Abstract
Female fertility is determined to a large extent by the quality (developmental competence) of the oocyte as reflected in its ability to undergo meiosis, be fertilized, and give rise to a healthy embryo. Growth of the mammalian oocyte is coordinated with that of the follicle that encloses it by the actions of signals that pass in both directions between the germline and somatic components. This review summarizes what is known about the roles played by two different modes of intrafollicular signalling in oogenesis: paracrine factors activating receptors on the opposite cell type, and direct sharing of small molecules throughout the follicle via gap junction channels. Recent evidence indicates that these two modes of signalling interact to regulate oocyte growth and granulosa cell proliferation, and that defects in either can contribute to female infertility.
Keywords: oogenesis, folliculogenesis, paracrine signalling, KITL, GDF9, BMP15, cumulus cell, granulosa cell, connexin, gap junction, CX37, CX43, intercellular communication
Introduction
Female fertility is determined to a large extent by the developmental competence of the oocyte as reflected in its ability to undergo meiosis, be fertilized, and give rise to a healthy embryo. Although paternal factors are certainly of importance in influencing developmental events post-fertilization, that influence is exerted only after activation of the embryonic genome, an event which, in mammals, occurs after one or more cleavage divisions of the zygote (depending on the species; reviewed by Kidder 1992). Thus the importance of events occurring within the developing ovarian follicle cannot be overstated.
The growing mammalian follicle consists of a single oocyte, one or more layers of surrounding somatic cells (follicle or granulosa cells), and an outer rim of theca cells separated from the granulosa cells by a basal lamina (Figure 1). The follicle grows through inward division of the outermost layer of granulosa cells, generating additional layers until a fluid-filled cavity, the antrum, develops (Da Silva-Buttkus et al. 2008). The antrum separates the granulosa cells into two subpopulations, the cumulus granulosa surrounding the oocyte and the mural granulosa adjacent to the basal lamina. Within the follicle, the oocyte grows and, prior to ovulation, develops competence to undergo meiosis and be fertilized. During each mammalian ovarian cycle, depending on the species, one or more growing follicles will become mature whereas others will undergo atresia (death by apoptosis; reviewed by Krysko et al. 2008). During their development, follicles pass from the primordial (non-growing) stage through primary (unilaminar) and secondary (multilaminar) growing stages until becoming tertiary (antral) follicles. It is about this time that meiotic competence is achieved (Sorensen and Wassarman 1976). A fully-grown antral follicle ready for ovulation is called a pre-ovulatory or Graafian follicle. After ovulation, the follicle remnant develops into a highly vascularised endocrine organ, the corpus luteum, the primary function of which is to produce progesterone to support the ensuing pregnancy.
Fig. 1.
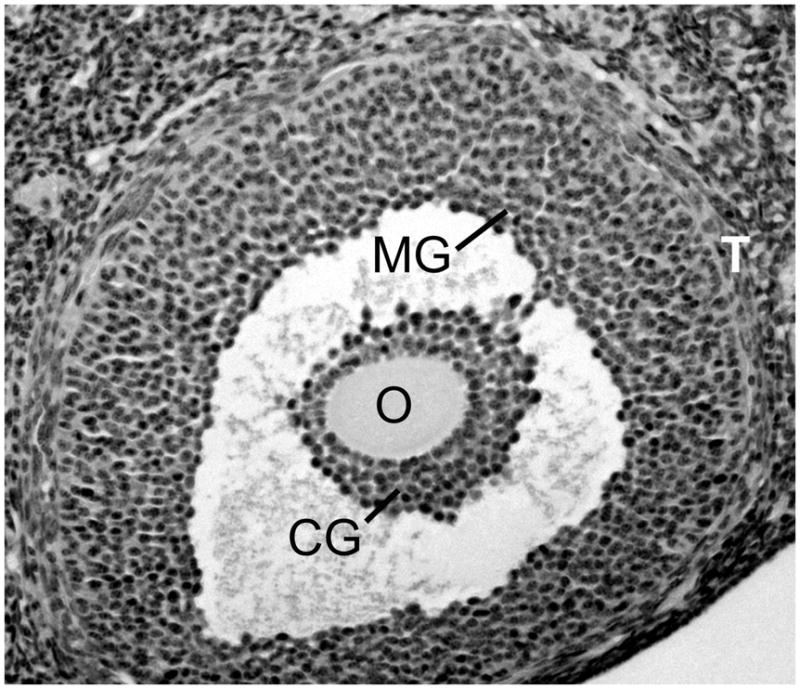
The ovarian follicle. The oocyte (O) is enclosed in a mass of cumulus granulosa cells (CG) separated from the mural granulosa cells (MG) by the antral cavity. The thecal cell layers (T) form the outer boundary of the follicle.
It has been known for many decades that ovarian folliculogenesis, oogenesis, and ovulation are regulated by hormones (gonadotropins) emanating from the pituitary gland (reviewed by Amsterdam et al. 2003). More recently, it has become apparent that this gonadotropin action on the ovary is mediated by intra-ovarian signals provided by multiple families of paracrine factors (for reviews see Conti et al. 2006; Drummond et al. 2003; Gilchrist et al. 2004a; Gilchrist et al. 2008; Kaivo-oja et al. 2006; Krysko et al. 2008; Kwintkiewicz and Giudice 2009; Ojeda et al. 2000; Pangas and Matzuk 2004; Silva et al. 2009; Thomas and Vanderhyden 2006; Vanderhyden 2002) as well as by direct cell-cell communication via gap junction channels (reviewed by Kidder 2005). In particular, bi-directional signals of both types are now understood to operate within the oocyte-granulosa cell complex to govern oocyte and follicle growth and differentiation, coordinating the developmental pathways of the two cell types (reviewed by Sugiura and Eppig 2005). It is this bi-directional communication, a critical process for ensuring oocyte developmental competence, that is the subject of this review.
Bi-directional Paracrine Signalling between Oocytes and Granulosa Cells
From the time of follicle formation, through folliculogenesis to ovulation, the organization and normal functioning of the ovary is heavily dependent upon close interactions between the germ cells and the surrounding somatic cells. Growing follicles support oocyte growth and the acquisition of meiotic and developmental competence, and granulosa cells have clearly established roles in supporting the growth of the oocyte (Brower and Schultz, 1982), regulating the progression of meiosis (Eppig 1991), and modulating the global transcriptional activity in the oocyte genome (De La Fuente and Eppig 2001). In turn, the oocyte influences several aspects of granulosa cell development including proliferation, differentiation, and extracellular matrix and steroid hormone production (reviewed by Gilchrist et al. 2008). The evidence for this cellular interdependence continues to accumulate, along with the identification of some of the paracrine signalling factors involved, which has considerably improved our knowledge of the molecular and cellular processes that control the development of healthy oocytes.
Granulosa cell regulation of oocyte development: KITL
Despite the clear evidence that granulosa cells are required for oocyte growth, very few granulosa-derived paracrine factors have been identified that support or promote this process. The most studied ligand-receptor system to be characterized for its role in mediating granulosa-oocyte interactions is KIT ligand (KITL) and the KIT tyrosine kinase receptor. In the postnatal ovary, both KIT receptors and KITL are expressed in developing and preovulatory follicles. KIT is found in oocytes at all stages of follicular development in mouse (Orr-Urtreger et al. 1990; Manova et al. 1990; Horie et al 1991) and human ovaries (Horie et al. 1993), and KITL is expressed in rat (Ismail et al. 1996), mouse (Manova et al. 1993; Motro et al. 1993) and human granulosa cells (Laitinen et al. 1995). In the mouse, KITL has been shown to stimulate oocyte growth (Packer et al. 1994; Thomas et al. 2005).
The evidence to date suggests that KITL is necessary and sufficient to induce primordial follicle development. In vitro studies support the possibility that KITL alone is sufficient for the initiation of follicle growth (Parrott and Skinner 1999; Jin et al. 2005a). Klinger and De Felici (2002) used a multistep culture system for mouse oocytes obtained from E15.5–E16.5 embryos, and found that KITL alone can induce the onset of growth, although it was not sufficient to fully activate the mechanisms governing the acquisition of meiotic competence. Given the evidence that oocytes control the rate of follicle growth (Eppig et al. 2002), it can be speculated that KITL activation of oocyte KIT receptors may trigger the molecular events in the oocyte that initiate its own growth as well as its ability to produce factors that stimulate granulosa cell proliferation.
Female mice with naturally occurring mutations in KIT or KITL have helped to reveal the importance of KIT activity in female fertility. The phenotypes of these mutants vary in their severity, from normal fertility to complete sterility, largely reflecting the extent of dysfunction caused by the mutation (Table 1). Mutations that reduce levels of KITL expression cause infertility: oocytes are present, although fewer in number, and follicular development is arrested (Kuroda et al. 1988; Huang et al. 1993). The importance of KITL activation of oocyte KIT receptors has also been demonstrated by the in vivo administration of antibodies blocking KIT activation at various times after birth. Blockade of KIT function disturbs the onset of primordial follicle development, primary follicle growth, follicular fluid formation in preantral follicles, and the penultimate stage of ovarian follicle maturation before ovulation (Yoshida et al. 1997). These results suggest that ovarian follicle growth is dependent on KIT at a time when functional receptors for follicle-stimulating hormone (FSH), critical for preantral follicle growth, are not yet expressed in mouse ovary.
Table 1.
