Abstract
Artificial limbs allow amputees to manipulate objects, but the loss of a limb severs the sensory link between a subject and objects they touch. A novel surgical technique we term targeted reinnervation (TR) allows severed cutaneous nerves to reinnervate skin on a different portion of the body. This technique provides a physiologically appropriate portal to the sensory pathways of the missing limb through the reinnervated skin. This study quantified the ability of three amputee subjects who had undergone TR surgery on the chest (two subjects) and upper arm (one subject) to discriminate changes in graded force on their reinnervated skin over a range of 1–4 N using a stochastic staircase approach. These values were compared to those from sites on their intact contralateral skin and index fingers, and from the chests and index fingers of a control population (n = 10). Weber’s ratio (WR) was used to examine the subjects’ abilities to discriminate between a baseline force and subsequent forces of different magnitudes. WRs of 0.22, 0.25, and 0.12 were measured on the reinnervated skin of the three TR subjects, whereas WRs of 0.25,0.23, and 0.12 were measured on their contralateral skin. TR subjects did not have substantially different WRs on their reinnervated versus their contralateral normal side and did not appear to exhibit a trend towards impaired sensation. No significant difference was found between the WR of the chest and index finger of the control subjects, which ranged between 0.09 and 0.21. WR of reinnervated skin for TR subjects were within the 95% confidence interval of the control group. These data suggest that subjects with targeted reinnervation have unimpaired ability to discriminate gradations in force.
Index Terms: Force difference limens, psychophysics, tactile resolution, targeted reinnervation
Amputation results in a loss of control to the missing limb, manipulation of the environment through the missing limb, and sensation from the missing limb. A recently developed surgical technique addresses these latter two losses by redirecting nerves previously innervating the amputated limb to new muscle and skin sites proximal to the amputation site. We term this surgical approach targeted reinnervation (TR) [1]–[4]. After residual nerves are transferred, the motor efferents reinnervate the denervated target muscles. The reinnervated muscles function as biological amplifiers for the limb nerve signals and provide new myoelectric control sites corresponding to lost movements of the amputated limb [5]. These physiologically appropriate myoelectric control sites allow the amputee to intuitively control a multifunction robotic limb by simply attempting the appropriate movements. In addition to the motor reinnervation, the sensory afferents of the peripheral nerves often reinnervate denervated skin overlying the nerve transfer sites. This sensory reinnervation creates a somatosensory expression of the missing limb on the reinnervated skin of the amputee [1]–[4]. When these individuals are touched on different parts of their reinnervated skin, they interpret the sensation as occurring on their missing hand and limb.
This study sought to quantify the reinnervated sensation of these subjects, specifically, their abilities to distinguish changes in force levels, using psychophysics. Although the human body constantly interacts with a variety of physical stimuli, only a few are detected by sensory receptors, and even fewer are perceived due to physical thresholds and cortical representation. Sensory psychophysics studies the relationship between these physical stimuli and the resulting perception, and proceeds from the foundation that all sensory systems convey four types of information for any given stimulation: modality, location, intensity, and timing [6]. For TR subjects, the modality (touch, temperature, etc.) and timing are presumably the same, but the set of sensory receptors activated by the stimulus are different, not only in quantity but potentially also in quality, since nerves once innervating glabrous skin now innervate hairy skin. Given the fact that the location has changed, the question arises as to how the perceived intensity has changed, if at all, both with respect to the previous sensory receptors and the cortical processing that created a meaningful representation of the activated receptors. Towards this end, perceived intensity on reinnervated skin must be compared both with glabrous skin on the hand and with non-reinnervated hairy skin. Weber’s ratio (WR) models the relationship between the physical magnitude of a stimulus and the perceived intensity of the stimulus as a linear relationship between the baseline stimuli and the difference limen of that baseline [7], [8]. Some studies report WR [7] whereas others only report difference limen [8].
