Abstract
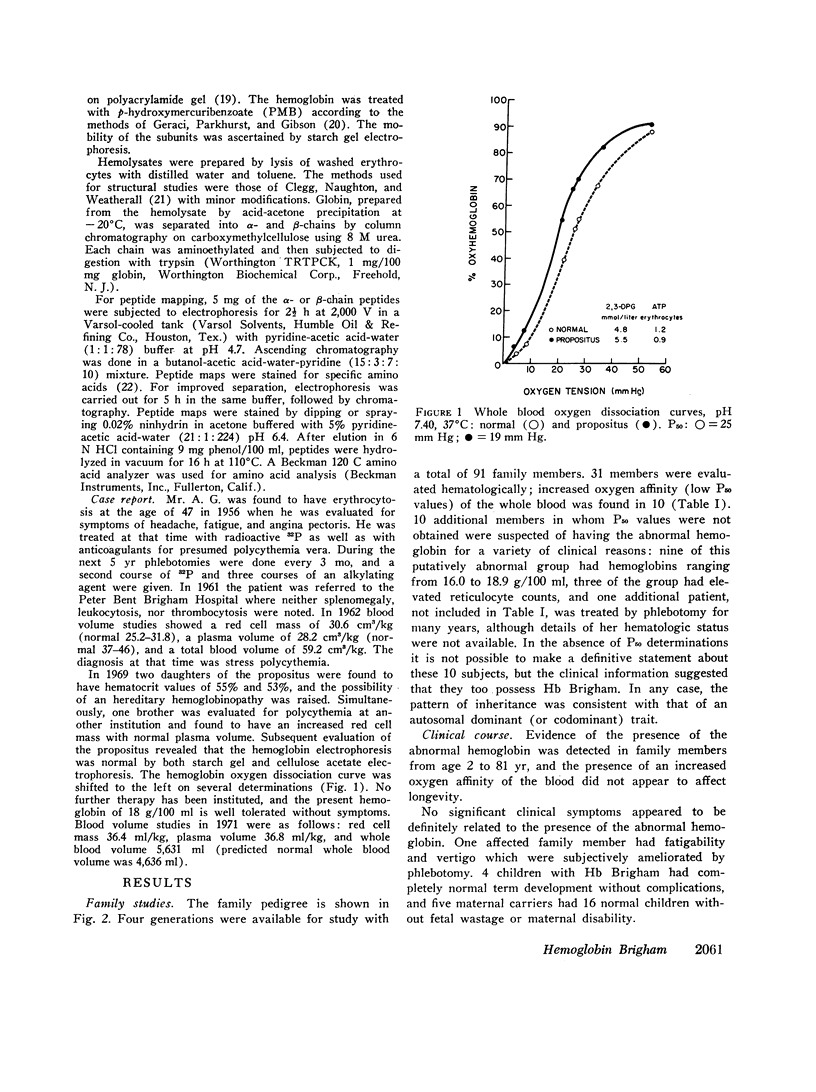
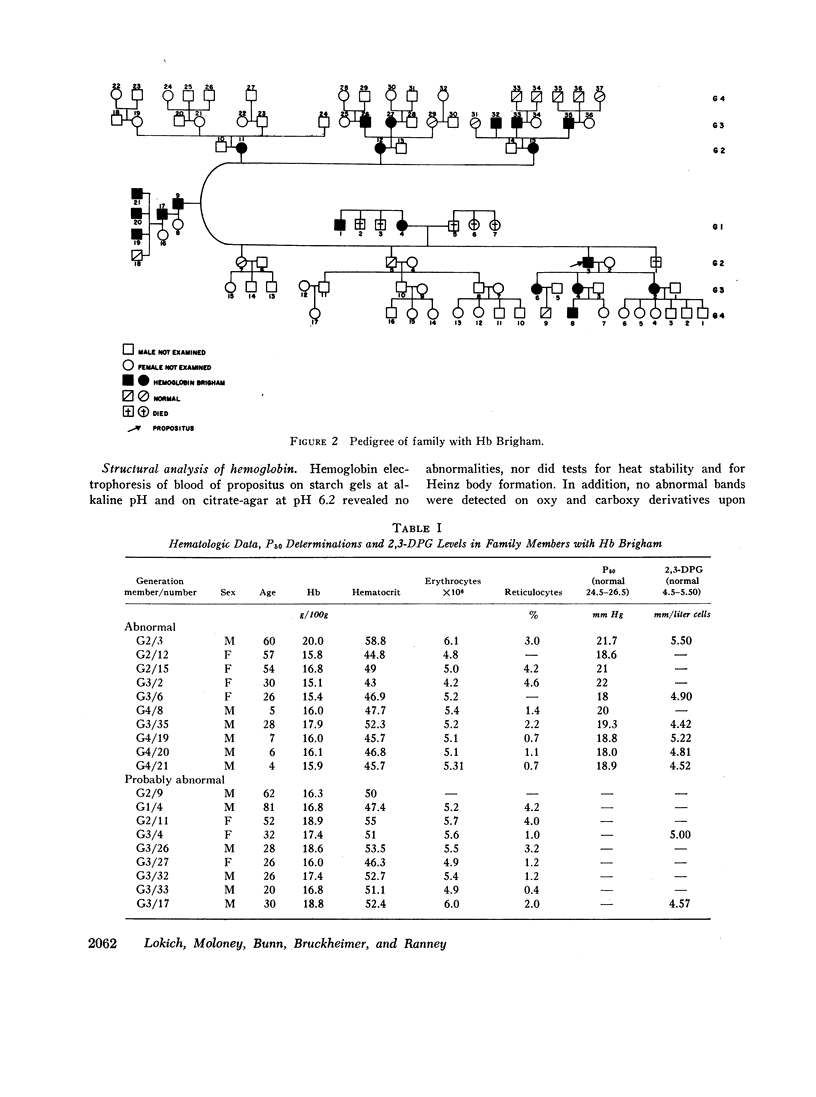
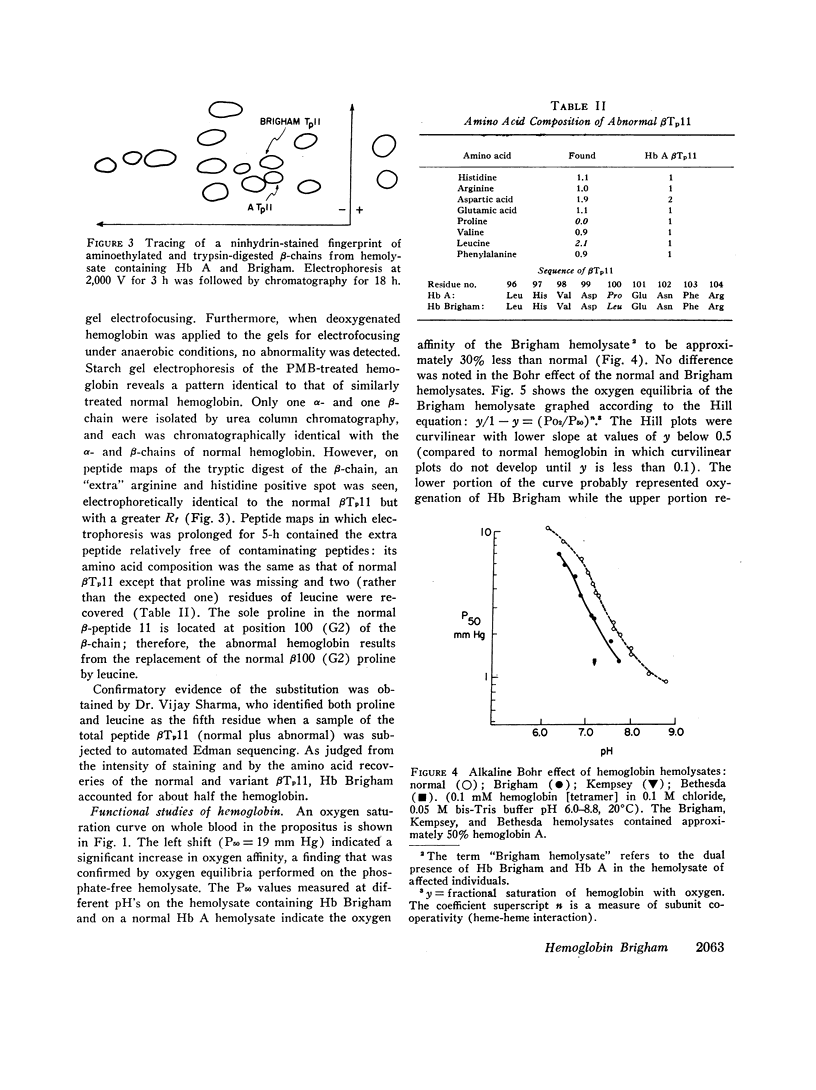
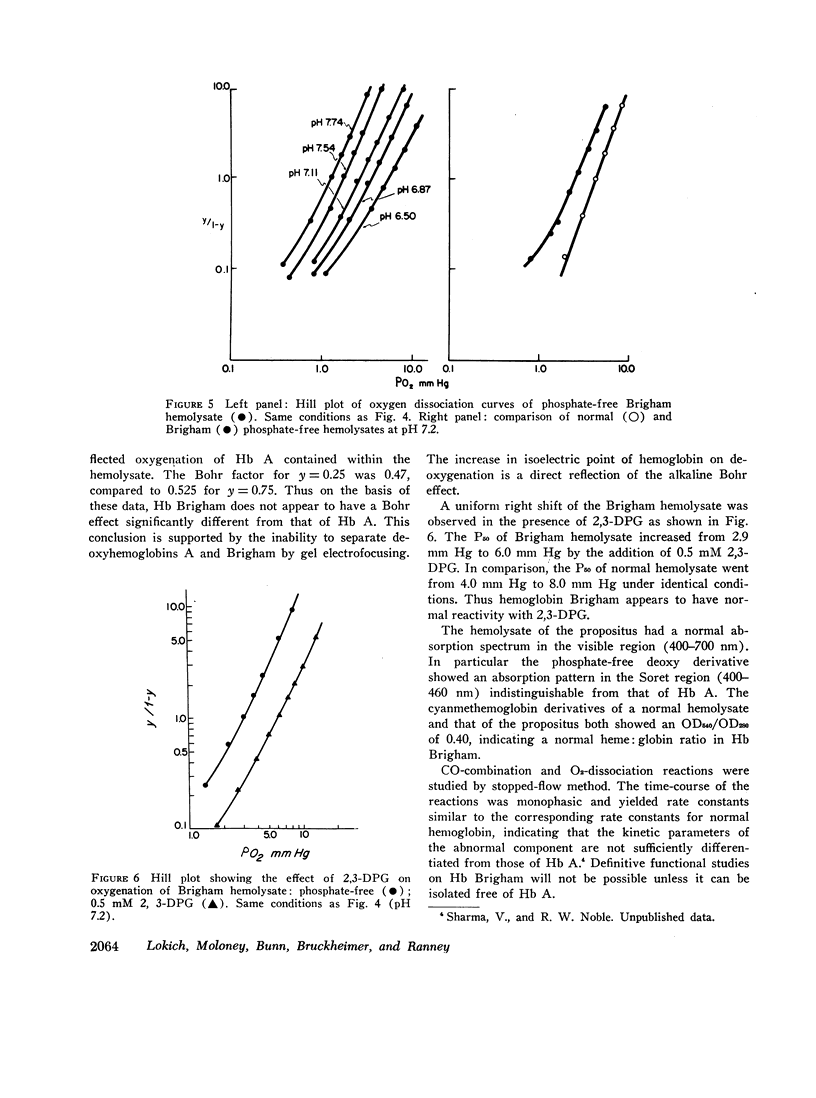
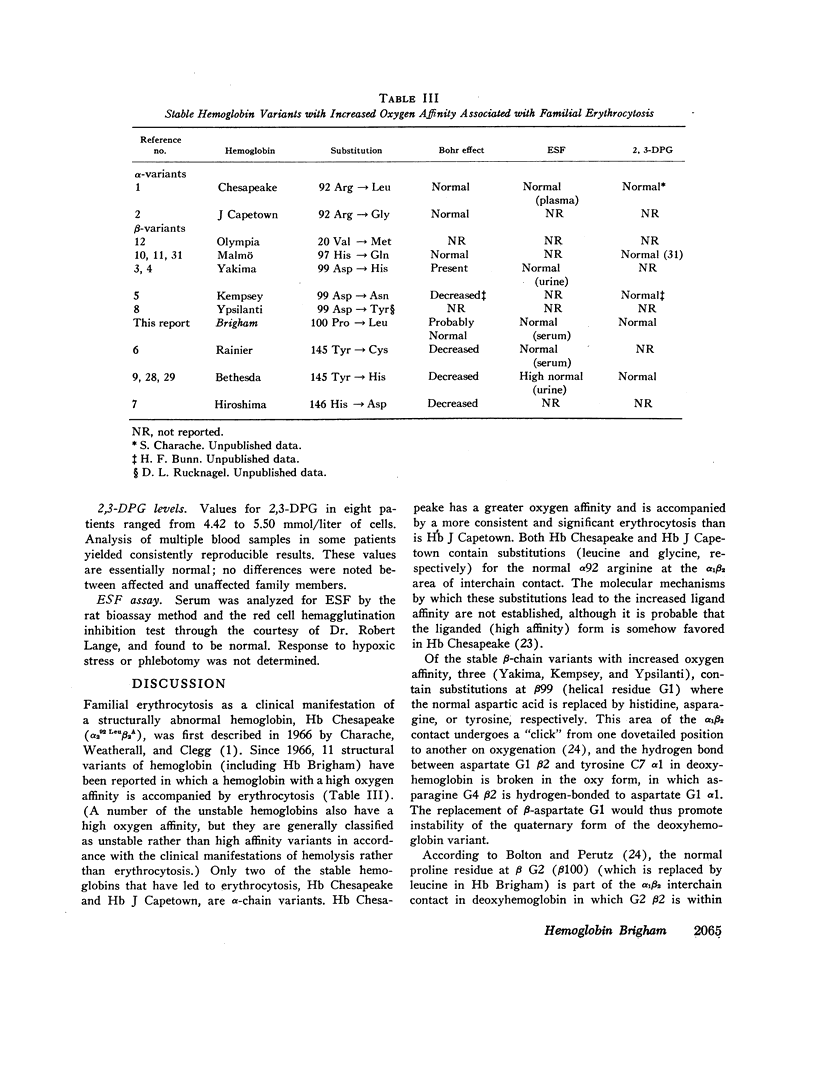
Erythrocytosis associated with the presence of a hemoglobin with increased oxygen affinity has been reported for 10 hemoglobin variants, most of which demonstrate altered electrophoretic mobility. Several members of a family were found to have erythrocytosis, and both the whole blood and the hemoglobin exhibited increased oxygen affinity. Phosphate-free hemoglobin solutions had a normal Bohr effect and reactivity to 2,3-diphosphoglycerate. The electrophoretic properties of the hemoglobin were normal, but on peptide mapping of a tryptic digest of the isolated β-chains, a normal βT11 peptide and an abnormal βT11 with greater Rf were seen. Analysis of the abnormal peptide showed the substitution of leucine for the normal proline at β100 (helical residue G2).
The hemoglobin variant, designated Hb Brigham, serves to emphasize the necessity for detailed evaluation of the structure and function of hemoglobin in familial erythrocytosis even with electrophoretically “normal” hemoglobin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson J. W., Hayashi A., Stamatoyannopoulos G., Burger W. F. Erythrocyte function and marrow regulation in hemoglobin Bethesda (beta-145 histidine). J Clin Invest. 