Abstract
Parasites and pathogens are possibly key evolutionary forces driving recognition systems. However, empirical evidence remains sparse. The ubiquitous pioneering ant Formica fusca is exploited by numerous socially parasitic ant species. We compared the chemical cue diversity, egg and nest mate recognition abilities in two Finnish and two UK populations where parasite pressure is high or absent, respectively. Finnish populations had excellent egg and nest mate discrimination abilities, which were lost in the UK populations. The loss of discrimination behaviour correlates with a loss in key recognition compounds (C25-dimethylalkanes). This was not owing to genetic drift or different ecotypes since neutral gene diversity was the same in both countries. Furthermore, it is known that the cuticular hydrocarbon profiles of non-host ant species remain stable between Finland and the UK. The most parsimonious explanation for the striking difference in the cue diversity (number of C25-dimethylalkanes isomers) between the UK and Finland populations is the large differences in parasite pressure experienced by F. fusca in the two countries. These results have strong parallels with bird (cuckoo) studies and support the hypothesis that parasites are driving recognition cue diversity.
Keywords: Fomica, parasites, recognition, dimethylalkanes
1. Introduction
Parasites and hosts engage in various coevolutionary arms races that are predicted to be a key force that influences many biological processes from speciation to recognition systems in particular [1–4]. Some of the best examples for the latter involve studies on cuckoos, since the interests of host and cuckoo are diametrically opposed. ‘Cuckoos’ are brood parasites that lay their eggs in the nest of a host species that then rear the cuckoos' offspring. This trait has evolved many times across various taxa including fishes [5], birds [6] and insects [7]. In birds, it is known that egg discrimination is a widely used tactic by hosts against cuckoos [6,8]. It has also been suggested that ant hosts could theoretically defend themselves against parasites through improved recognition abilities and that this should correlate with high parasite pressure [9]. However, the precise recognition abilities of social insects are also important in recognizing nest mates from non-nest mate conspecifics [10], and the respective roles of social parasitism and conspecific nest mate recognition in the origin and maintenance of recognition systems are unclear.
While social parasitism includes a wide range of interspecific relations [7], the most integrated social parasites in ants, and also highly harmful to the hosts, are those that invade the nests of other species and trick host workers into becoming their workforce. This behaviour takes three main forms: slave making, inquilinism and temporary social parasitism [11]. Slave-making ants continually raid other ant nests to steal their worker brood to refresh their workforce, whereas inquline queens live their whole lives inside a host colony. In temporary social parasitism, colony-founding queens invade a host colony, kill the resident queen and use the host workers to rear her brood, which progressively replaces the host worker population. Colony-foundation by temporary social parasitism is a common strategy among wood ants and their relatives (Formica rufa group, Formica exsecta group, Formica sanguinea). Especially monogynous (single queen per colony) and monodomous (single nest per colony) wood-ant colonies result from a successful take-over of a host colony [7,12–14]. Such forms are common in southern Finland ([12]; S. J. Martin, H. Helantera & F. P. Drijfhout 2005–2010, personal observations). By contrast, in the UK wood ants are rare, presumably because habitat loss and fragmentation have led to the extinction of many wood-ant species [15]. Colonies of the isolated populations of the few remaining species of wood ants possess many queens (polygyny) and colony establishment occurs more often by budding than parasitism [7]. Consequently, in the UK, interspecific parasitism has rarely been recorded [16,17]. The wood ants' main host is Formica fusca. This is a typical pioneer species that rapidly colonizes new areas of cleared forest and other disturbed habitats [18,19]. It is widely distributed throughout the Palaearctic region, including southern regions of Finland and England, so the F. fusca populations in Finland are under high parasitic pressure from wood ants, whereas those in the UK are not (figure 1).
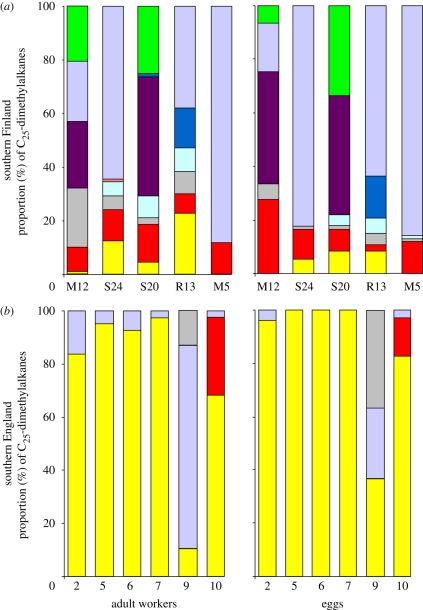
Figure 1.
Comparison of colony-specific cue diversity in regions where parasite pressure is (a) high (southern Finland) [20] and (b) low/absent (southern England). Each colony is represented by a single column in which each positional isomer of pentacosane dimethylalkane (x,y-diMeC25) is represented by a different colour and is based on the mean proportion of five workers per colony. Although F. sanguinea was absent from both English collection sites, small remnant populations do exist in southern England (see National Biodiversity Network Gateway, www.nbnorg.uk). Green, 3,11; purple, 3,13; dark blue, 5,17; orange, 5,15; violet, 5,13; light blue, 7,15; dark grey, 7,11; red, 9,13; yellow, 11,15.
