Abstract
The metabolic and kinetic responses to rapidly intravenously administered sodium acetoacetate (1.0 mmol/kg body wt) was studied after an overnight fast in 12 male and female adults weighing between 88 and 215% of average body weight. Blood was obtained before, during, and after the infusion for determination of circulating concentrations of immunoreactive insulin, glucose, acetoacetate, β-hydroxybutyrate and free fatty acids. In three obese subjects the studies were repeated after 3 and 24 days of total starvation.
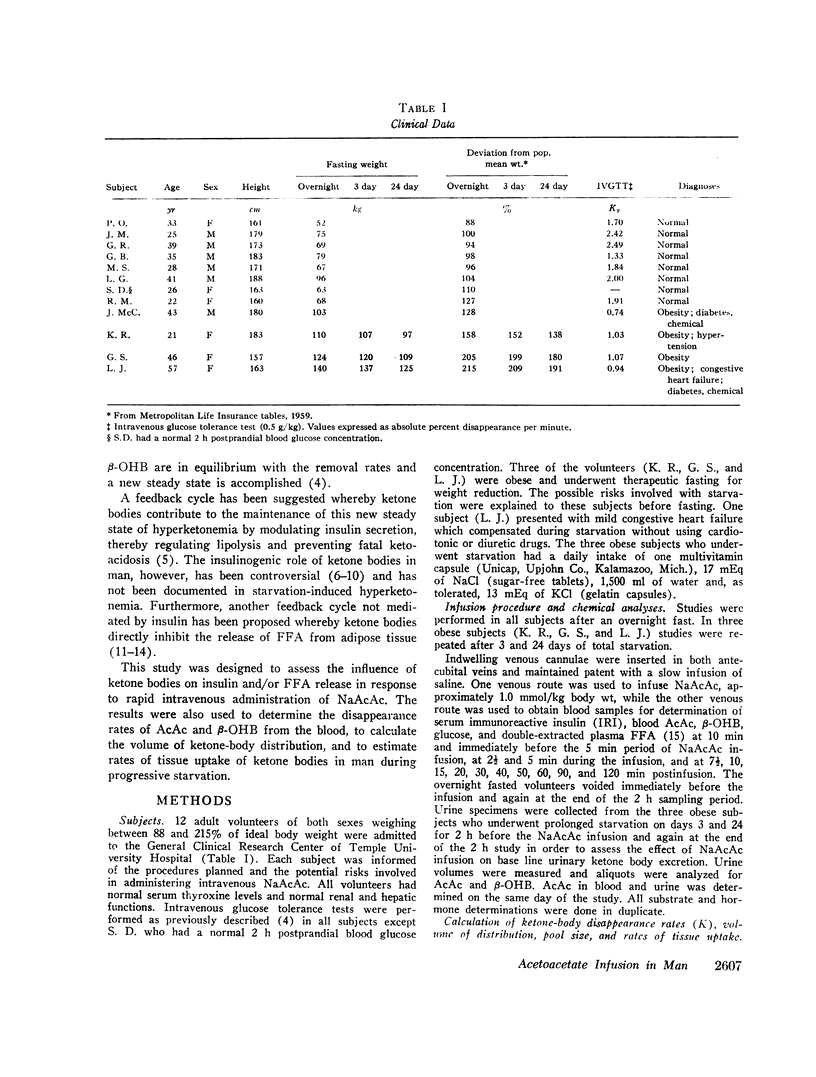
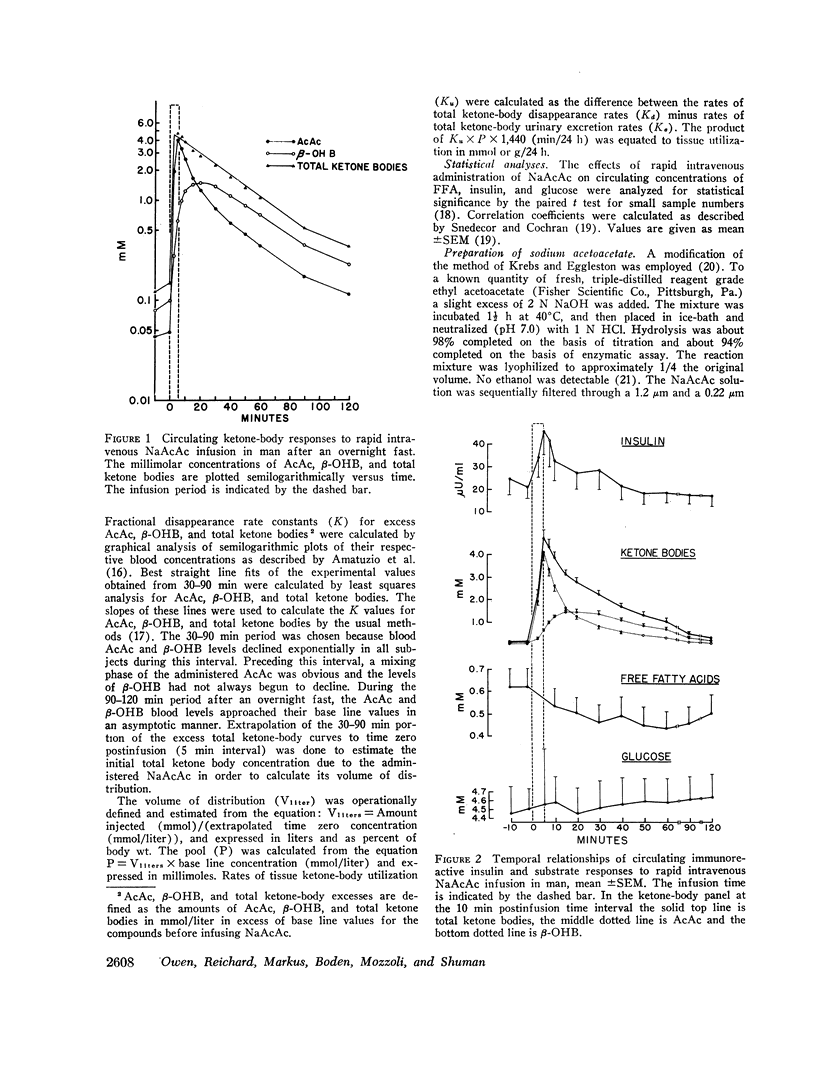
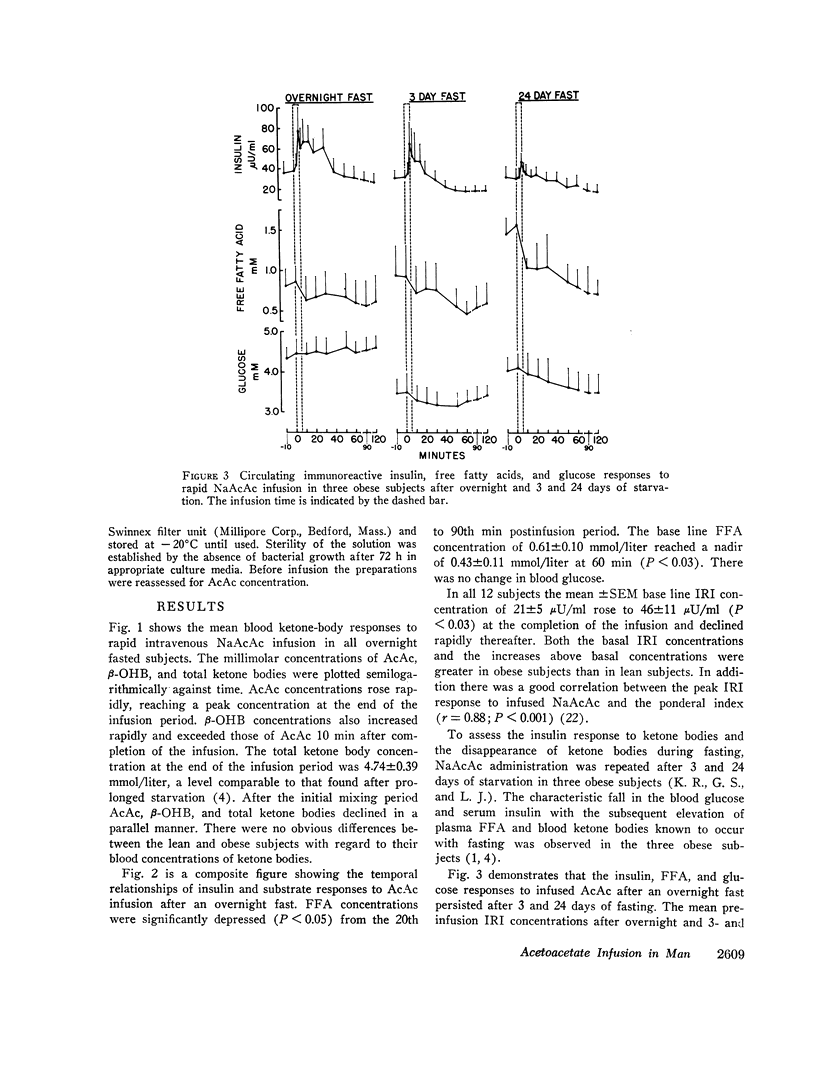
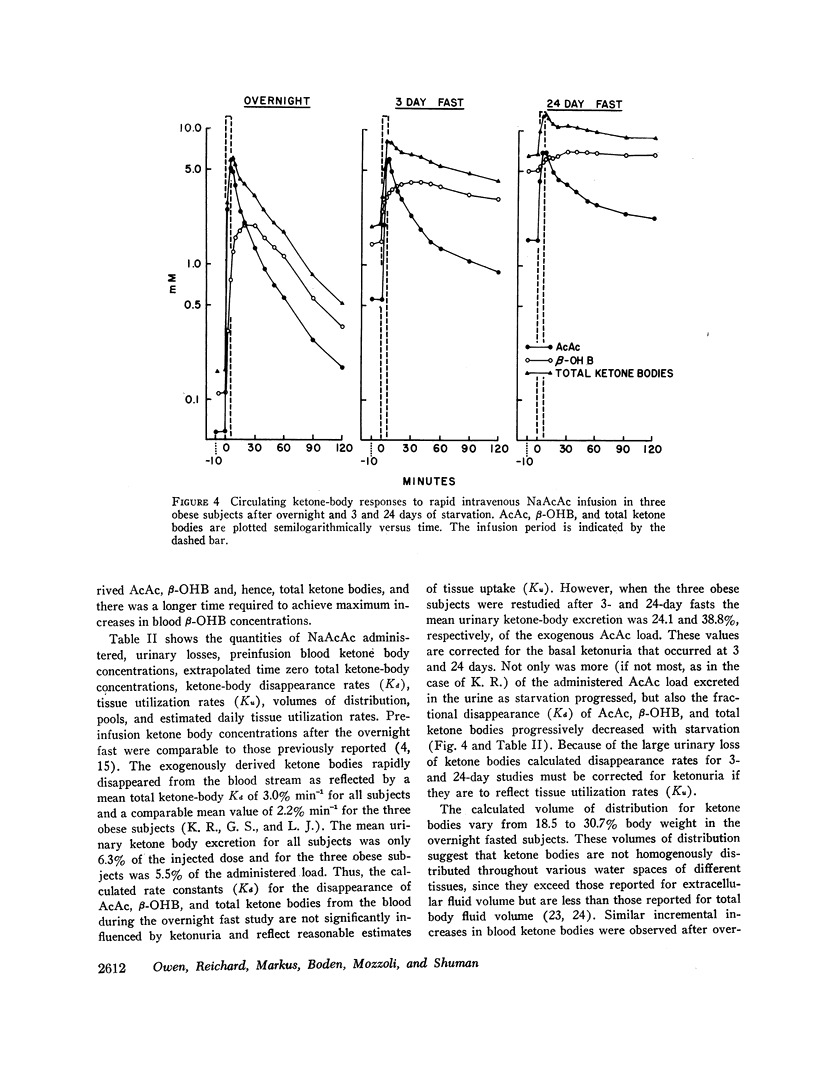
