Abstract
A prediction model, developed in the Framingham Heart Study (FHS), has been proposed for use in estimating a given individual’s risk of hypertension. We compared this model with systolic blood pressure (SBP) alone and age-specific diastolic blood pressure (DBP) categories for the prediction of hypertension. Participants in the Multi-Ethnic Study of Atherosclerosis, without hypertension or diabetes (n=3013), were followed for the incidence of hypertension (SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg and/or the initiation of antihypertensive medication). The predicted probability of developing hypertension between four adjacent study examinations, with a median of 1.6 years between examinations, was determined. The mean (standard deviation) age of participants was 58.5 (9.7) years and 53% were women. During follow-up, 849 incident cases of hypertension occurred. The c-statistic for the FHS model was 0.788 (95% CI: 0.773, 0.804) compared with 0.768 (95% CI: 0.751, 0.785; p=0.096 compared to the FHS model) for SBP alone and 0.699 (95% CI: 0.681, 0.717; p<0.001 compared to the FHS model) for age-specific DBP categories. The relative integrated discrimination improvement index for the FHS model versus SBP alone was 10.0% (95% CI: −1.7%, 22.7%) and versus age-specific DBP categories was 146% (95% CI: 116%, 181%). Using the FHS model, there were significant differences between observed and predicted hypertension risk (Hosmer-Lemeshow goodness of fit p<0.001); re-calibrated and best-fit models produced a better model fit (p=0.064 and 0.245, respectively). In this multi-ethnic cohort of U.S. adults, the FHS model was not substantially better than SBP alone for predicting hypertension.
Keywords: hypertension, epidemiology, systolic blood pressure, diastolic blood pressure, risk prediction
Introduction
Hypertension is a major risk factor for the incidence of cardiovascular and kidney disease(1–4). The identification of adults at high risk for incident hypertension is important for the cost-effective implementation of interventions. Randomized controlled trials have demonstrated the benefits of lifestyle modification and pharmaceutical therapy on the reduction of hypertension incidence(5–9).
To assist in the identification of individuals at high risk for hypertension, a prediction model was developed using data from Caucasian adult participants from the Framingham Heart Study (FHS)(10). This model includes multiple factors and was designed for use in clinical practice to identify patients at increased risk for the incidence of hypertension. While good performance of this model was noted within the FHS population, this equation has not been externally validated which is necessary before it can be recommended for widespread use. Often, there is substantial over- or under-estimation of event rates when risk prediction models are applied in populations different from that in which they were developed(11;12).
Therefore, we sought to determine the performance of the FHS hypertension risk prediction model using standard criteria in a contemporary, multi-ethnic population of U.S. adults(13). Additionally, we compared this model with the predictive ability of systolic blood pressure (SBP) categories alone and age-specific categories of diastolic blood pressure (DBP). Age-specific categories were modeled rather than DBP alone as the association between DBP and hypertension incidence is not uniform across age group. These analyses were conducted using longitudinal data on the incidence of hypertension among adults ≥ 45 years of age from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort.
Methods
Study Population
MESA enrolled 6,814 community dwelling adults who were 45 to 84 years of age at baseline (14). Participants from 4 race-ethnicity groups (Caucasian, African-American, Hispanic, and Asian - primarily of Chinese descent) were recruited from 6 U.S. communities: Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan, New York; and St. Paul, Minnesota. Participants were excluded if they had a history of clinically evident cardiovascular disease (CVD), were under treatment for cancer, pregnant, weighed more than 300 lbs, had significant cognitive deficits, were living in a nursing home or on the waiting list for a nursing home, had plans to leave the community within five years, only spoke a language other than English, Spanish, Cantonese or Mandarin, had a chest computed tomography (CT) scan in the previous year, or had any serious medical condition which would prevent long-term participation in MESA.