1972 Nov;51(11):2883–2888. doi: 10.1172/JCI107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson J. W., Parer J. T., Stamatoyannopoulos G. Erythrocytosis associated with hemoglobin Rainier: oxygen equilibria and marrow regulation. J Clin Invest. 1969 Aug;48(8):1376–1386. doi: 10.1172/JCI106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund S. The oxygen dissociation curve of whole blood containing haemoglobin Malmö. Scand J Haematol. 1972;9(4):377–386. doi: 10.1111/j.1600-0609.1972.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Bradley T. B., Davis W. E., Drysdale J. W., Burke J. F., Beck W. S., Laver M. B. Structural and functional studies on hemoglobin Bethesda (alpha2beta2 145His), a varient associated with compensatory erythrocytosis. J Clin Invest. 1972 Sep;51(9):2299–2309. doi: 10.1172/JCI107040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Meriwether W. D., Balcerzak S. P., Rucknagel D. L. Oxygen equilibrium of hemoglobin E. J Clin Invest. 1972 Nov;51(11):2984–2987. doi: 10.1172/JCI107125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Righetti P., Bunn H. F. The separation of human and animal hemoglobins by isoelectric focusing in polyacrylamide gel. Biochim Biophys Acta. 1971 Jan 19;229(1):42–50. doi: 10.1016/0005-2795(71)90315-1. [DOI] [PubMed] [Google Scholar]
- Easley C. W. Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta. 1965 Sep 13;107(2):386–388. doi: 10.1016/0304-4165(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Edwards M. J., Martin R. J. Mixing technique for the oxygen-hemoglobin equilibrium and Bohr effect. J Appl Physiol. 1966 Nov;21(6):1898–1902. doi: 10.1152/jappl.1966.21.6.1898. [DOI] [PubMed] [Google Scholar]
- Fairbanks V. F., Maldonado J. E., Charache S., Boyer S. H., 4th Familial erythrocytosis due to electrophoretically undetectable hemoglobin with impaired oxygen dissociation (hemoglobin Malmö, alpha 2 beta 2 97 gln). Mayo Clin Proc. 1971 Nov;46(11):721–727. [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Glynn K. P., Penner J. A., Smith J. R., Rucknagel D. L. Familial erythrocytosis. A description of three families, one with hemoglobin Ypsilanti. Ann Intern Med. 1968 Oct;69(4):769–776. doi: 10.7326/0003-4819-69-4-769. [DOI] [PubMed] [Google Scholar]
