Abstract
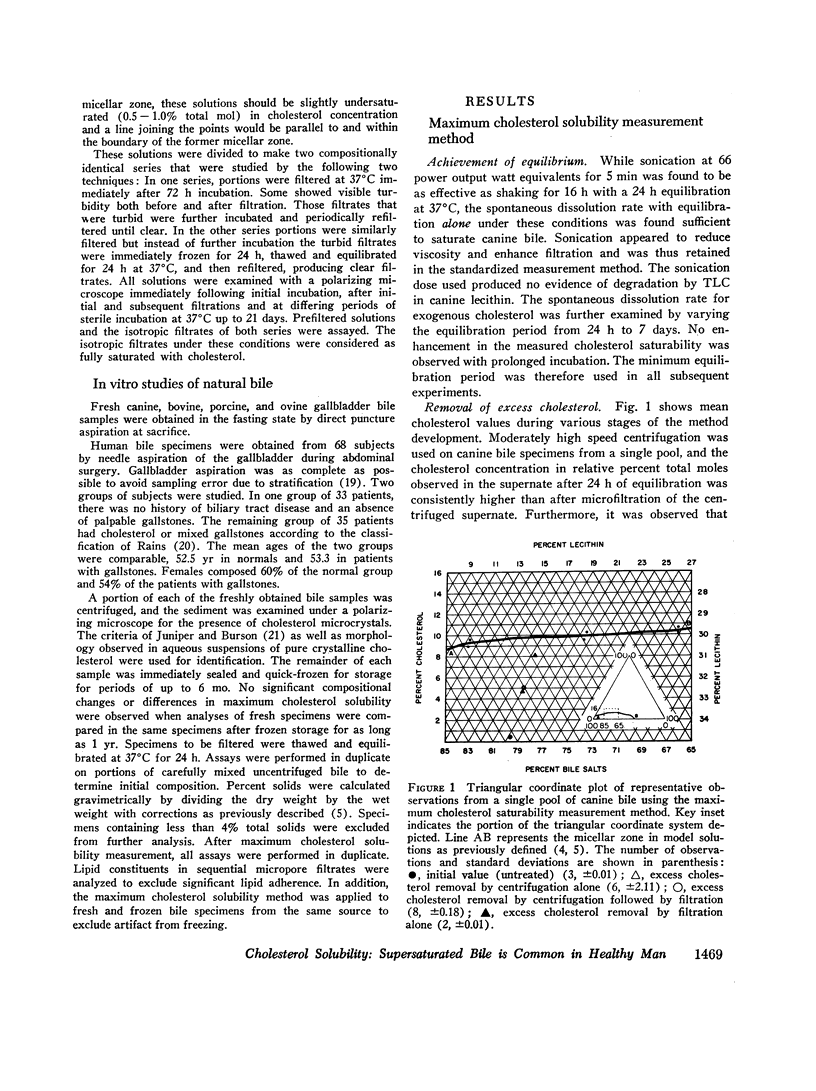
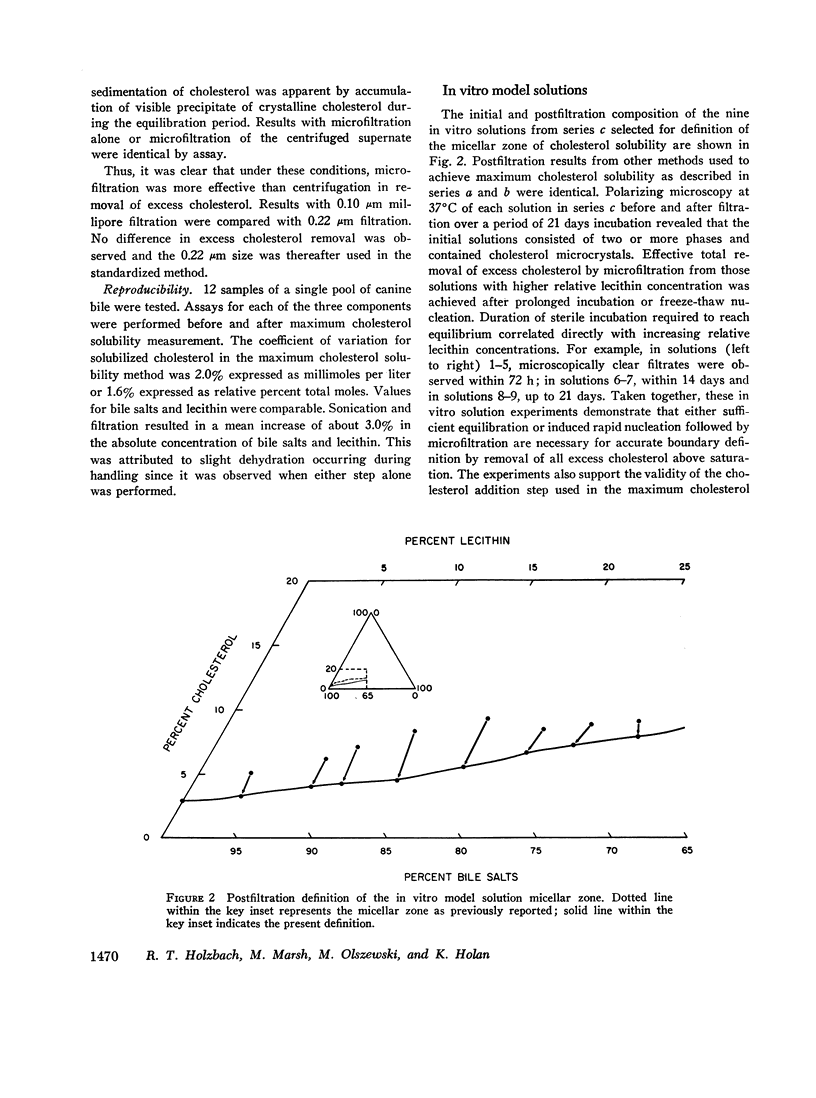
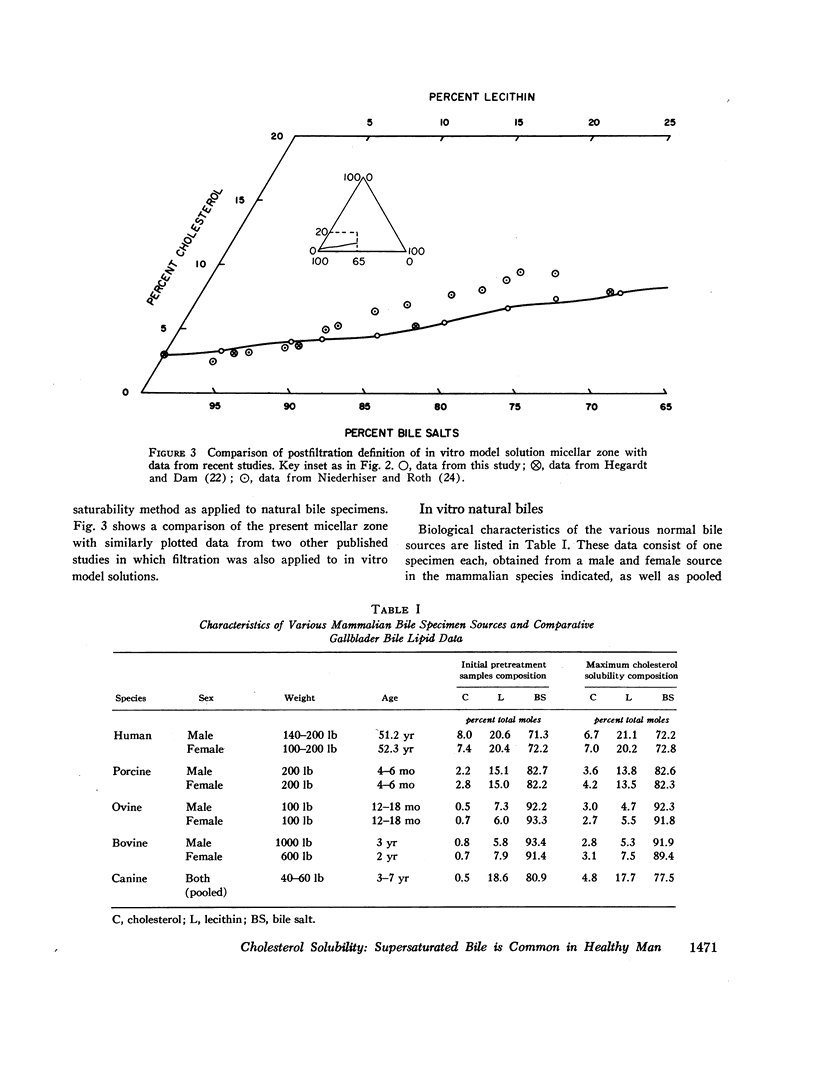
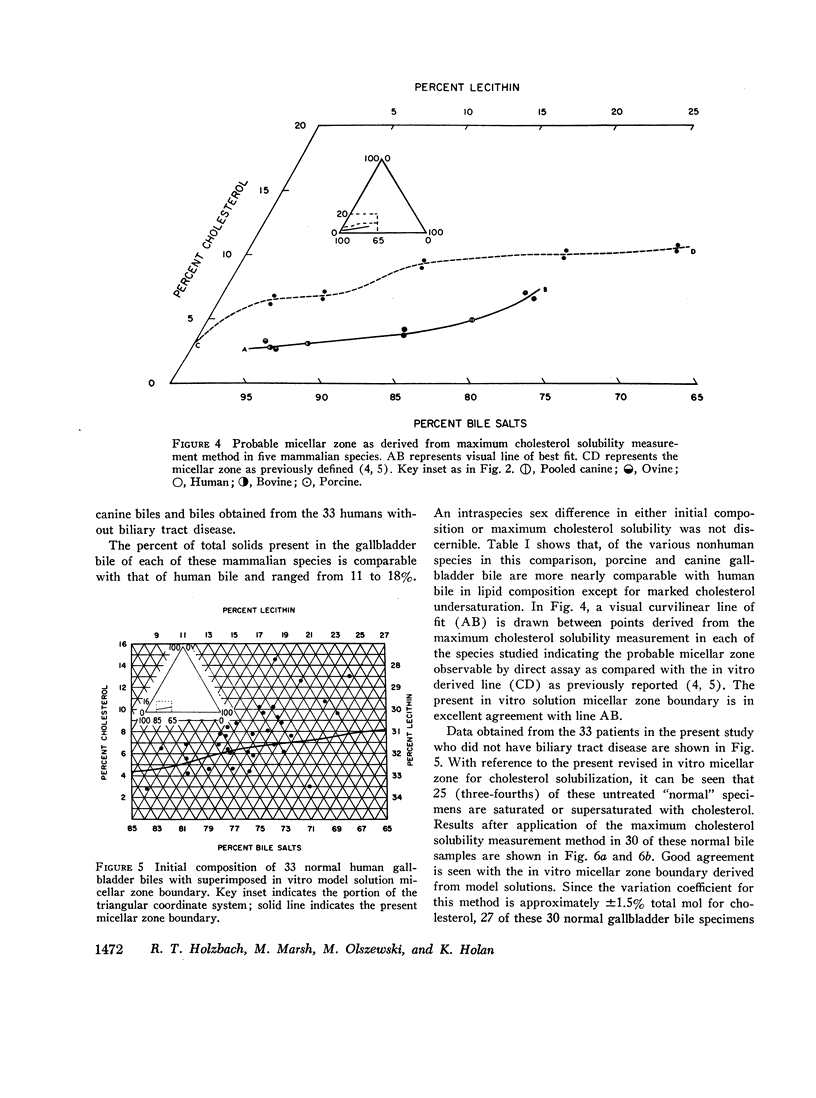
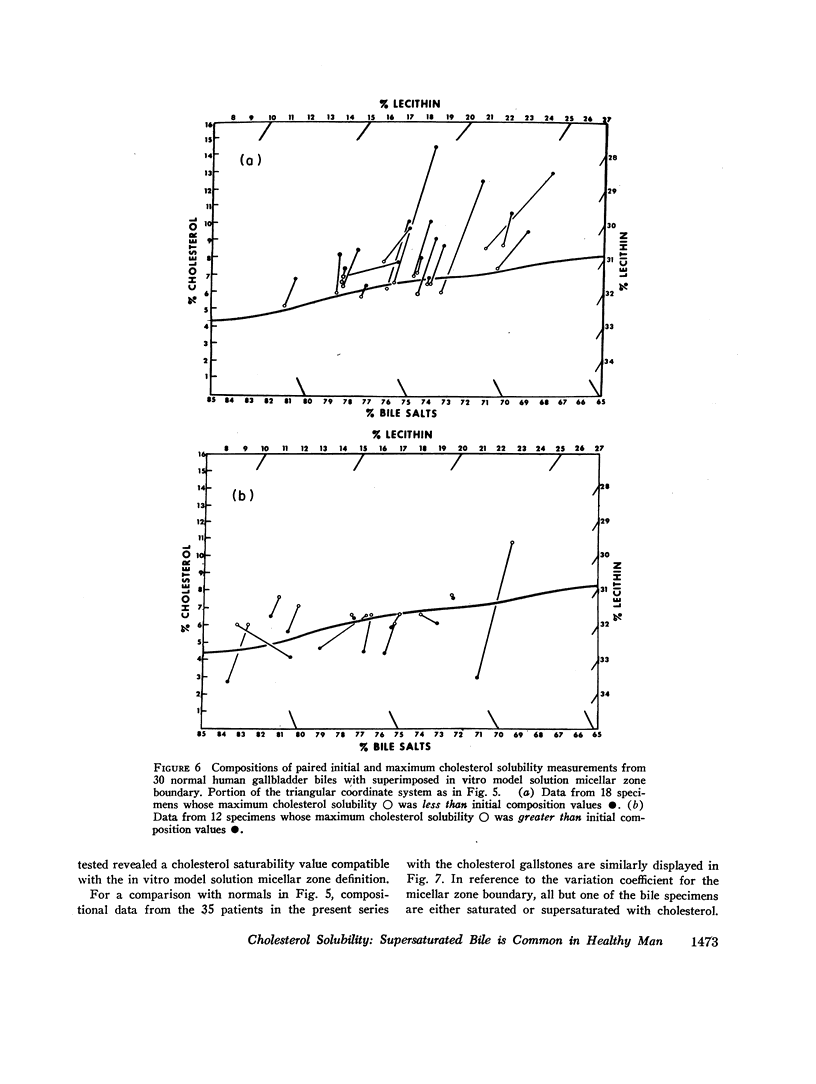
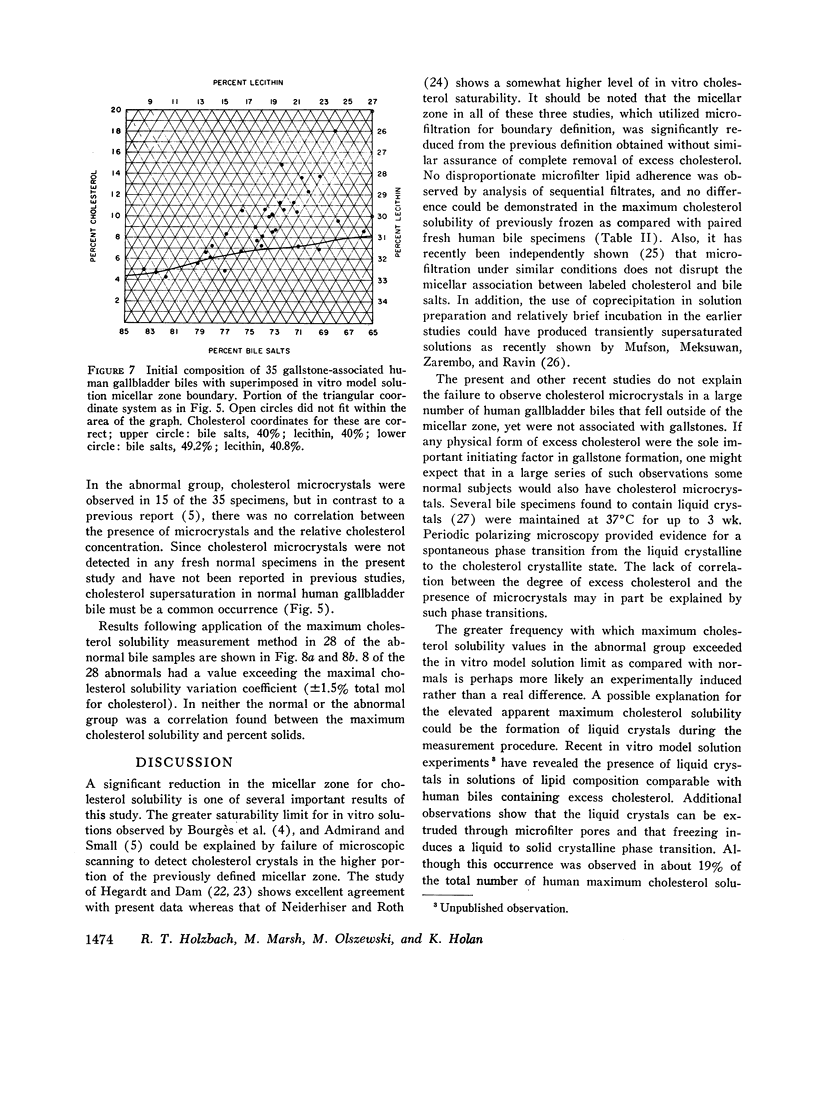
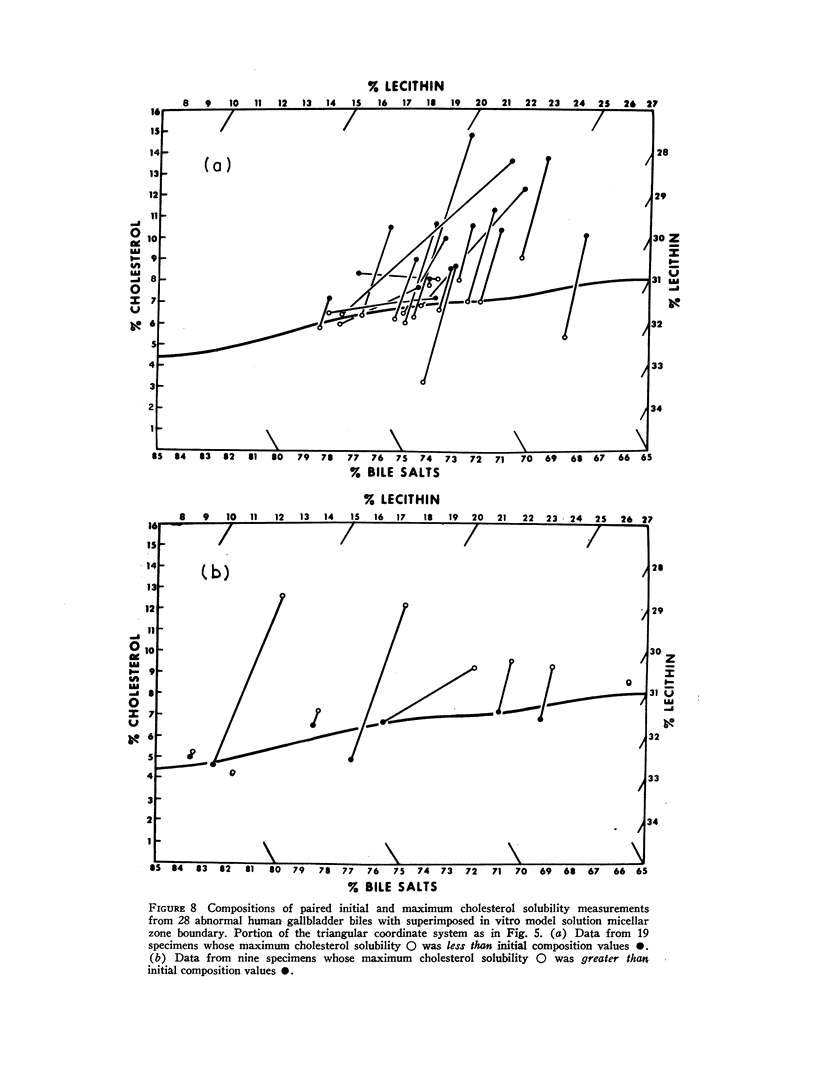
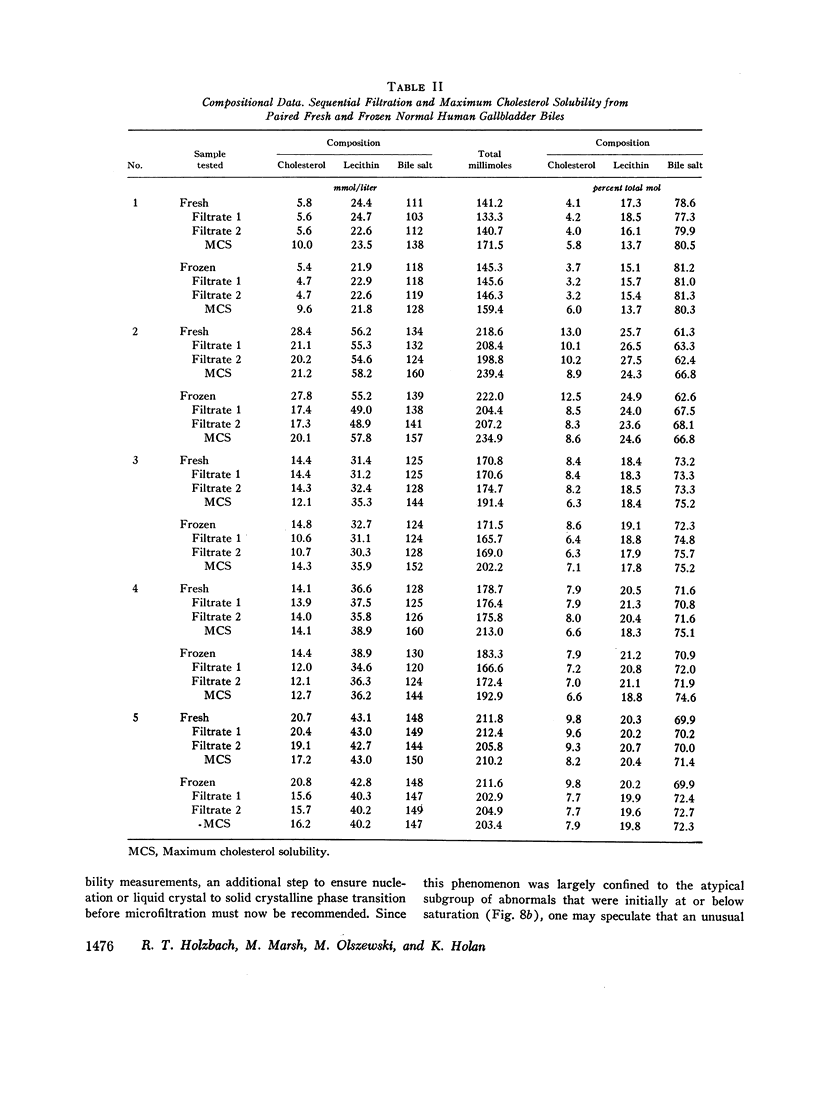
The development and validation of a direct method for measuring maximum cholesterol solubility in bile is described. Application of this method to five large mammalian species, including man, produced a micellar zone significantly smaller than that previously reported. Further studies on in vitro model solutions patterned after bile confirmed this new micellar zone. Thus, direct evidence demonstrates that the micellar zone boundary derived in vitro from model solutions is applicable to human gallbladder bile. Using