Abstract
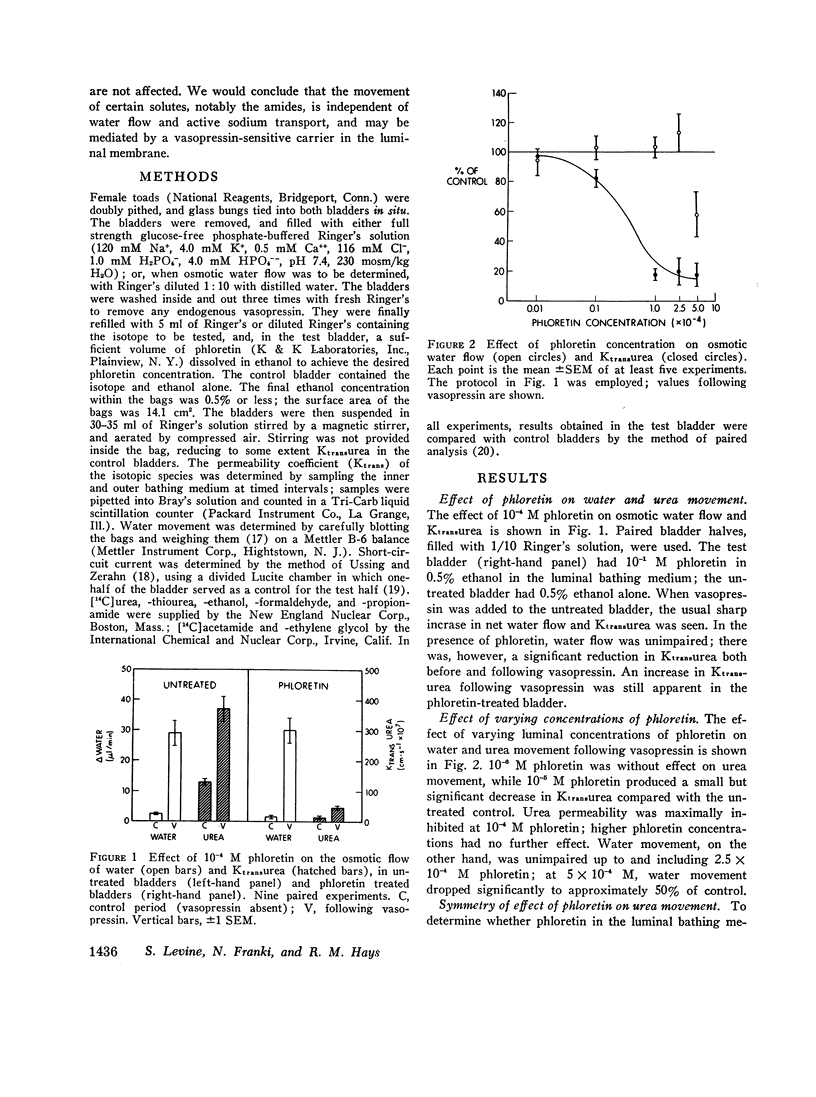
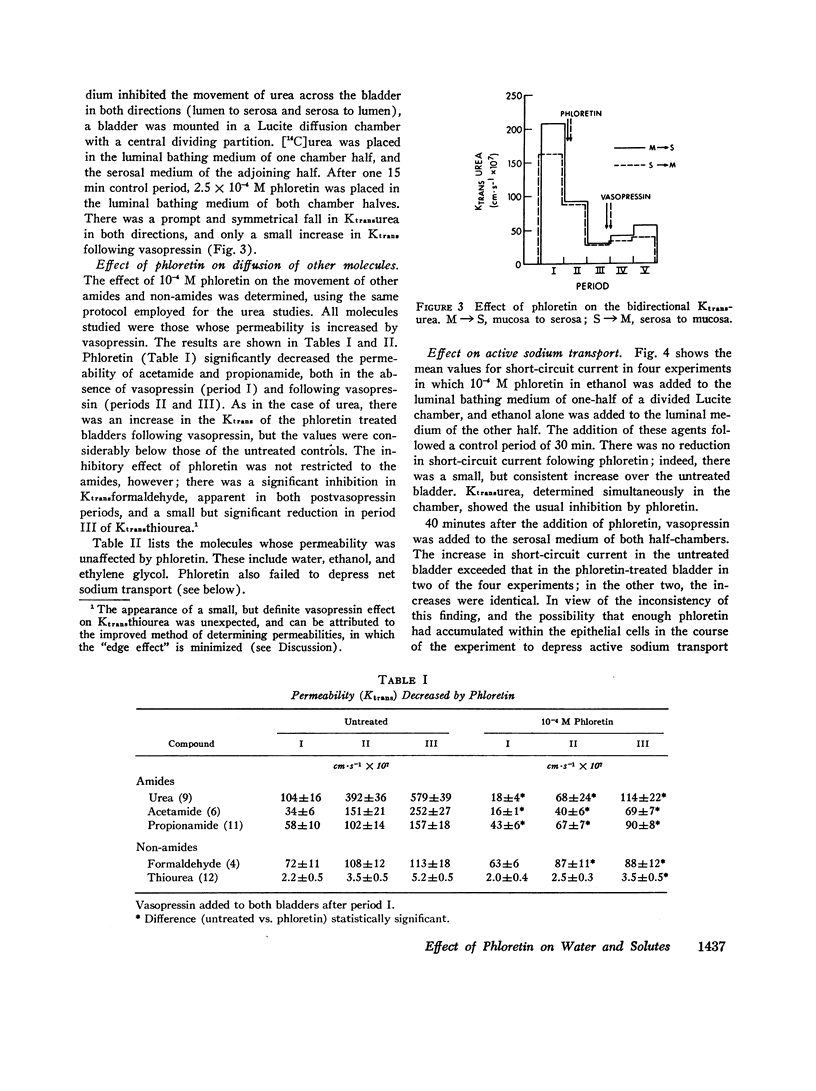
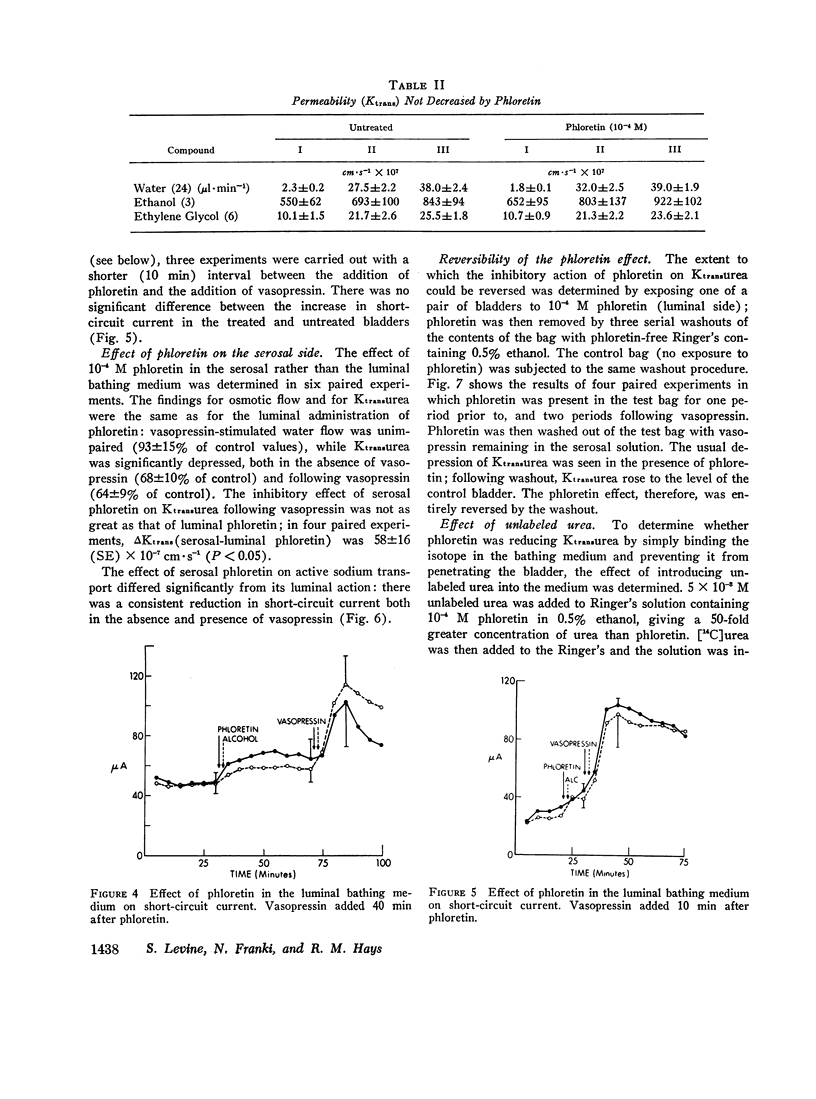
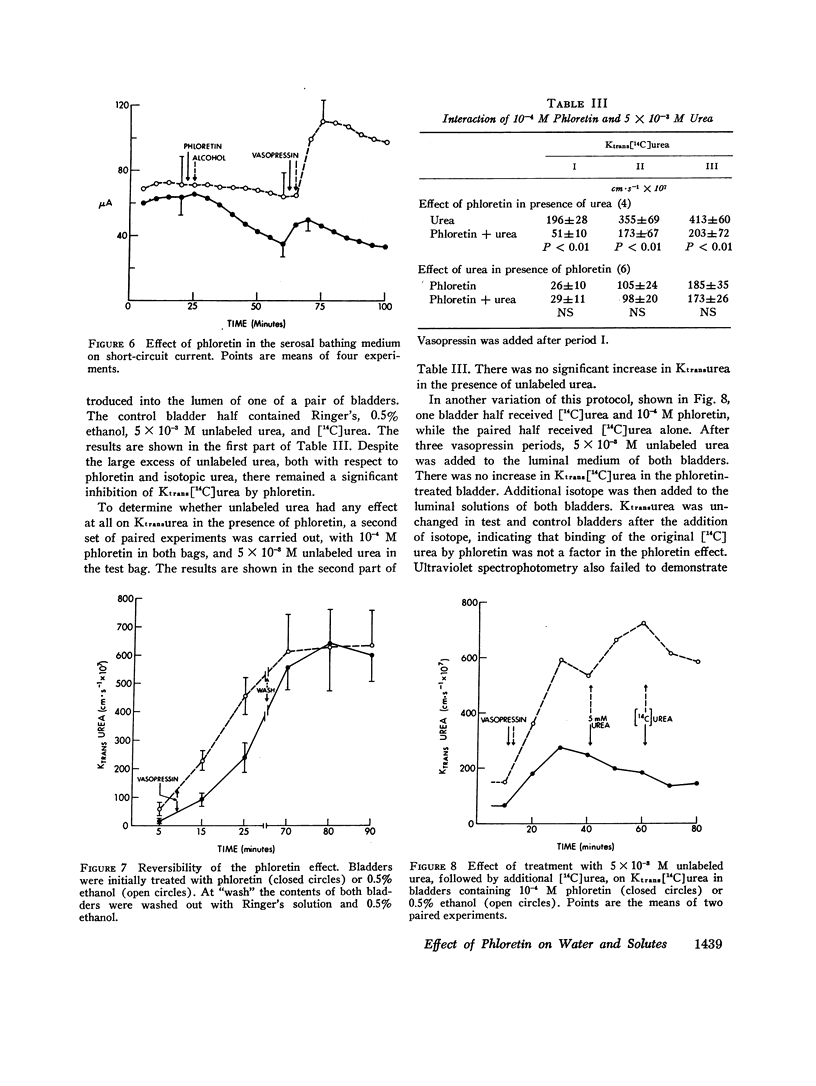
It is generally believed that urea crosses the cell membrane through aqueous channels, and that its movement across the membrane is accelerated in the direction of net water flow (solvent drag effect). The present report presents evidence for a vasopressin-sensitive pathway for the movement of urea, other amides, and certain non-amides, which is independent of water flow. Phloretin, when present at 10-4 M concentration in the medium bathing the luminal surface of the toad bladder, strongly inhibits the movement of urea, acetamide, and propionamide across the toad bladder, both in the absence and presence of vasopressin. The vasopressin-stimulated movement of formaldehyde and thiourea is also reduced. Osmotic water flow, on the other hand, is not affected; nor is the movement of ethanol and ethylene glycol, or the net transport of sodium. On the basis of these studies we would conclude that the movement of many, if not all, solutes across the cell membrane is independent of water flow, and that a vasopressin-sensitive carrier may be involved in the transport of certain solutes across the cell membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN B., USSING H. H. Solvent drag on non-electrolytes during osmotic flow through isolated toad skin and its response to antidiuretic hormone. Acta Physiol Scand. 1957 Jun 8;39(2-3):228–239. doi: 10.1111/j.1748-1716.1957.tb01425.x. [DOI] [PubMed] [Google Scholar]