- Greer J. Three-dimensional structure of abnormal human haemoglobins Chesapeake and J Capetown. J Mol Biol. 1971 Nov 28;62(1):241–249. doi: 10.1016/0022-2836(71)90143-4. [DOI] [PubMed] [Google Scholar]
- Hakim J., Boucherot J., Troube H., Boivin P. Red cell 2, 3 -diphosphoglycerate and adenosine triphosphate levels in patients with polycythemia vera. Rev Eur Etud Clin Biol. 1972 Jan;17(1):99–102. [PubMed] [Google Scholar]
- Hamilton H. B., Iuchi I., Miyaji T., Shibata S. Hemoglobin Hiroshima (beta-143 histidine--aspartic acid): a newly identified fast moving beta chain variant associated with increased oxygen affinity and compensatory erythremia. J Clin Invest. 1969 Mar;48(3):525–535. doi: 10.1172/JCI106010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. T., Osgood E. E., Brimhall B., Koler R. D. Hemoglobin Yakina. I. Clinical and biochemical studies. J Clin Invest. 1967 Nov;46(11):1840–1847. doi: 10.1172/JCI105674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novy M. J., Edwards M. J., Metcalfe J. Hemoglobin Yakina. II. High blood oxygen affinity associated with compensatory erythrocytosis and normal hemodynamics. J Clin Invest. 1967 Nov;46(11):1848–1854. doi: 10.1172/JCI105675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed C. S., Hampson R., Gordon S., Jones R. T., Novy M. J., Brimhall B., Edwards M. J., Koler R. D. Erythrocytosis secondary to increased oxygen affinity of a mutant hemoglobin, hemoglobin Kempsey. Blood. 1968 May;31(5):623–632. [PubMed] [Google Scholar]
- Smith L. L., Plese C. F., Barton B. P., Charache S., Wilson J. B., Huisman T. H. Subunit dissociation of the abnormal hemoglobins G Georgia ( 2 95Leu (G2) 2 ) and Rampa ( 2 95Ser (G2) 2 ). J Biol Chem. 1972 Mar 10;247(5):1433–1439. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Nute P. E., Adamson J. W., Bellingham A. J., Funk D. Hemoglobin olympia ( 20 valine leads to methionine): an electrophoretically silent variant associated with high oxygen affinity and erythrocytosis. J Clin Invest. 1973 Feb;52(2):342–349. doi: 10.1172/JCI107190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshire B. G., Clark K. G., Lorkin P. A., Lehmann H. Haemoglobin Denmark Hill 95 (G2) Pro-Ala, a variant with unusual electrophoretic and oxygen-binding properties. Biochim Biophys Acta. 1972 Oct 31;278(3):459–464. doi: 10.1016/0005-2795(72)90006-2. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Bernini L. F., Meera Khan P. Haemoglobin Rampa: Alpha 95 Pro--Ser. Biochim Biophys Acta. 1971 Apr 27;236(1):197–200. doi: 10.1016/0005-2795(71)90165-6. [DOI] [PubMed] [Google Scholar]