Unlike vertebrates, such as birds that discriminate an egg laid by a cuckoo from their own based on visual recognition cues, insects use chemical cues [10]. Recently, empirical evidence has shown that in ants particular groups of cuticular hydrocarbons act as nest mate recognition compounds [21–23], and presumably the same compounds are also used in discriminating against parasitic intruders. Therefore, social parasites have evolved a range of chemical strategies such as mimicry or the use of repellent or appeasement substances [11] to successfully invade a host colony. However, less is known about how ant social parasites are able produce brood that are reared and not destroyed by the host workers (but see [24]).
The ant F. fusca possesses nine different colony-specific pentacosane dimethylalkane isomers (C25-dimethylalkane). These are the only compounds that contain information necessary for nest mate recognition, i.e. vary both in number and proportion between colonies, but remain stable within colonies [20]. Dimethylalkanes represent over half of the 1000+ cuticular hydrocarbons of ants currently described [25] and are known to play a role in nest mate recognition [26]. These putative nest mate recognition compounds of F. fusca are the focus of this investigation as this allows us to investigate the hypothesis that selection imposed by parasites is necessary for maintenance of recognition cue diversity. We compared the C25-dimethylalkane diversity (number of isomers) of workers and eggs from F. fusca populations in areas of high (southern Finland) and no/low (southern England) parasitic pressure. In addition, for the recognition of adult individuals, F. fusca are also able to discriminate between visually indistinct conspecific nest mate and non-nest mate eggs [27,28]. Behavioural bioassays were used to establish the discriminatory abilities of the workers from the two countries, against workers and eggs from conspecific colonies. Both adults and eggs were tested since invading parasite queens need to overcome two barriers, entering the colony and getting their eggs accepted, in order to establish a new colony and so are two areas where they may be driving cue diversity. We predict that the lack of cue diversity leads to weakened nest mate and egg discrimination in the UK populations.
2. Material and methods
(a). Study sites and species
A total of 40 entire F. fusca colonies were collected from two locations in southern Finland (Raseborg & Gråkärr) and southern England (Thetford & New Forest) during April 2006 and 2007. At all four locations, F. fusca was the most common potential host species (S. J. Martin, H. Helantera & F. P. Drijfhout 2005–2010, personal observations), although none of the sampled colonies were parasitized. Colonies were maintained in the laboratory in silicon lined boxes for two weeks during which the bioassays were conducted. During this period, they were fed weekly with an artificial ant diet [29] and their nest material was kept moist. The comparison of chemical profiles of eggs and workers from Finland were obtained from five whole F. fusca colonies collected in 2005 from southern Finland close to the Raseborg site, whereas the data from the UK were obtained from the colonies collected during 2006.
(b). Egg bioassays
Eggs for the bioassays and chemical analysis were obtained by isolating the F. fusca queen for 24 h at room temperature in a darkened Petri dish. This ensured that the cuticular hydrocarbon profile of the eggs was not influenced by the workers that tend the eggs. Then two rows of eggs (control and test) were placed on a glass slide and placed inside the discriminator colony. Control eggs came from the discriminator colony queen and the test eggs were from a different colony in the population. The reaction of individual ants towards each egg was recorded as accepted or rejected. An accepted egg was one was that antennated and picked up in the worker's mandibles and then carried off into the colony, whereas a rejected egg was one that was clearly checked using the antenna but not picked up or picked up, antennated and then dropped. A total of 100 eggs from six different colonies were used for each species. The proportion of nest mate and non-nest mate eggs accepted were then compared using Fishers' exact test.
(c). Aggression bioassays
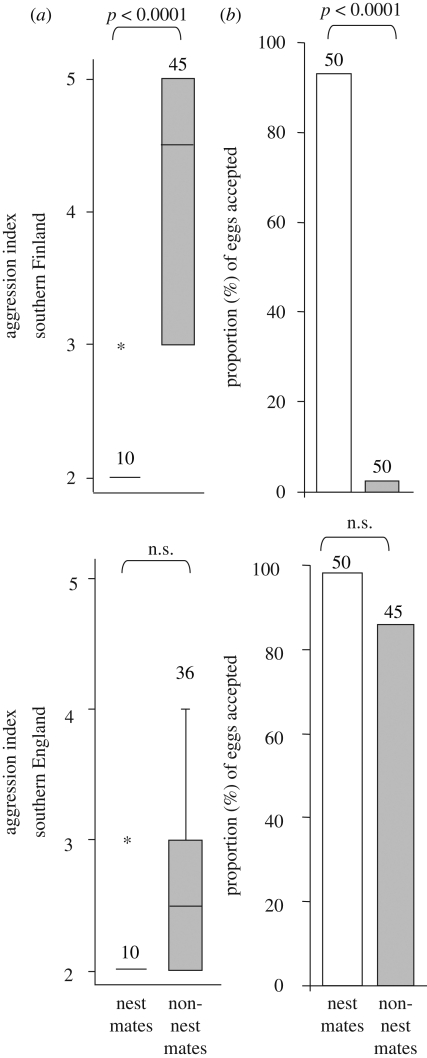
Aggression bioassays between nest mate or non-nest mate workers for colonies derived from the same populations were recorded after dyadic encounters in a neutral arena (Petri dish) using a five-point scale: 1, ignore; 2, antennation; 3, mandible gaping; 4, attack; 5, fighting. The highest scoring encounter during a 3 min period was recorded. Preliminary trials showed that the average time until individuals first encountered each other was 13 s, indicating that 3 min would be sufficient time for an encounter to take place. By using workers from six different colonies, up to 45 non-nest mate encounters and 10 nest mate encounters were recorded for each population. The Mann–Whitney U-test was used to compare the aggression indices between nest mates and non-nest mates from the two populations. The exact significance is reported.