Examples of known mutations in KIT or KITL in mice and their impact on female fertility
| Gene name | Phenotype | Mutation | Reference |
|---|---|---|---|
| Kit (white spotting) | Embryonic/perinatal death, fetal gonads lack primordial germ cells | Null mutation, deletion of transmembrane domain | Nocka et al. 1990 |
| Kit v | Neonatal death | Thr660→Met, reduced kinase activity | Nocka et al. 1990; Reith et al. 1990 |
| Kit44 | Viable, sterile | intron insertion, reduced mRNA level | Geissler et al. 1981; Geissler et al. 1988 |
| KitlSl (Steel) | Embryonic lethal | Null mutation | McCoshen and McCallion 1975 |
| KitlSl-d (Dickie) | Viable, sterile | Deletion of transmembrane domain, produces only KITL1 | Brannan et al. 1991; Flanagan et al. 1991 |
| KitlSl-pan (panda) | Viable, sterile | ~40 cM inversion, reduced mRNA expression | Beechey et al. 1985; Huang et al. 1993 |
| KitlSl-con (contrasted) | Viable, sterile | Mutation in non-coding region, reduced mRNA expression | Beechey and Searle 1983; Bedell et al. 1995 |
| KitlSl-t (Tutikawa) | Viable, sterile | Mutation undefined | Kohrogi et al. 1983; Kuroda et al. 1988 |
Binding of KITL to the KIT receptor leads to the activation of several signalling pathways that regulate cell survival/apoptosis, including those involving RAS, RAF, mitogen activated protein kinase, and AKT (Kinoshita et al. 1997; Wang et al. 1999). One of the most important downstream effectors of KIT activation in oocytes is phosphatidylinositol (PI) 3-kinase (PI3K), through which the signal is transduced into changes in expression of BAX and BCL2L1, important players in the apoptotic pathway (Jin et al. 2005b). Selective PI3K inhibitors block the anti-apoptotic effect of KITL in germ cells during fetal oogenesis (Morita et al. 1999), and mice expressing a mutant KIT receptor (KITY719F), which fails to interact with PI3K, have impaired follicle development at the early preantral stage (Kissel et al. 2000). PTEN (tumour suppressor phosphatase with tensin homology) negatively regulates PI3K signalling, and oocyte-specific knockout of Pten has recently been shown to result in activation of the entire primordial follicle pool and consequent premature ovarian failure (Reddy et al. 2008).
Downstream of PI3K, KIT activation by ligand-induced stimulation of growing oocytes has been shown to induce rapid phosphorylation and activation of AKT, and phosphorylation and functional suppression of the transcription factor FKHRL1 (FOXO3A), both of which are abolished by inhibition of PI3K (Reddy et al. 2005). Mice deficient in FKHRL1 exhibit female infertility in adult life, due to excessive activation of primordial to primary follicles, and have enlarged primary oocytes (Castrillon et al. 2003). From these studies, it appears that KITL-induced KIT activity leads to downstream PI3K/AKT signalling and suppression of FKHRL1 activity that promotes both oocyte growth and survival.
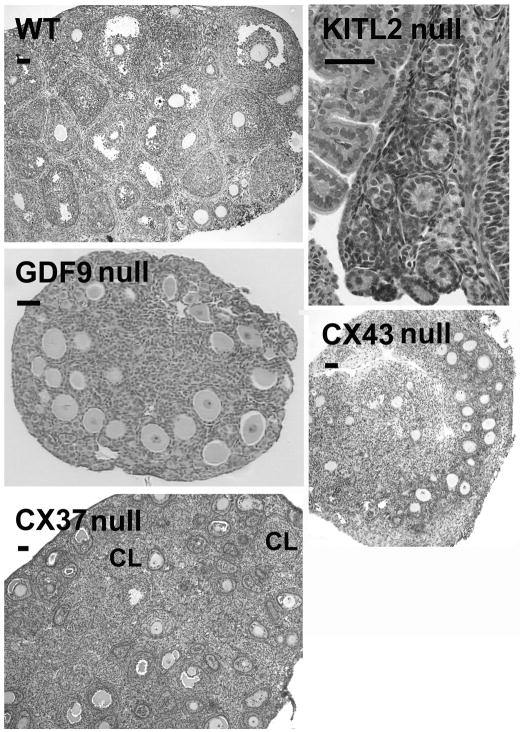
KITL is expressed in granulosa cells as either membrane-bound or soluble proteins arising from alternatively spliced mRNAs(Huang et al. 1992). Soluble KITL (KITL1) can be cleaved due to the presence of an 84 base pair exon (exon 6), which encodes a proteolytic cleavage site, allowing the extracellular domain to be released as a soluble product. Membrane-bound KITL (KITL2) lacks this exon, is not efficiently cleaved and thus remains more stably on the membrane(Huang et al. 1992). Several lines of evidence support the physiological importance of membrane-bound KITL2 in vivo. The most compelling evidence is provided by the viable KitlSl-d mutants in which the genomic regions encoding the transmembrane and cytoplasmic domains of KITL are deleted. These mice can only produce the secreted form of KITL, but they show all the pleiotropic defects, including infertility, seen in KitlSl mutants, which lack expression of both isoforms (Brannan et al. 1991; Flanagan et al. 1991). Females homozygous for the KitlSl allele are short-lived, but surviving juveniles are able to initiate folliculogenesis which is limited to primary stage follicles (see Figure 2). In contrast, mice that exclusively produce KITL2 are fertile (Tajima et al. 1998), suggesting that KITL2 may be the principal isoform required for oocyte development. Indeed, although both soluble and membrane-bound KITL have similar binding affinities for KIT receptors (Flanagan and Leder 1990), KITL2 has been reported to induce a more persistent activation of KIT receptor kinase than the soluble form of KITL(Miyazawa et al. 1995; Kurosawa et al. 1996). Experiments using oocytes in co-culture with fibroblasts expressing either KITL1 or KITL2 have confirmed that KITL2 is more effective in promoting oocyte growth (Thomas et al. 2008).
Fig. 2.
Comparison of folliculogenesis in juvenile mice either wildtype (WT) or lacking KITL2 (KitlSl−d/KitlSl−d), GDF9 (Gdf9−/Gdf9−), CX43 (Gja1−/Gja1−, C57BL/6 strain), or CX37 (Gja4−/Gja4−). In females lacking KITL2, GDF9, or CX43, folliculogenesis does not proceed beyond the primary stage. In mice lacking CX37, large antral and preovulatory follicles are lacking but small, atypical corpora lutea (CL) are present instead. Scale bars indicate 50 μm.
The network of intrafollicular paracrine factors that regulate ovarian function is modulated to a great extent by endocrine factors, most predominantly FSH, which is the key gonadotropic hormone that stimulates follicle growth. FSH regulates KITL expression in a biphasic manner, with low concentrations of FSH increasing the relative abundance of KITL2 and stimulating oocyte growth, whereas high FSH increases the KITL1/KITL2 ratio and fails to promote oocyte development (Thomas et al. 2005). The current evidence therefore suggests that FSH stimulates granulosa cell production of KITL2 which subsequently stimulates KIT receptor activation in oocytes and downstream PI3K/AKT signalling to promote oocyte growth and survival. However, it is now well established that the oocyte plays an active role in its own development and this is perhaps best exemplified by the observation that oocytes regulate the mRNA expression of KITL in granulosa cells, and do so in a stage-specific manner (Joyce et al. 1999, 2000). The interdependence of oocytes and granulosa cells on paracrine communication is therefore critical for the initiation and progression of oocyte and follicle growth.
Oocyte regulation of granulosa cell function: Oocyte factors
The physiological importance of the oocyte in folliculogenesis was originally suggested by an in vivo study in which ovectomy led to spontaneous transformation of the Graafian follicle into a corpus luteum (el-Fouly et al. 1970). These results indicated that the oocyte or oocyte-secreted factors contribute to the suppression of luteinization, the process of granulosa cell differentiation in response to the surge of luteinizing hormone (LH) that induces ovulation. This concept was supported by subsequent evidence provided by in vivo and in vitro experiments in rats, rabbits, and most recently mice showing that the removal, absence, or destruction of oocytes leads to granulosa cell luteinization (Nekola and Nalbandov 1971; Hubbard and Erickson 1988; Simon et al. 1997). Much of our understanding of the specific roles of the oocyte in regulating granulosa cell function was subsequently derived from studies in which the oocyte was removed from preantral follicles or oocyte-cumulus cell complexes. Co-culture experiments (Salustri et al. 1990a,b) and experiments using the procedure of oocytectomy (Buccione et al. 1990; Vanderhyden et al. 1990) revealed the absolute requirement for oocyte-secreted factors for successful cumulus expansion in mice. Cumulus expansion is an extensive rearrangement of the cytoskeleton through the assembly of actin microfilaments and the induction of hyaluronic acid synthesis which transform the tightly packed cumulus cells into a much larger mass of mucified cells. This expansion is required for optimal extrusion of the oocyte-cumulus cell mass from the follicle at ovulation, and it is now clear that this process is dependent on two signalling events: 1) stimulation by gonadotropins or epidermal growth factor-like peptides, and 2) a paracrine factor(s) secreted by the oocyte. The oocyte factor enables the cumulus cells to respond to the gonadotropic/EGF signal to induce expression of several transcripts (Has2, Tnfaip6, Ptx3, and Ptgs2) required for formation and stability of the extracellular matrix (Diaz et al. 2006; Dragovic et al. 2005, 2007).
Numerous studies have shown that oocytes are potent stimulators of granulosa cell proliferation, which can interact with other mitogens in a species-specific manner (Vanderhyden et al. 1992; reviewed by Gilchrist et al. 2008). Oocytes were also found to secrete paracrine factors that inhibit granulosa cell LH receptor expression (Eppig et al. 1997) and progesterone production in murine, porcine, and bovine cumulus cells (Vanderhyden et al. 1993; Vanderhyden and Tonary 1995; Coskun et al. 1995; Vanderhyden and Macdonald, 1998; Li et al. 2000), both indicative of a role in suppression of luteinization. A comprehensive catalog of the granulosa/cumulus cell genes or functions regulated by oocyte-secreted factors has recently been compiled (Gilchrist et al. 2008). While the use of oocytectomy demonstrated the ability of oocytes to promote granulosa cell proliferation and follicular integrity (Vanderhyden et al. 1992), the procedure also revealed that some aspects of granulosa cell development, notably differentiation into cumulus cells, were not modulated by oocyte-secreted paracrine factors, but required intimate contact with the oocyte, presumably communicating through gap junctions (Vanderhyden et al. 1990).
Oocytes cannot grow in isolation from their granulosa cells, although limited development is possible when in co-culture with soluble factors from granulosa cells (Cecconi and Colonna 1996). The molecular mechanisms by which granulosa cells support oocyte development are not well defined, although one interesting study has demonstrated that the factors necessary to support oocyte development are conserved between rats and mice (Eppig and Wigglesworth 2000). By forming chimeric reaggregated ovaries with interspecific exchange of somatic and germ cell components, the authors showed that mouse oocytes grown in rat follicles underwent fertilization and subsequent embryonic and fetal development. Interestingly, species-specific characteristics of the oocytes were retained, despite the xenogeneic follicular environment. Similar reaggregation experiments using oocytes and somatic cells from different stages of mouse follicles demonstrated that the rate of follicle development is controlled by the oocyte (Eppig et al. 2002). That same group has shown that oocytes can regulate the granulosa cell expression of glycolytic enzymes, and hypothesize that oocytes use this ability to regulate granulosa cell metabolism to orchestrate the rate of follicular development (Sugiura and Eppig 2005).
