating its activity with small molecules is a promising strategy for increasing bone mass. The rationale is blocking the ability of GSK3β to phosphorylate β-catenin would stabilize β-catenin and allow it to translocate to the nucleus where it can interact with lymphoid enhancer-binding factor (Lef)/T-cell factor 7 (Tcf7) transcription factors and regulate the expression of genes involved in bone regeneration. As predicted, several GSK inhibitors increase bone density.
Lithium is a well-characterized GSK3β inhibitor that stimulates osteoblast differentiation in vitro and bone regeneration in vivo. Clement-LaCroix and colleagues [31] administered lithium chloride (LiCl) for four weeks to Lrp5 knockout mice, which have significantly reduced bone mass [13]. LiCl restored trabecular bone mass to near wild-type levels in the Lrp5-deficient animals. LiCl also increased bone mass in animals with senile osteoporosis and ovariectomy-induced osteoporosis; moreover, it enhanced bone densities in wildtype animals. Increased bone mass was associated with reduced adiposity, suggesting that GSK3β inhibition might be acting on bone marrow-residing progenitor cells. In other studies, LiCl enhanced callus formation and fracture healing when administered to mice four days following trauma [32] and prevented myeloma-induced bone disease [33]. Lithium might also be effective at increasing bone mass in humans. Oral lithium is a pharmacologic agent that has been used for over 50 years to treat bipolar patients [34]. Epidemiological studies indicate decreased risks fracture risks [35], and reduced bone turnover in lithium users [36], although it has not been determined that this is a direct effect on bone cells or Wnt target genes and other epidemiological surveys dispute the protective effect of LiCl on fractures [37].
The orally active, small molecule GSK3α/β dual-inhibitor, 603281-31-8, is 500 times more selective for GSK3β than other kinases, and was also reported to increase bone mass [38–40]. It significantly increased mineral apposition rate, bone mineral density, trabecular area, trabecular thickness, and trabecular number in ovariectimized mice within 60 days [39]. Vertebral strength was also improved [39]. It is of interest, 603281-31-8 reduced adipogenesis in ovariectimized mice and elevated mRNA levels of osteoblast products, including biglycan, type 1 collagen, osteocalcin, alkaline phosphatase, osteonectin, and runt-related transcription factor 2 (Runx2), and increased the OPG: Receptor activator of NF-kB ligand (RANKL) ratio [40]. The authors concluded that the increased bone mass observed with the GSK3α/β inhibitor is probably mediated by an increase in bone formation with a small effect on bone resorption.
The observations that GSK3β inhibition improves bone mass in various rodent models and that lithium reduces fracture risk in humans demonstrate that targeting this enzyme is a promising anabolic therapy, at least for short-term use. While the long-standing use of lithium to treat bipolar disorders suggests that is relatively safe, further studies are necessary to confirm the protective effect of lithium on fractures. It is important to note that GSK3 is not a specialized repressor of β-catenin as it participates in many other intracellular signaling pathways, some of which can be oncogenic [41]. Cancer risk is not elevated in humans treated with lithium; however, 80% of mice expressing constitutively active β-catenin developed benign tumors in the ribs (osteomata) [20]. Perhaps a greater concern for skeletal health is that long-term lithium treatment is associated with increased PTH secretion and hypercalcemia (discussed further in [37]). For GSK3β inhibition to be an efficacious long-term anabolic therapy for bone regeneration in osteopenic or osteoporosis patients, ideal strategies would enable GSK3β inhibition to occur exclusively in osteoblast lineage cells. Finally, it is interesting that in addition to being a negative regulator of β-catenin, GSK3β is a positive regulator of Wnt signaling when it is at the membrane where it phosphorylates Lrp6 [42]. Thus GSK3β inhibitors probably promote signaling downstream of β-catenin but might make the cells less responsive to autocrine or paracrine Wnt signals emanating from cell surface receptors.
4.2. Secreted Inhibitors of Wnt Pathways
An alternative approach to targeting an intracellular component of anabolic Wnt pathways is to take aim at an extracellular antagonist(s). With more than 20 therapeutic monoclonal antibodies now used clinically to block a variety of signaling pathways and treat various conditions including cancer, inflammatory disease, macular degeneration and transplant rejection, it is highly feasible to augment Wnt signals by neutralizing natural suppressors with immunotherapy. Secreted Wnt inhibitors utilize two general mechanisms to antagonize Wnt signaling (Figures 2B and 2C). Dickkopf (Dkk)1, sclerostin, and Wnt modulator in surface ectoderm (Wise) bind to the Lrp5/6 co-receptors and competitively inhibit Wnts from associating with Lrp5/6. Conversely, secreted Frizzled-related proteins (Sfrps), Cerberus, and Wnt inhibitory factor-1 (Wif-1) interact directly with Wnts and/or Fzd receptors to hinder functional interactions between the ligand and receptor (reviewed in [9]).