(d). Chemical methods and analysis
Ten eggs and five workers were collected from each colony prior to the bioassays and placed in pairs (eggs) or individually (workers) into glass inserts containing 30 µl of hexane. After 10 min, the ants were removed and hexane allowed to evaporate; eggs were not removed. Vials were sealed and stored at 5°C. Just prior to the analysis, 30 µl of hexane was added to the vials and sample analysed on a HP 6890 GC (equipped with a HP-5MS column; length: 30 m; ID: 0.25 mm; film thickness: 0.25 µm) connected to a HP5973 MSD (quadrupole mass spectrometer with 70 eV electron impact ionization). Samples were injected in the splitless mode and the oven was programmed from 70°C to 200°C at 40°C min−1 and then from 200°C to 320°C at 25°C min−1 and held for 2 min at 320°C. Helium was used as the carrier gas, at a constant flow rate of 1 ml min−1. Cuticular hydrocarbons were characterized by the use of standard MS databases, diagnostic ions and their Kovats indices. Only the pentacosane dimethylalkanes (x,y-diMeC25) were subsequently analysed since it is known that only these compounds are colony specific [20]. In cases where C25-dimethylalkanes overlapped because of having similar retention times, a characteristic ion of a compound was used to delineate the area of the total ion chromatogram for integration. This introduced some margin of error, but this was expected to be equal across all samples analysed. In cases where isomers completely overlap (e.g. 3,11-diMeC25 and 3,13-diMeC25), peak area was divided according to the ratio in which the diagnostic ions were present. Owing to the small size of eggs relative to adult workers, the amount of C25-dimethylalkanes extracted was two orders of magnitude lower. Therefore, it can be difficult to determine proportions accurately and minor compounds on workers were often difficult to detect on the eggs. Owing to these detection problems, we did not expect to find a precise match in the proportions of x,y-diMeC25 compounds between eggs and workers, but did expect a close qualitative match between eggs and workers of the major compounds.
(e). Genetic methods and analysis
DNA extraction and microsatellite genotyping were carried out as described in Seppä et al. [30]. Gene diversities (Hsk) for 10 microsatellite loci (FL 20, FL12 [31], FE13, FE17, FE 19, FE21, FE51 [32] FY4, FY7 and FY13 [33]) were calculated in Fstat 2.9.3.2 [34] according to Nei [35]. The UK sample consisted of 29 individuals from 12 colonies in the two study populations. The gene diversity for each locus was calculated for the pooled sample of the two populations. The Finnish sample consisted of two populations (Raseborg, which was also used in the study of chemical diversity, 147 individuals from 30 colonies, six loci; and another population ca 300 km north, close to Hyytiälä forest station, 75 individuals from 22 colonies, 10 loci). Gene diversities were compared with a two-tailed t-test.
Signals of a population bottleneck in the distribution of allele frequencies were tested with the software Bottleneck [36]. Sign tests and Wilcoxon tests were carried out for the Infinite allele model, Stepwise mutation model and Two-phase model (four different runs for Two-phase model, with the proportions of stepwise mutations and variance for mutation step length in multi-step mutations at 70/30, 70/20, 80/20 and 90/10, respectively), and significant one-tailed p-values for heterozygosity excess were looked for. Furthermore, deviations from an L-shaped distribution of allele frequencies, which would indicate loss of rare alleles in bottlenecks, were looked for [36].
3. Results
(a). Cue diversity
In F. fusca, variation in the synthesis of nine different isomers of C25-dimethylalkanes is sufficient to produce unique colony profiles [20]. The C25-dimethylalkanes diversity (number of isomers per colony) was significantly different (t38 = −4.34, p < 0.0001) between the English (range 2–3, mean ± s.d. = 2.2 ± 0.4) and Finnish (range 2–7, mean ± s.d. = 3.5 ± 1.3) populations. Therefore, both isomer number and the variation in their proportions are greatly reduced in the two English F. fusca populations relative to the two Finnish populations (figure 1).
