Abstract
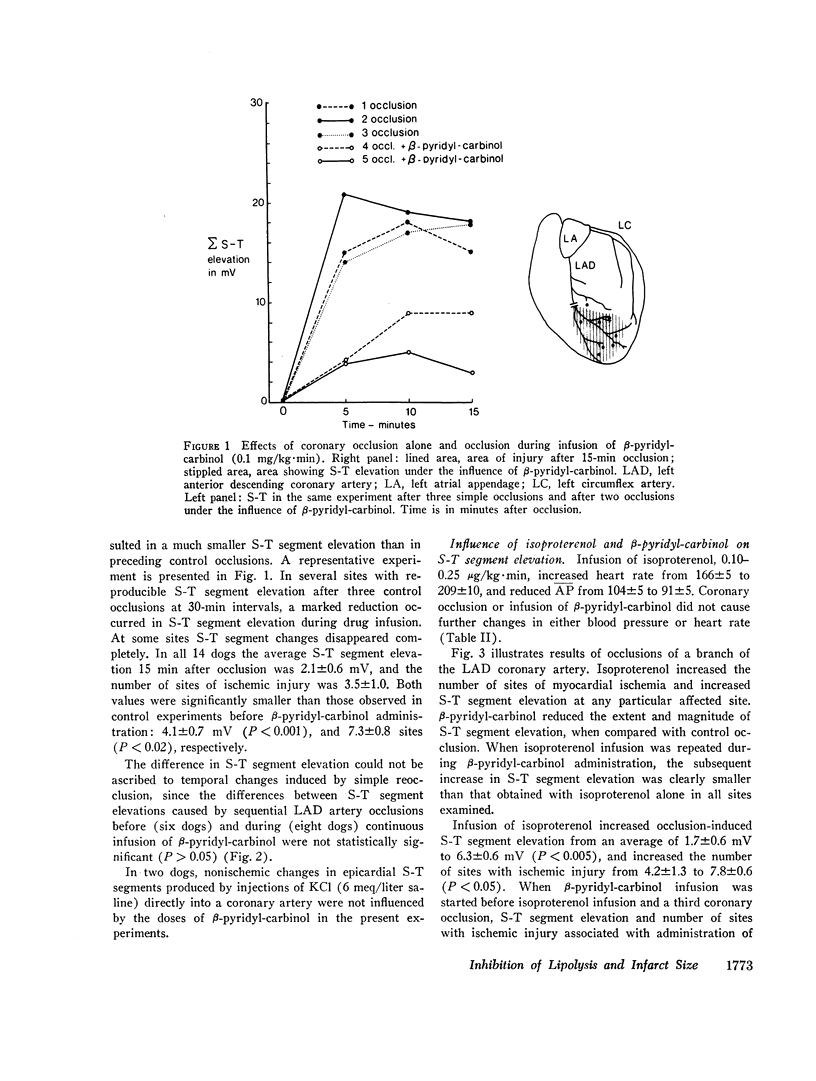
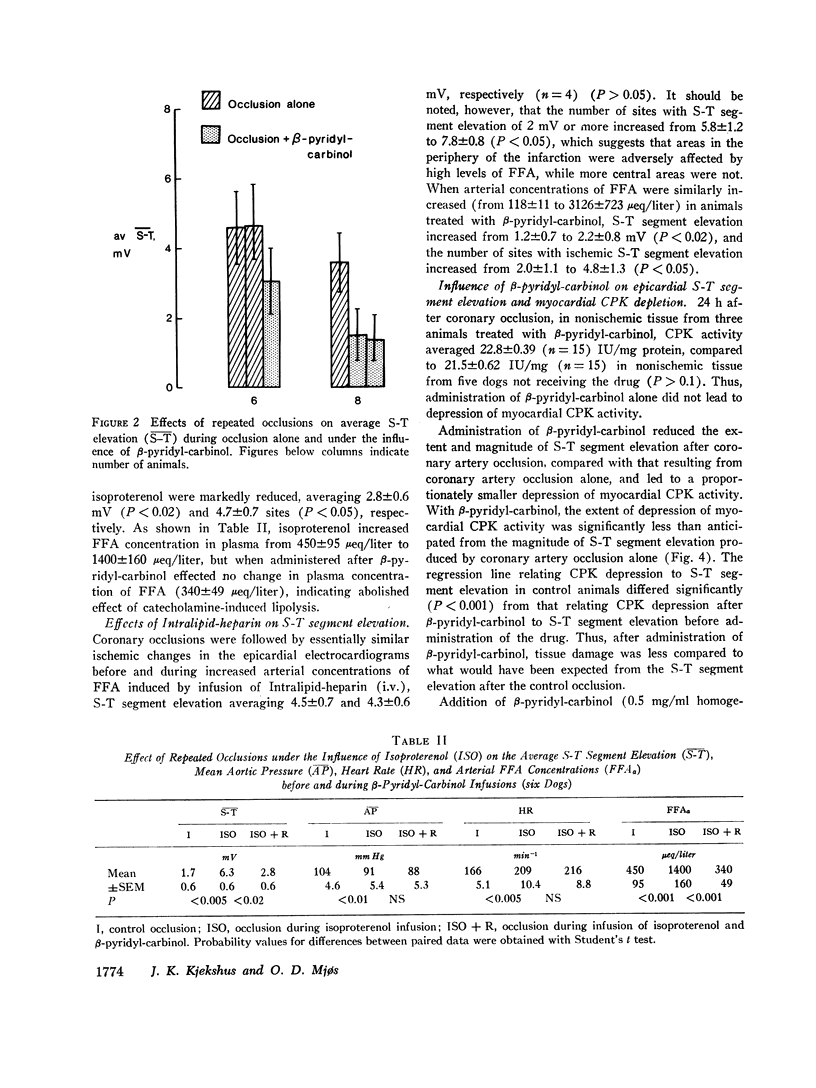
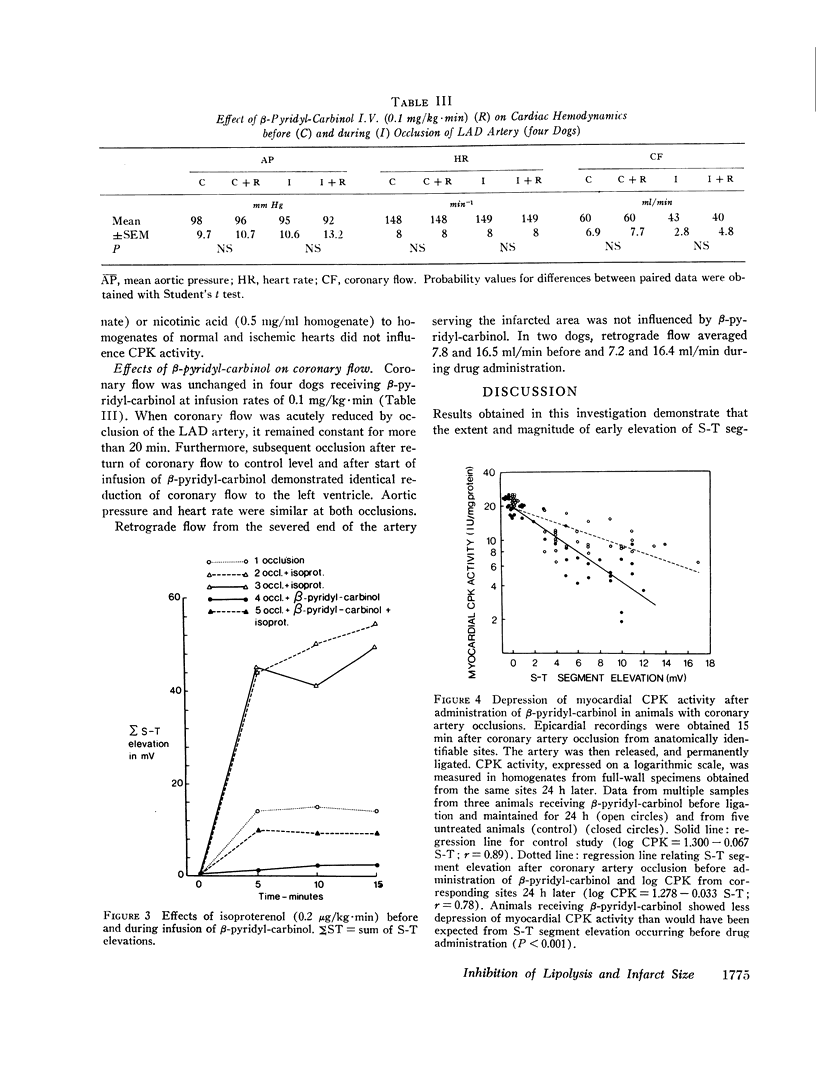
Recent studies have demonstrated a depressant effect of increased delivery of FFA to the hypoxic heart. Because catecholamines are released in acute myocardial infarction, it is likely that lipolytic activity is increased. The purpose of this study was to determine whether inhibition of hormone-sensitive lipases influence the extent and severity of myocardial ischemic injury produced by coronary occlusion. Myocardial infarction was produced by occlusion of the left anterior descending coronary artery in open-chest dogs. 15 min later a surface map of S-T segments was obtained with the use of 10-14 epicardial leads in the distribution area of the occluded artery. Average S-T segment elevation of all sites was used as an index of myocardial ischemic injury. Before coronary occlusion, the average S-T segment elevation was 0.3±0.2, which increased to 4.1±0.7 mV (SEM, 12 dogs) after occlusion. Inhibition of lipolytic activity by β-pyridyl-carbinol before repeated coronary occlusion reduced the occlusion-induced S-T segment elevation to 2.1±0.6 mV (P < 0.001). When arterial concentrations of FFA were raised by i.v. infusion of a triglyceride emulsion and heparin, average S-T segment elevation after coronary occlusion increased from 1.2±0.7 to 2.2±0.8 mV (P < 0.05) in animals treated with β-pyridyl-carbinol, which suggests an unfavorable effect of circulating FFA in this setting. Isoproterenol given before a repeated occlusion increased the severity and extent of the ischemic injury. The effect of isoproterenol on the occlusion-induced S-T segment elevation was reduced, however, when the lipolytic effect of the drug was inhibited by β-pyridyl-carbinol.
