Abstract
By transporting serotonin (5-HT) into neurons and other cells, serotonin transporter (SERT) modulates the action of 5-HT at cell surface receptors. SERT itself is modulated by several processes, including the cGMP signaling pathway. Activation of SERT by cGMP requires the cGMP-dependent protein kinase (PKG). Here we show that in HeLa cells lacking endogenous PKG, expression of PKGIα or PKGIβ was required for 8-bromoguanosine-3′,5′-cyclic monophosphate (8-Br-cGMP) to stimulate SERT phosphorylation and 5-HT influx. Catalytically inactive PKG mutants and wild-type PKGII did not support this stimulation. However, a mutant PKGII (G2A) that was not myristoylated substituted for functional PKGI, suggesting that myristoylation and subsequent membrane association blocked productive interaction with SERT. PKG also influenced SERT expression and localization. PKGI isoforms increased total and cell surface SERT levels, and PKGII decreased cell surface SERT without altering total expression. Remarkably, these changes did not require 8-Br-cGMP or functional kinase activity and were also observed with a SERT mutant resistant to activation by PKG. Both PKGIα and PKGIβ formed detergent-stable complexes with SERT, and this association did not require catalytic activity. The nonmyristoylated PKGII G2A mutant stimulated SERT expression similar to PKGI isoforms. These results suggest multiple mechanisms by which PKG can modulate SERT and demonstrate that the functional difference between PKG isoforms results from myristoylation of PKGII.
Keywords: Protein Kinase G (PKG), Protein Myristoylation, Protein-Protein Interactions, Signal Transduction, Serotonin Transporters, Isoform Selectivity
Introduction
Serotonin transporter (SERT),2 a member of the NSS or SLC6 family of sodium-coupled transporters, is a presynaptic plasma membrane protein responsible for reuptake of serotonin (5-HT) after its release by neurons. SERT is of particular interest because it is a major molecular target for antidepressant drugs and psychostimulants (1, 2). SERT is regulated by multiple signal transduction pathways involving cAMP, cGMP, calmodulin, p38 mitogen-activated protein kinase, and protein kinase C. Thus, understanding the signaling systems and molecular mechanisms underlying SERT regulation has been a major research focus (3–10).
A rare SERT mutation was found associated with multiple psychiatric disorders in several unrelated families (11, 12). Subsequent investigation suggested that the mutation I425V disrupted modulation of SERT activity by the cGMP signaling pathway (13, 14). SERT activation by cGMP was originally observed in RBL cells, where activation of adenosine A3 receptors led to nitric oxide production and an increase in endogenous 5-HT transport activity. This increase was sensitive to inhibitors of cGMP-dependent protein kinase (PKG) (7). Stimulation of nitric-oxide synthase by A3 activation increased the activity of NO-stimulated soluble guanylyl cyclase, leading to activation of PKG. Subsequent studies in cells transfected with SERT showed that generation of NO or addition of 8-bromoguanosine-3′,5′-cyclic monophosphate (8-Br-cGMP) led to increased SERT phosphorylation and increased activity (9, 13–18). Moreover, cGMP-dependent SERT phosphorylation and activation were blocked by mutation of Thr-276 near the cytoplasmic end of the fifth transmembrane helix (14, 15), not but by other serine or threonine mutations (15). These results are consistent with phosphorylation of SERT on Thr-276 by PKG. Furthermore, inhibitors of NO-stimulated soluble guanylyl cyclase or PKG prevented phosphorylation and stimulation (14, 15).
Several studies have addressed the mechanism by which PKG activates SERT activity. A consistent increase in Vmax was reported in response to the I425V mutation or PKG activation that was not accompanied by increased surface expression in several cell expression systems or synaptosomes (13, 15). In contrast, Zhu et al. found an increase in SERT activity and ligand binding in response to activation of A3 receptors or addition of 8-Br-cGMP that was potentiated by sildenafil, an inhibitor of cGMP phosphodiesterase (9, 16). The association of these effects with an increase in cell surface SERT labeling led the authors to conclude that PKG altered SERT subcellular localization. Furthermore, sensitivity to an inhibitor of p38 MAPK suggested the involvement of this kinase in SERT activation through an increase in catalytic activity (8, 9, 17). Although uncertainty remains concerning how PKG stimulates SERT activity, there is general agreement that such activation occurs and that it involves SERT phosphorylation, probably at Thr-276.
The I425V SERT mutant, which had higher activity than wild type, was not stimulated by activation of the cGMP signaling pathway (13, 14). The increased activity was attributed to a decreased rate of SERT dephosphorylation in the mutant, leading to significant levels of SERT phosphorylation and activation even at basal levels of cGMP (14). Consequently, inhibition of NO-stimulated soluble guanylyl cyclase or PKG in cells expressing SERT I425V (but not wild type) led to a decline in 5-HT transport as the mutant transporter was slowly dephosphorylated (14). Slower dephosphorylation of I425V was not due to a decrease in association of protein phosphatase 2A. This enzyme was found to associate to a greater degree with SERT activated by 8-Br-cGMP or by the I425V mutation (14).
PKG is a serine/threonine-specific protein kinase found in a variety of eukaryotes. Two PKG genes encode PKGI and PKGII. PKGI is encoded by two alternatively spliced mRNAs that produce the isoforms PKGIα and PKGIβ, which differ in their N terminus. PKG Iα and Iβ are soluble proteins, whereas PKGII, encoded by a separate gene, is anchored at the plasma membrane by myristoylation of Gly-2 after removal of the N-terminal methionine residue (19). Myristoylation and consequent membrane targeting of PKGII are required for its ability to phosphorylate several proteins including the cystic fibrosis transmembrane conductance regulator Cl− channel (20). Structurally, PKG is a homodimer consisting of a typical protein kinase domain at the C terminus, a regulatory domain that binds cGMP, and an N-terminal heptad leucine/isoleucine repeat responsible for dimerization and interaction with substrates (21–23). Both PKGI and PKGII are predominantly present in most brain regions and play critical roles in the regulation of brain function (24–26).
Steiner et al. recently investigated the interaction between PKG isoforms and SERT (27). Their work provided evidence for co-localization of PKGI (but not PKGII) with SERT in cell bodies and processes of RN46A cultured neurons. They also showed that inhibiting PKGI expression or function blocked the ability of 8-Br-cGMP to stimulate SERT activity and that PKGIα and SERT form a stable complex when transiently expressed in HEK-293 cells (27). Here we establish that the molecular basis of PKG specificity is the myristoylation of PKGII but not PKGI isoforms. Furthermore, we extend the previous findings to demonstrate that PKG has two isoform-specific effects on SERT: 1) an increase in 5-HT influx that requires a catalytically active kinase and a phosphate acceptor residue on SERT and 2) an effect of PKG on SERT expression that is independent of phosphorylation.