The major C25-dimethylalkanes extracted from workers and eggs from the same colony were the same in all Finnish and English colonies investigated (figure 2). Therefore, the C25-dimethylalkanes diversity was also significantly (t9 = −3.66, p < 0.005) lower in eggs laid in English than Finnish colonies.
Figure 2.
Comparison of colony-specific cue diversity between workers and eggs from (a) five Finnish and (b) six English colonies. Each colony is represented by a single column in which each positional isomer of pentacosane dimethylalkane (x,y-diMeC25) is represented by a different colour. Green, 3,11; purple, 3,13; dark blue, 5,17; orange, 5,15; violet, 5,13; light blue, 7,15; dark grey, 7,11; red, 9,13; yellow, 11,15.
(b). Egg and worker discrimination
At the behavioural level, workers form the Finnish F. fusca populations were significantly more aggressive (U = 34.5, p < 0.0001) towards non-nest mates than nest mates and accepted significantly more (p < 0.0001) nest mate than non-nest mate eggs. However, workers from the English F. fusca populations were not significantly (U = 111.5, p = 0.07) more aggressive towards non-nest mates than nest mates and did not accept significantly (p = 0.2) more nest mate than non-nest mate eggs (figure 3). The response of workers towards their colony nest mates and eggs was not significantly different (U = 50, p = 1 nest mates, p = 0.6 eggs) between the Finnish and English populations. However, the response of Finnish and English workers towards conspecific non-nest mates and eggs was significantly different (U = 290, p = <0.0001 non-nest mate, p < 0.0001 eggs) between the two populations (figure 3).
Figure 3.
The behavioural differences in (a) conspecific worker aggression and (b) egg acceptance between nest mates and non-nest mates between the southern Finland and southern England populations. The Mann–Whitney U-test and Fishers' exact test were used to test for significance differences in aggression and egg acceptance, respectively. Sample sizes are also given. The asterisk indicates an outlier.
(c). Gene diversity
We can rule out the possibility that fragmentation or a decrease in population size of the UK populations has caused a loss of variability by drift, which is possible even if there is still selection to maintain cue variation, since there was no difference in neutral gene diversity between the UK and Finnish populations (average gene diversity for microsatellite loci in the UK Hsk = 0.48, s.d. = 0.20, in Finland Hsk = 0.51, s.d. = 0.18, two-tailed t-test, d.f. = 24, t = 0.50, p = 0.63). Furthermore, no indication of a population bottleneck in the UK sample was found in the distribution of allele frequencies (all p-values for bottleneck tests >0.22, an L-shaped distribution of allele frequencies with 46% of the alleles with a frequency <10%).
4. Discussion
We demonstrate that recognition cue diversity is low in populations of the UK F. fusca and high in Finnish populations. We have ruled out that this is not owing to loss of underlying genetic variability by drift in the fragmented UK populations. Furthermore, species-specific hydrocarbon profiles of non-host Formica ants remained remarkably stable between Finland and the UK and are not expected to be influenced by ecological factors such as soil, vegetation or climate [37]. Therefore, the most parsimonious explanation for the difference in the cue diversity between the UK and Finland populations is the large differences in parasite pressure experienced by F. fusca in the two countries (figure 1). Therefore, parasite pressure appears to be a main driver of recognition cue diversity in this species of ant. This finding parallels, for example, that in weaverbirds, who in the absence of their cowbird parasites lose clutch specificity in egg appearance [8]. In the case of F. fusca, the lack of cue diversity is also reflected in accuracy of conspecific nest mate recognition.
The UK F. fusca population has lost all the 5,y-diMeC25 compounds and in the majority of colonies produce predominantly a single C25-dimethylalkane (11,15-diMeC25). Interestingly, the 11,15-dimethylalkanes are the most commonly produced dimethylalkanes among the ants [25]. However, much more information on the biosynthetic pathways is needed before we can comment on why some dimethylalkanes are lost and others retained.
Cuticular hydrocarbons in insects [38], including Formica ants [39], are at least partly genetically controlled as is the egg appearance (colour and spottiness) and egg discrimination behaviour in birds [40]. The similarity of adult and egg hydrocarbon profiles is widespread among the ants ([41,42]; this study), hornets [43] and bumble-bees (S. J. Martin, H. Helantera & F. P. Drijfhout 2009, unpublished data) but unusually not in honeybees [44] Correspondingly, ants, hornets and bumble-bees all possess heterospecific cuckoos but honeybees, until very recently, had no associated social parasites. Honeybees have poor egg discrimination abilities since queen-laid eggs can be moved successfully between colonies. This lack of egg recognition is a key factor which allowed the parasitic Capensis honeybees to get host honeybees to accept and rear their eggs [45]. In birds, pressure from brood parasites is correlated to both intra- and interspecific variation in the ability to discriminate against foreign eggs [6], but in social insects similar comparative predictions await testing, and it is not known whether recognition of nest mate eggs is limited to host species of social parasites.