Of the 6,814 MESA participants enrolled, those with prevalent hypertension at baseline (SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, current antihypertensive medication use, or a self-report of a diagnosis of hypertension; n=3337) were excluded from the current analyses. Additionally, among those without hypertension at baseline, we excluded participants with diabetes (fasting glucose ≥ 126 mg/dL or use of hypoglycemic drugs or insulin; n=223), missing information needed to calculate their hypertension risk (n=6) and who did not attend two sequential MESA study visits (n=235). Participants with diabetes were excluded to correspond with the population used in the development of the FHS hypertension risk prediction model. After these exclusions, n=3,013 participants who attended the baseline visit (years 2000 – 2002) and exam 2 study visits (years 2002 – 2004) were included in the current analysis. There were n=2,476 MESA participants who were free of hypertension and diabetes at exam 2 who attended exam 3 (years 2004 – 2006) and n=2,130 MESA participants who were free of hypertension and diabetes at exam 3 who attended exam 4 (years 2006 – 2008). Those excluded, primarily for having hypertension at baseline, were older (mean age = 65 versus 59 years), more likely to be African-American (34% versus 20%), and had a higher mean body mass index (29.2 versus 27.2 kg/m2). Written informed consent was obtained from all participants, and the study was approved by the Institutional Review Boards of all participating sites.
Risk Factor Measures
For the current analysis, risk factors for the incidence of hypertension are based on data collected at baseline and MESA exams 2 and 3. During each exam, standardized questionnaires were utilized to obtain information on demographics, cigarette smoking, medical conditions, and prescribed medication use. Body height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Parental history of hypertension was only assessed at exam 2.
Blood Pressure Measurements and Hypertension Ascertainment
Blood pressure measurements from all four MESA examinations conducted to date were used in the current analysis. After resting for 5 minutes in the seated position, participants’ blood pressure was measured three times at two-minute intervals using an automated oscillometric device (Dinamap Monitor Pro 100, GE Healthcare, Milwaukee, WI). Appropriate sized cuffs were utilized for blood pressure assessment. Blood pressure was defined as the average of the second and third readings. Participants were asked about their prior diagnoses of hypertension, and those responding affirmatively were also asked about their use of prescribed antihypertensive medications. Incident hypertension was defined as the first study visit, subsequent to baseline, at which the participant had SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or the initiation of treatment with antihypertensive medications. This is the same definition for hypertension used in developing the FHS hypertension risk prediction model(10). Pre-hypertension was defined, for those who were free of hypertension, as an SBP of 120 to 139 mmHg and/or a DBP of 80 to 89 mmHg.
Hypertension Risk Prediction Models
The FHS hypertension risk prediction model has the following components: SBP, sex, parental history of hypertension, BMI, cigarette smoking, and the interaction between DBP and age(10). These variables, with the exception of parental history of hypertension which was only assessed once, were updated at each study visit for the prediction of hypertension. The FHS hypertension risk prediction model applied an exponential function incorporating a linear combination of each of the aforementioned variables to estimate the probability of developing hypertension over the median 1.6 years of follow-up between each exam (Appendix 1). The SBP alone model included 7 SBP categories while the model comprised of age-specific levels of DBP included 20 categories (Appendix 1). The SBP alone and age-specific categories of DBP models used the same blood pressure categories published in the original report of the FHS hypertension risk prediction model(10).
Statistical Analyses
The prevalence or mean and standard deviation (SD) for each component of the hypertension risk prediction model at baseline was calculated, overall and by race-ethnicity. The percentage of participants developing hypertension at 1.6 years of follow-up, the median time between MESA study visits, was calculated overall, by race-ethnicity and for the individual components of the FHS risk prediction model. Participants remaining free of hypertension could contribute up to three periods at risk (i.e. between baseline and visit 2, visit 2 and visit 3, and visit 3 and visit 4. Additionally, using a repeated measures Poisson regression model and updating components at each exam, the relative risks for developing hypertension associated with race-ethnicity and components of the FHS risk prediction model were calculated. Next, the c-statistic was calculated for the FHS model, the SBP alone model, and the model of age-specific DBP categories(15). C-statistics were calculated for the overall population and for each race-ethnicity, separately. The statistical significance of differences in cstatistics for each model was compared using the method of DeLong, DeLong and Clarke-Pearson for correlated data (16).