the present criteria, normal human bile, in contrast to bile from other mammalian species, is commonly supersaturated with cholesterol. A male-female difference in bile composition is not demonstrable despite the well-established female preponderance of cholelithiasis. Bile from patients with cholesterol cholelithiasis has a micellar zone similar to normals but differs compositionally in that there is a greater excess of cholesterol above saturation. We conclude that cholesterol supersaturation may be a necessary but not solely sufficient cause for gallstone formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
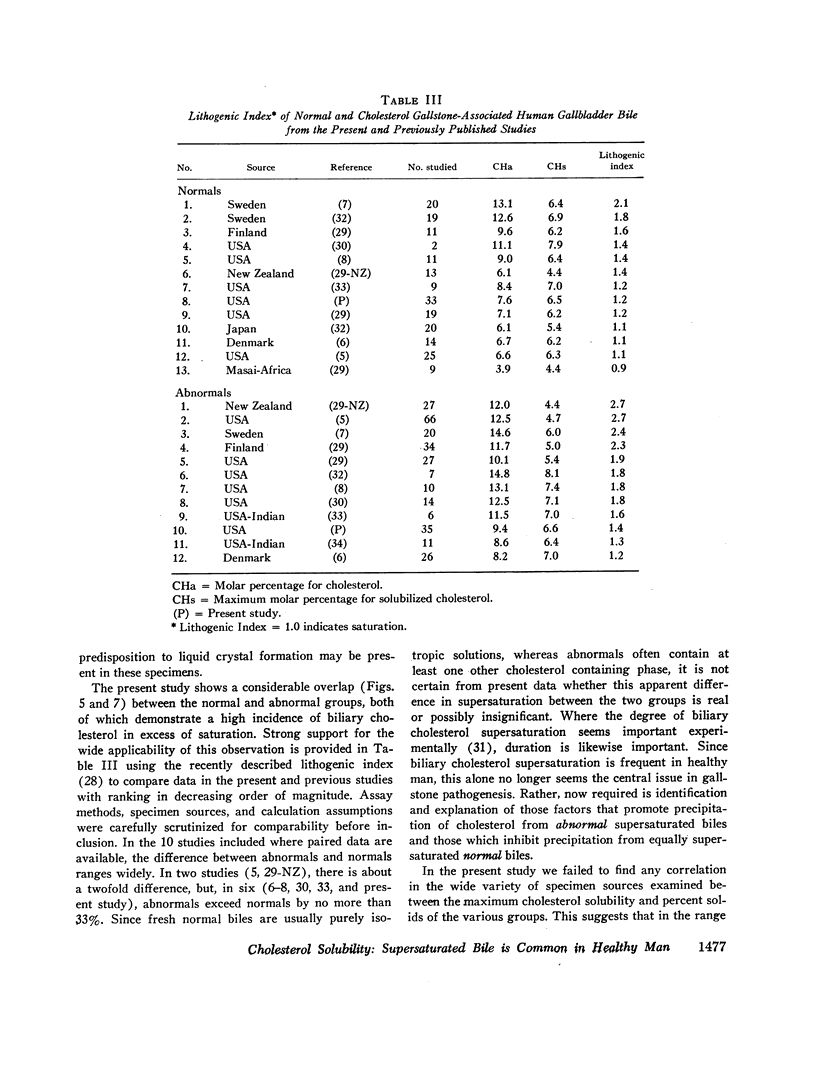
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bourgès M., Small D. M., Dervichian D. G. Biophysics of lipid associations. 3. The quaternary systems lecithin-bile salt-cholesterol-water. Biochim Biophys Acta. 1967 Oct 2;144(2):189–201. [PubMed] [Google Scholar]