Although there are strong parallels in the coevolutionary arms race driving egg recognition, insect cuckoo queens face a potentially bigger problem of invading the host colony occupied by tens to thousands of host workers, so several mechanisms may be employed. For example, studies with parasitic Polyergus queens suggest that they may be able to acquire chemical profiles from the host queens [46], or use appeasement chemicals [47], and thus at least partially surpass the usual nest mate recognition system during colony entry. Outside ants, in cuckoo bumble-bees, some species use chemical mimicry [48] while other use repellents [49] similar to those used by slave-making ants during raids [50]. Several species of bumble-bees have relatively simply cuticular hydrocarbon profiles and these are mimicked by host-specific cuckoo bumble-bees [48]. However, some bumble-bee hosts have a larger cue diversity making mimicry more difficult, and in these cases, the cuckoo bumble-bees are unique in their production of repellents in order to invade the host colony [48].
Because we understand the colony-specific recognition cues in both a social parasite (F. exsecta) and its host (F. fusca), it is now possible to study what determines a successful or failed nest invasion. Future studies should assess whether chemical similarity between parasite queens and hosts is necessary for both a successful entry to a host colony and subsequent egg acceptance, and what is the role of imprinting mechanisms of the host workers on eggs [28,51].
This study provides empirical evidence suggesting that in social insects the presence of parasites drives the diversity of recognition compounds. Maintaining diverse cues appears to be costly, since when parasite pressure is lacking cue diversity is greatly reduced, to a point where discrimination abilities are lost. Importantly, the lack of cue diversity is also reflected in the abilities to recognize conspecifics. This suggests that in F. fusca diverse cues are costly to produce and that the selection for rare cues from conspecific nest mate recognition [52] alone is not strong enough to maintain diversity. Similar loss of diversity has been observed in F. exsecta, where polygynous colonies have lost colony specificity of their Z9-alkene profiles ([23,53]), possibly because minimal encounter frequencies with non-nest mates make discrimination against conspecific nest mates unnecessary. However, our data from F. fusca are also compatible with the idea that kin recognition itself may select against rare cues (Crozier's paradox, [54,55]), and thus systems for kin recognition need external selection pressures, such as parasites [56], for the maintenance of cue variability. Since theoretical models that make different assumptions on the fitness effects of diverse cues make opposite predictions [52,54], work on the effects of cue diversity on recognition abilities and on the effects of encounter frequencies and recognition errors on fitness of colonies is required before alternative scenarios can be evaluated.
In summary, chemical egg recognition appears to have evolved in response to brood parasitism in ants and has strong parallels with visual egg recognition in bird (cuckoo) studies [6,57] and supports the hypothesis that parasites are driving recognition cue diversity [1]. The generality of the observed patterns, both at the levels of chemical diversity and behaviour, remains to be tested systematically in other systems where the parasite pressure varies geographically between host populations such as Leptothorax ants and their Harpagoxenus slave makers [58], or between closely related species. Furthermore, it remains open how diversity is maintained in social insects that do not have social parasites and how other factors, such as variation in kin structure, competition between conspecifics and non-social parasites play a role in maintenance of cue diversity and precise recognition abilities.
Acknowledgements
We thank Paul Schmid-Hempel of ETH Zurich, Roger Butlin and Duncan Jackson of Sheffield University for comments and Kalle Trontti, University of Helsinki, for help in genotyping. Also Liselotte Sundström of University of Helsinki for help with the original Finnish fieldwork funded from Academy of Finland grant (206505) and the two anonymous reviewers. This work was funded by NERC grants (NE/C512310/1 and NE/F018355/1) and grants from Academy of Finland (H.H.; grant numbers 213821, 121078).