After the overnight fast acetoacetate rose rapidly reaching a peak concentration at the end of the infusion; β-hydroxybutyrate concentrations also increased rapidly and exceeded those of acetoacetate 10 min postinfusion. Total ketone body concentration at the end of the infusion period was comparable to that found after prolonged starvation. After the initial mixing period, acetoacetate, β-hydroxybutyrate and total ketone bodies rapidly declined in a parallel manner. There were no obvious differences between the subjects with regard to their blood concentrations of ketone bodies. The mean plasma free fatty acid concentration decreased significantly during the 20th to 90th min postinfusion period; for example the control concentration of 0.61 mmol/liter fell to 0.43 mmol/liter at 60 min. In the three obese subjects studied repeatedly, fasting plasma free fatty acids decreased with acetoacetate infusion from 0.92 to 0.46 mmol/liter after the 3 day fast and from 1.49 to 0.71 mmol/liter after the 24 day fast. Acetoacetate infusion caused no changes in blood glucose concentration after an overnight fast. However, in the three obese subjects restudied after 3- and 24-day fasts blood glucose decreased, respectively, from 3.49 to 3.22 mmol/liter and from 4.07 to 3.49 mmol/liter. The mean serum insulin concentration in all subjects significantly increased from 21 to 46 μU/ml at the completion of the infusion and rapidly declined. In the three obese subjects restudied after 3- and 24-day fasts an approximate two-fold increase of serum insulin was observed after each acetoacetate infusion.