- Alvarado F. Hypothesis for the interaction of phlorizin and phloretin with membrane carriers for sugars. Biochim Biophys Acta. 1967 Jul 3;135(3):483–495. doi: 10.1016/0005-2736(67)90038-7. [DOI] [PubMed] [Google Scholar]
- BENTLEY P. J. The effects of neurohypophysial extracts on the water transfer across the wall of the isolated urinary bladder of the toad Bufo marinus. J Endocrinol. 1958 Sep;17(3):201–209. doi: 10.1677/joe.0.0170201. [DOI] [PubMed] [Google Scholar]
- Boylan J. W. A model for passive urea reabsorption in the elasmobranch kidney. Comp Biochem Physiol A Comp Physiol. 1972 May 1;42(1):27–30. doi: 10.1016/0300-9629(72)90361-1. [DOI] [PubMed] [Google Scholar]
- CHAN S. S., LOTSPEICH W. D. Comparative effects of phlorizin and phloretin on glucose transport in the cat kidney. Am J Physiol. 1962 Dec;203:975–979. doi: 10.1152/ajplegacy.1962.203.6.975. [DOI] [PubMed] [Google Scholar]
- Clapp J. R. Renal tubular reabsorption of urea in normal and protein-depleted rats. Am J Physiol. 1966 Jun;210(6):1304–1308. doi: 10.1152/ajplegacy.1966.210.6.1304. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Dobson J. G., Jr, Kidder G. W., 3rd Edge damage effect in in vitro frog skin preparations. Am J Physiol. 1968 Apr;214(4):719–724. doi: 10.1152/ajplegacy.1968.214.4.719. [DOI] [PubMed] [Google Scholar]
- FORSTER R. P. Active cellular transport of urea by frog renal tubules. Am J Physiol. 1954 Nov;179(2):372–377. doi: 10.1152/ajplegacy.1954.179.2.372. [DOI] [PubMed] [Google Scholar]