EXPERIMENTAL PROCEDURES
Materials
Recombinant VTF7-3 vaccinia virus encoding T7 RNA polymerase was prepared as described previously (28). 8-Br-cGMP, 5-HT, monoclonal M2 anti-FLAG antibody, anti-FLAG M2 affinity gel, and 3 × FLAG peptide were purchased from Sigma. Rp-8-pCPT-cGMPS was from Alexis. [3H]5-HT (27.1 Ci/mmol) and carrier-free 32Pi (8500–9120 Ci/mmol) were purchased from PerkinElmer Life Sciences. The expression plasmids for human vasodilator-stimulated phosphoprotein (VASP), PKGIα, PKGIα K390A, PKGIβ, PKGIβ K405A, PKGII, PKGII K482A, and mouse 16C2 monoclonal antibody against phospho-VASP were generous gifts from Dr. S. Lohmann (Institute for Clinical Biochemistry and Pathobiochemistry, University of Würzburg, Germany). Goat anti-PKGIα/β (C-17) and anti-PKGII (N-19) antibodies were from Santa Cruz Biotechnology. Mutants were generated using the QuikChange site-directed mutagenesis system (Stratagene). The mutated region was excised and subcloned back into the parental construct and confirmed by DNA sequencing. For SERT immunoprecipitation, a C-terminal FLAG-tagged hSERT construct in pcDNA 3.1 was generated. Tagged SERT did not differ in transport rate or kinetics from wild-type SERT.
Expression
HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified 5% CO2 incubator. Cells were plated in 96-well or 6-well culture plates and allowed to grow overnight. The confluent cells were infected with VTF7-3 and transiently transfected, using Lipofectin, with plasmids bearing hSERT or PKG cDNAs or both under the control of T7 promoter. Transfected cells were incubated for 20–22 h at 37 °C with 5% CO2 and used for further analysis. Protein concentration was determined with the Micro BCA protein assay reagent kit (Pierce).
5-HT Transport Assay
Transfected HeLa cells were incubated at room temperature with or without 100 μm 8-Br-cGMP treatment for 20 min. 5-HT influx was initiated by the addition of 20 nm [3H]5-HT in phosphate-buffered saline (PBS; 137 mm NaCl, 2.7 mm KCl, 4.3 mm Na2HPO4, and 1.4 mm KH2PO4, pH 7.3) containing 0.1 mm CaCl2 and 1 mm MgCl2 (PBS/CM), and the incubation was continued for 10 min. The assay was terminated by washing with ice-cold PBS buffer. The cells were then solubilized, and the extent of [3H]5-HT accumulation was determined by scintillation counting. Saturation kinetic analyses for 5-HT Km and Vmax were performed over a concentration range of 0.02–5 μm 5-HT by adding unlabeled 5-HT to a constant amount of [3H]5-HT.
Fractionation of Cytosolic and Membrane Components of Cells Expressing PKGII or Its Mutants
HeLa cells expressing WT PKGII or its mutants (G2A or K482A) were collected after overnight transfection and resuspended in PBS buffer. The cells were then lysed by two cycles of freeze-thawing and sonication, and the resulting homogenates were fractionated by centrifugation at 15,000 × g for 20 min at 4 °C. Cytosolic and membrane fractions were collected and stored at −80 °C in 0.1-ml aliquots for further use.
Biotinylation
Surface expression of SERT was determined using the membrane-impermeant biotinylation reagent sulfo-NHS-SS-biotin (Pierce) as described previously (29). In brief, cell surface proteins were labeled at 4 °C with sulfo-NHS-SS-biotin and isolated from the cell extracts with immobilized streptavidin (Pierce). SERT was detected in the pool of surface proteins by SDS-PAGE and Western blotting using SERT-specific antibody (provided generously by Dr. S. Ramamoorthy, Medical University of South Carolina). A HRP-conjugated anti-rabbit IgG was used to visualize the signal by Super Signal West Femto (Pierce). For comparing total SERT expression, the 68 kDa band of core-glycosylated SERT was quantified as described below. For comparisons of surface SERT, the 95 kDa band of mature SERT was quantified.
32P Incorporation
Approximately 4 × 105 cells co-expressing FLAG-tagged SERT and the indicated PKG isoforms or their inactive mutants were incubated with 1.0 mCi/ml carrier-free 32Pi for 60 min prior to the addition of PKG activators or inhibitors. After incubation for another 30 min with modulators, the cells were washed and then lysed with 800 μl of lysis buffer (25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, and 1% Triton X-100), and SERT was captured by 50 μl of anti-FLAG M2 affinity gel (Sigma, 50% suspension in lysis buffer). The gel was washed five times with lysis buffer and then eluted with 50 μl of 150 ng/μl 3 × FLAG peptide (Sigma). The entire eluted sample was applied to SDS-PAGE, and radiolabeled SERT was detected by autoradiography.
SDS-PAGE, Co-immunoprecipitation, and Immunoblotting
Samples of total lysates for gel electrophoresis contained 20 μg of protein, corresponding to ∼104 cells. For co-immunoprecipitations, samples prepared from ∼4 × 105 cells (800 μg) expressing FLAG-tagged SERT were lysed with lysis buffer, and the detergent extracts were incubated with 10 μg/ml anti-FLAG M2 antibody at 4 °C overnight, followed by incubating with 50 μl of protein G-Sepharose beads for an additional 1 h at 20 °C. The immunoadsorbents were washed five times with ice-cold lysis buffer before elution with 100 μl of SDS-PAGE sample buffer. Protein samples (50 μl) were separated by SDS-PAGE using a 4–15% linear gradient gel, transferred to a 0.2-μm nitrocellulose membrane (Bio-Rad), and probed with specific antibodies as indicated. Immunoreactive bands were visualized by chemiluminescence and quantified using a UVP Laboratory Imaging and Analysis System. This immunoprecipitation method typically leads to mild SERT aggregation and an anomalously low mobility on SDS-PAGE (compare Fig. 6 with Figs. 4 and 5).
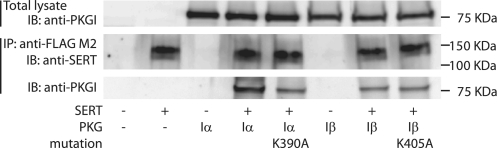
FIGURE 6.