Our study suggests that β-pyridyl-carbinol during acute coronary artery occlusion may be of importance in reducing the extent and severity of myocardial ischemic injury.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS J. R. IMPORTANCE OF FATTY ACID IN MYOCARDIAL METABOLISM. Circ Res. 1964 Nov;15:SUPPL 2–3108. [PubMed] [Google Scholar]
- Graham T. P., Jr, Ross J., Jr, Covell J. W., Sonnenblick E. H., Clancy R. L. Myocardial oxygen consumption in acute experimental cardiac depression. Circ Res. 1967 Aug;21(2):123–138. doi: 10.1161/01.res.21.2.123. [DOI] [PubMed] [Google Scholar]
- Henderson A. H., Most A. S., Parmley W. W., Gorlin R., Sonnenblick E. H. Depression of myocardial contractility in rats by free fatty acids during hypoxia. Circ Res. 1970 Apr;26(4):439–449. doi: 10.1161/01.res.26.4.439. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Herdson P. B., Sommers H. M. Structural and functional abnormalities in mitochondria isolated from ischemic dog myocardium. Lab Invest. 1969 Jun;20(6):548–557. [PubMed] [Google Scholar]
- Jennings R. B., Sommers H. M., Herdson P. B., Kaltenbach J. P. Ischemic injury of myocardium. Ann N Y Acad Sci. 1969 Jan 31;156(1):61–78. doi: 10.1111/j.1749-6632.1969.tb16718.x. [DOI] [PubMed] [Google Scholar]
