Abstract
Huntington's disease (HD) is an inherited neurodegenerative disorder caused by expanded polyglutamine repeats in the huntingtin (Htt) protein. Mutant Htt may damage and kill striatal neurons by a mechanism involving reduced production of brain-derived neurotrophic factor (BDNF) and increased oxidative and metabolic stress. Because electroconvulsive shock (ECS) can stimulate the production of BDNF and protect neurons against stress, we determined whether ECS treatment would modify the disease process and provide a therapeutic benefit in a mouse model of HD. ECS (50 mA for 0.2 s) or sham treatment was administered once weekly to male N171-82Q Htt mutant mice beginning at 2 months of age. Endpoints measured included motor function, striatal and cortical pathology, and levels of protein chaperones and BDNF. ECS treatment delayed the onset of motor symptoms and body weight loss and extended the survival of HD mice. Striatal neurodegeneration was attenuated and levels of protein chaperones (Hsp70 and Hsp40) and BDNF were elevated in striatal neurons of ECS-treated compared with sham-treated HD mice. Our findings demonstrate that ECS can increase the resistance of neurons to mutant Htt resulting in improved functional outcome and extended survival. The potential of ECS as an intervention in subjects that inherit the mutant Htt gene merits further consideration.
INTRODUCTION
Huntington's disease (HD) is an inherited neurological disorder that includes prominent motor, psychiatric and cognitive symptoms resulting from degeneration of neurons in the striatum and cerebral cortex (1). The genetic defect is the presence of expanded CAG repeats in the huntingtin gene resulting in long (>36) repeats of the amino acid glutamine in the huntingtin protein (Htt) (2,3). The function of normal Htt in neurons is unknown, although it may play roles in axonal and vesicle trafficking (4) and regulation of synaptic activity (5). Htt is widely expressed in neurons throughout the brain, and it remains unclear why striatal medium spiny neurons are particularly vulnerable to mutant Htt. The precise molecular mechanisms by which mutant Htt exerts its toxicity remain unknown, although several pathogenic mechanisms have been proposed including adverse actions on the mitochondrial function and neurotrophic signaling (6). In vulnerable neurons, mutant Htt forms intranuclear inclusions, and a similar protein aggregation process occurs when mutant Htt is expressed in cultured cells and transgenic mice (7,8).
One alteration that is implicated in the demise of striatal and cortical neurons in HD is a deficit in brain-derived neurotrophic factor (BDNF). Analyses of postmortem brain tissue samples from HD patients (9) and from multiple lines of Htt mutant mice (10,11) have demonstrated reduced levels of BDNF in the striatum and cerebral cortex. The reduction in BDNF levels may contribute to the degeneration of striatal and cortical neurons because BDNF can protect these neuronal populations against insults relevant to HD in cell culture and animal models (12–14). In addition, elevation of BDNF levels by overexpression of the BDNF gene in the striatum and cortex counteracts the neurodegenerative effects of mutant Htt and extends survival in mouse models of HD (11,15). Moreover, BDNF haploinsufficiency accelerates the neurodegenerative process in Htt mutant mice with the enkephalinergic striatal projection neurons being the most vulnerable to reduced BDNF levels (16). Mutant Htt impairs transcription of the BDNF gene by altering the interactions of the transcriptional regulators REST and cAMP response element-binding protein (CREB) with their DNA regulatory elements (17,18). Consistent with a role for reduced BDNF signaling in HD pathogenesis, dietary energy restriction (10) and environmental enrichment (19) increase BDNF levels in striatum and cortex and retard neurodegeneration in HD mouse models.
Electroconvulsive shock (ECS) is a clinical procedure that improves symptoms in many patients with severe depression (20). ECS treatment has also been reported to be effective in reducing psychiatric symptoms in patients with Alzheimer's (21) and Parkinson's (22) diseases. There have been a few case reports describing beneficial effects of ECS treatment on depressive symptoms in HD patients (23) and at least one report of improvement in motor symptoms in an HD patient (24). ECS might be expected to counteract the pathogenic actions of mutant Htt, because ECS is potent inducer of BDNF production (25,26). Here we report that ECS treatment suppresses the neurodegenerative process and extends the survival of Htt mutant mice by a mechanism involving up-regulation of the expression of protein chaperones and BDNF.
RESULTS
ECS ameliorates motor deficits and improves survival in N171-82Q HD mice
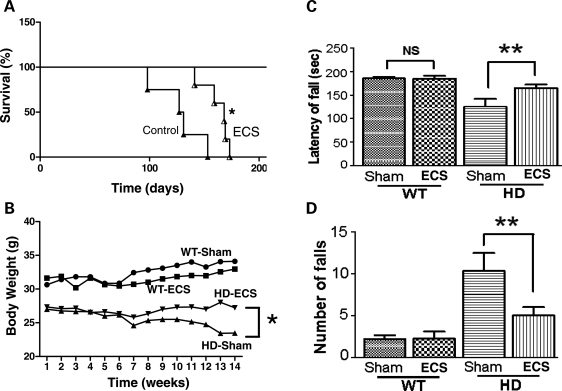
Previous studies showed that N171-82Q HD mice have an abbreviated lifespan of about 21–22 weeks; they exhibit motor deficits beginning at 14–16 weeks of age (27). To determine whether ECS provides significant clinical benefits in an animal model, we assessed the impact of ECS on the development of behavioral symptoms and the survival of male N171-82Q HD mice. Beginning at 2 months of age, 20 wild-type (WT) and 20 HD mice were randomly assigned to either an ECS treatment group or a sham control group (10 HD and 10 WT mice per group); ECS was administered once weekly. HD mice in the ECS group lived significantly longer, by an average of 2 weeks, compared with HD mice in the sham-treated group (Fig. 1A). Whereas all of the sham-treated mice had died by 22 weeks of age, only 30% of the HD mice subjected to ECS had died. No WT mice in either ECS- or sham-treated groups died during the 200-day course of this study.
Figure 1.
ECS treatment ameliorates motor deficits and extends survival of HD mice. (A) N171-82Q HD mice were treated once weekly with either ECS (n = 10) or sham control (n = 10) beginning at 2 months of age and deaths were recorded. The Kaplan–Meier analysis revealed a significantly greater survival of mice in the ECS group (P < 0.05). (B) Body weights of WT and HD mice in the ECS- and sham-treated groups. (C and D) Results of rotarod analysis of motor function (14 week-old mice) showing falling latency (C) and number of falls (D) for WT and HD mice in ECS- and sham-treated groups. Values are the mean and SEM (n = 10 mice per group). *P < 0.05, **P < 0.01.
Because unintended weight loss is a prominent feature in both HD patients and several transgenic mouse models of HD including N171-82Q HD mice (10,28), we measured body weights of all mice twice each week. The effects of ECS treatment on body weights of WT and HD mice are shown in Figure 1B. ECS treatment prevented the presymptomatic weight loss that occurred in sham-treated HD mice. ECS had no significant effect on body weight in WT mice; mice in the sham and ECS groups gained weight progressively throughout the course of the experiment (Fig. 1B).