Co-immunoprecipitation of PKGI with FLAG-tagged SERT. Cells expressing SERT with PKGIα and Iβ isoforms and mutants as indicated were solubilized, and a small portion of the lysates was analyzed for total expression of PKGI by Western blotting (IB) using anti-PKGI antibody (top panel). The remaining lysates were immunoprecipitated (IP) using an anti-FLAG M2 antibody as described under “Experimental Procedures,” and analyzed by Western blotting using SERT-specific antibody (middle panel) or anti-PKGI antibody (bottom panel). The blots shown are representatives of two experiments. In this co-immunoprecipitation procedure (different from the procedure used in Figs. 4 and 5), the principal SERT band migrates with an anomalously high apparent mass of 100–150 kDa.
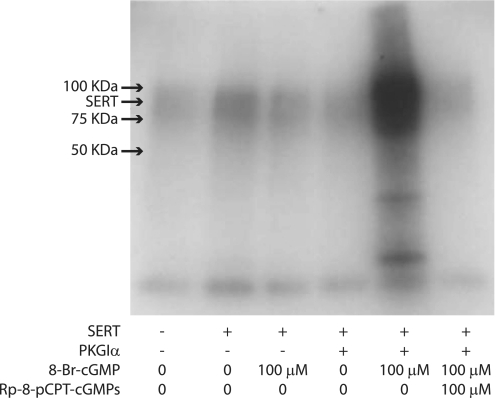
FIGURE 4.
PKGIα catalyzed 32P incorporation into SERT. HeLa cells expressing C-terminal FLAG-tagged SERT alone or with wild-type PKGIα were incubated with 1.0 mCi/ml 32Pi for 60 min, and then 8-Br-cGMP was added at a concentration of 100 μm as indicated and incubated for an additional 30 min. The specific PKG inhibitor, Rp-8-pCPT-cGMPs, was added where indicated at a concentration of 100 μm and incubated for 30 min prior to 8-Br-cGMP treatment. The cells were then washed and solubilized, and SERT was captured by immunoprecipitation as described under “Experimental Procedures.” 32P incorporation into SERT was detected by autoradiography. The mobility of the principal SERT band in Figs. 4 and 5 corresponds to a molecular mass of about 95 kDa.
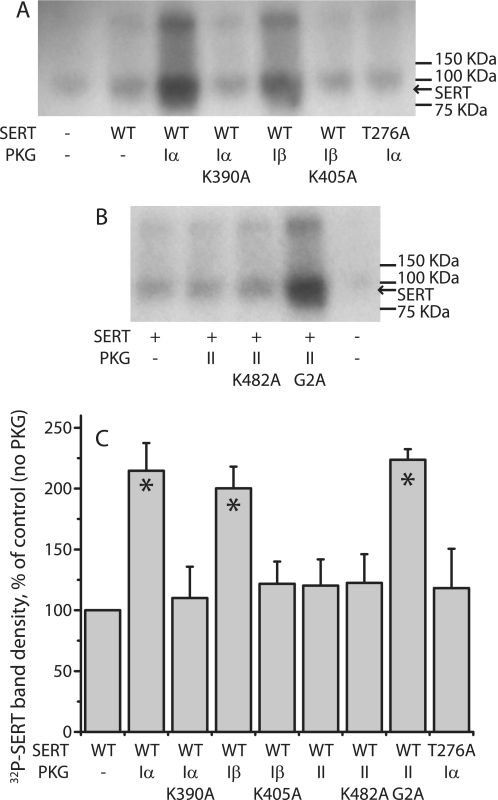
FIGURE 5.
PKG isoform specificity for SERT phosphorylation. A and B, HeLa cells were transfected with C-terminal FLAG-tagged SERT wild type or T276A together with PKGI (A) or PKGII (B) isoforms or catalytically inactive mutants. Cells were metabolically labeled with 32Pi for 60 min, 100 μm 8-Br-cGMP was added, and the cells were incubated for 30 min followed by immunoprecipitation as described in the Fig. 4 legend. The autoradiograms shown are representative of three experiments. C, quantitation of phospho-SERT band densities is shown. Relative intensities of 32P-labeled SERT bands were averages from three phosphorylation experiments. Values are expressed as mean ± S.E. (error bars). Asterisks indicate significant differences compared with SERT alone (p < 0.05).
Data Analysis
Nonlinear regression fits of experimental and calculated data were performed with Origin (OriginLab, Northampton, MA), which uses the Marquardt-Levenberg nonlinear least-squares curve-fitting algorithm. The statistical analysis given was from multiple experiments. Data with error bars represent the mean ± S.E. for multiple experiments. Statistical analyses comparing control and experimental conditions were performed using Student's paired t tests.
RESULTS
Functional Expression of Individual PKG Isoforms
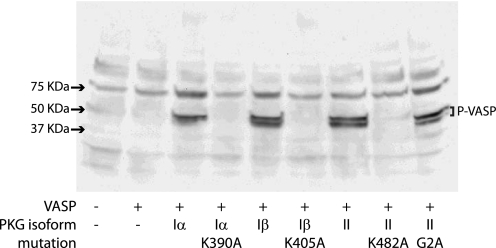
To investigate the action of individual PKG isoforms on SERT, we took advantage of the observation that cells in culture tend to lose expression of endogenous PKGs within a few cell divisions (30). Using RT-PCR and functional assays of PKG activity, we selected HeLa cells deficient in the expression of all PKG isoforms (data not shown) and then transfected cells with cDNAs encoding individual PKG isoforms and mutants. To confirm functional expression, we co-transfected each PKG cDNA together with cDNA encoding VASP, a substrate for all PKG isoforms. Cells co-expressing PKG and VASP were treated with 8-Br-cGMP, and PKG-dependent VASP phosphorylation was detected by immunoblotting with a monoclonal antibody (16C2) specific for phosphorylated VASP (31). 16C2 immunoreactivity indicates VASP phosphorylation at serines 157 and 239, two PKG-preferred phosphorylation sites in VASP (32). Immunoblotting analysis showed that all wild-type PKGs and a nonmyristoylated PKGII mutant, G2A, phosphorylated VASP (Fig. 1). In contrast, no phosphorylation was detected when VASP was co-transfected with catalytically inactive PKG mutants PKGIα K390A, PKGIβ K405A, and PKGII K482A (33). VASP phosphorylation was not observed in nontransfected cells or cells expressing VASP alone. Furthermore, PKG- and 8-Br-cGMP-dependent VASP phosphorylation in these cells was inhibited by the PKG specific inhibitor, Rp-8-pCPT-cGMPS (data not shown).