- Goldberg M., Wojtczak A. M., Ramirez M. A. Uphill transport of urea in the dog kidney: effects of certain inhibitors. J Clin Invest. 1967 Mar;46(3):388–399. doi: 10.1172/JCI105540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYS R. M., LEAF A. Studies on the movement of water through the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 May;45:905–919. doi: 10.1085/jgp.45.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupka R. M. Evidence for a carrier conformational change associated with sugar transport in erythrocytes. Biochemistry. 1971 Mar 30;10(7):1143–1148. doi: 10.1021/bi00783a007. [DOI] [PubMed] [Google Scholar]
- LEAF A., HAYS R. M. Permeability of the isolated toad bladder to solutes and its modification by vasopressin. J Gen Physiol. 1962 May;45:921–932. doi: 10.1085/jgp.45.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. The atachment of phloretin and analogues to human erythrocytes in connection with inhibition of sugar transport. J Biol Chem. 1959 Nov;234:3022–3026. [PubMed] [Google Scholar]
- Lippe C., Micelli S., Gallucci E. Amide and thio-amide permeability across the urinary bladder of Rana esculenta. Effect of posthypophyseal hormones. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):297–302. doi: 10.1016/0300-9629(72)90110-7. [DOI] [PubMed] [Google Scholar]
- MAFFLY R. H., HAYS R. M., LAMDIN E., LEAF A. The effect of neurohypophyseal hormones on the permeability of the toad bladder to urea. J Clin Invest. 1960 Apr;39:630–641. doi: 10.1172/JCI104078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey R. I., Farmer R. E. Inhibition of water and solute permeability in human red cells. Biochim Biophys Acta. 1970 Jul 7;211(1):104–106. doi: 10.1016/0005-2736(70)90130-6. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-NIELSEN B., O'DELL R. Effect of diet on distribution of urea and electrolytes in kidneys of sheep. Am J Physiol. 