4.2.1. Dickkopf (Dkk) 1
Dkk1 suppresses Wnt signaling by forming a ternary complex with Lrp5/6 and Kremen (Krm)1/2 (Figure 2C) [43, 44]. Disruption of the interaction between Lrp5/6 and Dkk1 prevents internalization and cell surface depletion of the putative Wnt co-receptors, Lrp5/6. Missense mutations in Lrp5 that cause high bone phenotypes and analogous changes in Lrp6 prevent Dkk1 from associating with the receptors [45, 46]. Molecular genetic studies in murine models support the negative role of the Dkk1/Krm complex in bone formation. Transgenic mice expressing Dkk1 from a type 1 collagen promoter had fewer osteoblasts and severe osteopenia [47], whereas deletion of a single Dkk1 allele increased bone mass without affecting bone resorption measures [48]. Studies with a hypomorphic Dkk1 mouse model demonstrated that just a 25% reduction in Dkk1 levels is sufficient to increase trabecular and cortical bone mass [49]. Deletion of both Krm1 and Krm2 also increased bone mass without significant changes in bone resorption markers [50]. Recently, the homeodomain transcription factor muscle segment homeodomain homeobox homolog 2 (Msx2) was shown to inhibit Dkk1 expression and transgenic overexpression of Msx2 from a broadly expressed promoter increased bone volume through enhanced canonical Wnt signaling [51]. Finally, suppression of Dkk1 by RNA interference alleviated osteoporosis caused by glucocorticoids and estrogen-deficiency [52, 53]. Together, these data strongly support the hypothesis that inhibition of the Dkk1/Krm complex is a promising strategy for promoting bone formation.
Several groups have tested Dkk1-neutralizing antibodies in various animal models and observed promising effects on bone density. Diarra and colleagues found that anti-Dkk1 reversed bone destruction in a tumor necrosis factor-induced rheumatoid arthritis model [54] by dose-dependently increasing bone formation rates, osteoblast numbers and OPG levels, while reducing osteoclast numbers. Interestingly, an increase in osteophytes (an osteoarthritis characteristic) was also noted. Meanwhile, Yaccoby et al tested humanized Dkk1-neutralizing antibodies in a SCID-rab mouse model of multiple myeloma because serum DKK1 levels are elevated in myeloma patients with osteolytic disease [55, 56]. In this model, anti-Dkk1 reduced the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts, increased the numbers of osteocalcin-positive osteoblasts, and reduced the number of myeloma cells on the subcutaneously implanted tumor-bearing bones. The Dkk1-neutralizing antibody also increased the bone mineral density of mouse femurs that did not contain tumors. The authors concluded that inhibiting Dkk1 promotes bone formation, reduces osteolysis, and suppresses multiple myeloma expansion [55]. These conclusions were verified by testing anti-Dkk1 in the 5T2MM murine model of multiple myeloma [57]. In a recent meeting presentation, Glantschnig and colleagues showed that subcutaneous administration of humanized anti-Dkk1 monoclonal antibodies dose-dependently increased bone mineral density within four weeks in six week-old mice and augmented bone mass within eight weeks in ovariectomized mice [58].
Although development of skeletal anabolic agents that inhibit Dkk1 is in the early stages, preliminary studies in mice suggest that Dkk1 is an attractive therapeutic target for inducing bone formation. To date, most drug development efforts have focused on creating Dkk1-neutralizing monoclonal antibodies. However, small molecules that disrupt the Dkk1-Lrp5/6 or Dkk1-Krm1/2 interaction offer another possibility given the high bone mass phenotypes observed in humans with LRP5 mutations that prevent it from associating with Dkk1. The expression of Dkk1 in multiple tissues might eventually limit the efficacy of Dkk1-targeted therapies; however, the observation that that just a 25% reduction in Dkk1 levels is sufficient to increase bone mass suggest that partial inhibition might be sufficient.