FIGURE 1.
VASP phosphorylation by functional PKG isoforms. PKG-deficient HeLa cells were transfected with cDNAs encoding VASP together with PKGIα, PKGIβ, PKGII, and various kinase mutants as indicated. The leftmost lane indicates nontransfected cells. After a 22-h incubation, the cells were incubated with 100 μm 8-Br-cGMP at 37 °C for 30 min prior to solubilization. Solubilized proteins were subjected to SDS-PAGE and Western blot analysis using an anti-phospho-VASP antibody. The blot shown is a representative of three experiments.
PKG Expression Was Required for 8-Br-cGMP Stimulation of 5-HT Transport
Fig. 2 shows that 8-Br-cGMP stimulated the transport rate (Vmax) for SERT, and this stimulation required co-expression of functional PKG. 8-Br-cGMP did not stimulate SERT activity in cells co-expressing inactive PKG mutants PKGIα K390A, PKGIβ K405A, or PKGII K482A, consistent with a requirement for phosphorylation in SERT activation. In contrast, 8-Br-cGMP did not activate SERT co-expressed with PKGII. However, the G2A mutant, which is not myristoylated, stimulated SERT in the presence of 8-Br-cGMP, suggesting that myristoylation blocked the ability of PKGII to activate SERT, possibly by anchoring PKGII to the membrane. No marked change in the Km for 5-HT was observed in these experiments (data not shown).
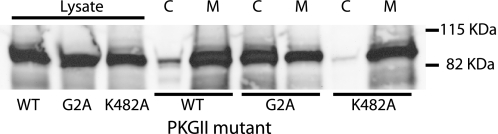
Myristoylation of PKGII occurs through modification of Gly-2 by the covalent attachment of myristate following removal of the initiator methionine residue by cellular methionyl aminopeptidases, a modification that promotes association of the enzyme with cell membranes (19). Mutation of Gly-2 to alanine blocks this modification and renders the mutant largely soluble. Fig. 3 shows Western blotting results demonstrating the effect of mutating Gly-2 and Lys-482 to alanine on the distribution of PKGII between cytosolic and membrane fractions after sedimentation of membranes from cell homogenates. Total expression levels of PKGII mutants, K482A and G2A, were comparable with that of wild type (leftmost three lanes). Wild type PKGII (WT) and K482A were highly enriched in the precipitate (M), with negligible amounts in the supernatant fraction (C). However, distribution of the G2A mutant was dramatically different, with the majority of enzyme found in the cytosolic supernatant fraction (C), although a significant amount of G2A was found associated with the membranes (M), possibly due to specific association with SERT and other membrane proteins as observed by Steiner et al. for PKGI isoforms (27).
FIGURE 3.
Membrane association of PKGII and its mutants. HeLa cells expressing wild-type PKGII and mutants G2A and K482A were fractionated into cytosolic and membrane fractions as described under “Experimental Procedures,” solubilized, and separated by SDS-PAGE. PKGII was detected by immunoblotting with a polyclonal antibody. The leftmost three lanes were homogenates from cells expressing wild-type PKGII and G2A and K482A mutants. The remaining lanes show the cytosolic (C) and membrane (M) fractions of wild-type (WT), G2A, and K482A. Each sample contained 20 μg of protein. The distribution of protein between cytosolic and membrane fractions was 126–180 μg for wild type, 148–163 μg for G2A, and 134–188 μg for K482A.
32P Incorporation into SERT by Co-expressed PKG
To determine which PKG isoforms phosphorylated SERT, we measured 32P incorporation into co-expressed SERT in cells metabolically labeled with 32PO43−. 32P-labeled, C-terminal FLAG-tagged SERT was measured by immunocapture with an anti-FLAG M2 affinity agarose gel and autoradiographic detection. In PKG-deficient cells, only basal phosphorylation of SERT was detected, and 8-Br-cGMP did not increase the phosphorylation level (Fig. 4). However, 32P incorporation was markedly increased by 8-Br-cGMP when SERT was co-expressed with PKGIα. Moreover, SERT phosphorylation by PKGIα was blocked by the PKG inhibitor Rp-8-pCPT-cGMPS. Of interest is that 32P incorporation occurred predominantly into the 95 kDa band representing mature glycosylated SERT and not the 60–66-kDa region where unglycosylated and core glycosylated SERT migrates (see also Figs. 8 and 9).
FIGURE 8.
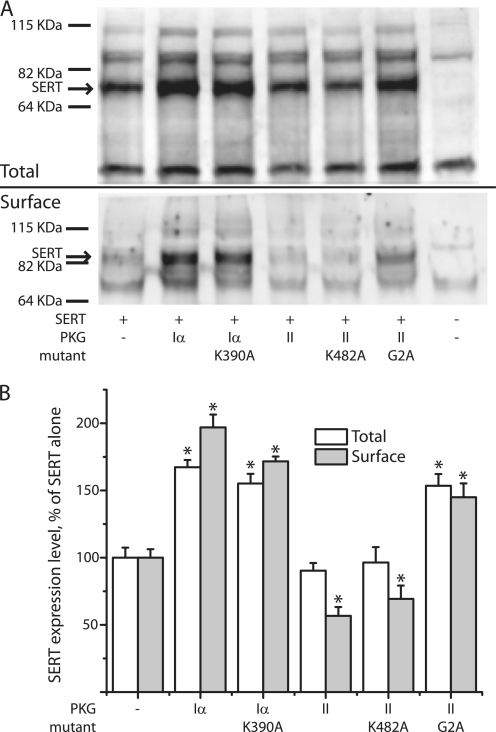
Effects of PKG isoforms on SERT expression. A, Western blots of SERT expression in cells co-expressing the indicated PKG isoforms and mutants are shown. Cells were transfected with equal amounts (1 μg/well) of SERT and PKG cDNAs. Total (upper panel) and cell surface (lower panel) SERT expression was detected by Western blotting with anti-SERT antibody. Total expression was measured in detergent extracts of whole cells. For measurement of surface expression, cells were treated with sulfo-NHS-SS-biotin to label cell surface proteins, solubilized, and the biotinylated fraction was extracted with streptavidin beads. The blots shown are representatives of three experiments. B, from the relative integrated density of SERT bands (the 68 kDa band for total SERT and the 95 kDa band for surface SERT), the expression levels of SERT were estimated as a percentage of SERT expression alone. Asterisks indicate values significantly different (p < 0.05) from that of control (SERT alone). Samples contained 20 μg total protein, corresponding to 104 cells. Samples of surface proteins contained all of the biotinylated protein recovered from an initial sample of 800 μg of protein, corresponding to 4 × 105 cells.