1959 Oct;197:856–860. doi: 10.1152/ajplegacy.1959.197.4.856. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-NIELSEN B., RABINOWITZ L. METHYLUREA AND ACETAMIDE: ACTIVE REABSORPTION BY ELASMOBRANCH RENAL TUBULES. Science. 1964 Dec 18;146(3651):1587–1588. doi: 10.1126/science.146.3651.1587. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-NIELSEN B., SHRAUGER C. R. HANDLING OF UREA AND RELATED COMPOUNDS BY THE RENAL TUBULES OF THE FROG. Am J Physiol. 1963 Sep;205:483–488. doi: 10.1152/ajplegacy.1963.205.3.483. [DOI] [PubMed] [Google Scholar]
- SHARP G. W., LEAF A. BIOLOGICAL ACTION OF ALDOSTERONE IN VITRO. Nature. 1964 Jun 20;202:1185–1188. doi: 10.1038/2021185a0. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen B., Robinson R. R. Contribution of urea to urinary concentrating ability in the dog. Am J Physiol. 1970 May;218(5):1363–1369. doi: 10.1152/ajplegacy.1970.218.5.1363. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen B., Truniger B., Rabinowitz L. Sodium-linked urea transport by the renal tubule of the spiny dogfish Squalus acanthias. Comp Biochem Physiol A Comp Physiol. 1972 May 1;42(1):13–25. doi: 10.1016/0300-9629(72)90360-x. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Schmidt-Nielsen B. Urea transport in the collecting duct of rats on normal and low protein diet. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;295(2):147–156. doi: 10.1007/BF00362746. [DOI] [PubMed] [Google Scholar]
- Walser M., Butler S. E., Hammond V. Reversible stimulation of sodium transport in the toad bladder by stretch. J Clin Invest. 1969 Sep;48(9):1714–1723. doi: 10.1172/JCI106137. [DOI] [PMC free article] [PubMed] [Google Scholar]