- Dam H. Determinants of cholesterol cholelithiasis in man and animals. Am J Med. 1971 Nov;51(5):596–613. doi: 10.1016/0002-9343(71)90284-1. [DOI] [PubMed] [Google Scholar]
- Dam H., Kruse I., Kallehauge H. E., Hartkopp O. E., Jensen M. K. Studies on human bile. I. Composition of bladder bile from cholelithiasis patients and surgical patients with normal bile compared with data for bladder bile of hamsters on different diets. Scand J Clin Lab Invest. 1966;18(4):385–404. doi: 10.3109/00365516609113159. [DOI] [PubMed] [Google Scholar]
- Holzbach R. T., Marsh M. E., Hallberg M. C. The effect of pregnancy on lipid composition of guinea pig gallbladder bile. Gastroenterology. 1971 Feb;60(2):288–293. [PubMed] [Google Scholar]
- ISAKSSON B. On the lipid constituents of bile from human gallbladder containing cholesterol gallstones; a comparison with normal human bladder bile. Acta Soc Med Ups. 1954 Sep 30;59(5-6):277–295. [PubMed] [Google Scholar]
- JUNIPER K., Jr, BURSON E. N., Jr Biliary tract studies. II. The significance of biliary crystals. Gastroenterology. 1957 Feb;32(2):175-208; discussion, 208-11. [PubMed] [Google Scholar]
- Marsh M., Holzbach R. T. Chromatographic separation of pure phosphatidylcholine from crude egg lecithin. Clin Chim Acta. 1973 Jan 10;43(1):87–90. doi: 10.1016/0009-8981(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Metzger A. L., Heymsfield S., Grundy S. M. The lithogenic index--a numerical expression for the relative lithogenicity of bile. Gastroenterology. 1972 Mar;62(3):499–501. [PubMed] [Google Scholar]