Next, the predicted probability of developing hypertension over 1.6 years was calculated using the FHS model, SBP alone and DBP with age and the relative integrated discrimination improvement index was calculated for the FHS model compared with models defined by SBP alone and the age-specific DBP categories. The relative integrated discrimination improvement is a measure of the average increase in sensitivity and specificity when comparing two prediction models (17).
To assess calibration, the predicted probability of hypertension from the FHS model was divided into deciles. By decile of predicted risk, the observed and mean predicted probability of developing hypertension over 1.6 years was calculated. Next, the predicted probability of developing hypertension was calculated after re-calibrating the FHS equation using the method described by D’Agostino(11). Finally, using the variables in the FHS equation, a best-fit model was generated. The predicted risk of hypertension before and after recalibration and using the best-fit model, separately, were compared to the observed incidence of hypertension via a modified Hosmer-Lemeshow goodness of fit chi-square test(18).
Three additional analyses were conducted. First, the c-statistic for pre-hypertension as well as the relative integrated discrimination improvement index for the FHS model versus pre-hypertension was assessed. Second, the c-statistic and relative integrated discrimination improvement index were evaluated for the FHS equation and SBP alone for developing the incidence of hypertension over 4.8 years of follow-up (i.e., without updating components of the equation). Finally, an analysis was conducted including individuals with diabetes. Statistical analyses were conducted with SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Overall, the mean age of participants was 58.5 ± 9.7 years; 53% were women, and 45%, 20%, 22%, and 13% were Caucasian, African American, Hispanic, and Asian, respectively (Table 1). There were 849 incident cases of hypertension – 360 between baseline and exam 2, 268 between exam 2 and 3, and 221 between exam 3 and 4. The cumulative incidence of hypertension at 1.6 years was 11.1% (Table 2). In a multivariable adjusted model including race-ethnicity and the FHS model components, African-American race-ethnicity, SBP, age-specific levels of DBP and BMI were associated with hypertension incidence.
Table 1.
Baseline levels of hypertension risk prediction model components in the Multi-Ethnic Study of Atherosclerosis (MESA), overall and by race-ethnicity
| Participant Characteristic |
Overall (n=3,013) |
Caucasian (n=1,353) |
African American (n=595) |
Hispanic (n=670) |
Asian (n=395) |
|---|---|---|---|---|---|
| Age, years | 58.5 (9.7) | 59.6 (9.8) | 57.8 (9.7) | 57.2 (9.5) | 58.1 (9.4) |
| % Women | 53 | 53 | 53 | 51 | 52 |
| SBP, mmHg | 114 (13) | 114 (13) | 117 (12) | 114 (13) | 112 (14) |
| DBP, mmHg | 69 (9) | 68 (9) | 71 (8) | 69 (9) | 69 (9) |
| BMI, kg/m2 | 27.2 (5.0) | 26.7 (4.6) | 29.0 (5.6) | 28.7 (4.7) | 23.4 (3.1) |
| BMI category, kg/m2 | |||||
| % <25 | 36 | 40 | 25 | 20 | 72 |
| % 25 – 29 | 39 | 39 | 39 | 49 | 25 |
| % ≥ 30 | 24 | 21 | 37 | 32 | 3 |
| % Current Smoker | 15 | 13 | 22 | 17 | 6 |
| Parental hypertension | |||||
| % None | 58 | 60 | 48 | 65 | 54 |
| % One | 34 | 33 | 39 | 29 | 39 |
| % Both | 8 | 7 | 13 | 6 | 7 |
Numbers in table are mean (standard deviation) or percent
Abbreviations: SBP – systolic blood pressure; DBP – diastolic blood pressure; BMI – body mass index.
Table 2.