- Kjekshus J. K., Maroko P. R., Sobel B. E. Distribution of myocardial injury and its relation to epicardial ST-segment changes after coronary artery occlusion in the dog. Cardiovasc Res. 1972 Sep;6(5):490–499. doi: 10.1093/cvr/6.5.490. [DOI] [PubMed] [Google Scholar]
- Kjekshus J. K., Mjos O. D. Effect of free fatty acids on myocardial function and metabolism in the ischemic dog heart. J Clin Invest. 1972 Jul;51(7):1767–1776. doi: 10.1172/JCI106978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjekshus J. K., Sobel B. E. Depressed myocardial creatine phosphokinase activity following experimental myocardial infarction in rabbit. Circ Res. 1970 Sep;27(3):403–414. doi: 10.1161/01.res.27.3.403. [DOI] [PubMed] [Google Scholar]
- Kurien V. A., Yates P. A., Oliver M. F. The role of free fatty acids in the production of ventricular arrhythmias after acute coronary artery occlusion. Eur J Clin Invest. 1971 Jan;1(4):225–241. doi: 10.1111/eci.1971.1.4.225. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Kjekshus J. K., Sobel B. E., Watanabe T., Covell J. W., Ross J., Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971 Jan;43(1):67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- Opie L. H., Norris R. M., Thomas M., Holland A. J., Owen P., van Noorden S. Failure of high concentrations of circulating free fatty acids to provoke arrhythmias in experimental myocardial infarction. Lancet. 1971 Apr 24;1(7704):818–822. doi: 10.1016/s0140-6736(71)91494-2. [DOI] [PubMed] [Google Scholar]
- Pasyk S., Bloor C. M., Khouri E. M., Gregg D. E. Systemic and coronary effects of coronary artery occlusion in the unanesthetized dog. Am J Physiol. 1971 Mar;220(3):646–654. doi: 10.1152/ajplegacy.1971.220.3.646. [DOI] [PubMed] [Google Scholar]