Loss of motor coordination is another hallmark that develops early and worsens as the disease progresses in HD patients and transgenic mouse models of HD (10,29). To determine the impact of ECS on motor function, we subjected 14-week-old mice to rotarod testing. Sham-treated HD mice exhibited highly significant motor dysfunction as indicated by lower falling latencies (time until falling) and greater numbers of falls compared with sham-treated WT mice (Fig. 1C and D). We found that falling latency was significantly greater and the frequency of falling was significantly less in ECS-treated compared with sham-treated HD mice (Fig. 1C and D). Collectively, these data demonstrate that ECS treatment promotes body weight maintenance, ameliorates the severity of motor deficits and extends survival of N171-82Q HD mice.
ECS ameliorates striatal degeneration in N171-82Q HD mice
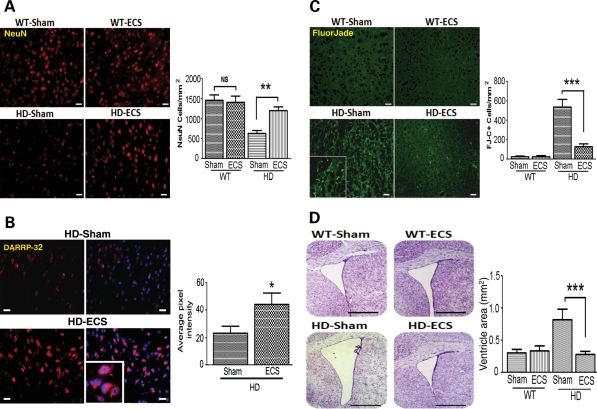
To determine whether improved motor function and extended survival of N171-82Q HD mice treated with ECS results from a slowed progression of the neurodegenerative process, we performed histological analyses to evaluate the neuronal loss in the striatum in brain tissue sections from ECS- and sham-treated HD and WT mice that were killed at 16 weeks of age (an early symptomatic time point). In N171-82Q HD mice, the neuronal loss occurs primarily in the striatum and cerebral cortex resulting in a corresponding enlargement of the lateral ventricles (10,27). Degenerating neurons in N171-82Q HD mice are found beginning at approximately 14 weeks of age (27), and so we evaluated the neuronal loss by NeuN and DARPP-32 immunostaining at 16 weeks of age when striatal degeneration is prominent. The number of NeuN-labeled neurons was significantly greater in the striatum of ECS-treated HD mice compared with sham control HD mice (Fig. 2A). DARPP-32 is a dopamine- and adenosine 3′:5′-monophosphate-regulated neuronal phosphoprotein and is expressed selectively in medium spiny projection neurons (30). The number of neurons labeled with DARPP-32 was also significantly greater in the striatum of ECS-treated HD mice in comparison to sham-treated HD mice (Fig. 2B), indicating that ECS rescues the degeneration of medium spiny projection neurons. Fluoro-Jade C staining was used to detect degenerating neurons in the striatum; the number of FlouroJade C-labeled degenerating striatal cells was significantly lower in ECS-treated HD mice compared with sham-treated HD mice (Fig. 2C).
Figure 2.
ECS treatment attenuates the degeneration of striatal neurons in HD mice. (A) Representative images of NeuN-stained cells (upper micrographs; scale bar = 250 µm) and results of counts of NeuN-positive cells (lower graph) in 16-week-old WT and N171-82Q HD mice in ECS- and sham-treated groups, **P< 0.01. (B) Representative images showing DARRP-2 immunoreactivity (red; scale bar = 20 µm) and results of densitometric analysis (right graph) in 16-week-old N171-82Q HD mice in ECS- and sham-treated groups. Values are the mean and SEM (n = 9–10 mice per group). *P< 0.05. (C) Representative images of FluorJade C (FJ-C)-stained (degenerating neurons) in the striatum (micrographs at left; scale bar = 250 µm) and results of counts of FJ-C stained cells (graph) in 16-week-old WT and HD mice in ECS- and sham-treated groups. ***P< 0.001. (D) Representative images of cresyl violet-stained coronal brain sections (left) and results of measurements of the area of the lateral ventricle (graph) in 16-week-old WT and HD mice in ECS- and sham-treated groups. ***P< 0.001. Scale bar = 250 µm.
Progressive striatal and cortical atrophy results in bilateral ventricular enlargement and flattening of the medial aspect of the striatum, accompanied by thinning of the cerebral cortex in HD mice (10). As expected, the lateral ventricle size in 16-week-old sham control HD mice was significantly greater than of age-matched WT mice (Fig. 2D). In contrast, the ventricles of 16-week-old ECS-treated HD mice were significantly smaller than the ventricles of sham-treated HD mice, and similar in size to WT mice (Fig. 2D). Collectively, these findings suggest that ECS attenuates brain atrophy by protecting striatal and cortical neurons against the neurodegenerative effects of mutant Htt.
ECS reduces Htt protein aggregation in N171-82Q HD mice
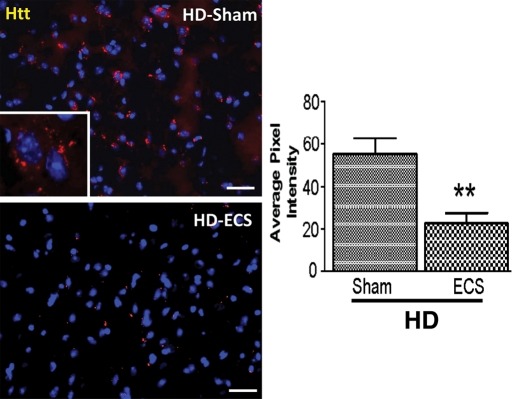
Mutant Htt forms abnormal intracellular aggregates in degenerating neurons in the striatum of N171-82Q HD mice (7–10) that are associated with neuronal dysfunction and death (31,32). We therefore determined whether ECS reduced neuronal degeneration by suppressing Htt aggregate formation. The density of Htt aggregates in the striatum, as measured by image analysis of brain sections immunostained with EM48 antibody, was significantly lower in ECS-treated HD mice in comparison with sham-treated HD mice (Fig. 3). ECS also markedly prevented Htt aggregate formation in the cortex of N171-82Q HD mice (Supplementary Material, Fig. S1). No Htt aggregate formation was detected in brain sections from WT mice (data not shown).
Figure 3.
ECS treatment attenuates Htt aggregation in striatum of HD mice. Representative images showing huntingtin (Htt) immunoreactive aggregates in the striatum (red; left micrographs; scale bar = 250 µm) stained with EM48 antibody and results of densitometric analysis of EM48 immunoreactivity (right graph) in 16-week-old N171-82Q HD mice in ECS- and sham-treated groups. Values are the mean and SEM (n = 9–10 mice per group). **P< 0.01.