4.2.2. Sclerostin
Sclerostin is the product of the SOST gene. Loss-of-function mutations in SOST cause autosomal recessive sclerosteosis, which is characterized by progressive bone thickening and a hyperostotic skeleton [59]. In addition, a homozygous 52 kb noncoding deletion of a SOST enhancer element that drives sclerostin expression in the skeleton was found in patients with Van Buchem disease another autosomal recessive high bone mass disease [60, 61]. SOST knockout mice have increased bone mineral density, bone volume, bone formation and bone strength [62], whereas transgenic mice overexpressing SOST are osteopenic [63]. Sclerostin is a secreted protein that seems to be primarily expressed in bone tissue, predominantly by osteocytes [63, 64] also by premature osteoclasts [65]. Mechanical loading and intermittent PTH treatment suppress sclerostin expression in osteocytes [66, 67], while RANKL and Macrophage colony stimulating factor 1 (CSF1) reduce its production by preosteoclasts [65]. Like Dkk1, sclerostin binds to Lrp5/6 and antagonizes canonical Wnt signaling [68–70]; however, it binds to different region of LRP5/6 than DKK1 and it does not mediate receptor internalization [69]. Recent reports suggest that it might also signal through Wnt–independent pathways [71, 72]. Because inactivating mutations in SOST cause high bone mass diseases in humans and animal models and sclerostin expression is seemingly restricted to bone cells, inhibiting sclerostin has emerged an attractive pursuit that is currently progressing through preclinical testing.
A sclerostin-neutralizing monoclonal antibody was developed and evaluated in several bone loss models. In a rodent model of postmenopausal osteoporosis, six month-old female rats were ovariectomized, aged for one year to allow for bone loss, and then treated with anti-sclerostin for five weeks. Anti-sclerostin antibodies reversed the estrogen-deficiency-induced bone loss by increasing bone formation rates without affecting bone resorption parameters and improving bone strength to levels greater than those found in non-ovariectomized rats [73]. The same group reported that anti-sclerostin increased bone formation, bone mineral density and bone strength in cynomologus monkeys after just two months [74]. Finally, in a single injection study, post-menopausal women who received a subcutaneous dose of anti-sclerostin had increased bone formation markers for at least three weeks following treatment. The single dose of anti-sclerostin was purportedly well tolerated in these women [75]. Taken together, these results indicated that sclerostin inhibition increases bone formation and suggest that anti-sclerostin represents a promising therapy for the anabolic treatment of diseases characterized by bone loss such as osteoporosis.
4.2.3. Sfrp1
Sfrp1 antagonizes canonical Wnt signaling either by interacting with Wnts to prevent them from associating with Fzd receptors or by binding directly to Fzd proteins to form a nonfunctional complex [76]. Despite a broad tissue expression profile, several lines of evidence suggest that inhibition of Sfrp1 stimulates canonical Wnt signaling and promotes bone accrual. Sfrp1 deficient mice have increased trabecular bone mineral density, volume, and mineral apposition, but no changes in cortical bone density [77]. Sfrp1 depletion also prevented age-associated bone loss and reduced body fat percentages [77]. Meanwhile, administration of recombinant human SFRP1 decreased proximal femur bone density and trabecular bone volume in rats [78]. Overexpression of SFRP1 in immortalized human osteoblasts suppressed canonical Wnt signaling by 70% and accelerated apoptosis, implicating Sfrp1 as a negative regulator of osteoblast and osteocyte survival [78].
Development and testing of Sfrp1 inhibitors is still in the early stages. Bodine and colleagues screened a large panel of compounds using a cell based reporter gene assay and identified several potential Sfrp1 antagonists [79]. Specifically, a diphenylsulfone sulfonamide was found to prevent Sfrp1-mediated apoptosis in preosteocytes in vitro and stimulate bone formation ex vivo [79]. In other studies, a commercially available Sfrp1 polyclonal antibody suppressed Porphyromonas gingivalis-induced periodontal bone loss, reduced osteoclastogenesis, and decreased inflammatory cell infiltration [80]. To our knowledge, the effect of Sfrp1 antibodies or inhibitors on in vivo bone parameters has yet to be reported.
For potential therapeutic targeting of Sfrp1 as a bone anabolic agent to advance, the molecular actions of Sfrp1 must be more clearly deciphered. Reports have indicated that some Sfrps interact with each other and may quench one another's activity thus promoting Wnt signaling. Furthermore, in the absence of Wnt ligands, it has been proposed that the interaction between Sfrps and Fzd might be sufficient to activate signal transduction [81]. Given the potential biphasic nature of Sfrps, further basic research is necessary before inhibition of Sfrp1 can emerge as a safe, efficacious bone anabolic agent.
5. Conclusion
Modulation of Wnt signaling pathways has emerged as a promising and feasible strategy to increase bone density. Small molecule inhibitors of the intracellular Wnt-β-catenin pathway regulator GSK3β increase bone mass, lower adiposity and reduce fracture risk. Neutralizing antibodies to secreted inhibitors of Wnt signaling such as Dkk1, Sfrp1 and sclerostin stimulate bone formation in animal models. Although these novel therapies offer much promise, systemic stimulation of Wnt pathways to enhance bone mass has potential risks and the long-term safety of these therapies must be determined. Tissue-specific targeting of new bone anabolic drugs is an important challenge that must be overcome to ensure safe and efficacious treatment of individuals with osteoporosis and/or fractures.