FIGURE 9.
SERT T276A expression was influenced by PKG isoforms. Total and surface expression of SERT T276A was measured as in Fig. 8. A, Western blots of total and surface expression of T276A co-expressed with PKGIα, the catalytically inactive mutant K390A and the nonmyristoylated PKGII mutant G2A. B, quantification of the Western blot results estimated as a percentage of SERT expression alone. Asterisks indicate values significantly different (p < 0.05) from that of control (T276A alone). C, cells co-expressing PKGIα with wild-type or T276A SERT incubated with or without 100 μm 8-Br-cGMP at 37 °C for 30 min. Transport was then measured as described under “Experimental Procedures” and was estimated as a percentage of SERT activity without 8-Br-cGMP treatment. Error bars indicate standard error of the means from three experiments. Asterisks indicate values significantly different (p < 0.05) from that of control (without 8-Br-cGMP treatment). Sample amounts were similar to those in Fig. 8.
Fig. 5 shows that PKGIα, PKGIβ, and the G2A mutant of PKGII all promoted 32P incorporation into SERT when stimulated with 8-Br-cGMP (Fig. 5A for PKGI, 5B for PKGII, and 5C for quantification). In contrast, co-expression of the inactive mutants, PKGIα K390A, PKGIβ K405A, and PKGII K482A, had little effect on 32P incorporation into SERT when stimulated with 8-Br-cGMP. Importantly, wild-type PKGII did not incorporate 32P into SERT in these experiments. These data are consistent with our observation of increased SERT transport activity in response to activation of functional cytosolic PKG isoforms (Fig. 2). PKGIα did not stimulate phosphorylation of the T276A mutant of SERT (Fig. 5, A and C), which is resistant to PKG activation (14), consistent with previous results (15).
FIGURE 2.
PKG requirement for 8-Br-cGMP stimulation of SERT Vmax. PKG-deficient HeLa cells transfected with SERT and individual PKG isoforms or their mutants as indicated were incubated for 30 min at 37 °C in the presence or absence of 100 μm 8-Br-cGMP. 5-HT influx was measured over a concentration range of 0.02–5 μm [3H]5-HT, and Km and Vmax values were determined. The results represent Vmax data from at least three experiments. Error bars indicate standard errors from at least three independent measurements. The asterisks indicate Vmax values significant increased by 8-Br-cGMP (p < 0.05, paired Student's t test).
Association of SERT with PKG1 Isoforms
Both PKGI isoforms, which responded to cGMP by phosphorylating and activating SERT, also associated with the transporter, as demonstrated by the immunoprecipitation results shown in Fig. 6. SERT was immunoprecipitated from lysates of cells co-expressing SERT and PKGIα or PKGIβ using an antibody against a FLAG tag attached to the SERT C terminus. PKGI expression in the lysate was confirmed with an anti-PKGI antibody (top panel), and SERT expression in the FLAG-immunoprecipitate was detected with an antibody against the SERT C terminus (middle panel). The bottom panel of Fig. 6 shows that not only the functional wild-type PKGI isoforms were detected in the SERT immunoprecipitates, as shown by Steiner et al. (27), but also the catalytically inactive mutants PKGIα K390A and PKGIβ K405A, demonstrating that the association of SERT with PKGI was not dependent on the ability of the enzyme to phosphorylate or activate SERT.
PKG Affected SERT Expression Levels
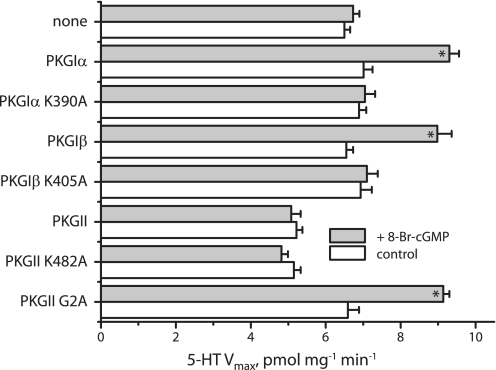
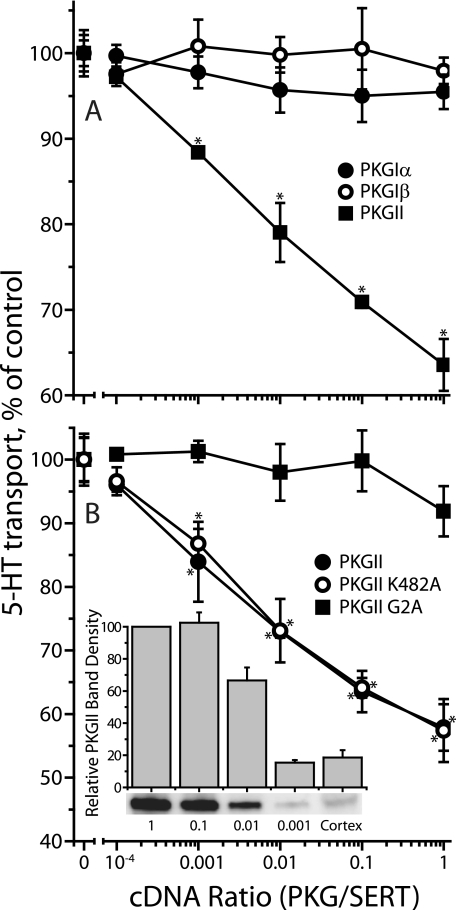
We noticed that co-expression of PKGII inhibited SERT activity despite its inability to support activation by 8-Br-cGMP (Fig. 2). Fig. 7A shows that, in the absence of 8-Br-cGMP, cells co-transfected with SERT and varying amounts of cDNA encoding PKGI isoforms transported 5-HT at rates similar to that of cells expressing SERT alone. However, PKGII co-expression markedly reduced 5-HT influx. This effect was not related to the kinase activity of PKGII, as it was also observed in the catalytically inactive K482A mutant (Fig. 7B). Inhibition was relieved by the G2A mutation (Fig. 7B), which prevents myristoylation of PKGII and renders it largely soluble (see Fig. 3). The amount of PKGII expression required for SERT inhibition was consistent with native expression in mouse frontal cortex. The inset to Fig. 7B shows that 103 dilution of PKGII cDNA led to an expression level less than or equal to the level in cortex. This degree of expression led to significant inhibition of SERT as shown in Fig. 7.