Cumulative incidence for developing hypertension at 1.6 years of follow-up and relative risk for hypertension by demographics and risk prediction model components at baseline in the Multi-Ethnic Study of Atherosclerosis (MESA)
| Participant Characteristic |
Number of events |
Time periods at risk* |
% developing hypertension at 1.6 years |
Relative risk† (95% CI) |
|---|---|---|---|---|
| Overall | 849 | 7,619 | 11.1% | NA |
| Race-ethnicity | ||||
| Caucasian | 351 | 3,514 | 10.0% | 1 (reference) |
| African American | 225 | 1,449 | 15.5% | 1.22 (1.05 – 1.41) |
| Hispanic | 188 | 1,634 | 11.5% | 1.10 (0.94 – 1.29) |
| Asian | 85 | 1,022 | 8.3% | 0.99 (0.81 – 1.22) |
| Sex | ||||
| Male | 403 | 3,580 | 11.3% | 1 (reference) |
| Female | 446 | 4,039 | 11.0% | 1.11 (0.97 – 1.26) |
| Systolic blood pressure, mmHg | ||||
| <110 | 96 | 3,148 | 3.1% | 1 (reference) |
| 110 – 114 | 75 | 1,126 | 6.7% | 1.98 (1.45 – 2.71) |
| 115 – 119 | 121 | 1,081 | 11.2% | 2.97 (2.26 – 3.91) |
| 120 – 124 | 106 | 768 | 13.8% | 3.42 (2.55 – 4.59) |
| 125 – 129 | 119 | 584 | 20.4% | 4.85 (3.69 – 6.39) |
| 130 – 134 | 153 | 491 | 31.2% | 7.10 (5.40 – 9.34) |
| 135 – 139 | 179 | 421 | 42.5% | 9.49 (7.27 – 12.4) |
| Diastolic blood pressure, mmHg by Age |
||||
| Age<50 years | ||||
| < 70 | 17 | 659 | 2.6% | 1 (reference) |
| 70 – 74 | 11 | 223 | 4.9% | 1.17 (0.57 – 2.42) |
| 75 – 79 | 29 | 193 | 15.0% | 2.46 (1.39 – 4.34) |
| 80 – 84 | 14 | 94 | 14.9% | 1.75 (0.90 – 3.41) |
| 84 – 89 | 12 | 35 | 34.3% | 2.86 (1.48 – 5.50) |
| Age 50 to 59 years | ||||
| < 70 | 68 | 1553 | 4.4% | 1.51 (0.90 – 2.55) |
| 70 – 74 | 43 | 673 | 6.4% | 1.35 (0.77 – 2.37) |
| 75 – 79 | 69 | 497 | 13.9% | 2.06 (1.20 – 3.53) |
| 80 – 84 | 51 | 253 | 20.2% | 2.29 (1.32 – 3.95) |
| 84 – 89 | 24 | 68 | 35.3% | 2.86 (1.58 – 5.17) |
| Age 60 to 69 years | ||||
| < 70 | 113 | 1,153 | 9.8% | 2.44 (1.46 – 4.05) |
| 70 – 74 | 51 | 472 | 10.8% | 1.87 (1.08 – 3.23) |
| 75 – 79 | 53 | 274 | 19.3% | 2.41 (1.39 – 4.17) |
| 80 – 84 | 34 | 141 | 24.1% | 2.48 (1.41 – 4.39) |
| 84 – 89 | 10 | 35 | 28.6% | 2.48 (1.26 –4.91) |
| Age ≥70 years | ||||
| < 70 | 150 | 903 | 16.6% | 3.13 (1.88 – 5.20) |
| 70 – 74 | 53 | 217 | 24.4% | 3.06 (1.81 – 5.20) |
| 75 – 79 | 25 | 113 | 22.1% | 2.56 (1.40 – 4.69) |
| 80 – 84 | 17 | 51 | 33.3% | 2.94 (1.57 – 5.51) |
| 84 – 89 | 5 | 12 | 41.7% | 3.21 (1.61 – 6.37) |
| Body mass index, kg/m2 | ||||
| <25 | 244 | 2,846 | 8.6% | 1 (reference) |
| 25–29 | 324 | 2,995 | 10.8% | 1.06 (0.91 – 1.23) |
| ≥ 30 | 281 | 1,778 | 15.8% | 1.35 (1.15 – 1.59) |
| Parental history of hypertension | ||||
| 0 | 497 | 4,384 | 11.3% | 1 (reference) |
| 1 | 279 | 2,626 | 10.6% | 1.04 (0.91 – 1.19) |
| 2 | 73 | 609 | 12.0% | 1.11 (0.89 – 1.37) |
| Current smoking | ||||
| No | 726 | 6,571 | 11.1% | 1 (reference) |
| Yes | 123 | 1,048 | 11.7% | 1.15 (0.97 – 1.36) |
Participants could contribute up to three periods at risk (i.e., between baseline and visit 2, visit 2 and visit 3, and visit 3 and visit 4).