6. Expert Opinion
Since the seminal discoveries earlier this decade that LRP5 controls bone density and strength [12, 15, 16], other Wnt signaling pathway components have been intensely examined (Table 1). Molecular and genetic studies have identified multiple mechanisms for bone mass regulation (i.e., osteoblast number, osteoclast maturation and bone resorption, and progenitor differentiation) by Wnt pathways. Several reports demonstrated that Lrp5 regulates bone mass by affecting osteoblast numbers; however, usage of several different conditional promoters indicate that this occurs via Wnt-dependent pathways in progenitor cells and via Wnt-independent endocrine pathways (e.g., gut-derived serotonin) in committed osteoblasts [19]. The affects of Wnts on progenitor cell specification to the osteoblast lineage are convincing. Adipogenesis is increased by the absence of Wnt5a or Wnt10b or by the presence of LiCl or Sfrp1, whereas β-catenin is a crucial factor for specifying osteoblastogenesis over chondrogenesis. However, β-catenin seems to have a different role in mature osteoblasts where it has no effect on osteoblast number but stimulates OPG production to block bone resorption and thereby increase overall bone density. Hypomorphic deletion of Lrp6 also increases bone resorption by augmenting RANKL expression. In sum, the data suggest that Wnts and the Lrp5/6 co-receptors affect osteoblast differentiation from progenitor cells and the coupling of bone formation with bone resorption.
The disparate mechanisms might be explained by several non-mutually exclusive factors. The first is receptor expression on osteoblastic cells. We know little about how the expression of Wnt receptors (e.g., Lrp5/6, Fzd1–10, Ror2, Ryk) fluctuates on the cell surface during the differentiation of a progenitor cell to a committed osteoblast or during the maturation of a committed osteoblast towards a lining cell or osteocyte. Perhaps Lrp6 or specific Fzds are sufficient to transmit Wnt survival signals and/or regulate gene expression in mature osteoblastic cells. Second, Wnts do not stimulate linear signaling cascades with specialized components. Wnts and their receptors are initiators that mobilize intracellular signaling molecules (e.g., β-catenin, GSK3β), which subsequently amplify and transmit the signal to the nucleus where gene expression programs are controlled. These intracellular molecules are not exclusive to the Wnt pathway and in fact regulate signals from other cell surface molecules, including receptor tyrosine kinases, G-protein coupled receptors, and E-cadherin. Thus, the range of outcomes from Wnt signals will contextually depend on receptor availability, cell maturation status, and other stimuli present in the environment.
Wnt pathways have been studied for nearly 30 years. The work begun with the Drosophila protein Wingless was quickly linked to human cancers, tissue development and regeneration, and more recently to tissue degeneration [82–84]. Overall, the vast efforts must be considered successful as our understanding of the cellular, biochemical and molecular events were translated into several therapeutic strategies for treating diseases, including systemic bone loss and critical-sized defects. As discussed in this review, modulating Wnt or Lrp5/6 signaling antagonists (e.g., GSK3β, Dkk1, sclerostin, Sfrp1) seems to be the most feasible approach for treating low bone mass or stimulating bone regeneration. The immense literature on Wnt signaling suggests that optimism should be cautiously tempered for a number of reasons. First and foremost is that unregulated activation of Wnt/β-catenin pathways is carcinogenic [84]. So far, the only mouse model that has developed skeletal tumors is one in which β-catenin is overexpressed from the collagen 1a1 (2.3) promoter throughout development [20]. These tumors are benign (osteomata), but occur in 80% of the animals. It has not been determined if these mice are more sensitive to classic tumor initiators, like loss of p53 or pRb. There is no indication of increased tumor risks in patients carrying activating LRP5 or inactivating SOST mutations, but these rare inherited diseases affect small cohorts.