Oocyte regulation of granulosa cell function: GDF9 and BMP15
The identity of the oocyte-secreted factors first hinted at by el-Fouly et al. in 1970 remained unknown for decades until the first candidate was revealed by the characterization of the oocyte-specific protein, growth-differentiation factor 9 (GDF9, McGrath et al. 1995). A second oocyte-specific member of the transforming growth factor β (TGFβ) superfamily, bone morphogenetic protein 15 (BMP15; also known as growth and differentiation factor 9b) was reported shortly thereafter (Dube et al. 1998; Laitinen et al. 1998). The subsequent demonstration that loss of expression of GDF9 or BMP15 has profound effects on ovarian function and fertility in mice and sheep (Dong et al. 1996; Galloway et al. 2000) reinvigorated this area of research and has resulted in a large variety of experimental models being investigated (reviewed by Gilchrist et al. 2008). As members of the TGFβ superfamily, the actions of GDF9 and BMP15 are mediated by members of the SMAD family of transcription factors (Kaivo-oja et al. 2006).
GDF9 is expressed in human and mouse ovaries and appears to be localized exclusively to oocytes at all stages of follicular growth, except primordial follicles, in neonatal and adult mice(Aaltonen et al. 1999, McGrath et al. 1995). In GDF9-deficient mouse ovaries, follicular development does not progress beyond the primary stage (Figure 2), but the oocytes within these follicles grow larger than normal(Dong et al. 1996; Ethier et al. 2005). Despite their size, the oocytes do not acquire full developmental competence(Elvin et al. 1999). The mutant ovaries also have elevated levels of Kitl mRNA(Elvin et al. 1999; Ethier et al. 2005), which suggests that GDF9 inhibits KITL expression and therefore may be the oocyte-secreted factor previously reported to regulate KITL expression (Joyce et al. 1999, 2000).
Significant species differences exist in the relative importance of BMP15 for fertility. In sheep, where this gene has been well studied, mutations in BMP15 have an interesting impact on fertility, with homozygous mutations resulting in infertility, whereas heterozygosity leads to an increased ovulation rate (Galloway et al. 2000). In contrast, Bmp15 null mice are fertile, suggesting a less critical role in rodent ovarian function (Yan et al. 2001). In the human, recombinant GDF9 has been shown to promote the development of primordial follicles to the secondary stage in culture, as well as to improve follicular survival(Hreinsson et al. 2002), suggesting the potential use of these factors in assisted reproduction technologies.
Initial investigations of GDF9 and BMP15 as potential candidates for the oocyte-secreted factors that regulate granulosa cell function raised more questions than answers (Vanderhyden et al. 2003), but recent strategies using neutralizing antibodies or inhibitors of specific signalling components have begun to provide clarification. Like the oocyte factor that is required for mouse cumulus expansion, GDF9 promotes the expansion of cumulus cells through induction of expression of Has2, Tnfaip6, Ptx3, and Ptgs2 (Elvin et al. 1999; Varani et al. 2002), but it appears that both GDF9 and BMP15 may be involved in this process (Dragovic et al. 2005; Gui and Joyce 2005; Yoshino et al. 2006). In rodents, both GDF9 and BMP15 promote proliferation of granulosa cells from small antral follicles(Hayashi et al. 1999; Otsuka et al. 2000; Vitt et al. 2000), and the growth-promoting actions of oocytes are mediated, at least in part, by GDF9 (Gilchrist et al. 2004b). Like oocytes, BMP15 has been reported to inhibit FSH-stimulated progesterone production by rat granulosa cells (Otsuka et al. 2000). However, both GDF9 and BMP15 are expressed as preproteins, and evidence that mouse oocytes do not secrete bioactive, processed BMP15 until just prior to ovulation (Yoshino et al. 2006) indicates that the progesterone-inhibiting actions of growing oocytes may not be due to BMP15.
It is clear that future studies will need to elucidate the cross-modulation between the TGFβ family members and the gonadotropins, the interplay between GDF9 and BMP15, and the regulatory factors that control their cleavage into active proteins. For example, evidence for interactions between GDF9, BMP15 and KITL has been reported: recombinant GDF9 inhibits Kitl mRNA expression in mouse preantral granulosa cells (Joyce et al. 2000), whereas BMP15 promotes Kitl expression in monolayers of granulosa cells from rat early antral follicles (Otsuka and Shimasaki 2002). Using intact murine oocyte-granulosa complexes in vitro, it has been shown that FSH regulates Bmp15 expression in a dose-dependent manner via KIT signalling (Thomas et al. 2005). The complexity of the oocyte-granulosa cell regulatory loops controlling follicular function will undoubtedly be compounded as the roles of additional factors are investigated, as exemplified by a recent study that showed cooperation between BMP15 and the oocyte-secreted fibroblast growth factor 8B (FGF8B) in regulating cumulus cell glycolysis (Sugiura et al. 2007).
Figure 3 summarizes our current understanding of the roles of TGFβ superfamily members in developing follicles. Although particular focus has been given in the past decade to members of this superfamily, and in particular to those factors that show relatively unique expression in the ovary, it should be noted that the role that these factors play in mediating oocyte-granulosa cell interactions is likely to become much more complex as we learn more about the regulation of their expression and proteolytic activation, the influence of hormones and follicular stage, and their interactions with other growth factors, as well as the species differences in the biological activity of these paracrine factors.
Fig. 3.
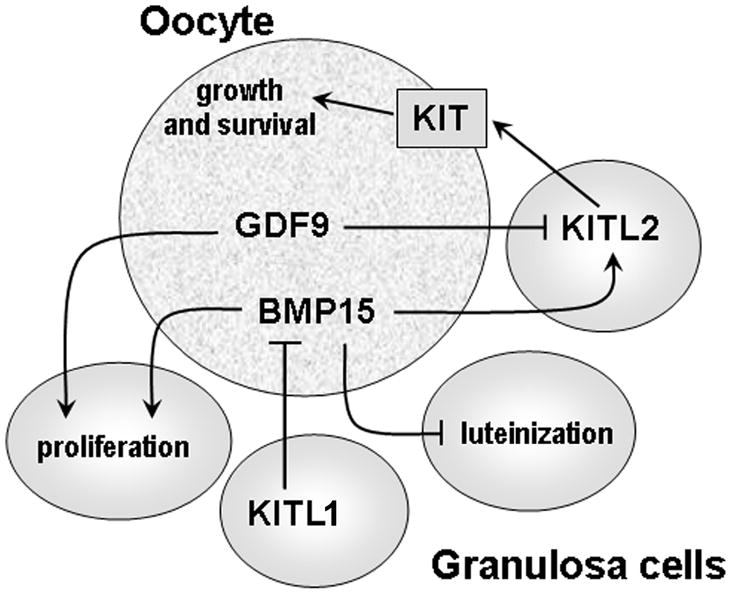
GDF9, BMP15 and KITL interactions during oocyte and follicular development. In rodents, KIT activation, primarily by KITL2, promotes oocyte growth and survival. Both GDF9 and BMP15 promote proliferation of granulosa cells from small antral follicles and BMP15 inhibits FSH-stimulated progesterone production and luteinization. GDF9 suppresses granulosa cell expression of both Kitl1 and Kitl2, whereas BMP15 stimulates Kitl expression in granulosa cells in both rats and mice. In turn, KITL inhibits Bmp15 expression in oocytes via KIT signalling.
Bi-directional Gap Junctional Communication between Oocytes and Granulosa Cells
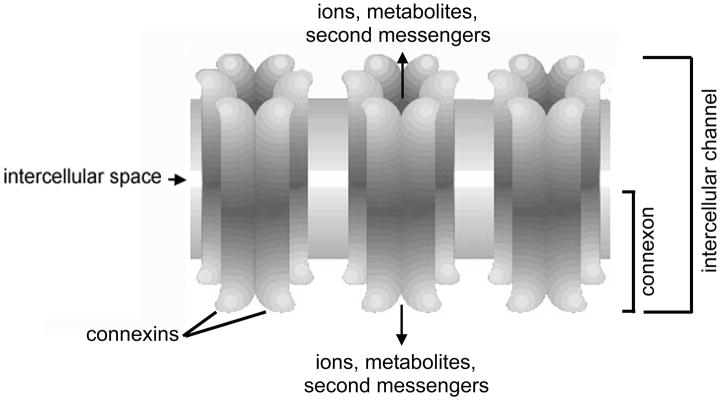
Gap junctions are specialized structures occurring at points of very close cell-cell contact; they consist of arrays of intercellular channels that allow direct sharing of small (less than ~ 1 kD) molecules between the cells (for a comprehensive review of gap junction structure, function, and regulation, see Harris 2001). Each intercellular channel consists of two hemichannels (connexons) that are docked end-to-end, with each cell contributing one hemichannel/connexon (Figure 4). Connexons are hexameric structures assembled from a large family of subunit proteins called connexins. Gap junction channels formed of different connexins are differentially permeable to molecules based on size and molecular charge. Furthermore, they are subject to regulation by different factors such as intracellular pH, divalent cations, and protein kinases. This diversity is proposed to tailor the channels for distinct physiological roles reflecting their expression in different cell types.
Fig. 4.
Basic elements of the gap junction. Each connexon (hemichannel) consists of six connexins oligomerized to form a cylindrical channel in the plasma membrane. When connexons from adjacent cells dock end-to-end, an intercellular channel is formed. A cluster of such intercellular membrane channels constitutes a gap junction. A variety of inorganic ions, small metabolites, and second messengers are able to pass through gap junctions channels, with restrictions on size and charge being imposed by the connexin composition.