All variables listed in table were included in a single multivariable Poisson regression model.
The c-statistic for the incidence of hypertension was 0.788 (95% CI: 0.773, 0.804) for the FHS model and 0.768 (95% CI: 0.751, 0.785; p=0.096 compared to the FHS) for the SBP model and 0.699 (95% CI: 0.681, 0.717; p<0.001 compared to the FHS) for the model of age-specific DBP categories (Table 3). The c-statistic was highest for Asians and lowest for Caucasians and Hispanics for each model. The relative integrated discrimination improvement for the FHS model compared to SBP alone was 10.0% (95% CI: −1.7%, 22.7%), and compared to the DBP with age model was 146% (95% CI: 116%, 181%).
Table 3.
C-statistic for the Framingham Heart Study hypertension risk prediction model versus models of systolic blood pressure alone and diastolic blood pressure by age group in the Multi-Ethnic Study of Atherosclerosis (MESA).
| c-statistic (95% CI) | |||
|---|---|---|---|
| Ethnicity | FHS model | SBP model | DBP with age model |
| Overall | 0.788 (0.773 – 0.804) | 0.768 (0.751 – 0.785) | 0.699 (0.681 – 0.717) |
| Caucasian | 0.782 (0.757 – 0.808) | 0.753 (0.726 – 0.780) | 0.699 (0.671 – 0.728) |
| African-American | 0.795 (0.765 – 0.825) | 0.761 (0.729 – 0.794) | 0.715 (0.680 – 0.750) |
| Hispanic | 0.783 (0.749 – 0.818) | 0.752 (0.714 – 0.789) | 0.692 (0.652 – 0.733) |
| Asian | 0.882 (0.848 – 0.915) | 0.837 (0.795 – 0.879) | 0.758 (0.714 – 0.803) |
Abbreviations: FHS – Framingham Heart Study, SBP – systolic blood pressure, DBP – diastolic blood pressure
Each p-value > 0.05 comparing SBP to the FHS model, overall and for each race-ethnicity.
Each p-value <0.001 comparing DBP with age to the FHS model, overall and for each race-ethnicity.
The Framingham model includes age, sex, body mass index, current smoking, family history of hypertension and systolic and diastolic blood pressure modeled using the equation-based system (see methods for additional details). The systolic blood pressure model uses systolic blood pressure modeled in 7 categories (<110, 110 – 114, 115 – 119, 120 – 124, 125 – 129, 130 – 134 and 135 – 139 mmHg). The diastolic blood pressure with age model includes 20 categories (diastolic blood pressure levels of <70, 70 – 74, 75 – 79, 80 – 84, and 85 – 89 mmHg for each age group <50, 50 – 59, 60 – 69, and ≥ 70 years). The SBP model includes the 7 SBP categories. The DBP with age model includes the 20 diastolic blood pressure with age categories.