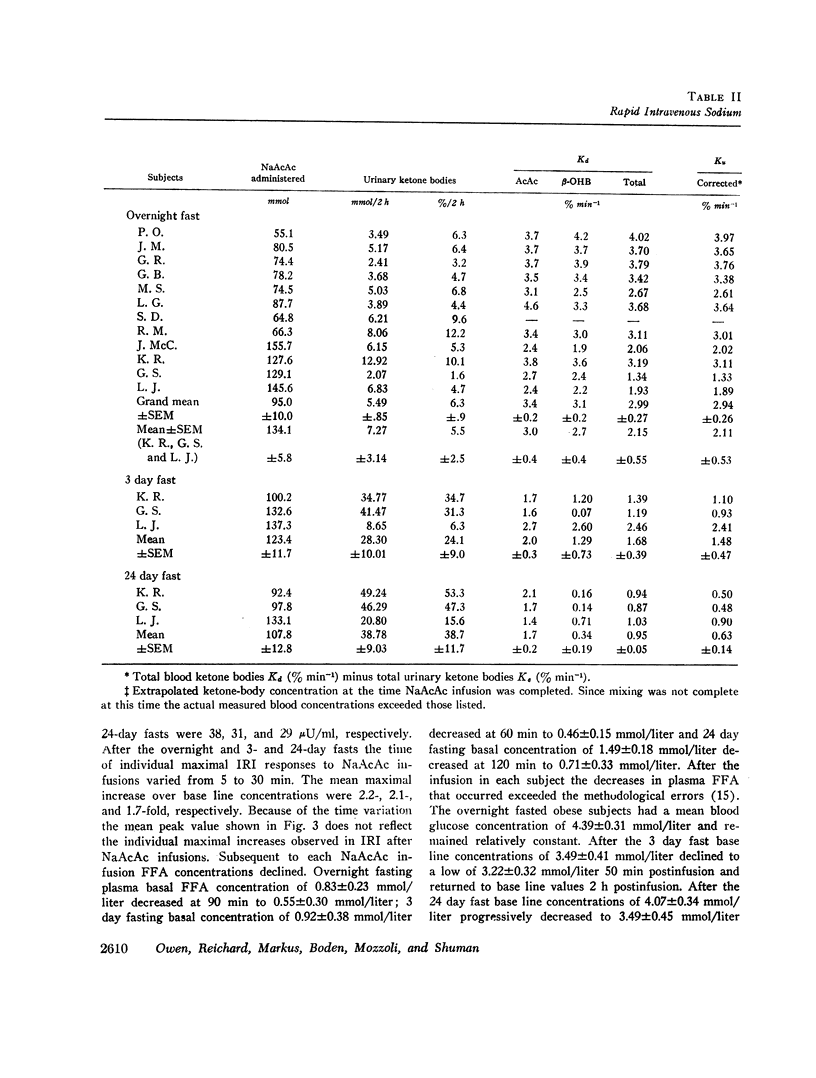
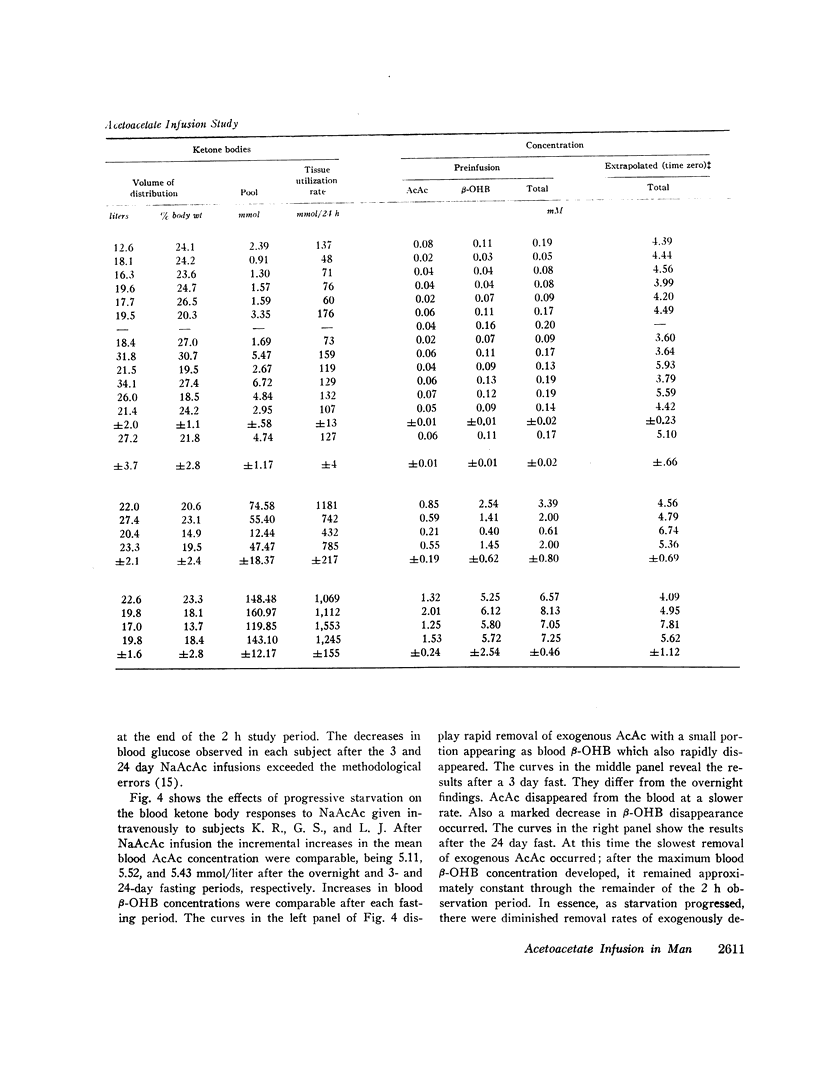
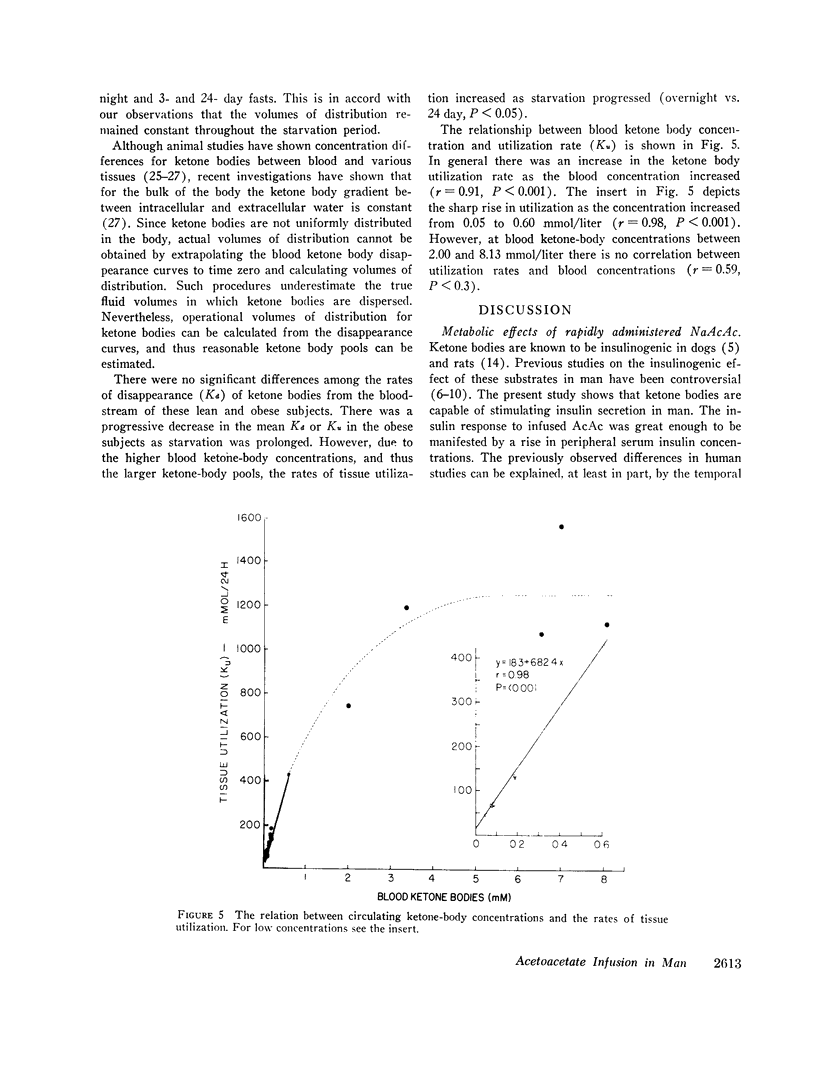
The mean fractional utilization rate of exogenously derived ketone bodies for all 12 subjects after an overnight fast was 2.9% min-1. In the three obese subjects studied after an overnight, 3 and 24 day fast the mean fractional utilization rates were 2.1%, 1.5%, and 0.6% min-1, respectively. Ketone body volumes of distribution in the overnight fasted subjected varied from about 18% to 31% of body wt, suggesting that ketone bodies are not homogenously distributed in the body water. In the three obese subjects restudied after 3- and 24-day fasts volumes of distribution remained approximately constant. When total ketone body concentrations in the blood were below 2.0 mmol/liter, there was a linear relationship between ketone body utilization rates and ketone body concentrations; no correlation was found when blood concentrations were higher.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMATUZIO D. S., STUTZMAN F. L., VANDERBILT M. J., NESBITT S. Interpretation of the rapid intravenous glucose tolerance test in normal individuals and in mild diabetes mellitus. J Clin Invest. 1953 May;32(5):428–435. doi: 10.1172/JCI102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasse E. O., Havel R. J. Evidence for an effect of inulin on the peripheral utilization of ketone bodies in dogs. J Clin Invest. 1971 Apr;50(4):801–813. doi: 10.1172/JCI106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasse E. O. Inhibition of free fatty acid oxidation by acetoacetate in normal dogs. Eur J Clin Invest. 