In addition to cancer, systemic stimulation of Wnt pathways to enhance bone mass has other concerns. It was recently discovered that germline inactivating mutations in a gene encoding for a Wnt pathway inhibitor, Wilms Tumor on the X (WTX), cause X-linked sclerosing bone dysplasia [85]. Thus, if used during development, molecules that activate Wnt signaling may cause developmental defects in a variety of tissues, including sclerosis or craniosynostosis in skeletal tissues. In adults, vascular calcification is a risk because Wnts are overexpressed and Wnt/Lrp signaling pathways are activated in calcified vasculature [86, 87]. A third risk is hyperparathyroidism and hypercalcemia, which develops in 25% of patients that are treated with LiCl [37]. Fourth, prolonged activation of Wnt-β-catenin signaling pathways in skeletal tissues produces osteoarthritis (OA) symptoms. SFRP3 (FRZB) variants with diminished ability to antagonize Wnts are common in OA patients [88]; whereas DKK1 serum levels are associated with reduced risk of joint space narrowing [89]. Dkk1-neutralizing antibodies produced osteophytes in mouse joints after an inflammatory stimulus [54] and recently it was demonstrated that constitutive overexpression of active β-catenin in mature chondrocytes caused progressive OA and osteophytes [90].
Much work remains to be done in clinical and basic research laboratories to optimize current biologics and develop tissue-specific treatments. Broad, genome-scale functional screens will likely uncover previously unappreciated modifiers of Wnt signaling and might identify new therapeutic targets and their importance to bone biology must be empirically determined. Meanwhile, the receptor specificity of Wnt ligands, receptor expression patterns, and the ligand specificity of secreted antagonists must be defined. An important question to be answered is what is the most efficient cellular target of compounds that target the Wnt pathways. If it is an osteoblast progenitor cell, the effectiveness of Wnt anabolics might be limited in older patients that have fewer bone marrow mesenchymal stem cells than younger people. Proper dosing regimens and delivery vehicles must be determined identified to prevent unwanted side effects. Of the therapies mentioned in this review, the sclerostin antibodies ostensibly hold the most promise because sclerostin seems to predominantly secreted by cells in bone; thus, targeting it would theoretically have few side effects. It is likely that systemic treatments for osteoporosis that revolve around activating the Wnt pathway will be used for short-periods of time, perhaps alternating with the anti-resorptive agents, as is the current practice with the only approved anabolic, PTH.
Acknowledgments
We thank Dr. Sundeep Khosla for thoughtful comments. Grants AR50074 and AR48147 from the NIAMS/NIH made this publication possible.
Abbreviations
- APC
Adenomatous polyposis coli
- BMP
Bone morphogenic protein
- BMU
Basic multicellular unit
- CSF1
Macrophage colony stimulating factor
- Dkk
Dickkopf
- Fzd
Frizzled
- GSK
Glycogen synthase kinase
- JNK
c-Jun N-terminal kinase
- Krm
Kremen
- LiCl
Lithium Chloride
- Lrp
Low-density lipoprotein receptor-related protein
- OA
Osteoarthritis
- OPG
Osteoprotegerin
- OPPG
Osteoporosis-pseudoglioma syndrome
- PCP
Planar cell polarity
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PTH
Parathyroid hormone
- RANKL
Receptor activator of NF-kappaB ligand
- SCID-rab
Severe combined immunodeficient-rabbit
- Scl
Sclerostin
- SFRP
Secreted Frizzled-related protein
- Wif
Wnt inhibitory factor
- Wise
Wnt modulator in surface ectoderm
- Wnt
Wingless-type MMTV integration site
References
- 1.Dennison E, Mohamed MA, Cooper C. Epidemiology of osteoporosis. Rheum Dis Clin North Am. 2006;32(4):617–29. doi: 10.1016/j.rdc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 2.United States Department of Health and Human Services . Bone health and osteoporosis: A report of the surgeon general. U.S. Department of Health and Human Servises, Office of the Surgeon General; Rockville, MD: 2004. [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. Jama. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Drake MT, Clarke BL, Khosla S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–45. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30(3):312–21. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 6.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in england and wales. Bone. 2001;29(6):517–22. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Merchan EC, Forriol F. Nonunion: General principles and experimental data. Clin Orthop Relat Res. 2004;(419):4–12. [PubMed] [Google Scholar]
- 8.Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 2008;118(2):421–8. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 10.van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1(35):re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- 11.Mikels AJ, Nusse R. Purified wnt5a protein activates or inhibits beta-catenin-tcf signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong Y, Slee RB, Fukai N, et al. Ldl receptor-related protein 5 (Lrp5) affects bone accrual and eye development. Cell. 2001;107(4):513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]; * * This report showed that mutations in the Wnt-coreceptor, Lrp5, are linked to the low bone mass disease, OPPG.