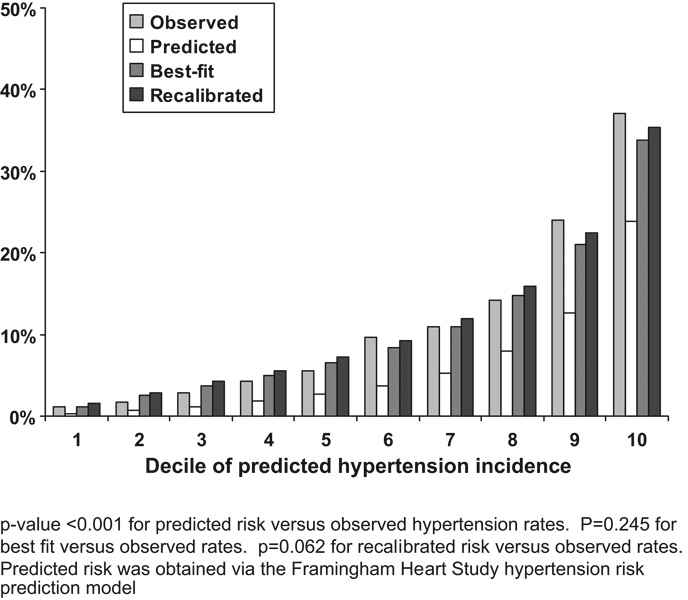
The FHS prediction model under-estimated the risk of hypertension in all deciles of predicted risk (p<0.001; Figure 1). The predicted risk for hypertension after recalibration and using a best-fit model was markedly closer to the observed risk of hypertension (p=0.062 and p=0.245, respectively).
Figure 1.
Probability of hypertension incidence over 1.6 years by decile of predicted risk in the Multi-Ethnic Study of Atherosclerosis (MESA).
Pre-hypertension and hypertension incidence
At baseline, 1092 MESA participants (36% of those without hypertension) had pre-hypertension. Among participants with and without pre-hypertension, 23.6% and 5.3%, respectively, developed hypertension during follow-up. The c-statistic for pre-hypertension predicting incident hypertension was 0.701 (95% CI: 0.684, 0.717) which was significantly lower than the c-statistics for the FHS model and SBP alone (each p<0.001). The c-statistics for pre-hypertension predicting hypertension were 0.685 (95% CI: 0.658, 0.711), 0.690 (95% CI: 0.658, 0.722), 0.685 (95% CI: 0.649, 0.721), and 0.778 (95% CI: 0.732, 0.824) among Caucasians, African-Americans, Hispanics and Asians, respectively. The relative integrated discrimination improvement was 78% (95% CI: 58%, 99%) for the FHS model compared to pre-hypertension.
Including individuals with diabetes
During follow-up, 122 incident cases of hypertension over 497 intervals of follow-up (24.6%) occurred among MESA participants with diabetes. The c-statistic was markedly similar including individuals with diabetes and was 0.782 (95% CI: 0.768, 0.797), 0.763 (95% CI: 0.747, 0.778), and 0.689 (95% CI: 0.672, 0.706) for the FHS model, SBP alone, and DBP and age, respectively.
Hypertension incidence over 4.8 years of follow-up
The c-statistic was similar when evaluating the incidence of hypertension over 4.8 years of follow-up (0.792 [95% CI: 0.775, 0.807] for the FHS model, 0.773 [95% CI: 0.775, 0.791] for SBP alone, and 0.691 [95% CI: 0.671, 0.711] for DBP and age). Additionally, the relative integrated discrimination improvement for incident hypertension over 4.8 years was 9.1% (95% CI: −1.6%, 20.8%) for the FHS model compared to SBP alone.
DISCUSSION
In the current analysis of data from a multi-ethnic community-based sample ≥ 45 years of age, we evaluated the FHS hypertension risk prediction model for the incidence of hypertension over 1.6 years of follow-up. We report that this model provides good discrimination and, after recalibration, good fit compared to the observed incidence rates. However, using SBP alone provided similar discrimination when compared to the FHS model, which was derived using multiple variables. Specifically, the FHS model provided small, and not statistically significant, improvements in the c-statistic over using SBP alone. Also, the relative improvement discrimination index suggests that the FHS model does not have substantially improved sensitivity and specificity when compared to SBP alone.