1970 Nov;1(3):155–160. doi: 10.1111/j.1365-2362.1970.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Balasse E. O., Ooms H. A., Lambilliotte J. P. Evidence for a stimulatory effect of ketone bodies on insulin secretion in man. Horm Metab Res. 1970 Nov;2(6):371–372. doi: 10.1055/s-0028-1096822. [DOI] [PubMed] [Google Scholar]
- Balasse E., Ooms H. A. Changes in the concentrations of glucose, free fatty acids, insulin and ketone bodies in the blood during sodium beta-hydroxybutyrate infusions in man. Diabetologia. 1968 Jun;4(3):133–135. doi: 10.1007/BF01219433. [DOI] [PubMed] [Google Scholar]
- Bates M. W., Krebs H. A., Williamson D. H. Turnover rates of ketone bodies in normal, starved and alloxan-diabetic rats. Biochem J. 1968 Dec;110(4):655–661. doi: 10.1042/bj1100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björntorp P., Scherstén T. Effect of beta-hydroxybutyrate on lipid mobilization. Am J Physiol. 1967 Mar;212(3):683–687. doi: 10.1152/ajplegacy.1967.212.3.683. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes. 1971 Dec;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- FAJANS S. S., FLOYD J. C., Jr, KNOPF R. F., CONN J. W. A COMPARISON OF LEUCINE- AND ACETOACETATE-INDUCED HYPOGLYCEMIA IN MAN. J Clin Invest. 1964 Oct;43:2003–2008. doi: 10.1172/JCI105074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCKSON J. R., OOMS H. A., BELLENS R., CONARD V., BASTENIE P. A. Physiologic significance of the intravenous glucose tolerance test. Metabolism. 1962 May;11:482–500. [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B. Inhibition by -hydroxybutyrate of lipolysis induced by sympathetic nerve activity in canine subcutaneous adipose tissue in situ. Metabolism. 