- 13.Kato M, Patel MS, Levasseur R, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a wnt coreceptor. J Cell Biol. 2002;157(2):303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmen SL, Giambernardi TA, Zylstra CR, et al. Decreased BMD and limb deformities in mice carrying mutations in both lrp5 and lrp6. J Bone Miner Res. 2004;19(12):2033–40. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- 15.Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the ldl receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–9. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in ldl-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 17.Akhter MP, Wells DJ, Short SJ, et al. Bone biomechanical properties in lrp5 mutant mice. Bone. 2004;35(1):162–9. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Babij P, Zhao W, Small C, et al. High bone mass in mice expressing a mutant Lrp5 gene. J Bone Miner Res. 2003;18(6):960–74. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 19.Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–37. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]; **The study challenges the notion that Lrp5 regulates bone mass by transmitting Wnt signals in mature osteoblasts and shows that Lrp5 indirectly affects bone mass by regulation serotonin synthesis in the gut.
- 20.Glass DA, 2nd, Bialek P, Ahn JD, et al. Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of hedgehog and wnt signaling in osteoblast development. Development. 2005;132(1):49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 22.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8(5):727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Rodda SJ, McMahon AP. Distinct roles for hedgehog and canonical wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 25.Takada I, Mihara M, Suzawa M, et al. A histone lysine methyltransferase activated by non-canonical wnt signalling suppresses ppar-gamma transactivation. Nat Cell Biol. 2007;9(11):1273–85. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 26.Bennett CN, Ouyang H, Ma YL, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22(12):1924–32. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- 27.Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324–9. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study demonstrated that a Wnt ligand, Wnt10b, affects osteoblast differentiation at the expense of adipogenesis.
- 28.Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ. Topgal mice show that the canonical wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005;20(7):1103–13. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- 29.Silkstone D, Hong H, Alman BA. Beta-catenin in the race to fracture repair: In it to wnt. Nat Clin Pract Rheumatol. 2008;4(8):413–9. doi: 10.1038/ncprheum0838. [DOI] [PubMed] [Google Scholar]
- 30.Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 31.Clement-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406–11. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This manuscript show that Lithium chloride induces bone formation and reduces adiposity in several mouse models of low bone mass.
- 32.Chen Y, Whetstone HC, Lin AC, et al. Beta-catenin signaling plays a disparate role in different phases of fracture repair: Implications for therapy to improve bone healing. PLoS Med. 2007;4(7):e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards CM, Edwards JR, Lwin ST, et al. Increasing wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111(5):2833–42. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livingstone C, Rampes H. Lithium: A review of its metabolic adverse effects. J Psychopharmacol. 2006;20(3):347–55. doi: 10.1177/0269881105057515. [DOI] [PubMed] [Google Scholar]
- 35.Vestergaard P, Rejnmark L, Mosekilde L. Reduced relative risk of fractures among users of lithium. Calcif Tissue Int. 2005;77(1):1–8. doi: 10.1007/s00223-004-0258-y. [DOI] [PubMed] [Google Scholar]
- 36.Zamani A, Omrani GR, Nasab MM. Lithium's effect on bone mineral density. Bone. 2009;44(2):331–4. doi: 10.1016/j.bone.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Wilting I, de Vries F, Thio BM, et al. Lithium use and the risk of fractures. Bone. 2007;40(5):1252–8. doi: 10.1016/j.bone.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 38.Engler TA, Henry JR, Malhotra S, et al. Substituted 3-imidazo[1,2-a]pyridin-3-yl- 4-(1,2,3,4-tetrahydro-[1,4]diazepino-[6,7,1-hi]indol-7-yl)pyrrole-2,5-dion es as highly selective and potent inhibitors of glycogen synthase kinase-3. J Med Chem. 2004;47(16):3934–7. doi: 10.1021/jm049768a. [DOI] [PubMed] [Google Scholar]
- 39.Kulkarni NH, Onyia JE, Zeng Q, et al. Orally bioavailable gsk-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res. 2006;21(6):910–20. doi: 10.1359/jbmr.060316. [DOI] [PubMed] [Google Scholar]
- 40.Kulkarni NH, Wei T, Kumar A, et al. Changes in osteoblast, chondrocyte, and adipocyte lineages mediate the bone anabolic actions of pth and small molecule gsk-3 inhibitor. J Cell Biochem. 2007;102(6):1504–18. doi: 10.1002/jcb.21374. [DOI] [PubMed] [Google Scholar]
- 41.Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: Properties, functions, and regulation. Chem Rev. 2001;101(8):2527–40. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 42.Zeng X, Tamai K, Doble B, et al. A dual-kinase mechanism for wnt co-receptor phosphorylation and activation. Nature. 2005;438(7069):873–7. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao B, Wu W, Davidson G, et al. Kremen proteins are dickkopf receptors that regulate wnt/beta-catenin signalling. Nature. 2002;417(6889):664–7. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 44.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 45.Balemans W, Devogelaer JP, Cleiren E, Piters E, Caussin E, Van Hul W. Novel lrp5 missense mutation in a patient with a high bone mass phenotype results in decreased dkk1-mediated inhibition of wnt signaling. J Bone Miner Res. 2007;22(5):708–16. doi: 10.1359/jbmr.070211. [DOI] [PubMed] [Google Scholar]