In the FHS, as well as in MESA, level of SBP was identified as a major risk factor for incident hypertension. In the current analysis, the percentage of MESA participants developing hypertension at 1.6 years of follow-up was less than 4% among individuals with a SBP < 110 mmHg and increased in a graded relation to over 40% among those with a SBP of 135 to 139 mmHg at baseline. In conjunction with our finding that SBP alone was similar to a multivariable model for the prediction of hypertension, this strong association suggests that SBP alone may be sufficient for identifying individuals at high risk for developing hypertension within the next few years. This finding highlights the importance of blood pressure elevations among individuals without hypertension. Prior studies have demonstrated a continuous graded association between SBP and the incidence of cardiovascular and renal diseases (2;19;20) down to levels well below 140 mmHg.
The high prevalence of hypertension (29.3% in 1999–2004(21)) among U.S. adults, and its key role in CVD and chronic kidney disease, suggests the ability to identify high risk individuals is critically important. SBP is incorporated into global CVD risk prediction models. In the JNC-7 guidelines, pre-hypertension has been defined to help clinicians identify individuals at high risk for hypertension(22). In the current analysis, the predictive ability of pre-hypertension was low when compared to either the FHS model or SBP alone. This suggests a more refined risk stratification approach, perhaps using SBP categories, may be worthwhile to identify individuals for aggressive interventions to prevent hypertension.
Among adults with an SBP of 130 to 139 mmHg, a randomized trial of candesartan versus placebo showed a 16% relative risk reduction in the incidence of hypertension(9). Additionally, lifestyle changes have demonstrated strong benefits in the prevention of hypertension(5;23). Given the high incidence of hypertension and graded association between SBP and hypertension incidence, population-based strategies for lowering blood pressure may yield large risk reduction benefits(24). Also, aggressive interventions for individuals with elevated SBP may be warranted.
The current analysis highlights the need for external validation of risk prediction models prior to their implementation into clinical practice. Before risk prediction models are widely disseminated, their properties need to be evaluated in the populations which they will be utilized. Without validation, physicians cannot be confident that the absolute risk of disease outcomes provided by the model is appropriate for using when making clinical decisions. While the FHS model performed reasonably well in the current study, SBP alone also maintained a high predictive value for hypertension.
In the current study, the FHS risk prediction model substantially under-estimated the risk for hypertension. However, this discrepancy can be corrected through the process of recalibration. This finding suggests that if one is to apply the FHS hypertension risk prediction model, the model should be recalibrated. To do this, cross-sectional data on the components of the risk prediction model and data on the hypertension incidence are needed. Details of this process have been described by D’Agostino previously(11).
In addition to SBP, African-American ethnicity, higher BMI levels, and age-specific DBP levels were associated with an increased risk for hypertension. However, these factors did not substantially or statistically significantly improve the discrimination above SBP alone. Although these factors may be important in the pathogenesis of hypertension, they add little information when considered from a risk prediction perspective. Other factors not included in the FHS hypertension risk prediction model (e.g., dietary factors, physical activity, chronic kidney disease) have been associated with an increased risk for hypertension(6;25–27). It may be worthwhile to consider these factors in future risk prediction models.
The results of the current analysis should be considered in the context of certain limitations. As is common in large epidemiological studies, the incidence of hypertension was based on blood pressure measurements taken on a single date. Also, the FHS risk prediction model was developed in a younger population (ages 20 to 69 years, mean age 42 years) than MESA (ages ≥ 45 years, mean age 58.5 years). This may have influenced the calibration of the FHS model. Furthermore, the role of risk factors for hypertension may be age-dependent. For example, among young adults, African-Americans have a higher incidence of hypertension than Caucasians. Although the performance of the FHS hypertension risk prediction model as well as SBP alone may be different in adults < 45 years of age, we did not have data available to evaluate this question. Almost 50% of MESA study participants had developed hypertension by the time of their baseline study visit. Despite these limitations, the data set used for the current analyses has many strengths. These include the large ethnically diverse population enrolled, detailed clinical and metabolic characterization of the cohort, and active follow-up for incident hypertension in MESA. A substantial number of MESA participants (n=849) developed hypertension providing ample power for the current analysis. Also, all data were collected following standardized protocols by trained study staff.