1972 Feb;21(2):125–131. doi: 10.1016/0026-0495(72)90064-9. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Balasse E. O., Segel N., Basso L. V. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970 Nov;49(11):2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Alberti K. G., Houghton C. R., Williamson D. H., Krebs H. A. The effect of acetoacetate on plasma insulin concentration. Biochem J. 1971 Nov;125(2):541–544. doi: 10.1042/bj1250541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman D. E., Senior B., Goodman H. M. Anti-lipolytic effects of beta-hydroxybutyrate. Metabolism. 1969 Nov;18(11):906–915. doi: 10.1016/0026-0495(69)90031-6. [DOI] [PubMed] [Google Scholar]
- Jenkins D. J., Hunter W. M., Goff D. V. Ketone bodies and evidence for increased insulin secretion. Nature. 1970 Jul 25;227(5256):384–385. doi: 10.1038/227384a0. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- LJUNGGREN H., IKKOS D., LUFT R. Studies on body composition. II. Body fluid compartments and exchangeable potassium in obese females. Acta Endocrinol (Copenh) 1957 Jun;25(2):199–208. [PubMed] [Google Scholar]
- Loridan L., Senior B. Effects of infusion of ketones in children with ketotic hypoglycemia. J Pediatr. 1970 Jan;76(1):69–74. doi: 10.1016/s0022-3476(70)80132-9. [DOI] [PubMed] [Google Scholar]
- MADISON L. L., MEBANE D., UNGER R. H., LOCHNER A. THE HYPOGLYCEMIC ACTION OF KETONES. II. EVIDENCE FOR A STIMULATORY FEEDBACK OF KETONES ON THE PANCREATIC BETA CELLS. J Clin Invest. 