At the onset of follicle growth in the mammalian ovary, gap junctions already connect oocytes with granulosa cells and granulosa cells with each other (Simon et al. 1997; Juneja et al. 1999; Pérez-Armendariz et al. 2003). These junctional connections couple the developing follicle into a functional syncytium from that point onward, allowing all cells inside the basal lamina to directly share metabolites and signalling molecules subject to the restrictions imposed by the properties of the constituent connexins. This intrafollicular metabolic coupling is required for oocyte growth as well as meiotic maturation as evidenced by the fact that its disruption retards or even arrests growth and maturation (Simon et al. 1997; Ackert et al. 2001; Johnson et al., 2007). Indeed, many of the molecules known to be transferred to the growing oocyte from the granulosa cells via gap junction channels are nutritional in nature: these include amino acids, glucose, and ribonucleosides (reviewed by Eppig 1991; Eppig et al. 1996; Sugiura and Eppig 2005) as summarized in Table 2. The gap junctional connection also allows the granulosa cells to use their own ion transport mechanisms to regulate the pH of the oocyte, a capacity the oocyte itself does not possess until fully grown (FitzHarris and Baltz 2006; FitzHarris et al. 2007). Importantly, signals that regulate meiotic maturation of fully-grown oocytes pass through the oocyte-granulosa cell gap junctions (reviewed by Edry et al. 2006): the current view is that LH causes MAP kinase-dependent phosphorylation and disruption of the gap junctions coupling the mural with the cumulus granulosa cells, restricting the flow of meiosis-arresting cGMP into the oocyte (Norris et al. 2008, 2009; Sasseville et al. 2009). Signalling in the opposite direction, the growing oocyte influences the differentiation of the surrounding granulosa cells via the gap junctional pathway: disruption of oocyte-granulosa cell gap junctional coupling in the mouse causes loss of the oocyte and, consequently, premature luteinisation of the granulosa cells such that a mature preovulatory follicle is never formed (Simon et al. 1997).
Table 2.
Evidence for specific inorganic ions and organic molecules passing from granulosa cells to oocytes via gap junctions.*
| Ion or molecule | Reference |
|---|---|
| Na+, Cl− | Arellano et al. 2002 |
| cAMP | Bornslaeger and Schultz 1985; Salustri et al. 1985 |
| cGMP | Norris et al. 2009 |
| various ribonucleosides | Heller and Schultz 1980; Moor et al. 1980; Heller et al. 1981; Colonna and Mangia 1983 |
| various amino acids | Colonna and Mangia 1983; Haghighat and Van Winkle 1990; Eppig et al. 2005 |
| 2-deoxyglucose | Brower and Schultz 1982 |
| choline | Moor et al. 1980; Heller et al. 1981 |
| inositol | Moor et al. 1980 |
For organic molecules, where transfer to the oocyte was monitored using radioisotopic labeling, it remains possible that the molecule passing through the oocyte-granulosa cell gap junctions was actually a metabolite of the molecule taken up by the granulosa cells.
Roles of specific connexins in oogenesis: evidence from genetically modified mice
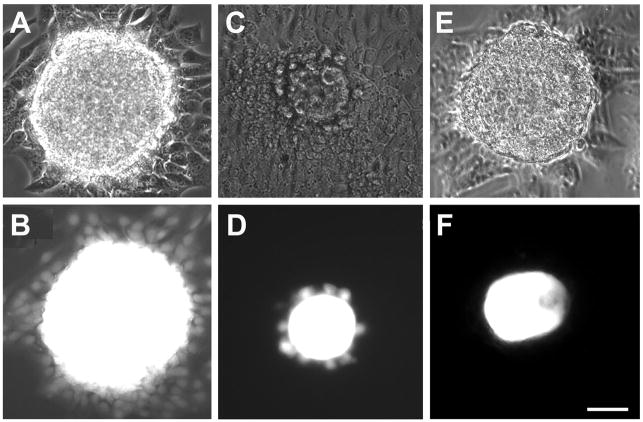
Much of our knowledge of the roles of gap junctional communication in oogenesis and other developmental processes has come from analysis of knockout mice lacking specific connexins. Of the ~20 connexins encoded in mammalian genomes, only connexin37 (CX37) and connexin43 (CX43) have been unequivocally demonstrated to play critical roles in oogenesis. CX43 is continuously expressed in fetal mouse ovaries from the onset of ovarian differentiation (Juneja et al. 1999; Pérez-Armendariz et al. 2003). In growing follicles, its expression increases in response to the rising level of FSH; it subsequently declines in response to the LH surge that triggers the onset of meiosis and ovulation (reviewed by Granot and Dekel 2002). CX43 forms numerous large gap junctions between granulosa cells which are therefore highly coupled metabolically until LH causes the closure of the channels via MAP kinase-dependent phosphorylation of CX43 (Norris et al. 2008). Because its ablation by gene targeting completely abolished coupling among the granulosa cells (see Figure 5), it was concluded that CX43 alone contributes to gap junction channels, at least in preantral granulosa cells (Gittens et al. 2003; Tong et al. 2006). Ablation of CX43 did not, however, disrupt coupling between oocytes and granulosa cells indicating that this connexin, although present at or very near the oocyte surface (Wong et al. 2000; Kidder and Mhawi 2002), does not normally contribute to gap junctions at that location at a level that is essential for supporting oogenesis (Veitch et al. 2004; Gittens and Kidder 2005). Folliculogenesis is disrupted in CX43 null mutant ovaries (Figure 2) with slowing of both oocyte and follicle growth, indicating a requirement for gap junctional coupling among the granulosa cells via CX43-containing channels to sustain granulosa cell proliferation (Ackert et al. 2001; Tong et al. 2006). Oocytes obtained from CX43-deficient follicles were determined to be morphologically abnormal and developmentally incompetent: they are vacuolated, their zonae pellucidae are poorly developed, cortical granules are absent, they fail to achieve meiotic competence, and they can not be fertilized in vitro (Ackert et al. 2001). These findings reinforce the importance of a well coupled, growing granulosa cell population for growth and differentiation of the oocyte. It should be noted that, in later stages of follicle development in the mouse and in follicles of other mammalian species, additional connexins have been identified in granulosa cells, but none has yet been shown to play an essential role in folliculogenesis or oogenesis (Kidder 2005).
Fig. 5.
Gap junctions couple oocytes and granulosa cells into a functional syncytium. A, C, and E are phase contrast micrographs of cultured oocyte-granulosa cell complexes while B, D, and F are the corresponding fluorescence micrographs. (A,B) The existence of gap junctional coupling is revealed by spreading of fluorescent dye (Lucifer yellow), injected into the oocyte, throughout the surrounding granulosa cell population of a wildtype complex. (C,D) In CX43 null mutant follicles, dye injected into the oocyte is restricted from spreading beyond the first layer of granulosa cells due to the absence of gap junctions in the granulosa cells. (E,F) In CX37 null mutant follicles, the injected dye fails to pass into the granulosa cells owing to the absence of gap junctions linking them with the oocyte. Scale bars, 50 μm. Micrographs from Veitch et al. (2004) and Li et al. (2007) are republished with permission from the Company of Biologists Ltd.
Although the weight of evidence indicates that CX43 does not contribute to the gap junctions connecting oocytes and granulosa cells of the mouse, this does not necessarily mean that it is not expressed in oocytes and makes no direct contribution to oocyte quality. Gershon et al. (2008) generated mice in which the Gja1 gene encoding this connexin was specifically deleted in oocytes (there was also considerable loss from granulosa cells, but folliculogenesis and ovulation were not affected). As with the germline Gja1 knockout (Veitch et al. 2004), coupling between oocytes and granulosa cells was not disrupted. Post-fertilization development to the blastocyst stage of embryos derived from the CX43-depleted oocytes was normal. Surprisingly, however, some of those blastocysts failed to implant, resulting in an almost 40% reduction of litter size. It is important to note that CX43 is expressed in the embryo from the 2-cell stage onward (De Sousa et al. 1993) and that CX43-null mutant blastocysts, obtained by crossing Gja1−/+ males with Gja1−/+ females and thus possessing a 50% complement of oogenetic CX43, implant normally despite lacking the ability to express CX43 embryonically (Reaume et al. 1995; De Sousa et al. 1997). Thus it is possible that oogenetic CX43, although not participating in gap junction formation at the oocyte surface, contributes to oocyte quality in a way that becomes manifest at the time of implantation of the resulting embryo. What that contribution might be- and why it can not be provided by embryonic expression of CX43- must await further experimentation, but additional functions for connexins, independent of gap junction formation, have been demonstrated in other cell types (reviewed by Goodenough and Paul 2003; Stout et al. 2004).
One of the additional ways that connexins have been proposed to contribute to developmental or physiological processes is via undocked connexons residing in non-junctional plasma membrane regions. It has been suggested that regulated opening of such gap junction “hemichannels” could allow small signalling molecules to exit the cell to the extracellular space where they can interact with receptors on nearby cells that are not necessarily coupled with the source cell via gap junctions. For example, there is accumulating evidence that ATP released via opening of gap junction hemichannels can activate purinergic receptors on neighbouring cells, providing a pathway for the propagation of intercellular Ca2+ waves (reviewed by Evans et al. 2006). This could have relevance for signalling within developing follicles since extracellular ATP is known to act through P2 purinergic receptors to induce Ca2+ release within granulosa cells (Tai et al. 2000), an effect that in turn leads to a Ca2+ increase in the oocyte (Webb et al. 2002). These considerations led to the hypothesis that the essential function of CX43 in oocyte and follicle development is to enable intercellular signalling via hemichannel release of ATP rather than (or in addition to) via gap junctional communication. This hypothesis was tested by Tong et al. (2007), who constructed recombinant, reaggregated mouse ovaries in which wildtype oocytes were combined with granulosa cells homozygously expressing either wildtype CX43 or a mutant form that assembles into functional plasma membrane hemichannels but is incapable of docking to form intercellular gap junction channels (both forms of CX43 were shown to allow regulated release of ATP from the cells). The result was that granulosa cells exclusively assembling CX43 hemichannels were not capable of supporting folliculogenesis. Thus, whatever role might be played by CX43 hemichannels in oocyte and follicle development, they can not supplant the need for CX43 gap junction channels. Whether CX43 hemichannel activity exists in the oocyte and is essential for post-fertilization events remains to be determined.