ECS normalizes levels of heat-shock proteins in N171-82Q HD mice
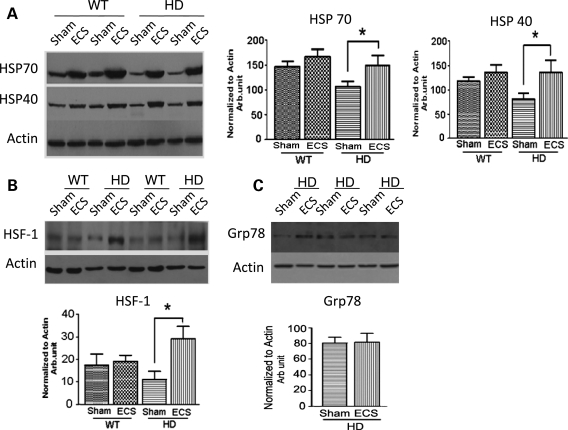
Heat-shock proteins (Hsps) are protein chaperones that prevent misfolding and aggregation of newly synthesized mutant proteins and damaged normal proteins (33). Recent findings suggest that levels of several Hsps decrease in striatal neurons in HD, possibly as the result of sequestration by mutant Htt (34,35). Additional findings suggest that mutant Htt can sequester heat-shock factor 1 (HSF1), a transcription factor responsible for the induction of many Hsps (36). To determine whether reduced aggregation may be attributed to increased expression of Hsps, we measured Hsp70 and Hsp40 in mice that were given a final ECS or sham treatment at 13 weeks of age and killed 1 week later. Levels of both protein chaperones were significantly lower in the striatum of sham-treated HD mice in comparison with sham-treated WT mice (Fig. 4A). This decrease in protein chaperones precedes striatal degeneration that is prominent at 16 weeks (Fig. 2A–C). ECS treatment markedly increased the levels of both Hsp70 and Hsp40 in the striatum (Fig. 4A and Supplementary Material, Fig. S2) and cortex (Supplementary Material, Fig. S3) of HD mice. Note that a trend towards increased Hsp70 and Hsp40 was present in the striatum of WT mice; however, significance was not reached. The elevation of Hsps in the striatum of HD mice was associated with an increase in the HSF1 protein (Fig. 4B). In contrast, the level of the glucose-regulated protein Grp78 was not significantly different in the striatum of ECS-treated HD mice in comparison with sham-treated HD mice (Fig. 4C). These results suggest that ECS treatment selectively increases the expression of Hsps in N171-82Q HD mice, possibly by a HSF1-mediated mechanism.
Figure 4.
ECS elevates levels of adaptive stress response proteins in the striatum of HD mice. (A) A representative immunoblot (left) and results of densitometric analysis (right) of Hsp70 and Hsp40 protein levels in striatal tissue samples from ECS- and sham-treated HD and WT mice. *P< 0.05. (B and C) An immunoblot (upper) and results of densitometric analysis (lower) of HSF-1 (B) and Grp78 (C) protein levels in striatal tissue samples from the indicated groups of mice. Values are the mean and SEM (n = 8–10 mice per group). *P< 0.05.
ECS restores BDNF levels in the striatum of N171-82Q HD mice
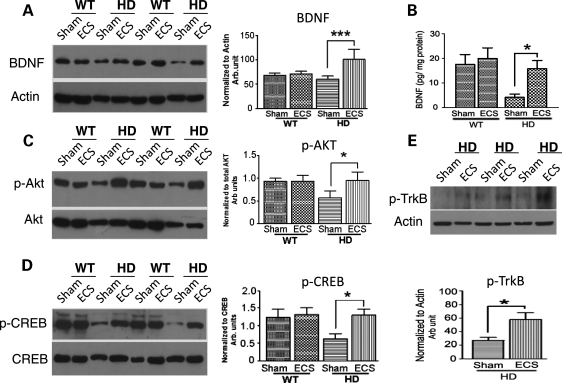
Previous studies have implicated reduced trophic support as a major pathway contributing to striatal degeneration in HD (37). The transcription of the bdnf gene has been reported to be impeded by mutant Htt protein, and BDNF protein levels are reduced in the striatum of HD patients and transgenic HD mice (9–11,18). ECS can increase BDNF levels acutely (within minutes to a few hours) in the hippocampus and cerebral cortex of normal mice, and this effect of ECS is potentiated by prior ECS treatments (25). We therefore determined the effects of ECS on BDNF levels in the striatum of N171-82Q HD mice that were given a final ECS or sham treatment at 13 weeks of age and killed 1 week later. BDNF protein levels, measured by both immunoblot (Fig. 5A) and enzyme-linked immunosorbent assay (ELISA) (Fig. 5B) analyses, were significantly lower in the striatum of HD mice in comparison with WT mice. BDNF levels were significantly greater in ECS-treated HD mice in comparison with sham-treated HD mice (Fig. 5A and B).
Figure 5.
ECS ameliorates deficits in levels of BDNF, and activated Akt and CREB, in the striatum of HD mice. (A) A representative immunoblot (left) and results of densitometric analysis (right) of BDNF protein levels in striatal tissue samples from ECS- and sham-treated HD and WT mice. *P< 0.001. (B) BDNF protein levels, measured by ELISA analysis, in samples of striatal tissue from ECS- and sham-treated HD and WT mice. *P< 0.05. (C) A representative immunoblot (left) and results of densitometric analysis (right) of phospho-Akt protein levels in striatal tissue samples from ECS- and sham-treated HD and WT mice. *P< 0.05. (D) A representative immunoblot (left) and results of densitometric analysis of phospho-CREB protein (right) levels in striatal tissue samples from ECS- and sham-treated HD and WT mice. *P< 0.05. (E) A representative immunoblot (upper) and results of densitometric analysis of phospho-TrkB protein (lower) levels in striatal tissue samples from ECS- and sham-treated HD and WT mice. Values are the mean and SEM (n = 8–10 mice per group). *P< 0.05.
We next measured striatal levels of activated (phosphorylated) Akt and CREB, a kinase and transcription factor involved in BDNF signaling and BDNF transcription induction, respectively. The levels of p-Akt and p-CREB were significantly lower in the striatum of sham-treated HD mice compared with WT mice (Fig. 5C and D). In contrast, levels of p-Akt and p-CREB were similar in the striatum of ECS-treated HD mice and WT mice (Fig. 5C and D), suggesting that ECS can prevent the impairment of Akt- and CREB-mediated signaling caused by mutant Htt. Levels of phosphorylated tropomyosin-related kinase B (TrkB), the high-affinity BDNF receptor, were higher in the striatum of ECS-treated HD mice in comparison with sham-treated HD mice (Fig. 5E) further suggesting that the activation of TrkB receptor signaling upon binding of BDNF is involved in the increase of p-Akt and p-CREB in the striatum of ECS-treated HD mice.