1964 Mar;43:408–415. doi: 10.1172/JCI104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Guest M. J., Foster D. W. Ketone body metabolism in the ketosis of starvation and alloxan diabetes. J Biol Chem. 1970 Sep 10;245(17):4382–4390. [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Markus H., Sarshik S., Mozzoli M. Relationship between plasma and muscle concentrations of ketone bodies and free fatty acids in fed, starved and alloxan-diabetic states. Biochem J. 1973 Jun;134(2):499–506. doi: 10.1042/bj1340499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Reichard G. A., Jr Human forearm metabolism during progressive starvation. J Clin Invest. 1971 Jul;50(7):1536–1545. doi: 10.1172/JCI106639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer C. C. Some re-evaluations of the build and blood pressure study, 1959 as related to ponderal index, somatotype and mortality. N Engl J Med. 1966 Feb 3;274(5):254–259. doi: 10.1056/NEJM196602032740505. [DOI] [PubMed] [Google Scholar]
- Söling H. D., Zahlten R., Reimold W. V., Willms B. Utilization of ketone bodies by adipose tissue and its regulation by carbohydrate metabolism. Horm Metab Res. 1970 Mar;2(2):56–63. doi: 10.1055/s-0028-1095108. [DOI] [PubMed] [Google Scholar]
- WERK E. E., Jr, McPHERSON H. T., HAMRICK L. W., Jr, MYERS J. D., ENGEL F. L. Studies on ketone metabolism in man. I. A method for the quantitative estimation of splanchnic ketone production. J Clin Invest. 1955 Aug;34(8):1256–1267. doi: 10.1172/JCI103172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R., KREBS H. A. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961 Sep;80:540–547. doi: 10.1042/bj0800540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]