References
- 1.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Hedrick P. W., Kim K. J. 2000. Genetics of complex polymorphisms: parasites and maintenance of the major histocompatibility complex variation. In Evolutionary genetics: from molecules to morhphology (eds Singh R. S., Krimbas C. B.), pp. 204–234 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Mallon E., Schmid-Hempel P. 2004. Behavioural interactions, kin and disease susceptibility in the bumblebee Bombus terrestris. J. Evol. Biol. 17, 829–833 10.1111/j.1420-9101.2004.00717.x (doi:10.1111/j.1420-9101.2004.00717.x) [DOI] [PubMed] [Google Scholar]
- 4.Nash D. R., Als T. D., Maile R., Jones G. R., Boomsma J. J. 2008. A mosaic of chemical coevolution in a large blue butterfly. Science 319, 88–90 10.1126/science.1149180 (doi:10.1126/science.1149180) [DOI] [PubMed] [Google Scholar]
- 5.Ochi H., Onchi T., Yanagisawa Y. 2001. Alloparental care between catfishes in Lake Tanganyika. J. Fish Biol. 59, 1279–1286 10.1111/j.1095-8649.2001.tb00191.x (doi:10.1111/j.1095-8649.2001.tb00191.x) [DOI] [Google Scholar]
- 6.Davies N. B. 2000. Cuckoos, cowbirds and other cheats. London, UK: T. & A. D. Posyer [Google Scholar]
- 7.Hölldobler B., Wilson E. O. 1990. The ants. Berlin, Germany: Springer-Verlag [Google Scholar]
- 8.Lahti D. C. 2005. Evolution of bird eggs in the absence of cuckoo parasitism. Proc. Natl Acad. Sci. USA 102, 18 057–18 062 10.1073/pnas.0508930102 (doi:10.1073/pnas.0508930102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies N. B., Bourke A. F. G., Brooke M. de L. 1989. Cuckoos and parasitic ants: interspecific brood parasitism as an evolutionary arms race. Trends Ecol. Evol. 4, 274–278 10.1016/0169-5347(89)90202-4 (doi:10.1016/0169-5347(89)90202-4) [DOI] [PubMed] [Google Scholar]
- 10.Blomquist G. J., Bagneres G. 2010. Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 11.Lenoir A., D'Ettorre P. D., Errard C., Hefetz A. 2001. Chemical ecology and social parasitism. Annu. Rev. Entomol. 46, 573–599 10.1146/annurev.ento.46.1.573 (doi:10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 12.Collingwood C. A. 1979. The Formicidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomol. Scand. 8, 1–174 [Google Scholar]
- 13.Czechowski W., Radchenko A., Czechowska W. 2002. The ants (Hymenoptera, Formicidae) of Poland. Warsaw, Poland: Museum and Institute of Zoology, Polish Academy of Sciences [Google Scholar]
- 14.Seifert B. 2007. Ants of Central and Northern Europe. Tauer, Germany: Lutra-Verlags-und Vertriebsgesellschaft [Google Scholar]
- 15.Punttila P. 1996. Succession, forest fragmentation, and the distribution of wood ants. Oikos 75, 291–298 10.2307/3546252 (doi:10.2307/3546252) [DOI] [Google Scholar]
- 16.Donisthorpe H. 1927. British ants: their life history and classification. London, UK: Routledge & Sons [Google Scholar]
- 17.Sudd J. H., Douglas J. M., Gaynard T., Murray D. M., Scockdale J. M. 1977. The distribution of wood-ants Formica lugubris in a northern English forest. Ecol. Entomol. 2, 301–313 10.1111/j.1365-2311.1977.tb00895.x (doi:10.1111/j.1365-2311.1977.tb00895.x) [DOI] [Google Scholar]
- 18.Savolainen R. 1990. Colony success of the submissive ant Formica fusca within territories of the dominant Formica polyctena. Ecol. Entomol. 15, 79–85 10.1111/j.1365-2311.1990.tb00786.x (doi:10.1111/j.1365-2311.1990.tb00786.x) [DOI] [Google Scholar]
- 19.Hannonen M., Helanterä H., Sundström L. 2004. Habitat age, breeding system and kinship in the ant Formica fusca. Mol. Ecol. 13, 1579–1588 10.1111/j.1365-294X.2004.02136.x (doi:10.1111/j.1365-294X.2004.02136.x) [DOI] [PubMed] [Google Scholar]
- 20.Martin S. J., Helanterä H., Drijfhout F. P. 2008. Colony-specific hydrocarbons identify nest mates in two species of Formica ant. J. Chem. Ecol. 34, 1072–1080 10.1007/s10886-008-9482-7 (doi:10.1007/s10886-008-9482-7) [DOI] [PubMed] [Google Scholar]
- 21.Akino T., Yamamura K., Wakamura S., Yamaoka R. 2004. Direct behavioural evidence for hydrocarbons as nest mate recognition cues in Formica japonica (Hymenoptera: Formicidae). Appl. Entomol. Zool. 39, 381–387 10.1303/aez.2004.381 (doi:10.1303/aez.2004.381) [DOI] [Google Scholar]
- 22.Ozaki M., Wada-Katsumata A., Fujikawa K., Iwasaki M., Yokohari F., Satoji Y., Nisimura T., Yamaoka R. 2005. Ant nest mate and non-nest mate discrimination by a chemosensory sensillium. Science 309, 311–315 10.1126/science.1105244 (doi:10.1126/science.1105244) [DOI] [PubMed] [Google Scholar]