FIGURE 7.
Effect of PKG co-expression on SERT activity. HeLa cells were transfected with a constant amount of SERT cDNA (0.125 μg/well in 96-well plates) with a varying amount of cDNA (0–0.125 μg/well) encoding (A) PKGIα (filled circles), PKGIβ (open circles), or PKGII (filled squares) or (B) PKGII wild type (open circles), PKGII K482A (filled squares) or PKGII G2A (filled circles). After incubation for 22 h, SERT transport activity was assayed in the absence of 8-Br-cGMP by adding 20 nm [3H]5-HT and incubating for 10 min at room temperature as described under “Experimental Procedures.” The results represent data from three experiments with triplicate determinations. Error bars indicate S.E. of the means from the three determinations. Points marked with an asterisk were found to be statistically different (p < 0.05) from control (SERT alone). Inset, expression of PKGII in HeLa cells compared with native expression in mouse cerebral cortex. Samples (20 μg, corresponding to ∼104 cells) of the transfected cells used in B, along with the same amount of cell homogenate from mouse cerebral cortex, were separated by SDS-PAGE, and stained for PKGII immunoreactivity. The bands below the columns are from a typical measurement, and the columns and error bars represent means ± S.E. from quantification of three experiments.
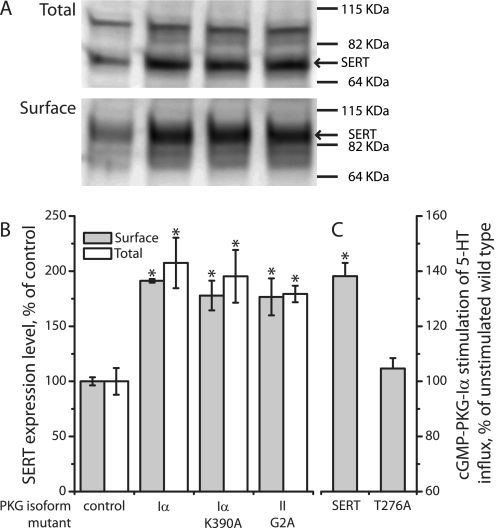
Further investigation revealed that PKGI isoforms also influenced SERT expression. As shown in Fig. 8, co-expression with PKGIα increased total and surface expression of SERT although it did not increase activity in the absence of 8-Br-cGMP (Fig. 7A). The decrease in SERT activity observed with membrane-associated PKGII expression (Fig. 7) was apparently due to a decrease in SERT surface expression with little effect on total expression (Fig. 8). These changes did not require functional kinase activity as they were also observed with the inactive mutants PKGIα K390A and PKGII K482A. Consistent with its ability to phosphorylate and activate SERT, the nonmyristoylated G2A mutant of PKGII increased SERT expression like the soluble PKGIα. PKGIβ effects on SERT expression were similar to those of PKGIα (data not shown).
Effect of PKG Co-expression on the SERT T276A Mutant
Previously, we showed that activation of PKG did not stimulate transport activity and phosphorylation of SERT mutant T276A (14, 15). To examine the effect of PKG isoforms on expression of the mutant, we co-expressed SERT T276A with PKGIα, catalytically inactive PKGIα K390A, and PKGII G2A. As reported previously for endogenous PKG in CHO-1 cells (15), 32P incorporation into T276A was not stimulated by 8-Br-cGMP when co-expressed with PKGIα (Fig. 5), and transport was not increased (Fig. 9C). However, expression analysis indicated that co-expression of PKGIα, PKGIα K390A, and PKGII G2A all increased both total and cell surface expression levels of T276A (Fig. 9, A and B).
DISCUSSION
The results presented here establish the basis of isoform selectivity in the PKG-dependent phosphorylation and activation of SERT. Furthermore, the data demonstrate that interaction between SERT and isoforms of PKG alters expression and localization of the transporter independent of SERT phosphorylation. These studies extend previous observations of SERT co-localization and association with PKGIα (27) by demonstrating that both PKGI isoforms associate with and activate SERT, that myristoylation of PKGII leads to its inability stimulate SERT activity, and that PKGI and PKGII have opposite effects on SERT surface expression and activity.
Our results suggest that PKG has two effects on SERT: stimulation of SERT activity and alteration of SERT expression. The 8-Br-cGMP-dependent stimulation of SERT activity was associated with SERT phosphorylation (Figs. 2 and 5A). However, the alteration of SERT expression was independent of the ability of PKG to phosphorylate SERT. A similar effect on SERT expression was also found with the catalytically inactive mutants (Figs. 7 and 8).
Regulation of SERT by cGMP signaling involves many factors, including a cell stimulus that leads to generation of cGMP, the activation of PKG, and subsequent phosphorylation of SERT, followed by termination of the signal by dephosphorylation. Previous studies have led to the understanding that activation of PKG is followed by SERT phosphorylation and stimulation of transport activity, either by increased catalytic activity (13, 15) or surface expression (9, 17). The present results along with another recently published report (27) lead to the conclusion that PKG is an essential element in SERT activation. In cells lacking PKG, cGMP did not stimulate 5-HT transport or SERT phosphorylation, and the catalytic activity of PKG was required for this stimulation (Figs. 2 and 5). Furthermore, PKG associated in a complex with the transporter (Fig. 6), suggesting direct phosphorylation of the transporter. Moreover, as previously noted (14, 15), mutation of Thr-276 blocked both phosphorylation and activation of SERT by PKG (Figs. 5 and 9), consistent with this residue serving as the phosphate acceptor. However, Thr-276 is not in a consensus sequence for PKG phosphorylation and was shown to be in a relatively inaccessible position near the cytoplasmic end of the fifth transmembrane helix of SERT (29, 34). Protein kinases prefer extended peptides as substrates, making Thr-276 an unexpected site for phosphorylation. Therefore, we consider it premature to conclude that PKG phosphorylates Thr-276 or even that PKG phosphorylates SERT directly.