Perspectives
In this contemporary multi-ethnic study of U.S. adults ≥ 45 years of age, the FHS hypertension risk prediction model demonstrated good discrimination. However, this model’s performance was not substantially or statistically significantly better than SBP modeled alone. Future studies in other populations, especially adults < 45 years of age, are needed to further validate this hypertension risk prediction model. If appropriate, the development and validation of new hypertension risk models may be warranted. Given the high incidence of hypertension among U.S. adults as well as the strong association between SBP, below 140 mmHg, and the subsequent incidence of hypertension, individualized and population-wide approaches to lower SBP are needed.
Acknowledgments
Sources of Funding
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors have potential conflicts to disclose.
Reference List
- 1.He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138:211–219. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 2.Klag MJ, Whelton P, Randall B, Neaton J, Brancati F, Ford C, Shulman N, Stamler J. Blood pressure and End-stage renal disease in men. NEJM. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 3.Whelton P, Klag MJ. Hypertension as a risk factor for renal disease: Review of clinical and epidemiological evidence. Hypertension. 1989;13:I19–I27. doi: 10.1161/01.hyp.13.5_suppl.i19. [DOI] [PubMed] [Google Scholar]
- 4.Stamler J, Stamler R, Neaton J. Blood pressure, systolic and diastolic, and cardiovascular risks. Archives of Internal Medicine. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 5.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 6.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 7.Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38:1112–1117. doi: 10.1161/hy1101.093424. [DOI] [PubMed] [Google Scholar]
- 8.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N DASH Collaborative Research Group. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 9.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH, Jr, Messerli FH, Oparil S, Schork MA. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 10.Parikh NI, Pencina MJ, Wang TJ, Benjamin EJ, Lanier KJ, Levy D, D'Agostino RB, Sr, Kannel WB, Vasan RS. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Intern Med. 2008;148:102–110. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 11.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 12.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–224. doi: 10.1016/j.jacc.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Wilson PW. Challenges to improve coronary heart disease risk assessment. JAMA. 2009;302:2369–2370. doi: 10.1001/jama.2009.1765. [DOI] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 17.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW, Hosmer T, Le CS, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–980. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds K, Gu D, Muntner P, Kusek JW, Chen J, Wu X, Duan X, Chen CS, Klag MJ, Whelton PK, He J. A population-based, prospective study of blood pressure and risk for end-stage renal disease in China. J Am Soc Nephrol. 2007;18:1928–1935. doi: 10.1681/ASN.2006111199. [DOI] [PubMed] [Google Scholar]
- 20.Kengne AP, Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Gu DF, Suh I, Woodward M. Systolic blood pressure, diabetes and the risk of cardiovascular diseases in the Asia-Pacific region. J Hypertens. 2007;25:1205–1213. doi: 10.1097/HJH.0b013e3280dce59e. [DOI] [PubMed] [Google Scholar]
- 21.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 23.Whelton PK, He J, Appel LJ. Treatment and prevention of hypertension. In: Manson JE, Ridker PM, Gaziano JM, Hennekens CH, editors. Prevention of myocardial infarction. New York: Oxford University Press; 1996. pp. 154–171. [Google Scholar]
- 24.Rose G. Strategy of prevention: lessons from cardiovascular disease. Br Med J (Clin Res Ed) 1981;282:1847–1851. doi: 10.1136/bmj.282.6279.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelton PK, Buring J, Borhani NO, Cohen JD, Cook N, Cutler JA, Kiley JE, Kuller LH, Satterfield S, Sacks FM. The effect of potassium supplementation in persons with a high-normal blood pressure. Results from phase I of the Trials of Hypertension Prevention (TOHP). Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Ann Epidemiol. 1995;5:85–95. doi: 10.1016/1047-2797(94)00053-v. [DOI] [PubMed] [Google Scholar]
- 26.Kestenbaum B, Rudser KD, de Boer IH, Peralta CA, Fried LF, Shlipak MG, Palmas W, Stehman-Breen C, Siscovick DS. Differences in kidney function and incident hypertension: the multi-ethnic study of atherosclerosis. Ann Intern Med. 2008;148:501–508. doi: 10.7326/0003-4819-148-7-200804010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, Karanja N, Lin PH DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]