- 46.Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by dkk1 form a common mechanism by which high bone mass-associated missense mutations in lrp5 affect canonical wnt signaling. Mol Cell Biol. 2005;25(12):4946–55. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Sarosi I, Cattley RC, et al. Dkk1-mediated inhibition of wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–66. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Morvan F, Boulukos K, Clement-Lacroix P, et al. Deletion of a single allele of the dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–45. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 49.MacDonald BT, Joiner DM, Oyserman SM, et al. Bone mass is inversely proportional to dkk1 levels in mice. Bone. 2007;41(3):331–9. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellwanger K, Saito H, Clement-Lacroix P, et al. Targeted disruption of the wnt regulator kremen induces limb defects and high bone density. Mol Cell Biol. 2008;28(15):4875–82. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA. Msx2 exerts bone anabolism via canonical wnt signaling. J Biol Chem. 2008;283(29):20505–22. doi: 10.1074/jbc.M800851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang FS, Ko JY, Lin CL, Wu HL, Ke HJ, Tai PJ. Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomorphological study in ovariectomized rats. Bone. 2007;40(2):485–92. doi: 10.1016/j.bone.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Wang FS, Ko JY, Yeh DW, Ke HC, Wu HL. Modulation of dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology. 2008;149(4):1793–801. doi: 10.1210/en.2007-0910. [DOI] [PubMed] [Google Scholar]
- 54.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156–63. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]; * This report shows that Dkk1-neutralizing antibodies increase bone formation in an inflamed joint.
- 55.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr. Antibody-based inhibition of dkk1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109(5):2106–11. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian E, Zhan F, Walker R, et al. The role of the wnt-signaling antagonist dkk1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 57.Heath DJ, Chantry AD, Buckle CH, et al. Inhibiting dickkopf-1 (dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2008 doi: 10.1359/jbmr.081104. In press. [DOI] [PubMed] [Google Scholar]
- 58.Glantschnig H, Hampton R, Wei N, et al. Fully human anti-dkk1 antibodies increase bone formation and resolve osteopenia in mouse models of estrogen-deficiency induced bone loss. J Bone Miner Res. 2008;23:S60. [Google Scholar]
- 59.Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin Genet. 2003;63(3):192–7. doi: 10.1034/j.1399-0004.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 60.Loots GG, Kneissel M, Keller H, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in van buchem disease. Genome Res. 2005;15(7):928–35. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the sost gene in patients with van buchem disease. J Med Genet. 2002;39(2):91–7. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–9. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 63.Winkler DG, Sutherland MK, Geoghegan JC, et al. Osteocyte control of bone formation via sclerostin, a novel bmp antagonist. Embo J. 2003;22(23):6267–76. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Bezooijen RL, Roelen BA, Visser A, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical bmp antagonist. J Exp Med. 2004;199(6):805–14. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves wnt/bmp signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105(52):20764–9. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of sost/sclerostin. J Biol Chem. 2008;283(9):5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 67.Bellido T, Ali AA, Gubrij I, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: A novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–83. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 68.Semenov M, Tamai K, He X. Sost is a ligand for Lrp5/Lrp6 and a wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–5. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Zhang Y, Kang H, et al. Sclerostin binds to Lrp5/6 and antagonizes canonical wnt signaling. J Biol Chem. 2005;280(20):19883–7. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 70.Ellies DL, Viviano B, McCarthy J, et al. Bone density ligand, sclerostin, directly interacts with Lrp5 but not Lrp5G171V to modulate wnt activity. J Bone Miner Res. 2006;21(11):1738–49. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- 71.Caverzasio J. Wnt/Lrp5-independent inhibition of osteoblastic cell differentiation by sclerostin. J Bone Miner Res. 2008;23:S72. [Google Scholar]
- 72.Grabenstaetter T, Sakane Y, Jacobi C, et al. Sost blocks gsk3-beta inhibitor-induced alkaline phosphatase activity. J Bone Miner Res. 2008;23:S251. [Google Scholar]
- 73.Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass and bone strength in a rat model of postmenopausal osteoporosis *. J Bone Miner Res. 2008 doi: 10.1359/jbmr.081206. In press. [DOI] [PubMed] [Google Scholar]; **This report demonstrates that Scleostin antibodies increase bone density in a pre-clinical osteoporosis model.