- Mufson D., Meksuwan K., Zarembo J. E., Ravin L. J. Cholesterol solubility in lecithin-bile salt systems. Science. 1972 Aug 25;177(4050):701–702. doi: 10.1126/science.177.4050.701. [DOI] [PubMed] [Google Scholar]
- Nakayama F. Cholesterol-holding capacity of bile in relation to gallstone formation. Clin Chim Acta. 1966 Aug;14(2):171–176. doi: 10.1016/0009-8981(66)90083-0. [DOI] [PubMed] [Google Scholar]
- Nakayama F., Van der Linden W. Bile from gallbladder harbouring gallstone: can it indicate stone formation? Acta Chir Scand. 1970;136(7):605–610. [PubMed] [Google Scholar]
- Nakayama F., van der Linden W. Bile composition: Sweden versus Japan. Its possible significance in the difference in gallstone incidence. Am J Surg. 1971 Jul;122(1):8–12. doi: 10.1016/0002-9610(71)90337-0. [DOI] [PubMed] [Google Scholar]
- Neiderhiser D. H., Roth H. P. Cholesterol solubilization by solutions of bile salts and bile salts plus lecithin. Proc Soc Exp Biol Med. 1968 May;128(1):221–225. doi: 10.3181/00379727-128-32983. [DOI] [PubMed] [Google Scholar]
- Neiderhiser D. H., Roth H. P. Effect of phospholipase A on cholesterol solubilization by lecithin in a bile salt solution. Gastroenterology. 1970 Jan;58(1):26–31. [PubMed] [Google Scholar]
- Olszewski M. F., Holzbach R. T., Saupe A., Brown G. H. Liquid crystals in human bile. Nature. 1973 Mar 30;242(5396):336–337. doi: 10.1038/242336a0. [DOI] [PubMed] [Google Scholar]
- Osuga T., Portman O. W. Experimental formation of gallstones in the squirrel monkey. Proc Soc Exp Biol Med. 1971 Mar;136(3):722–726. doi: 10.3181/00379727-136-35350. [DOI] [PubMed] [Google Scholar]
- Porter H. P., Saunders D. R. Isolation of the aqueous phase of human intestinal contents during the digestion of a fatty meal. Gastroenterology. 1971 Jun;60(6):997–1007. [PubMed] [Google Scholar]