DISCUSSION
HD is a progressive neurodegenerative disease for which there is no effective therapy. Because HD is caused by a mutation in the gene encoding Htt, genetic testing can identify patients before they become symptomatic, thus offering the possibility of early interventions that delay or prevent the onset of the disease. In the present study, we show that once-weekly ECS treatments delay disease onset and improve survival by an average of 2 weeks which is comparable with the reported increase in survival in N171-82Q HD mice that were either maintained on an intermittent fasting dietary restriction regimen (10) or treated with antidepressant drugs (38,39). Histological analyses demonstrated a reduction in the neuron loss in the striatum of ECS-treated HD mice compared with sham-treated HD mice. These results in an animal model of HD suggest that ECS treatment can counteract the pathogenic actions of mutant Htt, thereby preserving the viability and function of striatal neurons.
To elucidate the mechanism by which ECS treatment ameliorates cellular pathology and extends lifespan, we examined neuroprotective proteins that inhibit apoptotic biochemical cascades and preserve the cellular ability to adapt to stress. ECS treatment resulted in elevated levels of the protein chaperones Hsp70 and Hsp40 in the striatum of HD mice. Studies in which levels of Hsp70 or Hsp40 are selectively increased or decreased have shown that these two chaperones can protect neurons against insults relevant to HD including excitotoxins, metabolic stress and mutant Htt (40,41).
The expanded polyglutamine stretch in mutant Htt is thought to trigger a conformational change that leads to partial unfolding or misfolding which, if not corrected by molecular chaperones, can lead to abnormal proteolysis and protein aggregation (42,43). Histopathological comparison showed that ECS treatment resulted in a significant reduction in striatal and cortical Htt aggregation. Misfolding and aggregation of mutant Htt are therapeutic targets since they are early molecular events in the pathogenic cascades that underlie the neurological dysfunction in transgenic HD mice (32). A variety of molecular chaperones have been demonstrated to exert therapeutic effects against various experimental models of the polyglutamine diseases, including HD (44–46). Here we showed that once-weekly ECT treatment increases Hsp70 and Hsp40 that likely played a significant role in suppressing Htt misfolding and aggregation. Indeed, we found that the ECS treatment reduces Htt aggregation in striatal (Fig. 3) and cortical (data not shown) cells of HD mice suggesting that ECS interrupts the disease process at an early stage. The ECS-induced increase of Hsp70 and Hsp40 is likely mediated by HSF1, a stress-responsive transcriptional regulator that has been shown to suppress polyglutamine aggregate formation in cellular and mouse models (47).
We found that striatal BDNF levels were markedly reduced in HD mice compared with WT mice, and that BDNF levels were significantly increased in HD mice that had been maintained on long-term ECS treatment compared with sham control HD mice. BDNF is a neurotrophin that plays critical roles in synaptic plasticity and neuronal survival in many brain regions including those affected in HD. Previous studies have shown that endogenous BDNF (16), and BDNF delivered directly or by transgenesis (11–14), can protect striatal and cortical neurons in experimental models of HD. Because BDNF transcription has been reported to be impeded by the misfolded Htt protein and because Hsps reduce the accumulation of misfolded Htt protein, our data suggest that elevation of Hsps may contribute in part to the restoration of the BDNF level in ECS-treated HD mice. Furthermore, as the majority of striatal BDNF is synthesized by cortical neurons (48), the data also implies that mitigation of cortical aggregate formation may lessen the HD's adverse effects on the basal ganglia.
The elevation of BDNF levels may mediate, at least in part, the retardation of disease onset and extension of survival by ECS in N171-82Q HD mice. Consistent with the latter possibility, it was reported that paroxetine and seratraline, two other anti-depressant treatments that increase BDNF levels in the striatum and cortex of HD mice, also delay disease onset and extend survival in N171-82Q HD mice (38,39). BDNF may protect neurons against excitotoxic, metabolic and oxidative stress believed to be involved in the death of neurons in HD. Indeed, BDNF can protect neurons against glutamate receptor-mediated excitotoxicity (14,49) energetic/mitochondrial stress (12,13) and oxidative insults (50). By maintaining the survival and function of striatal neurons, BDNF levels could improve motor control in HD mice as reduction in BDNF levels advances the age of onset and exacerbates the severity of motor dysfunction (16,18). Hence, although ECS treatment may induce the expression of a plethora of plasticity-related genes (51), the elevation of BDNF is likely responsible for ameliorating the behavioral and neuropathological phenotype in N171-82Q HD mice.
Apart from protecting vulnerable neurons, ECS has been shown to facilitate neurogenesis through upregulation of BDNF and other growth factors (52). Although not addressed in the present study, it is possible that increased neurogenesis which functions to replace lost or damaged striatal neurons may contribute to ECS-induced beneficial effects. It has been demonstrated that induction of neurogenesis can slow disease progression in transgenic HD mice (15). One functional consequence of neurogenesis is learning and memory (53), and impaired learning has also been described in different transgenic mouse models of HD (54). Previous studies showed that ECS treatment increased the total number of synapses in adult male rat hippocampus (55,56). Because a reduction in BDNF levels has been described in the hippocampus of transgenic HD mice (57) and because this neurotrophin has been shown to rescue the deficits in long-term potentiation of synaptic transmission (a cellular correlate of learning and memory) in hippocampal slices from transgenic HD mice (58), it will be interesting to determine whether ECS treatment prevents cognitive impairments in transgenic HD mice.
Interestingly, ECS did not have significant effects on levels of Hsp70, Hsp40 and BDNF in the striatum of WT mice, although there were clear trends towards elevated levels of each of these proteins. Previous studies have shown that BDNF mRNA levels are increased acutely (hours to a few days) in the hippocampus following ECS (59). Other studies have found that BDNF mRNA levels in the hippocampus are elevated 2-fold in rats that had received daily ECS treatments for a 10-day period (60). Another study found that BDNF protein levels were significantly increased in the cortex and striatum of rats after 10 daily ECS treatments (60). In our study, the mice were treated with ECS once weekly for 8 weeks using a 50 mA, 0.2 s ECS stimulus. Our ECS treatment was therefore less frequent than previous studies, which could explain the lesser effect of our treatment regimen on BDNF levels compared with more frequent ECS treatments. Striatal neurons in HD mice may be more sensitive to a low ECS treatment because of their lower threshold for excitability, and hence exhibit a greater increase in levels of BDNF and protein chaperones.
In addition to mitigating both gross brain and neuronal atrophy, ECS treatment also ameliorated the progressive body weight loss in N171-82Q HD mice. The basis for the weight loss in HD mice is not yet clear. BDNF and its receptor, TrkB, play prominent roles in food intake and energy metabolism regulation through central mechanisms involving the hypothalamus (61). The potential role of BDNF in regulation of energy metabolism was first discovered through generation of BDNF ± mice which display an obese phenotype (62,63). Subsequently, mutations in bdnf and trkB have been identified in some obese patients (64,65). Genetic ablation of bdnf in the hypothalamus of adult mice results in hyperphagic behavior and obesity (66). Emerging evidence suggests that disturbed functions of the hypothalamus may contribute to some signs and symptoms associated with metabolic alterations in HD patients (67). Further studies are needed to examine whether the body weight loss and its amelioration by ECS involve altered expression of BDNF in the hypothalamus of HD mice.