In contrast to the gap junctions linking granulosa cells with each other, those linking the oocyte with surrounding granulosa cells are composed of CX37: immunostaining for this connexin in the mouse revealed distinct gap junction plaque-like structures on the oocyte surface, beneath the zona pellucida (Simon et al. 1997; Veitch et al. 2004). CX37 could be seen in this location from the onset of follicle growth. Ablation of CX37 removed all gap junctions from the oocyte surface and disrupted oocyte-granulosa cell coupling (Figure 5), but there was no effect on coupling among the granulosa cells (Simon et al. 1997; Veitch et al. 2004). Mice lacking CX37 are viable and the males are fertile but in the females, folliculogenesis proceeds until a late preantral stage when oocyte growth ceases prematurely. The vast majority of null mutant oocytes failed to undergo nuclear envelop breakdown when released from the follicles, indicating that their growth ceases before acquisition of full meiotic competence (Simon et al. 1997; Carabatsos et al. 2000). Eventually, the oocytes degenerate and the mutant ovaries become populated with small corpora lutea, indicating that the granulosa cells had differentiated prematurely to become luteal cells (Figure 2). Thus it is likely that signals passing from the oocyte to the granulosa cells via CX37 channels are required to maintain the differentiated state of the granulosa cells, preventing them from luteinizing before ovulation.
The different locations of CX37 and CX43 gap junction channels in the oocyte-granulosa cell complex, taken together with the distinct biophysical, regulatory, and permeability properties of these two channel types (summarized in Li et al. 2007), suggest that different molecules pass through the channels and/or the channels are differentially regulated in these two locations. If so, then a change in the connexin composition of the oocyte-granulosa cell gap junctions might be expected to impair oogenesis. Li et al. (2007) tested this hypothesis by generating mice in which the gap junctions coupling growing oocytes with adjacent granulosa cells were assembled from CX43 instead of CX37. Despite this “connexin switch”, oogenesis occurred normally and the females were fertile. Since CX43 channels are less selective (i.e. permeable to a wider range of molecules) than CX37 channels, it is possible that the only requirement for gap junction channels coupling oocytes with granulosa cells is that they are permeable to a specific set of key molecules involved in oocyte growth, meiotic control, and regulation of granulosa cell function.
To summarize, gap junctional communication within the developing follicle, both between oocytes and granulosa cells and among the granulosa cells themselves, maintains the follicle in a functionally integrated state. Ions and/or molecules passing from the granulosa cells into the oocyte are required for oocyte growth, maturation, and survival while ions and/or molecules passing from the oocyte into the granulosa cells are required for maintaining the latter in the appropriate differentiated state until LH triggers ovulation and luteinisation. The challenge now is to identify the key ions and molecules passing through this functional syncytium and to determine what precise roles they play in contributing to oocyte developmental competence and regulating granulosa cell proliferation and differentiation.
Integration of Signalling Pathways within the Oocyte-Granulosa Cell Complex
The evidence summarized above makes it clear that both paracrine signalling and gap junctional communication within the developing ovarian follicle are essential for oogenesis. Do these two very different types of signalling pathway interact? Recent evidence suggests that they do, although we are only beginning to understand how the interaction occurs.
Gap junctional communication is required for optimal response to paracrine factors in various cell types including ovarian granulosa cells. In the early mammalian embryo, NODAL signalling is preferentially activated in the left lateral plate of the mesoderm while being repressed by FGF8 in the right lateral plate, establishing left-right asymmetry. Feistel and Blum (2008) concluded that attenuation of gap junctional communication within the left lateral plate is required for NODAL activation whereas its maintenance in the right lateral plate is required for FGF8 action. In osteoblasts, Lima et al. (2009) showed that that CX43 is required for the cells to respond to FGF2 by expressing osteocalcin. The results of both of these studies are consistent with the hypothesis that the role of gap junctions is to relay signals downstream of the FGF receptor to neighbouring cells, thus coordinating the cells’ response.
The same interpretation has been applied to results of experiments examining the relationship between GDF9 signalling and gap junctional communication in developing follicles (Gittens et al., 2005). As mentioned above, the principal effect of CX43 loss in the ovary is attenuation of granulosa cell proliferation. On the C57BL/6 genetic background in the mouse, the effects of deleting the genes encoding CX43 and GDF9 are essentially the same: follicles cease growing in the primary stage (Dong et al. 1996; Ackert et al. 2001; Figure 2). This comparison implies that the GDF9 signalling pathway and CX43-mediated gap junctional communication interact, a possibility that was first explored by analysis of the effects of each knockout on the other gene’s expression (Gittens et al., 2005). It was found that CX43 gene expression and gap junctional communication are unaffected by the loss of GDF9, and GDF9 expression persists in the absence of CX43. However, the granulosa cells failed to respond normally to oocyte-derived GDF9 when CX43 null mutant follicles were cultured in vitro, resulting in a reduced rate of proliferation. Proliferation could be restored to the wildtype level by addition of recombinant GDF9 to the culture medium. Thus it was concluded that the proliferation deficit of CX43 null mutant granulosa cells is due at least in part to reduced responsiveness of the population to oocyte-derived GDF9. Given that follicle growth results in the outer layers of granulosa cells being at increasing distance from the oocyte and thus being exposed to lower concentrations of GDF9, it is proposed that gap junctions are required for relaying GDF9-induced signalling molecules from the proximal to the distal granulosa cells, coordinating proliferation throughout the follicle.
Implications for Female Fertility
Given the importance of oocyte-granulosa cell intercommunication in ensuring the developmental competence of rodent and other animal oocytes, it is important to determine if the same is true in the human. There are several suggestions in the literature that the paracrine factors discussed in this review are important for human female fertility. Increased KITL in follicular fluid in patients undergoing in vitro fertilization (IVF) has been correlated with successful pregnancies (Smikle et al. 1998). A decrease in GDF9 mRNA has been reported in polycystic ovary disease, a common cause of female infertility (Teixeira Filho et al. 2002), suggesting a potential role in human folliculogenesis. Rare mutations in both GDF9 and BMP15 contribute to premature ovarian failure (premature menopause) (Di Pasquale et al. 2004, 2006; Dixit et al. 2005, 2006; Laissue et al. 2006), and GDF9 mutations are associated with dizygotic twinning (Montgomery et al. 2004; Palmer et al. 2006). There is also some evidence that certain polymorphisms in the BMP15 gene can predispose women undergoing fertility treatment to over-respond to FSH (Moron et al. 2006).
To date, there is no direct evidence linking mutations in connexin-encoding genes with human female infertility. There is, however, evidence associating CX43 expression and the strength of gap junctional coupling among cumulus granulosa cells with the success of IVF. Wang et al. (2009) measured CX43 level and intercellular electrical conductance provided by gap junction channels in cultured cumulus cells obtained from patients undergoing IVF. Pooled cumulus cells were obtained from the oocytes retrieved from each patient. There was a significant positive association between pregnancy rate and either the level of expression of CX43 or the strength of gap junctional intercellular conductance: those patients scoring above the population mean for either measurement were more likely to become pregnant from the procedure. Assuming that these parameters reflect the situation within the developing follicle before oocyte retrieval, the results imply that CX43-mediated gap junctional coupling among granulosa cells is a determinant of oocyte developmental competence in humans as it is in mice.
It is possible, perhaps even likely, that there are defects in intrafollicular signalling pathways affecting human female fertility that have gone un-noticed because they render the patient subfertile, but not infertile. As an example, a recent analysis (Tong et al. 2009) of oogenesis in mice that model the human dysmorphogenesis syndrome, oculodentodigital dysplasia (ODDD), revealed subfertility that, with one exception, has not been reported in human females with the same syndrome. ODDD is caused by mutations in the GJA1 gene that encodes CX43. The ODDD patient population exhibits a broad and variable range of health problems, reflecting the widespread expression of CX43 in the bodies of mammals, in addition to a consistent set of eye, teeth, and digitalabnormalities (reviewed by Laird 2008). The mutant mouse line, which exhibits all of the diagnostic features of human ODDD, carries a dominant mutation (the Gja1Jrt allele) that causes a single amino acid substitution (G60S) in CX43 (Flenniken et al. 2005). The females of this mutant strain, while not infertile, produce smaller litters than their wildtype littermates (Tong et al. 2009). The reduction in litter size is at least partially due to a reduction in the number of follicles reaching the preovulatory stage. Thus while CX43 knockout ovaries produce few if any mature follicles (Ackert et al. 2001; Tong et al 2006), Gja1Jrt/+ ovaries produce a reduced number (~30% of wildtype), rendering the females subfertile (Tong et al 2009). These findings imply that ODDD human females could have undetected subfertility related to an intra-ovarian signalling deficit, and that such females merit closer gynaecological assessment.
Acknowledgments
Work from the Kidder laboratory cited in this review was funded by grants from the Medical Research Council of Canada, the Canadian Institutes of Health Research (CIHR), and the Childrens’ Health Research Institute, London, Ontario. Work from the Vanderhyden laboratory was funded as part of the Program on Oocyte Health under the Healthy Gametes and Great Embryos Strategic Initiative of the CIHR Institute of Human Development, Child and Youth Health.