- Russell I. S., Wheeler M. B., Freake R. The composition of human gall-stones. Br J Surg. 1968 Mar;55(3):161–168. doi: 10.1002/bjs.1800550302. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Schuster C. R., Jr Psychological approaches to opiate dependence and self-administration by laboratory animals. Fed Proc. 1970 Jan-Feb;29(1):2–5. [PubMed] [Google Scholar]
- Small D. M., Bourgès M. C., Dervichian D. G. The biophysics of lipidic associations. I. The ternary systems: lecithin-bile salt-water. Biochim Biophys Acta. 1966 Dec 7;125(3):563–580. [PubMed] [Google Scholar]
- Small D. M., Rapo S. Source of abnormal bile in patients with cholesterol gallstones. N Engl J Med. 1970 Jul 9;283(2):53–57. doi: 10.1056/NEJM197007092830201. [DOI] [PubMed] [Google Scholar]
- TERA H. Sedimentation of bile constituents. Ann Surg. 1963 Mar;157:468–472. doi: 10.1097/00000658-196303000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnberg L. A., Anthony-Mote A. The quantitative determination of bile salts in bile using thin-layer chromatography and 3 alpha-hydroxysteroid dehydrogenase. Clin Chim Acta. 1969 May;24(2):253–259. doi: 10.1016/0009-8981(69)90321-0. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C. C., Jr, Juttijudata P., Swell L. Bile-rich duodenal fluid as an indicator of biliary lipid composition and its applicability to detection of lithogenic bile. Am J Dig Dis. 1971 Sep;16(9):797–802. doi: 10.1007/BF02239307. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C., Jr, Swell L. Significance of the liver in the production of lithogenic bile in man. Gastroenterology. 1970 Jul;59(1):62–69. [PubMed] [Google Scholar]