A functional BDNF polymorphism (BDNF Val66Met) was reported to influence the vulnerability to various psychiatric disorders (68). There has been a resurgence of interest in the use of ECS for the treatment of drug-refractive psychiatric disorders, and it is considered safe and effective for the treatment of depression in the elderly, including those with co-morbidities (56). ECS treatments often result in long-lasting clinical improvements in psychiatric symptoms which are correlated with the increased BDNF level (69–71). Several reports describe the beneficial effects of ECS in relieving depression in HD patients (23,72–74) who, in general, have higher suicide rates relative to those with other medical and neurodegenerative diseases (75,76). However, there have been no controlled studies of ECS treatment in symptomatic HD patients, nor any attempts to delay the onset of HD with periodic ECS treatments. Our preclinical findings demonstrate for the first time that ECS treatment slows the progression of the neurodegenerative process caused by mutant Htt in an animal model of HD. ECS treatment resulted in increase in the expression of several adaptive cellular stress response proteins which may promote neuronal survival and plasticity, and so forestall the neurodegenerative process resulting in a delay in the disease onset and life extension. The present findings have significant implications for preventive treatment strategies for individuals that carry the mutant Htt gene.
MATERIALS AND METHODS
Mice and ECS treatment
HD-N171-82Q mice were purchased from the Jackson Laboratories (Bar Harbor, ME, USA) and were maintained on the B6C3F1 background; offspring were identified by PCR analysis using genomic DNA extracted from tail biopsies to distinguish transgene-bearing mice from their WT littermates (27). Mice were maintained under usual laboratory conditions that included ad libitum feeding and drinking, in a non-enriched environment (77). Experiments were performed on 2-month-old male mice that were assigned randomly to control and ECS treatment groups. All procedures were approved by the National Institute on Aging Animal Care and Use Committee. ECS was delivered to mice under isoflurane inhalation anesthesia once a week via bilateral ear clip electrodes using the Ugo Basile, ECS Unit 7801. The stimulus current was 50 mA and the stimulus duration was 0.2 s (78). The presence of tonic seizures immediately after the shock was confirmed by observing the extension of all limbs and forward head extension that normally last for about 10–15 s in each cohort regardless of the genotype. The sham groups were handled identically to the ECS-treated rats except no current was applied. ECS- and sham-treated mice were returned to their cages 10 min afterwards.
Rotarod test
Motor coordination and balance were evaluated at weekly intervals throughout the course of the study using an accelerating rotary rod apparatus (Columbus Instrument, OH, USA) as described previously (10). To exclude the possibility that ECS may negatively impact motor performance, rotarod performance was performed 3 days after ECS. Mice were trained to use the rotarod apparatus during a 2 min trial (4 rpm) on the day before the first day of testing. On test days, mice were placed on the rotarod for three trials for a maximal 4 min at accelerating speeds from 4 to 40 rpm and maintenance at 40 rpm after 4 min. Each trial was separated by a 30 min rest period to alleviate stress and fatigue. Latency to fall and falling times for each mouse were recorded by a trained observer blind to the treatment group of the mice.
Survival study
Once motor symptoms appeared the HD mice were examined twice daily in the early morning and late afternoon by an investigator blinded as to treatment group of the mice. Mice unable to right themselves after being placed on their backs, and unable to initiate movement after being gently prodded for 10 min (79) were euthanized; their age at this point was considered the age of death. Deaths that occurred overnight were recorded the next morning.
Tissue preparation and histologic analysis
Immediately after being anesthetized with an overdose of isoflurane, mice were perfused transcardially with saline, followed by 4% paraformaldehyde (PFA) in PBS (pH 7.4). Brains were post-fixed in 4% PFA for 24 h, and then cryoprotected in 30% sucrose/PBS for 48 h. Serial coronal sections (10μm thickness) cut through the entire striatum with a freezing microtome (Microm HM 505 N) were collected on slides. Nissl and Fluorojade C staining were performed as previously described (80) to determine surviving and degenerating neurons, respectively. For Fluoro-Jade staining, the slides were immersed for 3 min in 100% ethanol, for 1 min in 70% ethanol, and for 1 min in distilled water and then incubated in a solution containing 0.01% Fluoro-Jade (Histo-Chem, Inc., Jefferson, AR, USA) and 0.1% acetic acid (1:10) for 30 min on a shaker. After three 10 min washes, the slides were cover slipped.
Immunohistochemistry
Serially cut coronal tissue sections were blocked with 5% normal serum in 0.1% Triton X-100 in PBS for 30 min at room temperature followed by incubation overnight at 4°C with primary antibodies against N-terminal Htt protein (EM48, Chemicon; 1:400 dilution), NeuN (Chemicon; 1:400 dilution) and DARPP-32 (Abcam; 1;500 dilution). Tissue sections were incubated with Alexa568- or Alexa488-conjugated secondary antibodies (1:200, Molecular Probes) appropriate for the specific primary antibodies. The sections were then washed with 0.05% Tween-20 in phosphate-buffered saline for 1 h and counterstained with 4′,6′-diamidino-2-phenylindole (50 ng/ml) for 30 s, washed and mounted in FluorSave medium (Calbiochem).
Quantification of Htt aggregates and neurons
Images of EM48-positive neuronal inclusions and DARPP-32, Nissl or Fluorojade C-stained neurons were obtained by scanning 10–12 coronal sections spread over the anterior–posterior extent of the striatum (inter-section distance: 400 µm), using a 20× objective on a Nikon 80i Research Upright Microscope equipped with image acquisition software (QimagingRetiga 2000). All images were segmented using the same light threshold, mask smoothing and object size filters. Total area of pixel intensity was measured with the automated measurement tools in IP lab software (BD Biosciences Bio-imaging). The total density was averaged and expressed as normalized, corrected OD. Labeled cells were counted throughout the depth of the section for four adjacent fields of each section. All brain specimens were coded and analyses were performed by an investigator blinded as to the genotype and treatment group of the mice.
Immunoblotting
Methods for protein quantification, electrophoretic separation and transfer to nitrocellulose membranes were described previously (62). Membranes were incubated in blocking solution (5% milk in Tween Tris-buffered saline; TTBS) overnight at 4°C followed by a 1 h incubation in primary antibody diluted in blocking solution at room temperature. Membranes were then incubated for 1 h in secondary antibody conjugated to horseradish peroxidase (Vector Laboratories) and bands were visualized using a chemiluminiscence detection kit (ECL, Amersham). Membranes were stripped and re-probed with the actin antibody to verify and normalize protein loading (50 µg total protein, unless stated otherwise). For immunodetection of blots, enhanced chemiluminescence (ECL, Amersham) was applied. Immunoreactive bands were quantified using NIH Image software. Information on the primary antibodies used in this study, including the source, dilution and molecular weight of the antigen, can be found in Supplementary Material, Table S1.