References
- Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppa L, Louhio H, Tuuri T, Sjoberg J, Butzow R, Hovata O, Dale L, Ritvos O. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84:2744–2750. doi: 10.1210/jcem.84.8.5921. [DOI] [PubMed] [Google Scholar]
- Ackert CL, Gittens JEI, O’Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233:258–270. doi: 10.1006/dbio.2001.0216. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Tajima K, Frajese V, Seger R. Analysis of signal transduction stimulated by gonadotropins in granulosa cells. Mol Cell Endocrinol. 2003;202:77–80. doi: 10.1016/s0303-7207(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Martínez-Torres A, Garay E. Ionic currents activated via purinergic receptors in the cumulus cell-enclosed mouse oocyte. Biol Reprod. 2002;67:837–846. doi: 10.1095/biolreprod.102.003889. [DOI] [PubMed] [Google Scholar]
- Bedell MA, Brannan CI, Evans EP, Copeland NG, Jenkins NA, Donovan PJ. DNA rearrangements located over 100 kb 5′ of the Steel (Sl)-coding region in Steel-panda and Steel-contrasted mice deregulate Sl expression and cause female sterility by disrupting ovarian follicle development. Genes Dev. 1995;9:455–470. doi: 10.1101/gad.9.4.455. [DOI] [PubMed] [Google Scholar]
- Beechey CVB, Searle AG. Male-fertile black-eyed white at Sl locus. Mouse News Lett. 1985;73:17. [Google Scholar]
- Beechey CV, Searle AG. Contrasted, a steel allele in the mouse with intermediate effects. Genet Res. 1983;42:183–191. doi: 10.1017/s0016672300021649. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Schultz RM. Regulation of mouse oocyte maturation: effect of elevating cumulus cell cAMP on oocyte cAMP levels. Biol Reprod. 1985;33:698–704. doi: 10.1095/biolreprod33.3.698. [DOI] [PubMed] [Google Scholar]
- Brannan CI, Lyman SD, Williams DE, Eisenman J, Anderson DM, Cosman D, Bedell MA, Jenkins NA, Copeland NG. Steel-Dickie mutation encodes a c-kit ligand lacking transmembrane and cytoplasmic domains. Proc Natl Acad Sci USA. 1991;88:4671–4674. doi: 10.1073/pnas.88.11.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower PT, Schultz RM. Intercellular communication between granulosa cells and mouse oocytes: existence and possible nutritional role during oocyte growth. Dev Biol. 1982;90:144–153. doi: 10.1016/0012-1606(82)90219-6. [DOI] [PubMed] [Google Scholar]
- Brower PT, Schultz RM. Intercellular communication between granulosa cells and mouse oocytes: existence and possible nutritional role during oocyte growth. Dev Biol. 1982;90:144–153. doi: 10.1016/0012-1606(82)90219-6. [DOI] [PubMed] [Google Scholar]
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol. 1990;138:16–25. doi: 10.1016/0012-1606(90)90172-f. [DOI] [PubMed] [Google Scholar]
- Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226:167–179. doi: 10.1006/dbio.2000.9863. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Cecconi S, Colonna R. Influence of granulosa cells and of different somatic cell types on mammalian oocyte development in vitro. Zygote. 1996;4:305–307. doi: 10.1017/s0967199400003294. [DOI] [PubMed] [Google Scholar]
- Colonna R, Mangia F. Mechanisms of amino acid uptake in cumulus-enclosed mouse oocytes. Biol Reprod. 1983;28:797–803. doi: 10.1095/biolreprod28.4.797. [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol. 2006;20:715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- Coskun S, Uzumcu M, Lin YC, Friedman CI, Alak BM. Regulation of cumulus cell steroidogenesis by the porcine oocyte and preliminary characterization of oocyte-produced factor(s) Biol Reprod. 1995;53:670–675. doi: 10.1095/biolreprod53.3.670. [DOI] [PubMed] [Google Scholar]
- Da Silva-Buttkus P, Jayasooriya GS, Mora JM, Mobberley M, Ryder TA, Baithun M, Stark J, Franks S, Hardy K. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J Cell Sci. 2008;121:3890–3900. doi: 10.1242/jcs.036400. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol. 2001;229:224–236. doi: 10.1006/dbio.2000.9947. [DOI] [PubMed] [Google Scholar]
- De Sousa PA, Valdimarsson G, Nicholson BJ, Kidder GM. Connexin trafficking and the control of gap junction assembly in mouse preimplantation embryos. Development. 1993;117:1355–1367. doi: 10.1242/dev.117.4.1355. [DOI] [PubMed] [Google Scholar]
- De Sousa PA, Juneja SC, Caveney S, Houghton FD, Davies TC, Reaume AG, Rossant J, Kidder GM. Normal development of preimplantation mouse embryos deficient in gap junctional coupling. J Cell Sci. 1997;110:1751–1758. doi: 10.1242/jcs.110.15.1751. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet. 2004;75:106–111. doi: 10.1086/422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, Einaudi S, Radetti G, Russo G, Sacco M, Wasniewska M, Cole T, Beck-Peccoz P, Nelson LM, Persani L. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab. 2006;91:1976–1979. doi: 10.1210/jc.2005-2650. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, O’Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol. 2006;299:91–104. doi: 10.1016/j.ydbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao L, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty B, Singh L. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause. 2005;12:749–754. doi: 10.1097/01.gme.0000184424.96437.7a. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty B, Singh L. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet. 2006;119:408–415. doi: 10.1007/s00439-006-0150-0. [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Armstrong DT, Gilchrist RB. Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology. 2005;146:2798–2806. doi: 10.1210/en.2005-0098. [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB. Oocyte-secreted factor activation of SMAD 2/3 signalling enables initiation of mouse cumulus cell expansion. Biol Reprod. 2007;76:848–857. doi: 10.1095/biolreprod.106.057471. [DOI] [PubMed] [Google Scholar]
- Drummond AE, Dyson M, Le MT, Ethier JF, Findlay JK. Ovarian follicle populations of the rat express TGF-β signalling pathways. Mol Cell Endocrinol. 2003;202:53–57. doi: 10.1016/s0303-7207(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- Edry I, Sela-Abramovich S, Dekel N. Meiotic arrest of oocytes depends on cell-to-cell communication in the ovarian follicle. Mol Cell Endocrinol. 2006;252:102–106. doi: 10.1016/j.mce.2006.03.009. [DOI] [PubMed] [Google Scholar]
- el-Fouly MA, Cook B, Nekola M, Nalbandov AV. Role of the ovum in follicular luteinization. Endocrinology. 1970;87:288–293. [PubMed] [Google Scholar]
- Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicular defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol. 1999;13:1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. BioEssays. 1991;13:569–574. doi: 10.1002/bies.950131105. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K. Development of mouse and rat oocytes in chimeric reaggregated ovaries after interspecific exchange of somatic and germ cellcomponents. Biol Reprod. 2000;63:1014–1023. doi: 10.1095/biolreprod63.4.1014. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O’Brien M, Wigglesworth K. Mammalian oocyte growth and development in vitro. Mol Reprod Dev. 1996;44:260–273. doi: 10.1002/(SICI)1098-2795(199606)44:2<260::AID-MRD17>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56:976–894. doi: 10.1095/biolreprod56.4.976. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci USA. 2002;99:2890–2894. doi: 10.1073/pnas.052658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod. 2005;73:351–357. doi: 10.1095/biolreprod.105.041798. [DOI] [PubMed] [Google Scholar]
- Ethier JF, Thomas FH, Vanderhyden BC. Initiation of oocyte growth in growth and differentiation factor-9 (GDF-9) deficient mice precedes both initiation of follicle development and the increase in Kit ligand (KL) mRNA expression. Biol Reprod. 2005;(Special Issue) Abstract 420. [Google Scholar]
- Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feistel K, Blum M. Gap Junctions relay FGF8-mediated right-sided repression of Nodal in rabbit. Dev Dynam. 2008;237:3516–3527. doi: 10.1002/dvdy.21535. [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Baltz JM. Granulosa cells regulate intracellular pH of the murine growing oocyte via gap junctions: development of independent homeostasis during oocyte growth. Development. 2006;133:591–599. doi: 10.1242/dev.02246. [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Siyanov V, Baltz JM. Granulosa cells regulate oocyte intracellular pH against acidosis in preantral follicles by multiple mechanisms. Development. 2007;134:4283–4295. doi: 10.1242/dev.005272. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990;63:185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JEI, Gong XQ, Kelsey LB, Lounsbury C, Moreno L, Nieman BJ, Peterson K, Qu D, Roscoe W, Shao Q, Tong D, Veitch GIL, Voronina I, Vukobradovic I, Wood G, Zhu Y, Zirngibl R, Aubin JE, Bai D, Bruneau B, Grynpas M, Henderson J, Henkelman RM, McKerlie C, Sled JG, Stanford WL, Laird DW, Kidder GM, Adamson SL, Rossant J. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development. 2005;132:4375–4386. doi: 10.1242/dev.02011. [DOI] [PubMed] [Google Scholar]
- Galloway M, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- Geissler EN, McFarland EC, Russell ES. Analysis of pleiotropism at the dominant white-spotting (W) locus of the house mouse: a description of ten new W alleles. Genetics. 1981;97:337–361. doi: 10.1093/genetics/97.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Gershon E, Plaks V, Aharon I, Galiani D, Reizel Y, Sela-Abramovich S, Granot I, Winterhager E, Dekel N. Oocyte-directed depletion of connexin43 using the Cre-LoxP system leads to subfertility in female mice. Dev Biol. 2008;313:1–12. doi: 10.1016/j.ydbio.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte–somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004a;82–83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Cranfield M, Jeffery LA, Amato F, Scott SJ, Myllymaa S, Kaivo-Oja N, Lankinen H, Mottershead DG, Groome NP, Ritvos O. Immunoneutralization of growth differentiation factor 9 reveals it partially accounts for mouse oocyte mitogenic activity. Biol Reprod. 2004b;71:732–739. doi: 10.1095/biolreprod.104.028852. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- Gittens JEI, Kidder GM. Differential contributions of connexin37 and connexin43 to oogenesis revealed in chimeric reaggregated mouse ovaries. J Cell Sci. 2005;118:5071–5078. doi: 10.1242/jcs.02624. [DOI] [PubMed] [Google Scholar]
- Gittens JEI, Mhawi AA, Lidington D, Ouellette Y, Kidder GM. Functional analysis of gap junctions in ovarian granulosa cells: distinct role for connexin43 in early stages of folliculogenesis. Am J Physiol-Cell Physiol. 2003;284:C880–C887. doi: 10.1152/ajpcell.00277.2002. [DOI] [PubMed] [Google Scholar]