- 23.Martin S. J., Vitikainen E., Helanterä H., Drijfhout F. P. 2008. Chemical basis of nest mate recognition in the ant Formica exsecta. Proc. R. Soc. B 275, 1271–1278 10.1098/rspb.2007.1708 (doi:10.1098/rspb.2007.1708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson C. A., Topoff H., Vander Meer R. K., Lavine B. 2005. Do these eggs smell funny to you?: an experimental study of egg discrimination by hosts of the social parasite Polyergus breviceps (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 57, 245–255 10.1007/s00265-004-0851-0 (doi:10.1007/s00265-004-0851-0) [DOI] [Google Scholar]
- 25.Martin S. J., Drijfhout F. P. 2009. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 35, 1151–1161 10.1007/s10886-009-9695-4 (doi:10.1007/s10886-009-9695-4) [DOI] [PubMed] [Google Scholar]
- 26.Guerrieri F. J., Nehring V., Nielsen J., Giovanni Galizia C., d'Ettorre P. 2009. Ants recognise foes not friends. Proc. R. Soc. B 276, 2461–2468 10.1098/rspb.2008.1860 (doi:10.1098/rspb.2008.1860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helanterä H., Sundström L. 2007. Worker policing and nest mate recognition in the ant Formica fusca. Behav. Ecol. Sociobiol. 61, 1143–1149 10.1007/s00265-006-0327-5 (doi:10.1007/s00265-006-0327-5) [DOI] [Google Scholar]
- 28.Helanterä H., Martin S. J., Ratnieks F. L. W. 2007. Prior experience with eggs laid by non-nest mate queens induces egg acceptance errors in ant workers. Behav. Ecol. Sociobiol. 62, 223–228 10.1007/s00265-007-0456-5 (doi:10.1007/s00265-007-0456-5) [DOI] [Google Scholar]
- 29.Bhatkar A., Whitcomb W. H. 1970. Artificial diet for rearing various species of ants. Florida Entomol. 53, 229–232 10.2307/3493193 (doi:10.2307/3493193) [DOI] [Google Scholar]
- 30.Seppä P., Helanterä H., Chernenko A., Trontti K., Punttila P., Sundström L. 2009. Population genetics of the black ant Formica lemani (Hymenoptera: Formicidae). Biol. J. Linn. Soc. 97, 247–258 10.1111/j.1095-8312.2009.01192.x (doi:10.1111/j.1095-8312.2009.01192.x) [DOI] [Google Scholar]
- 31.Chapuisat M. 1996. Characterization of microsatellite loci in Formica lugubris B and their variability in other species. Mol. Ecol. 5, 599–601 10.1111/j.1365-294X.1996.tb00354.x (doi:10.1111/j.1365-294X.1996.tb00354.x) [DOI] [PubMed] [Google Scholar]
- 32.Gyllenstrand N., Gertsch P. J., Pamilo P. 2002. Polymorphic microsatellite DNA markers in the ant Formica exsecta. Mol. Ecol. Notes 2, 67–69 10.1046/j.1471-8286.2002.00152.x (doi:10.1046/j.1471-8286.2002.00152.x) [DOI] [Google Scholar]
- 33.Hasegawa E., Imai S. 2004. Characterization of microsatellite loci in red wood ants Formica (s. str.) spp. and the related genus Polyergus. Mol. Ecol. Notes 4, 200–203 10.1111/j.1471-8286.2004.00614.x (doi:10.1111/j.1471-8286.2004.00614.x) [DOI] [Google Scholar]
- 34.Goudet J. 2001. Fstat. See http://www.unil.ch/izea/softwares/fstat.html
- 35.Nei M. 1987. Molecular evolutionary genetics. New York, NY: Columbia University Press [Google Scholar]
- 36.Cornuet J. M., Luikart G. 1996. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144, 2001–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin S. J., Helanterä H., Drijfhout F. P. 2008. Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol. J. Linn. Soc. 95, 131–140 10.1111/j.1095-8312.2008.01038.x (doi:10.1111/j.1095-8312.2008.01038.x) [DOI] [Google Scholar]
- 38.Dallerac R., Labeur C., Jallon J.-M., Knipple D. C., Roelofs W. L., Wicker-Thomas C. 2000. A Δ9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 97, 9449–9454 10.1073/pnas.150243997 (doi:10.1073/pnas.150243997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beye M., Neumann P., Moritz R. F. A. 2004. Nest mate recognition and the genetic gestalt in the mound-building ant Formica polyctena. Insectes Soc. 44, 49–58 10.1007/s000400050022 (doi:10.1007/s000400050022) [DOI] [Google Scholar]
- 40.Martín-Gálvez D., Soler J. J., Martínez J. G., Krupa A. P., Richard M., Soler M., Møller A. P., Burke T. 2006. A quantitative trait locus for recognition of foreign eggs in the host of a brood parasite. J. Evol. Biol. 19, 543–550 10.1111/j.1420-9101.2005.01002.x (doi:10.1111/j.1420-9101.2005.01002.x) [DOI] [PubMed] [Google Scholar]
- 41.Endler A., Liebig J., Hölldobler B. 2006. Queen fertility, egg marking and colony size in the ant Camponotus floridanus. Behav. Ecol. Sociobiol. 59, 490–499 10.1007/s00265-005-0073-0 (doi:10.1007/s00265-005-0073-0) [DOI] [Google Scholar]