The finding that PKGI isoforms associate with SERT is meaningful in the context of other enzymes in the cGMP signaling pathway. PP2A was found to associate with SERT, and the association was modulated by cGMP-dependent phosphorylation and by PKC (14, 35). Neuronal nitric oxide synthase was shown to interact with the SERT C terminus and to be activated by the transport of 5-HT (36). With the addition of PKGI, it seems increasingly likely that a regulatory complex containing all of the enzymes of the cGMP signaling pathway is associated with SERT. This would allow production of the highly diffusible messengers NO and cGMP in amounts that could be effective locally but not in other parts of the cell. It remains to be seen whether additional components such as guanylyl cyclase and phosphodiesterase are also associated in the same complex. However, it may be significant that the predominant form of SERT phosphorylated is the mature glycosylation product associated with the plasma membrane (Fig. 4) and not the immature forms that largely remain intracellular (Figs. 8 and 9).
Previous work from Steiner et al. (27) indicated that PKGI, but not PKGII, co-localized with SERT in serotonergic neurons and that siRNA directed against PKGI prevented 8-Br-cGMP stimulation of SERT activity in HeLa cells. The results presented here confirm the requirement for PKGI in the stimulation of 5-HT influx and also demonstrate that wild-type PKGI but not PKGII activates SERT (Fig. 2). However, our results differ from those of Steiner et al. in that the previous work observed a difference between PKGIα and PKGIβ, with only the former associating with SERT. The results presented here indicate that both PKGI isoforms are equally capable of phosphorylating and activating SERT (Figs. 2 and 5) and of associating with the transporter in extracts from co-transfected HeLa cells (Fig. 6). The reason for the difference in results is not immediately apparent, although a weak band of co-precipitated PKGIβ was visible in the previous work, which utilized HEK-293T cells. Our results, which used the vaccinia-T7 expression system in HeLa cells, do not implicate the alternatively spliced N-terminal region of PKGI in the specificity of its interaction with SERT.
The findings presented here provide an unexpected explanation for the inability of PKGII to phosphorylate and activate SERT. Replacement of Gly-2 with alanine prevents the myristoylation that normally follows removal of Met-1 (19). This mutation increased PKGII solubility (Fig. 3) and resulted in a form of PKGII that was similar to PKGI in phosphorylating and activating SERT (Figs. 2 and 5). The ability of PKGII to phosphorylate SERT is not surprising in light of the high sequence similarity between the catalytic domains of PKGI and PKGII (37). However, myristoylation of PKGII was required for activating other physiologically important substrates such as cystic fibrosis transmembrane conductance regulator (20) and epithelial calcium channel (38). In the case of SERT phosphorylation, however, we found the opposite, leading to the conclusion that myristoylation prevents the productive interaction of PKGII with SERT, possibly due to specific spatial constraints for phosphorylation that cannot be met when the N terminus of PKGII is anchored to the membrane.
Despite the inability of wild-type PKGII to phosphorylate and activate SERT, we found a dramatic decrease in 5-HT influx when PKGII was co-expressed with SERT (Fig. 7). This decrease was independent of kinase activity and resulted from decreased abundance of SERT on the cell surface (Fig. 8). Co-expression of PKGI had the opposite effect on SERT, increasing both total and surface expression of the transporter (Fig. 8). These effects on expression were clearly distinct from the ability of PKG isoforms to phosphorylate SERT. Catalytically inactive kinase mutants were similar to the corresponding wild types, which had the same effects on SERT T276A, itself insensitive to activation by 8-Br-cGMP (Figs. 7–9).
The ability of PKG to influence SERT expression independent of phosphorylation is consistent with the observation that wild-type and catalytically inactive PKGI mutants associated equally well with SERT (Fig. 6). This differs from PP2A, a protein phosphatase that associated more strongly under conditions when SERT was phosphorylated (14). Although the most dramatic effects on SERT expression followed overexpression of PKG isoforms, activation of endogenously expressed PKG increased SERT activity in several cell lines and synaptosomes (7, 9, 13, 15), and physiological expression levels of PKGII led to decreased SERT activity (Fig. 7).
Although PKGI co-expression increased total and cell surface SERT expression, it did not increase 5-HT transport in the absence of 8-Br-cGMP. It has been argued, based on increased ligand binding in response to 8-Br-cGMP, that SERT activation by PKG represents increased abundance on the cell surface (9). However, other studies found that PKG activation by 8-Br-cGMP increased the Vmax for SERT without an increase in surface expression (13, 15). Thus, the possibility also exists that PKG-dependent SERT phosphorylation increases the catalytic transport rate. We now report that co-expression of PKG increased SERT surface expression with no change in transport rate (Fig. 8 versus Fig. 2), indicating a complex regulatory interaction between the two proteins that will require further study to understand completely.
Acknowledgments
We thank Suzanne M. Lohmann (University of Wuerzburg, Germany) for helpful discussions and reagents and Benjamin Turk (Yale University Department of Pharmacology) for critical reading.
This work was supported, in whole or in part, by National Institutes of Health Grant DA008213 (to G. R.).
- SERT
- serotonin transporter
- 8-Br-cGMP
- 8-bromoguanosine-3′,5′-cyclic monophosphate 5-HT, 5-hydroxytryptamine
- PKG
- cyclic GMP-dependent protein kinase
- Rp-8-pCPT-cGMPS
- 8-(4-chlorophenylthio)guanosine 3′,5′-cyclic monophosphorothioate, Rp-isomer
- VASP
- vasodilator-stimulated phosphoprotein.