Statistical analysis
Kaplan–Meier survival data were analyzed using the log-rank test for trend. All other data were analyzed using one-way ANOVA with Dunnett's post hoc test for pairwise comparisons or Student's t-test as appropriate. These statistical analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). P-values ≤ 0.05 were considered significant.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
FUNDING
This research was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health, and by NIH grant (1R21NS066265-01) to S.L.C.
REFERENCES
- 1.Haddad M.S., Cummings J.L. Huntington's disease. Psychiatr. Clin. North Am. 1997;20:791–807. doi: 10.1016/s0193-953x(05)70345-2. [DOI] [PubMed] [Google Scholar]
- 2.Gusella J.F., Wexler N.S., Conneally P.M., Naylor S.L., Anderson M.A., Tanzi R.E., Watkins P.C., Ottina K., Wallace M.R., Sakaguchi A.Y., et al. A polymorphic DNA marker genetically linked to Huntington's disease. Nature. 1983;306:234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- 3.Wexler N.S., Rose E.A., Housman D.E. Molecular approaches to hereditary diseases of the nervous system: Huntington's disease as a paradigm. Annu. Rev. Neurosci. 1991;14:503–529. doi: 10.1146/annurev.ne.14.030191.002443. [DOI] [PubMed] [Google Scholar]
- 4.Velier J., Kim M., Schwarz C., Kim T.W., Sapp E., Chase K., Aronin N., DiFiglia M. Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Exp. Neurol. 1998;152:34–40. doi: 10.1006/exnr.1998.6832. [DOI] [PubMed] [Google Scholar]
- 5.Smith R., Brundin P., Li J.Y. Synaptic dysfunction in Huntington's disease: a new perspective. Cell Mol. Life Sci. 2005;62:1901–1912. doi: 10.1007/s00018-005-5084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuccato C., Valenza M., Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol. Rev. 2010;90:905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- 7.Cooper J.K., Schilling G., Peters M.F., Herring W.J., Sharp A.H., Kaminsky Z., Masone J., Khan F.A., Delanoy M., Borchelt D.R., et al. Truncated N-terminal fragments of huntingtin with expanded glutamine repeats form nuclear and cytoplasmic aggregates in cell culture. Hum. Mol. Genet. 1998;7:783–790. doi: 10.1093/hmg/7.5.783. [DOI] [PubMed] [Google Scholar]
- 8.Martindale D., Hackam A., Wieczorek A., Ellerby L., Wellington C., McCutcheon K., Singaraja R., Kazemi-Esfarjani P., Devon R., Kim S.U., et al. Length of huntingtin and its polyglutamine tract influences localization and frequency of intracellular aggregates. Nat. Genet. 1998;18:150–154. doi: 10.1038/ng0298-150. [DOI] [PubMed] [Google Scholar]
- 9.Zuccato C., Marullo M., Conforti P., MacDonald M.E., Tartari M., Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington's disease. Brain Pathol. 2008;18:225–238. doi: 10.1111/j.1750-3639.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan W., Guo Z., Jiang H., Ware M., Li X.J., Mattson M.P. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl Acad. Sci. USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gharami K., Xie Y., An J.J., Tonegawa S., Xu B. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington's disease phenotypes in mice. J. Neurochem. 2008;105:369–379. doi: 10.1111/j.1471-4159.2007.05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng B., Mattson M.P. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- 13.Nakao N., Kokaia Z., Odin P., Lindvall O. Protective effects of BDNF and NT-3 but not PDGF against hypoglycemic injury to cultured striatal neurons. Exp. Neurol. 1995;131:1–10. doi: 10.1016/0014-4886(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 14.Bemelmans A.P., Horellou P., Pradier L., Brunet I., Colin P., Mallet J. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington's disease, as demonstrated by adenoviral gene transfer. Hum. Gene Ther. 1999;10:2987–2997. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- 15.Cho S.R., Benraiss A., Chmielnicki E., Samdani A., Economides A., Goldman S.A. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J. Clin. Invest. 2007;117:2889–2902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canals J.M., Pineda J.R., Torres-Peraza J.F., Bosch M., Martin-Ibanez R., Munoz M.T., Mengod G., Ernfors P., Alberch J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J. Neurosci. 2004;24:7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nucifora F.C., Jr, Sasaki M., Peters M.F., Huang H., Cooper J.K., Yamada M., Takahashi H., Tsuji S., Troncoso J., Dawson V.L., et al. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science. 2001;291:2423–2428. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 18.Zuccato C., Tartari M., Crotti A., Goffredo D., Valenza M., Conti L., Cataudella T., Leavitt B.R., Hayden M.R., Timmusk T., et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat. Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
- 19.Spires T.L., Grote H.E., Varshney N.K., Cordery P.M., van Dellen A., Blakemore C., Hannan A.J. Environmental enrichment rescues protein deficits in a mouse model of Huntington's disease, indicating a possible disease mechanism. J. Neurosci. 2004;24:2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne N.A., Prudic J. Electroconvulsive therapy: part I. A perspective on the evolution and current practice of ECT. J. Psychiatr. Pract. 2009;15:346–368. doi: 10.1097/01.pra.0000361277.65468.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutor B., Rasmussen K.G. Electroconvulsive therapy for agitation in Alzheimer disease: a case series. J. ECT. 2008;24:239–241. doi: 10.1097/YCT.0b013e3181587416. [DOI] [PubMed] [Google Scholar]
- 22.Moellentine C., Rummans T., Ahlskog J.E., Harmsen W.S., Suman V.J., O'Connor M.K., Black J.L., Pileggi T. Effectiveness of ECT in patients with parkinsonism. J. Neuropsychiatry Clin. Neurosci. 1998;10:187–193. doi: 10.1176/jnp.10.2.187. [DOI] [PubMed] [Google Scholar]
- 23.Lewis C.F., DeQuardo J.R., Tandon R. ECT in genetically confirmed Huntington's disease. J. Neuropsychiatry Clin. Neurosci. 1996;8:209–210. doi: 10.1176/jnp.8.2.209. [DOI] [PubMed] [Google Scholar]
- 24.Beale M.D., Kellner C.H., Gurecki P., Pritchett J.T. ECT for the treatment of Huntington's disease: a case study. Convuls. Ther. 1997;13:108–112. [PubMed] [Google Scholar]