- Prinzmetal M., Ishikawa K., Nakashima M., Oishi H., Ozkan E., Wakayama J., Baines J. M. Correlation between intracellular and surface electrograms in acute myocardial ischemia. J Electrocardiol. 1968;1(2):161–166. doi: 10.1016/s0022-0736(68)80023-8. [DOI] [PubMed] [Google Scholar]
- RAAB W. THE NONVASCULAR METABOLIC MYOCARDIAL VULNERABILITY FACTOR IN "CORONARY HEART DISEASE". FUNDAMENTALS OF PATHOGENESIS, TREATMENT, AND PREVENTION. Am Heart J. 1963 Nov;66:685–706. doi: 10.1016/0002-8703(63)90327-2. [DOI] [PubMed] [Google Scholar]
- Raaflaub J. Zur Umwandlung von beta-Pyridylcarbinol in Nicotinsäure im tierischen Organismus. Experientia. 1966 Apr 15;22(4):258–259. doi: 10.1007/BF01900948. [DOI] [PubMed] [Google Scholar]
- Rees J. R., Redding V. J. Anastomotic blood flow in experimental myocardial infarction. A new method, using 133-Xenon clearance, for repeated measurements during recovery. Cardiovasc Res. 1967 Apr;1(2):169–178. doi: 10.1093/cvr/1.2.169. [DOI] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- SAYEN J. J., SHELDON W. F., PEIRCE G., KUO P. T. Polarographic oxygen, the epicardial electrocardiogram and muscle contraction in experimental acute regional ischemia of the left ventricle. Circ Res. 1958 Nov;6(6):779–798. doi: 10.1161/01.res.6.6.779. [DOI] [PubMed] [Google Scholar]
- TROUT D. L., ESTES E. H., Jr, FRIEDBERG S. J. Titration of free fatty acids of plasma: a study of current methods and a new modification. J Lipid Res. 1960 Apr;1:199–202. [PubMed] [Google Scholar]