- Gittens JEI, Barr KJ, Vanderhyden BC, Kidder GM. Interplay between paracrine signalling and gap junctional communication in ovarian follicles. J Cell Sci. 2005;118:113–122. doi: 10.1242/jcs.01587. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexin channels. Nature Rev Mol Cell Biol. 2003;4:1–10. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Granot I, Dekel N. The ovarian gap junction protein connexin43: regulation by gonadotropins. Trends Endocrinol Metab. 2002;13:310–313. doi: 10.1016/s1043-2760(02)00623-9. [DOI] [PubMed] [Google Scholar]
- Gui LM, Joyce IM. RNA interference evidence that growth differentiation factor-9 mediates oocyte regulation of cumulus expansion in mice. Biol Reprod. 2005;72:195–199. doi: 10.1095/biolreprod.104.033357. [DOI] [PubMed] [Google Scholar]
- Haghighat N, Van Winkle LJ. Developmental change in follicular cell-enhanced amino acid uptake into mouse oocytes that depends on intact gap junctions and transport system gly. J Exp Zool. 1990;253:71–82. doi: 10.1002/jez.1402530110. [DOI] [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: Biophysics fills the gap. Quart Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- Hayashi M, McGee EA, Min G, Klein C, Rose UM, van Duin M, Huseh AJ. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology. 1999;140:1236–1244. doi: 10.1210/endo.140.3.6548. [DOI] [PubMed] [Google Scholar]
- Heller DT, Schultz RM. Ribonucleoside metabolism by mouse oocytes: metabolic cooperativity between the fully grown oocyte and cumulus cells. J Exp Zool. 1980;214:355–364. doi: 10.1002/jez.1402140314. [DOI] [PubMed] [Google Scholar]
- Heller DT, Cahill DM, Schultz RM. Biochemical studies of mammalian oogenesis: Metabolic cooperativity between granulosa cells and growing mouse oocytes. Dev Biol. 1981;84:455–464. doi: 10.1016/0012-1606(81)90415-2. [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura I, Taii S, Narimoto K, Noda Y, Nishikawa S, Nakayama H, Fujita J, Mori T. The expression of c-kit protein during oogenesis and early embryonic development. Biol Reprod. 1991;45:547–552. doi: 10.1095/biolreprod45.4.547. [DOI] [PubMed] [Google Scholar]
- Horie K, Fujita J, Takakura K, Kanzaki H, Suginami H, Iwai M, Nakayama H, Mori T. The expression of c-kit protein in human adult and fetal tissues. Human Reprod. 1993;8:1955–1962. doi: 10.1093/oxfordjournals.humrep.a137967. [DOI] [PubMed] [Google Scholar]
- Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ, Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab. 2002;87:316–321. doi: 10.1210/jcem.87.1.8185. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the Kit-ligand: KL-1 and KL-2. Mol Biol Cell. 1992;3:349–362. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P. The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol. 1993;157:100–109. doi: 10.1006/dbio.1993.1115. [DOI] [PubMed] [Google Scholar]
- Hubbard GM, Erickson GF. Luteinizing hormone-dependent luteinization and ovulation in the hypophysectomized rat: a possible role for the oocyte? Biol Reprod. 1988;39:183–1194. doi: 10.1095/biolreprod39.1.183. [DOI] [PubMed] [Google Scholar]
- Ismail R, Okawara J, Fryer JN, Vanderhyden BC. Hormonal regulation of the ligand for c-kit in the ovary and its effects on spontaneous oocyte meiotic maturation. Mol Reprod Dev. 1996;43:458–469. doi: 10.1002/(SICI)1098-2795(199604)43:4<458::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Jin X, Han CS, Yu FO, Wei P, Hu ZY, Liu YX. Anti-apoptotic action of stem cell factor on oocytes in primordial follicles and its signal transduction. Mol Reprod Dev. 2005a;70:82–90. doi: 10.1002/mrd.20142. [DOI] [PubMed] [Google Scholar]
- Jin X, Han CS, Zhang XS, Yuan JX, Hu ZY, Liu YX. Signal transduction of stem cell factor in promoting early follicle development. Mol Cell Endocrinol. 2005b;229:3–10. doi: 10.1016/j.mce.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Johnson MT, Freeman EA, Gardner DK, Hunt PA. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod. 2007;77:2–8. doi: 10.1095/biolreprod.106.059899. [DOI] [PubMed] [Google Scholar]
- Joyce IM, Pendola FL, Wigglesworth K, Eppig JJ. Oocyte regulation of Kit Ligand expression in mouse ovarian follicles. Dev Biol. 1999;214:342–353. doi: 10.1006/dbio.1999.9437. [DOI] [PubMed] [Google Scholar]
- Joyce IM, Clark AT, Pendola FL, Eppig JJ. Comparison of recombinant growth differentiation factor-9 and oocyte regulation of Kit Ligand messenger ribonucleic acid expression in mouse ovarian follicles. Biol Reprod. 2000;63:1669–1675. doi: 10.1095/biolreprod63.6.1669. [DOI] [PubMed] [Google Scholar]
- Juneja SC, Barr KJ, Enders GC, Kidder GM. Defects in the germ line and gonads of mice lacking connexin43. Biol Reprod. 1999;60:1263–1270. doi: 10.1095/biolreprod60.5.1263. [DOI] [PubMed] [Google Scholar]
- Kaivo-oja N, Jeffery LA, Ritvos O, Mottershead DG. Smad signalling in the ovary. Reprod Biol Endocrinol. 2006;4(21) doi: 10.1186/1477-7827-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder GM. The genetic program for preimplantation development. Dev Genet. 1992;13:319–325. doi: 10.1002/dvg.1020130502. [DOI] [PubMed] [Google Scholar]
- Kidder GM. Roles of gap junctions in ovarian folliculogenesis: implications for female infertility. In: Winterhager E, editor. Gap Junctions in Development and Disease. Heidelberg, Germany: Springer Verlag; 2005. pp. 223–237. [Google Scholar]
- Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123:613–620. doi: 10.1530/rep.0.1230613. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shirouzu M, Kamiya A, Hashimoto K, Yokoyama S, Miyajima A. Raf/MAPK and rapamycin-sensitive pathways mediate the anti-apoptotic function of p21ras in IL-3-dependent hematopoietic cells. Oncogene. 1997;15:619–627. doi: 10.1038/sj.onc.1201234. [DOI] [PubMed] [Google Scholar]
- Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow SR, Manova K, Besmer P. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signalling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 2000;19:1312–1326. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger FG, De Felici M. In vitro development of growing oocytes from fetal mouse oocytes: stage-specific regulation by stem cell factor and granulosa cells. Dev Biol. 2002;244:85–95. doi: 10.1006/dbio.2002.0592. [DOI] [PubMed] [Google Scholar]
- Kohrogi T, Yokoyama M, Taguchi T, Kitamura Y, Tutikawa K. Effect of the Slt mutant allele on the production of tissue mast cells in mice. J Hered. 1983;74:375–377. doi: 10.1093/oxfordjournals.jhered.a109814. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Diez-Fraile A, Criel G, Svistunov AA, Vandenabeele P, D’Herde K. Life and death of female gametes during oogenesis and folliculogenesis. Apotosis. 2008;13:1065–1087. doi: 10.1007/s10495-008-0238-1. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Terada N, Nakayama H, Matsumoto K, Kitamura Y. Infertility due to growth arrest of ovarian follicles in Sl/Slt mice. Dev Biol. 1988;126:71–79. doi: 10.1016/0012-1606(88)90240-0. [DOI] [PubMed] [Google Scholar]
- Kurosawa K, Miyazawa K, Gotoh A, Katagiri T, Nishimaki J, Ashman LK, Toyama K. Immobilized anti-Kit monoclonal antibody induces ligand-independent dimerization and activation of steel factor receptor: biologic similarity with membrane-bound form of steel factor rather than its soluble form. Blood. 1996;87:2235–2243. [PubMed] [Google Scholar]
- Kwintkiewicz J, Guidice LC. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Sem Reprod Med. 2009;27:43–51. doi: 10.1055/s-0028-1108009. [DOI] [PubMed] [Google Scholar]
- Laird DW. Closing the gap on autosomal dominant connexin-26 and connexin-43 mutants linked to human disease. J Biol Chem. 2008;283:2997–3001. doi: 10.1074/jbc.R700041200. [DOI] [PubMed] [Google Scholar]
- Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R, Dewailly D, Reyss AC, Jeffery L, Bachelot A, Massin N, Fellous M, Veitia RA. Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur J Endocrinol. 2006;154:739–744. doi: 10.1530/eje.1.02135. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Rutanen EM, Ritvos O. Expression of c-kit ligand messenger ribonucleic acids in human ovaries and regulation of their steady state levels by gonadotropins in cultured granulosa-luteal cells. Endocrinology. 1995;136:4407–4414. doi: 10.1210/endo.136.10.7545103. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Vuojolainen K, Jaatinen R, Ketola I, Aaltonen J, Lehtonen E, Heikinheimo M, Ritvos O. A novel growth differentiation factor-9 (GDF-9) related factor is co-expressed with GDF-9 in mouse oocytes during folliculogenesis. Mech Dev. 1998;78:135–140. doi: 10.1016/s0925-4773(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Li R, Norman RJ, Armstrong DT, Gilchrist RB. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod. 2000;63:839–845. doi: 10.1095/biolreprod63.3.839. [DOI] [PubMed] [Google Scholar]
- Li TY, Colley D, Barr KJ, Yee SP, Kidder GM. Rescue of oogenesis in CX37-null mutant mice by oocyte-specific replacement with CX43. J Cell Sci. 2007;120:4117–4125. doi: 10.1242/jcs.03488. [DOI] [PubMed] [Google Scholar]
- Lima F, Niger C, Hebert C, Stains JP. Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2–dependent mechanism. Mol Biol Cell. 2009;20:2697–2708. doi: 10.1091/mbc.E08-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manova K, Nocka K, Besmer P, Bachvarova RF. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990;110:1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- Manova K, Huang EJ, Angeles M, De Leon V, Sanchez S, Pronovost SM, Besmer P, Bachvarova RF. The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev Biol. 1993;157:85–99. doi: 10.1006/dbio.1993.1114. [DOI] [PubMed] [Google Scholar]
- McCoshen JA, McCallion DJ. A study of the primordial germ cells during their migratory phase in Steel mutant mice. Experientia. 1975;31:589–590. doi: 10.1007/BF01932475. [DOI] [PubMed] [Google Scholar]
- McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Williams DA, Gotoh A, Mishimaki J, Broxmeyer HE, Toyama K. Membrane-bound steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]
- Montgomery GW, Zhao ZZ, Marsh AJ, Mayne R, Treloar SA, James M, Martin NG, Boomsma DI, Duffy DL. A deletion mutation in GDF9 in sisters with spontaneous DZ twins. Twin Res. 2004;7:548–555. doi: 10.1375/1369052042663823. [DOI] [PubMed] [Google Scholar]
- Moor RM, Smith MW, Dawson RMC. Measurement of intercellular coupling between oocytes and cumulus cells using intracellular markers. Exp Cell Res. 1980;126:15–29. doi: 10.1016/0014-4827(80)90466-8. [DOI] [PubMed] [Google Scholar]