- 25.Nibuya M., Morinobu S., Duman R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altar C.A., Whitehead R.E., Chen R., Wortwein G., Madsen T.M. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol. Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 27.Schilling G., Becher M.W., Sharp A.H., Jinnah H.A., Duan K., Kotzuk J.A., Slunt H.H., Ratovitski T., Cooper J.K., Jenkins N.A., et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum. Mol. Genet. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- 28.Djousse L., Knowlton B., Cupples L.A., Marder K., Shoulson I., Myers R.H. Weight loss in early stage of Huntington's disease. Neurology. 2002;59:1325–1330. doi: 10.1212/01.wnl.0000031791.10922.cf. [DOI] [PubMed] [Google Scholar]
- 29.Thompson P.D., Berardelli A., Rothwell J.C., Day B.L., Dick J.P., Benecke R., Marsden C.D. The coexistence of bradykinesia and chorea in Huntington's disease and its implications for theories of basal ganglia control of movement. Brain. 1988;111:223–244. doi: 10.1093/brain/111.2.223. [DOI] [PubMed] [Google Scholar]
- 30.Hemmings H.C., Jr, Greengard P. DARPP-32, a dopamine-regulated phosphoprotein. Prog. Brain Res. 1986;69:149–159. doi: 10.1016/s0079-6123(08)61056-0. [DOI] [PubMed] [Google Scholar]
- 31.Moulder K.L., Onodera O., Burke J.R., Strittmatter W.J., Johnson E.M., Jr. Generation of neuronal intranuclear inclusions by polyglutamine-GFP: analysis of inclusion clearance and toxicity as a function of polyglutamine length. J. Neurosci. 1999;19:705–715. doi: 10.1523/JNEUROSCI.19-02-00705.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies S.W., Turmaine M., Cozens B.A., DiFiglia M., Sharp A.H., Ross C.A., Scherzinger E., Wanker E.E., Mangiarini L., Bates G.P. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 33.Wickner S., Maurizi M.R., Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science. 1999;286:1888–1893. doi: 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- 34.Hay D.G., Sathasivam K., Tobaben S., Stahl B., Marber M., Mestril R., Mahal A., Smith D.L., Woodman B., Bates G.P. Progressive decrease in chaperone protein levels in a mouse model of Huntington's disease and induction of stress proteins as a therapeutic approach. Hum. Mol. Genet. 2004;13:1389–1405. doi: 10.1093/hmg/ddh144. [DOI] [PubMed] [Google Scholar]
- 35.Muchowski P.J., Schaffar G., Sittler A., Wanker E.E., Hayer-Hartl M.K., Hartl F.U. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl Acad. Sci. USA. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang M.C., Chen H.M., Lai H.L., Chen H.W., Chou S.Y., Chen C.M., Tsai F.J., Chern Y. The A2A adenosine receptor rescues the urea cycle deficiency of Huntington's disease by enhancing the activity of the ubiquitin-proteasome system. Hum. Mol. Genet. 2009;18:2929–2942. doi: 10.1093/hmg/ddp230. [DOI] [PubMed] [Google Scholar]
- 37.Strand A.D., Baquet Z.C., Aragaki A.K., Holmans P., Yang L., Cleren C., Beal M.F., Jones L., Kooperberg C., Olson J.M., et al. Expression profiling of Huntington's disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J. Neurosci. 2007;27:11758–11768. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan W., Guo Z., Jiang H., Ladenheim B., Xu X., Cadet J.L., Mattson M.P. Paroxetine retards disease onset and progression in Huntingtin mutant mice. Ann. Neurol. 2004;55:590–594. doi: 10.1002/ana.20075. [DOI] [PubMed] [Google Scholar]
- 39.Duan W., Peng Q., Masuda N., Ford E., Tryggestad E., Ladenheim B., Zhao M., Cadet J.L., Wong J., Ross C.A. Sertraline slows disease progression and increases neurogenesis in N171–82Q mouse model of Huntington's disease. Neurobiol. Dis. 2008;30:312–322. doi: 10.1016/j.nbd.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai Y., Du L., Dunsmore K.E., Jenkins L.W., Wong H.R., Clark R.S. Selectively increasing inducible heat shock protein 70 via TAT-protein transduction protects neurons from nitrosative stress and excitotoxicity. J. Neurochem. 2005;94:360–366. doi: 10.1111/j.1471-4159.2005.03212.x. [DOI] [PubMed] [Google Scholar]
- 41.Tantucci M., Mariucci G., Taha E., Spaccatini C., Tozzi A., Luchetti E., Calabresi P., Ambrosini M.V. Induction of heat shock protein 70 reduces the alteration of striatal electrical activity caused by mitochondrial impairment. Neuroscience. 2009;163:735–740. doi: 10.1016/j.neuroscience.2009.06.070. [DOI] [PubMed] [Google Scholar]
- 42.Wanker E.E. Protein aggregation and pathogenesis of Huntington's disease: mechanisms and correlations. J. Biol. Chem. 2000;381:937–942. doi: 10.1515/BC.2000.114. [DOI] [PubMed] [Google Scholar]
- 43.Sittler A., Lurz R., Lueder G., Priller J., Lehrach H., Hayer-Hartl M.K., Hartl F.U., Wanker E.E. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington's disease. Hum. Mol. Genet. 2001;10:1307–1315. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- 44.Warrick J.M., Chan H.Y., Gray-Board G.L., Chai Y., Paulson H.L., Bonini N.M. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat. Genet. 1999;23:425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- 45.Wacker J.L., Zareie M.H., Fong H., Sarikaya M., Muchowski P.J. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat. Struct. Mol. Biol. 2004;11:1215–1222. doi: 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- 46.Vacher C., Garcia-Oroz L., Rubinsztein D.C. Overexpression of yeast hsp104 reduces polyglutamine aggregation and prolongs survival of a transgenic mouse model of Huntington's disease. Hum. Mol. Genet. 2005;14:3425–3433. doi: 10.1093/hmg/ddi372. [DOI] [PubMed] [Google Scholar]
- 47.Mejimoto M., Takaki E., Hayashi T., Kitaura Y., Tanaka Y., Inouye S., Nakai A. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J. Biol. Chem. 2005;280:34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- 48.Altar C.A., Cai N., Bliven T., Juhasz M., Conner J.M., Acheson A.L., Lindsay R.M., Wiegand S.J. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 49.Duan W., Guo Z., Mattson M.P. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J. Neurochem. 2001;76:619–626. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- 50.Mattson M.P., Lovell M.A., Furukawa K., Markesbery W.R. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J. Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- 51.Sun W., Park K.W., Choe J., Rhyu I.J., Kim I.H., Park S.K., Choi B., Choi S.H., Park S.H., Kim H. Identification of novel electroconvulsive shock-induced and activity-dependent genes in the rat brain. Biochem. Biophys. Res. Commun. 2005;327:848–856. doi: 10.1016/j.bbrc.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 52.Ploski J.E., Newton S.S., Duman R.S. Electroconvulsive seizure-induced gene expression profile of the hippocampus dentate gyrus granule cell layer. J. Neurochem. 2006;99:1122–1132. doi: 10.1111/j.1471-4159.2006.04156.x. [DOI] [PubMed] [Google Scholar]