- 74.Ominsky M, Stouch B, Doellgast G, et al. Administration of sclerostin monoclonal antibodies to female cynomolgus monkeys results in increased bone mineral density and bone strength. J Bone Miner Res. 2006;21:S44. [Google Scholar]
- 75.Padhi D, Stouch B, Jang G, et al. Anti-sclerostin antibody increases markers of bone formation in healthy postmenopausal women. J Bone Miner Res. 2007;22(Suppl. 1):S37. [Google Scholar]
- 76.Kawano Y, Kypta R. Secreted antagonists of the wnt signalling pathway. J Cell Sci. 2003;116(Pt 13):2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 77.Bodine PV, Zhao W, Kharode YP, et al. The wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18(5):1222–37. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 78.Wang FS, Lin CL, Chen YJ, et al. Secreted frizzled-related protein 1 modulates glucocorticoid attenuation of osteogenic activities and bone mass. Endocrinology. 2005;146(5):2415–23. doi: 10.1210/en.2004-1050. [DOI] [PubMed] [Google Scholar]
- 79.Moore WJ, Kern JC, Bhat R, et al. Modulation of wnt signaling through inhibition of secreted frizzled-related protein i (sfrp-1) with n-substituted piperidinyl diphenylsulfonyl sulfonamides. J Med Chem. 2009;52(1):105–16. doi: 10.1021/jm801144h. [DOI] [PubMed] [Google Scholar]
- 80.Li CH, Amar S. Inhibition of sfrp1 reduces severity of periodontitis. J Dent Res. 2007;86(9):873–7. doi: 10.1177/154405910708600913. [DOI] [PubMed] [Google Scholar]
- 81.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond wnt inhibition: New functions of secreted frizzled-related proteins in development and disease. J Cell Sci. 2008;121(Pt 6):737–46. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 82.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 83.De Ferrari GV, Papassotiropoulos A, Biechele T, et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104(22):9434–9. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Polakis P. The many ways of wnt in cancer. Curr Opin Genet Dev. 2007;17(1):45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 85.Jenkins ZA, van Kogelenberg M, Morgan T, et al. Germline mutations in Wtx cause a sclerosing skeletal dysplasia but do not predispose to tumorigenesis. Nat Genet. 2009;41(1):95–100. doi: 10.1038/ng.270. [DOI] [PubMed] [Google Scholar]
- 86.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine wnt signals. J Clin Invest. 2005;115(5):1210–20. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rajamannan NM, Nealis TB, Subramaniam M, et al. Calcified rheumatic valve neoangiogenesis is associated with vascular endothelial growth factor expression and osteoblast-like bone formation. Circulation. 2005;111(24):3296–301. doi: 10.1161/CIRCULATIONAHA.104.473165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loughlin J, Dowling B, Chapman K, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A. 2004;101(26):9757–62. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lane NE, Nevitt MC, Lui LY, de Leon P, Corr M. Wnt signaling antagonists are potential prognostic biomarkers for the progression of radiographic hip osteoarthritis in elderly caucasian women. Arthritis Rheum. 2007;56(10):3319–25. doi: 10.1002/art.22867. [DOI] [PubMed] [Google Scholar]
- 90.Zhu M, Tang D, Wu Q, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holmen SL, Zylstra CR, Mukherjee A, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280(22):21162–8. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 92.Ahmed Y, Nouri A, Wieschaus E. Drosophila Apc1 and Apc2 regulate wingless transduction throughout development. Development. 2002;129(7):1751–62. doi: 10.1242/dev.129.7.1751. [DOI] [PubMed] [Google Scholar]
- 93.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the wnt signaling pathway, forms a complex with gsk-3beta and beta-catenin and promotes gsk-3beta-dependent phosphorylation of beta-catenin. Embo J. 1998;17(5):1371–84. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu HM, Jerchow B, Sheu TJ, et al. The role of axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132(8):1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X, Liu P, Liu W, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37(9):945–52. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 96.Albers J, Gebauer M, Schulze J, et al. Mice lacking the wnt receptor frizzled-9 display osteopenia caused by decreased bone formation. J Bone Miner Res. 2008;23:S3. [Google Scholar]
- 97.Kugimiya F, Kawaguchi H, Ohba S, et al. Gsk-3beta controls osteogenesis through regulating runx2 activity. PLoS ONE. 2007;2(9):e837. doi: 10.1371/journal.pone.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zylstra C, Wan C, VanKoevering K, et al. Osteoblast-specific deletion of Lrp6 reveals distinct roles for Lrp5 and Lrp6 in bone development. J Bone Miner Res. 2008;23:S2. [Google Scholar]
- 99.Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13(6):709–17. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]