REFERENCES
- 1. Murphy D. L., Andrews A. M., Wichems C. H., Li Q., Tohda M., Greenberg B. (1998) J. Clin. Psychiatry 59, Suppl. 15, 4–12 [PubMed] [Google Scholar]
- 2. Stahl S. M. (1998) J. Affect. Disorders 51, 215–235 [DOI] [PubMed] [Google Scholar]
- 3. Qian Y., Galli A., Ramamoorthy S., Risso S., DeFelice L. J., Blakely R. D. (1997) J. Neurosci. 17, 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramamoorthy S., Cool D. R., Mahesh V. B., Leibach F. H., Melikian H. E., Blakely R. D., Ganapathy V. (1993) J. Biol. Chem. 268, 21626–21631 [PubMed] [Google Scholar]
- 5. Jayanthi L. D., Ramamoorthy S., Mahesh V. B., Leibach F. H., Ganapathy V. (1994) J. Biol. Chem. 269, 14424–14429 [PubMed] [Google Scholar]
- 6. Ciccone M. A., Timmons M., Phillips A., Quick M. W. (2008) Neuropharmacology 55, 763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller K. J., Hoffman B. J. (1994) J. Biol. Chem. 269, 27351–27356 [PubMed] [Google Scholar]
- 8. Zhu C. B., Carneiro A. M., Dostmann W. R., Hewlett W. A., Blakely R. D. (2005) J. Biol. Chem. 280, 15649–15658 [DOI] [PubMed] [Google Scholar]
- 9. Zhu C. B., Hewlett W. A., Feoktistov I., Biaggioni I., Blakely R. D. (2004) Mol. Pharmacol. 65, 1462–1474 [DOI] [PubMed] [Google Scholar]
- 10. Ramamoorthy S., Giovanetti E., Qian Y., Blakely R. D. (1998) J. Biol. Chem. 273, 2458–2466 [DOI] [PubMed] [Google Scholar]
- 11. Ozaki N., Goldman D., Kaye W. H., Plotnicov K., Greenberg B. D., Lappalainen J., Rudnick G., Murphy D. L. (2003) Mol. Psychiatry 8, 933–936 [DOI] [PubMed] [Google Scholar]
- 12. Delorme R., Betancur C., Wagner M., Krebs M. O., Gorwood P., Pearl P., Nygren G., Durand C. M., Buhtz F., Pickering P., Melke J., Ruhrmann S., Anckarsäter H., Chabane N., Kipman A., Reck C., Millet B., Roy I., Mouren-Simeoni M. C., Maier W., Råstam M., Gillberg C., Leboyer M., Bourgeron T. (2005) Mol. Psychiatry 10, 1059–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kilic F., Murphy D. L., Rudnick G. (2003) Mol. Pharmacol. 64, 440–446 [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y. W., Gesmonde J., Ramamoorthy S., Rudnick G. (2007) J. Neurosci. 27, 10878–10886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramamoorthy S., Samuvel D. J., Buck E. R., Rudnick G., Jayanthi L. D. (2007) J. Biol. Chem. 282, 11639–11647 [DOI] [PubMed] [Google Scholar]
- 16. Zhu C. B., Hewlett W. A., Francis S. H., Corbin J. D., Blakely R. D. (2004) Eur. J. Pharmacol. 504, 1–6 [DOI] [PubMed] [Google Scholar]
- 17. Prasad H. C., Zhu C. B., McCauley J. L., Samuvel D. J., Ramamoorthy S., Shelton R. C., Hewlett W. A., Sutcliffe J. S., Blakely R. D. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 11545–11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu C. B., Steiner J. A., Munn J. L., Daws L. C., Hewlett W. A., Blakely R. D. (2007) J. Pharmacol. Exp. Ther. 322, 332–340 [DOI] [PubMed] [Google Scholar]
- 19. Vaandrager A. B., Ehlert E. M., Jarchau T., Lohmann S. M., de Jonge H. R. (1996) J. Biol. Chem. 271, 7025–7029 [DOI] [PubMed] [Google Scholar]
- 20. Vaandrager A. B., Smolenski A., Tilly B. C., Houtsmuller A. B., Ehlert E. M., Bot A. G., Edixhoven M., Boomaars W. E., Lohmann S. M., de Jonge H. R. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lincoln T. M., Komalavilas P., Boerth N. J., MacMillan-Crow L. A., Cornwell T. L. (1995) Adv. Pharmacol. 34, 305–322 [DOI] [PubMed] [Google Scholar]
- 22. Vaandrager A. B., de Jonge H. R. (1996) Mol. Cell. Biochem. 157, 23–30 [DOI] [PubMed] [Google Scholar]
- 23. Lohmann S. M., Vaandrager A. B., Smolenski A., Walter U., De Jonge H. R. (1997) Trends Biochem. Sci. 22, 307–312 [DOI] [PubMed] [Google Scholar]
- 24. Geiselhöringer A., Gaisa M., Hofmann F., Schlossmann J. (2004) FEBS Lett. 575, 19–22 [DOI] [PubMed] [Google Scholar]
- 25. el-Husseini A. E., Bladen C., Vincent S. R. (1995) J. Neurochem. 64, 2814–2817 [DOI] [PubMed] [Google Scholar]
- 26. de Vente J., Asan E., Gambaryan S., Markerink-van Ittersum M., Axer H., Gallatz K., Lohmann S. M., Palkovits M. (2001) Neuroscience 108, 27–49 [DOI] [PubMed] [Google Scholar]
- 27. Steiner J. A., Carneiro A. M., Wright J., Matthies H. J., Prasad H. C., Nicki C. K., Dostmann W. R., Buchanan C. C., Corbin J. D., Francis S. H., Blakely R. D. (2009) Mol. Brain 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blakely R. D., Clark J. A., Rudnick G., Amara S. G. (1991) Anal. Biochem. 194, 302–308 [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y. W., Rudnick G. (2005) J. Biol. Chem. 280, 30807–30813 [DOI] [PubMed] [Google Scholar]
- 30. Smolenski A., Burkhardt A. M., Eigenthaler M., Butt E., Gambaryan S., Lohmann S. M., Walter U. (1998) Naunyn-Schmiedebergs Arch. Pharmacol. 358, 134–139 [DOI] [PubMed] [Google Scholar]
- 31. Smolenski A., Bachmann C., Reinhard K., Hönig-Liedl P., Jarchau T., Hoschuetzky H., Walter U. (1998) J. Biol. Chem. 273, 20029–20035 [DOI] [PubMed] [Google Scholar]
- 32. Butt E., Abel K., Krieger M., Palm D., Hoppe V., Hoppe J., Walter U. (1994) J. Biol. Chem. 269, 14509–14517 [PubMed] [Google Scholar]
- 33. Gambaryan S., Butt E., Marcus K., Glazova M., Palmetshofer A., Guillon G., Smolenski A. (2003) J. Biol. Chem. 278, 29640–29648 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y. W., Rudnick G. (2006) J. Biol. Chem. 281, 36213–36220 [DOI] [PubMed] [Google Scholar]
- 35. Bauman A. L., Apparsundaram S., Ramamoorthy S., Wadzinski B. E., Vaughan R. A., Blakely R. D. (2000) J. Neurosci. 20, 7571–7578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chanrion B., Mannoury la Cour C., Bertaso F., Lerner-Natoli M., Freissmuth M., Millan M. J., Bockaert J., Marin P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 8119–8124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vaandrager A. B., Hogema B. M., de Jonge H. R. (2005) Front. Biosci. 10, 2150–2164 [DOI] [PubMed] [Google Scholar]
- 38. Hoenderop J. G., Vaandrager A. B., Dijkink L., Smolenski A., Gambaryan S., Lohmann S. M., de Jonge H. R., Willems P. H., Bindels R. J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 6084–6089 [DOI] [PMC free article] [PubMed] [Google Scholar]