- Morita Y, Manganaro TF, Tao XJ, Martimbeau S, Donahoe PK, Tilly JL. Requirement for phosphatidylinositol-30-kinase in cytokine-mediated germ cell survival during fetal oogenesis in the mouse. Endocrinology. 1999;140:941–949. doi: 10.1210/endo.140.2.6539. [DOI] [PubMed] [Google Scholar]
- Moron FJ, de Castro F, Royo JL, Montoro L, Mira E, Saez ME, Real LM, Gonzalez A, Manes S, Ruiz A. Bone morphogenetic protein 15 (BMP15) alleles predict over-response to recombinant follicle stimulation hormone and iatrogenic ovarian hyperstimulation syndrome (OHSS) Pharmacogenet Genom. 2006;16:485–495. doi: 10.1097/01.fpc.0000215073.44589.96. [DOI] [PubMed] [Google Scholar]
- Motro B, Bernstein A. Dynamic changes in ovarian c-kit and Steel expression during the estrous reproductive cycle. Dev Dynamics. 1993;197:69–79. doi: 10.1002/aja.1001970107. [DOI] [PubMed] [Google Scholar]
- Nekola MV, Nalbandov AV. Morphological changes of rat follicular cells as influenced by oocytes. Biol Reprod. 1971;4:154–160. doi: 10.1093/biolreprod/4.2.154. [DOI] [PubMed] [Google Scholar]
- Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Romero C, Tapia V, Dissen GA. Neurotrophic and cell-cell dependent control of early follicular development. Mol Cell Endocrinol. 2000;163:67–71. doi: 10.1016/s0303-7207(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Avivi A, Zimmer Y, Givol D, Yarden Y, Lonai P. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development. 1990;109:911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shiimasaki S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J Biol Chem. 2000;275:39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc Natl Acad Sci USA. 2002;99:8060–8065. doi: 10.1073/pnas.122066899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AI, Hsu YC, Besmer P, Bachvarova RF. The ligand of the c-kit receptor promotes oocyte growth. Dev Biol. 1994;161:194–205. doi: 10.1006/dbio.1994.1020. [DOI] [PubMed] [Google Scholar]
- Palmer JS, Zhao ZZ, Hoekstra C, Hayward NK, Webb PM, Whiteman DC, Martin NG, Boomsma DI, Duffy DL, Montgomery GW. Novel variants in growth differentiation factor 9 in mothers of dizygotic twins. J Clin Endocrinol Metab. 2006;91:4713–4716. doi: 10.1210/jc.2006-0970. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Matzuk MM. Genetic models for transforming growth factor β superfamily signalling in ovarian follicle development. Mol Cell Endocrinol. 2004;225:83–91. doi: 10.1016/j.mce.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Pérez-Armendariz EM, Sáez JC, Bravo-Moreno JF, López-Olmos V, Enders GC, Villalpando I. Connexin43 is expressed in mouse fetal ovary. Anat Rec Part A. 2003;271A:360–367. doi: 10.1002/ar.a.10040. [DOI] [PubMed] [Google Scholar]
- Reaume A, De Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281:160–170. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Reith AD, Rottapel R, Giddens E, Brady C, Forrester L, Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990;4:390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- Salustri A, Petrungaro S, De Felici M, Conti M, Siracusa G. Effect of follicle-stimulating hormone on cyclic adenosine monophosphate level and on meiotic maturation in mouse cumulus cell-enclosed oocytes cultured in vitro. Biol Reprod. 1985;33:797–802. doi: 10.1095/biolreprod33.4.797. [DOI] [PubMed] [Google Scholar]
- Salustri A, Ulisse S, Yanagishita M, Hascall VC. Hyaluronic acid synthesis by mural granulosa cells and cumulus cells in vitro is selectively stimulated by a factor produced by oocytes and by transforming growth factor-beta. J Biol Chem. 1990a;265:19517–19523. [PubMed] [Google Scholar]
- Salustri A, Yanagishita M, Hascall VC. Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH-stimulated cumulus cells. Dev Biol. 1990b;138:26–32. doi: 10.1016/0012-1606(90)90173-g. [DOI] [PubMed] [Google Scholar]
- Sasseville M, Gagnon MC, Guillemette C, Sullivan R, Gilchrist RB, Richard FJ. Regulation of gap junctions in porcine cumulus-oocyte complexes: contributions of granulosa cell contact, gonadotropins, and lipid rafts. Mol Endocrinol. 2009;23:700–710. doi: 10.1210/me.2008-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JRV, Figueiredo JR, van den Hurk R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology. 2009;71:1193–1208. doi: 10.1016/j.theriogenology.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- Smikle CB, Dandekar PV, Schriock ED, Givens CR. Elevated ovarian follicular fluid stem cell factor concentrations are associated with improved pregnancy rates in in-vitro fertilization cycles. Fertil Steril. 1998;69:70–72. doi: 10.1016/s0015-0282(97)00422-6. [DOI] [PubMed] [Google Scholar]
- Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol. 1976;50:531–536. doi: 10.1016/0012-1606(76)90172-x. [DOI] [PubMed] [Google Scholar]
- Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Current Opinion Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Eppig JJ. Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fert Dev. 2005;17:667–674. doi: 10.1071/rd05071. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O’Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- Tai CJ, Kang SK, Cheng KW, Choi KC, Nathwani PS, Leung PC. Expression and regulation of P2U-purinergic receptor in human granulosa-luteal cells. J Clin Endocrinol Metab. 2000;85:1591–1597. doi: 10.1210/jcem.85.4.6558. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Moore MA, Soares V, Ono M, Kissel H, Besmer P. Consequences of exclusive expression in vivo of kit ligand lacking the major proteolytic cleavage site. Proc Natl Acad Sci USA. 1998;95:11903–11908. doi: 10.1073/pnas.95.20.11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira Filho FL, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, Shimasaki S, Erickson GF. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1337–1344. doi: 10.1210/jcem.87.3.8316. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Vanderhyden BC. Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol and Endocrinol. 2006;4:19. doi: 10.1186/1477-7827-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas FH, Ethier J-F, Shimasaki S, Vanderhyden BC. Follicle-stimulating hormone regulates oocyte growth by modulation of expression of oocyte and granulosa cell factors. Endocrinology. 2005;146:941–949. doi: 10.1210/en.2004-0826. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Ismail RS, Jiang J-Y, Vanderhyden BC. Kit ligand 2 promotes murine oocyte growth in vitro. Biol Reprod. 2008;78:167–175. doi: 10.1095/biolreprod.106.058529. [DOI] [PubMed] [Google Scholar]
- Tong D, Gittens JEI, Kidder GM, Bai D. Patch clamp study reveals that the importance of connexin43-mediated gap junctional communication for ovarian folliculogenesis is strain-specific in the mouse. Am J Physiol-Cell Physiol. 2006;290:C290–C297. doi: 10.1152/ajpcell.00297.2005. [DOI] [PubMed] [Google Scholar]
- Tong D, Li TY, Naus KE, Bai D, Kidder GM. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci. 2007;120:4016–4024. doi: 10.1242/jcs.011775. [DOI] [PubMed] [Google Scholar]
- Tong D, Colley D, Thoo R, Li TY, Plante I, Laird DW, Bai D, Kidder GM. Oogenesis defects in a mutant mouse model of oculodentodigital dysplasia. Disease Models Mech. 2009;2:157–167. doi: 10.1242/dmm.000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhyden BC. Molecular basis of ovarian development and function. Frontiers in Bioscience. 2002;7:2006–2022. doi: 10.2741/A895. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Macdonald EA. Mouse oocytes regulate granulosa cell steroidogenesis throughout follicular development. Biol Reprod. 1998;59:1296–1301. doi: 10.1095/biolreprod59.6.1296. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Tonary AM. Differentialregulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by a factor(s) secreted by the oocyte. Biol Reprod. 1995;53:1243–1250. doi: 10.1095/biolreprod53.6.1243. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol. 1990;140:307–317. doi: 10.1016/0012-1606(90)90081-s. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Telfer EE, Eppig JJ. Mouse oocytes promote proliferation of granulosa cells from preantral and antral follicles in vitro. Biol Reprod. 1992;46:1196–1204. doi: 10.1095/biolreprod46.6.1196. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Cohen JN, Morley P. Mouse oocytes regulate granulosa cell steroidogenesis. Endocrinology. 1993;133:423–426. doi: 10.1210/endo.133.1.8319589. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Macdonald EA, Nagyova E, Dhawan A. Evaluation of members of the TGFbeta superfamily as candidates for the oocyte factors that control mouse cumulus expansion and steroidogenesis. Reprod Suppl. 2003;61:55–70. [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–67. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- Veitch GI, Gittens JEI, Shao Q, Laird DW, Kidder GM. Selective assembly of connexin37 into gap junctions at the oocyte/granulosa cell interface. J Cell Sci. 2004;117:2699–2707. doi: 10.1242/jcs.01124. [DOI] [PubMed] [Google Scholar]
- Vitt UA, Hayashi M, Klein C, Hsueh AJ. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–377. doi: 10.1095/biolreprod62.2.370. [DOI] [PubMed] [Google Scholar]
- Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is upregulated by the phosphatidylinositol 30-kinase/factor complex containing CREB. Mol Cell Biol. 1999;19:6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Tong D, El-Gehani F, Tekpetey FR, Kidder GM. Connexin expression and gap junctional coupling in human cumulus cells: contribution to embryo quality. J Cell Molec Med. 2009;13:972–984. doi: 10.1111/j.1582-4934.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb RJ, Bains H, Cruttwell C, Carroll J. Gap-junctional communication in mouse cumulus-oocyte complexes: Implications for the mechanism of meiotic maturation. Reproduction. 2002;123:41–52. doi: 10.1530/rep.0.1230041. [DOI] [PubMed] [Google Scholar]
- Wong J, Luckers L, Okawara Y, Pelletier RM, Taketo T. Follicular development and atresia in the B6. YTIR sex-reversed mouse embryo. Biol Reprod. 2000;63:756–762. doi: 10.1095/biolreprod63.3.756. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI. Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol. 1997;184:122–137. doi: 10.1006/dbio.1997.8503. [DOI] [PubMed] [Google Scholar]
- Yoshino O, McMahon HE, Sharma S, Shimasaki S. A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci USA. 2006;103:10678–10683. doi: 10.1073/pnas.0600507103. [DOI] [PMC free article] [PubMed] [Google Scholar]