- 53.Deng W., Aimone J.B., Gage F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Raamsdonk J.M., Pearson J., Slow E.J., Hossain S.M., Leavitt B.R., Hayden M.R. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J. Neurosci. 2005;25:4169–4180. doi: 10.1523/JNEUROSCI.0590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart C., Reid I. Electroconvulsive stimulation and synaptic plasticity in the rat. Brain Res. 1993;620:139–141. doi: 10.1016/0006-8993(93)90280-z. [DOI] [PubMed] [Google Scholar]
- 56.Chen F., Madsen T.M., Wegener G., Nyengaard J.R. Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. Eur. Neuropsychopharmacol. 2009;19:329–338. doi: 10.1016/j.euroneuro.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Lynch G., Kramar E.A., Rex C.S., Jia Y., Chappas D., Gall C.M., Simmons D.A. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington's disease. J. Neurosci. 2007;27:4424–4434. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simmons D.A., Rex C.S., Palmer L., Pandyarajan V., Fedulov V., Gall C.M., Lynch G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc. Natl Acad. Sci. USA. 2009;106:4906–4911. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zetterström T.S., Pei Q., Grahame-Smith D.G. Repeated electroconvulsive shock extends the duration of enhanced gene expression for BDNF in rat brain compared with a single administration. Brain Res. Mol. Brain Res. 1998;57:106–110. doi: 10.1016/s0169-328x(98)00077-1. [DOI] [PubMed] [Google Scholar]
- 60.Altar C.A., Whitehead R.E., Chen R., Wörtwein G., Madsen T.M. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol. Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 61.Xu B.E.H., Goulding K., Zang D., Cepoi R.D., Cone K.R., Jones L.H., Tecott L.H., Reichardt L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kernie S.G., Liebl D.J., Parada L.F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rios M., Fan G., Fekete C., Kelly J., Bates B., Kuehn R., Lechan R.M., Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 64.Yeo G.S., Connie Hung C.C., Rochford J., Keogh J., Gray J., Sivaramakrishnan S., O'Rahilly S., Farooqi I.S. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 65.Friedel S., Horro F.F., Wermter A.K., Geller F., Dempfle A., Reichwald K., Smidt J., Bronner G., Konrad K., Herpertz-Dahlmann B., et al. Mutation screen of the brain derived neurotrophic factor gene (BDNF): identification of several genetic variants and association studies in patients with obesity eating disorders and attention-deficit/hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;132:96–99. doi: 10.1002/ajmg.b.30090. [DOI] [PubMed] [Google Scholar]
- 66.Unger T.J., Calderon G.A., Bradley L.C., Sena-Esteves M., Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aziz N.A., Swaab D.F., Pijl H., Roos R.A. Hypothalamic dysfunction and neuroendocrine and metabolic alterations in Huntington's disease: clinical consequences and therapeutic implications. Rev. Neurosci. 2007;18:223–251. doi: 10.1515/revneuro.2007.18.3-4.223. [DOI] [PubMed] [Google Scholar]
- 68.Pregelj P., Nedic G., Paska A.V., Zupanc T., Nikolac M., Balažic J., Tomori M., Komel R., Seler D.M., Pivac N. The association between brain-derived neurotrophic factor polymorphism (BDNF Val66Met) and suicide. J Affect Disord. 2010 doi: 10.1016/j.jad.2010.07.001. [Epub ahead of print, 26 July] [DOI] [PubMed] [Google Scholar]
- 69.Bocchio-Chiavetto L., Zanardini R., Bortolomasi M., Abate M., Segala M., Giacopuzzi M., Riva M.A., Marchina E., Pasqualetti P., Perez J., et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur. Neuropsychopharmacol. 2006;16:620–624. doi: 10.1016/j.euroneuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Marano C.M., Phatak P., Vemulapalli U.R., Sasan A., Nalbandyan M.R., Ramanujam S., Soekadar S., Demosthenous M., Regenold W.T. Increased plasma concentration of brain-derived neurotrophic factor with electroconvulsive therapy: a pilot study in patients with major depression. J. Clin. Psychiatry. 2007;68:512–517. doi: 10.4088/jcp.v68n0404. [DOI] [PubMed] [Google Scholar]
- 71.Taylor S.M. Electroconvulsive therapy, brain-derived neurotrophic factor, and possible neurorestorative benefit of the clinical application of electroconvulsive therapy. J. ECT. 2008;24:160–165. doi: 10.1097/YCT.0b013e3181571ad0. [DOI] [PubMed] [Google Scholar]
- 72.Piccinni A., Del Debbio A., Medda P., Bianchi C., Roncaglia I., Veltri A., Zanello S., Massimetti E., Origlia N., Domenici L., et al. Plasma brain-derived neurotrophic factor in treatment-resistant depressed patients receiving electroconvulsive therapy. Eur. Neuropsychopharmacol. 2009;19:349–355. doi: 10.1016/j.euroneuro.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 73.Evans D.L., Pedersen C.A., Tancer M.E. ECT in the treatment of organic psychosis in Huntington's disease. Convuls. Ther. 1987;3:145–150. [PubMed] [Google Scholar]
- 74.Ranen N.G., Peyser C.E., Folstein S.E. ECT as a treatment for depression in Huntington's disease. J. Neuropsychiatry Clin. Neurosci. 1994;6:154–159. doi: 10.1176/jnp.6.2.154. [DOI] [PubMed] [Google Scholar]
- 75.Druss B., Pincus H. Suicidal ideation and suicide attempts in general medical illnesses. Arch. Intern. Med. 2000;160:1522–1526. doi: 10.1001/archinte.160.10.1522. [DOI] [PubMed] [Google Scholar]
- 76.Farrer L.A. Suicide and attempted suicide in Huntington disease: implications for preclinical testing of persons at risk. Am. J. Med. Genet. 1986;24:305–311. doi: 10.1002/ajmg.1320240211. [DOI] [PubMed] [Google Scholar]
- 77.Martin B., Ji S., Maudsley S., Mattson M.P. ‘Control' laboratory rodents are metabolically morbid: why it matters. Proc. Natl Acad. Sci. USA. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barichello T., Bonatto F., Feier G., Martins M.R., Moreira J.C., Dal-Pizzol F., Izquierdo I., Quevedo J. No evidence for oxidative damage in the hippocampus after acute and chronic electroshock in rats. Brain Res. 2004;1014:177–183. doi: 10.1016/j.brainres.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 79.Ferrante R.J., Andreassen O.A., Jenkins B.G., Dedeoglu A., Kuemmerle S., Kubilus J.K., Kaddurah-Daouk R., Hersch S.M., Beal M.F. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J. Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmued L.C., Stowers C.C., Scallet A.C., Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.