- 42.Lommelen E., Johnson C. A., Drijfhout F. P., Billen J., Gobin B. 2008. Egg marking in facultatively queenless ant Gnamptogenys striatula: the source and mechanism. J. Insect Physiol. 54, 727–736 10.1016/j.jinsphys.2008.02.002 (doi:10.1016/j.jinsphys.2008.02.002) [DOI] [PubMed] [Google Scholar]
- 43.Martin S. J., Takahashi J., Ono M., Drijfhout F. P. 2008. Is the social parasite Vespa dybowskii using chemical transparency to get her eggs accepted? J. Insect Physiol. 54, 700–707 10.1016/j.jinsphys.2008.01.010 (doi:10.1016/j.jinsphys.2008.01.010) [DOI] [PubMed] [Google Scholar]
- 44.Martin S. J., Chaline N., Jones G., Ratnieks F. L. W. 2004. Role of hydrocarbons in egg recognition in the honeybee. Physiol. Entomol. 29, 395–399 10.1111/j.0307-6962.2004.00404.x (doi:10.1111/j.0307-6962.2004.00404.x) [DOI] [Google Scholar]
- 45.Martin S. J., Beekman M., Wossler T. C., Ratnieks F. L. W. 2002. Parasitic Cape honeybee workers, Apis mellifera capensis, evade policing. Nature 415, 163–165 10.1038/415163a (doi:10.1038/415163a) [DOI] [PubMed] [Google Scholar]
- 46.Johnson C. A., Vander Meer R. K., Lavine B. 2001. Changes in the cuticular hydrocarbon profile of the slave-maker ant queen, Polyergus breviceps Emery, after killing a formica host queen (Hymenoptera: Formicidae). J. Chem. Ecol. 27, 1787–1804 10.1023/A:1010456608626 (doi:10.1023/A:1010456608626) [DOI] [PubMed] [Google Scholar]
- 47.Mori A., Visicchio R., Sledge M. F., Grasso D. A., Le Moli F., Turillazzi S., Spencer S., Jones G. R. 2000. Behavioural assays testing the appeasement allomone of Polyergus rufescens queens during host-colony usurpation. Ethol. Ecol. Evol. 12, 315–322 [Google Scholar]
- 48.Martin S. J., Carruthers J. M., Williams P. H., Drijfhout F. P. In press Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J. Chem. Ecol. (doi:10.1007/s10886-010-9805-3) [DOI] [PubMed] [Google Scholar]
- 49.Zimma B. O., Ayasse M., Tengö J., Ibarra F., Schulz C., Francke W. 2003. Do social parasitic bumblebees use chemical weapons? (Hymenoptera, Apidae). J. Comp. Physiol. A 189, 769–775 10.1007/s00359-003-0451-x (doi:10.1007/s00359-003-0451-x) [DOI] [PubMed] [Google Scholar]
- 50.Tsuneoka Y., Akino T. 2009. Repellent effect on Formica workers of queen Dufour's gland secretion of the obligatory social parasite ant, Polyergus samurai (Hymenoptera: Formicidae). Appl. Entomol. Zool. 44, 133–141 10.1303/aez.2009.133 (doi:10.1303/aez.2009.133) [DOI] [Google Scholar]
- 51.Helanterä H., Ratnieks F. L. W. 2009. Two independent mechanisms of egg recognition in worker Formica fusca ants. Behav. Ecol. Sociobiol. 63, 573–580 10.1007/s00265-008-0692-3 (doi:10.1007/s00265-008-0692-3) [DOI] [Google Scholar]
- 52.Ratnieks F. L. W. 1991. The evolution of genetic cue diversity in social Hymenoptera. Am. Nat. 137, 202–226 10.1086/285154 (doi:10.1086/285154) [DOI] [Google Scholar]
- 53.Martin S. J., Helanterä H., Kiss K., Lee Y. R., Drijfhout F. P. 2009. Polygyny reduces rather than increases nest mate discrimination cue diversity in Formica exsecta ants. Insect. Soc 56, 375–383 10.1007/s00040-009-0035-z (doi:10.1007/s00040-009-0035-z) [DOI] [Google Scholar]
- 54.Crozier R. H. 1986. Genetic clonal recognition abilities in marine-invertebrates must be maintained by selection for something else. Evolution 40, 1100–1101 10.2307/2408769 (doi:10.2307/2408769) [DOI] [PubMed] [Google Scholar]
- 55.Rousset F., Roze D. 2007. Constraints on the origin and maintenance of genetic kin recognition. Evolution 61, 2320–2330 10.1111/j.1558-5646.2007.00191.x (doi:10.1111/j.1558-5646.2007.00191.x) [DOI] [PubMed] [Google Scholar]
- 56.Gardner A., West S. A. 2007. Social evolution: the decline and fall of genetic kin recognition. Curr. Biol. 17, R810–R812 10.1016/j.cub.2007.07.030 (doi:10.1016/j.cub.2007.07.030) [DOI] [PubMed] [Google Scholar]
- 57.Aviles J. M., Moller A. 2003. Meadow pipit (Anthus pratensis) egg appearance in cuckoo (Cuculus canorus) sympatric and allopatric populations. Bio. J. Linn. Soc. 79, 543–549 10.1046/j.1095-8312.2003.00208.x (doi:10.1046/j.1095-8312.2003.00208.x) [DOI] [Google Scholar]
- 58.Foitzik S., Fischer B., Heinze J. 2003. Arms races between social parasites and their hosts: geographic patterns of manipulation and resistance. Behav. Ecol. 14, 80–88 10.1093/beheco/14.1.80 (doi:10.1093/beheco/14.1.80) [DOI] [Google Scholar]