Characterizing the sensation ability of reinnervated skin may inform future attempts to provide sensory feedback to prosthesis users. Due to the limited dexterity of commercially available prostheses in the near future, previous studies attempting to restore sensation have almost exclusively focused on representing grip force [9]–[12]. This study similarly chose force as the stimulus modality. Perception of force intensity may be somewhat different from other stimuli, in that whereas other stimuli such as two-point discrimination and point localization are more sensitive on glabrous skin than on the trunk [13], pressure thresholds for glabrous skin are not appreciably different from hairy skin [13], [14]. No previous literature has explored the extension of this finding to force difference limens. Thus, the goal of this study was to evaluate the force difference limens in TR subjects, and if it was found that force difference limens differed between glabrous skin and hairy skin, to determine whether the resulting values corresponded better to the glabrous skin due to cortex representation or to hairy skin due to the sensory receptors.
I. Materials and Methods
This study examined three amputee subjects (SD1, SD2, and TH3) who had undergone the TR surgery and developed sensory reinnervation. Ten able-bodied subjects were also tested. All procedures were performed with informed consent and approved by the Northwestern University Institutional Review Board.
A. General Protocol
Force stimuli were applied to the testing sites on the subjects with a Kinea Designs (Evanston, IL) haptic tactor system. This device applies force to the skin through a rounded 10-mm-diameter acrylic plunger head. The tactor maintains constant contact with the skin and constant force by tracking the applied force within a workspace of 12 mm. The tactor can achieve 8.8 N maximum force, with a resolution 0.004 N. The tactor device was designed expressly to provide a tactile interface for a prosthetic limb. The force and position of the stimulator were controlled by a Matlab (Mathworks, Natick, MA) Simulink file and interfaced with the tactor through a data acquisition card (Measurement Computing PCI-DAS 1602/16). Audible noise produced by the stimulator was negligible. The tactor was mounted on a micromanipulator (Narishige MM-3) that was attached to an adjustable armature (Leica Microsystems, Bannockburn, IL). The tactor was positioned normal to the skin surface when applying force stimuli. To prevent distraction during testing, subjects wore noise cancellation headphones (Bose QuietComfort 2). Audio cues for testing were played through the headphones when force was applied.
Testing locations were shaved when necessary. All subjects were seated in an adjustable chair. When points on the chest were tested, subjects rested with their arms at their sides. When the index finger was tested, the hand was pronated 90° and rested against a gel mold. The index fingernail was affixed to a metal dome with cyanoacrylate adhesive. When the upper arm was tested, the subject’s arm was extended and placed on a rest that was parallel to the floor.
A two-alternative forced-choice paradigm was used to measure the subjects’ abilities to discriminate small differences in force. Force stimuli were presented in pairs consisting of the standard (S) force and a modified (M) force (either higher or lower than the standard), as shown in Fig. 1. Subjects were required to state which of the two forces they believed was larger. The order in which the S and M stimuli were presented (SM or MS) was randomized for each pair. As opposed to alternative testing strategies, which present the standard stimulus first for every trial, this procedure (referred to as the SM/MS procedure) ensures a consistent choice paradigm by eliminating the potential for the subject to acquire a memory of the standard force over time [15].
Fig. 1.
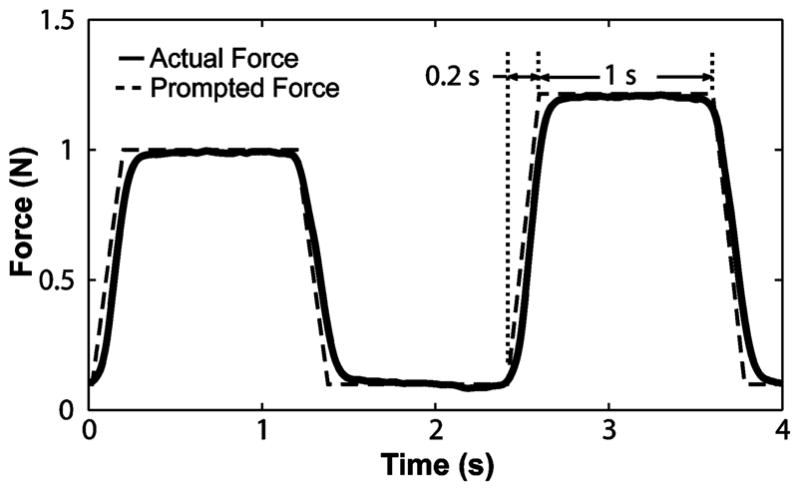
Force trace of a single trial. The order of the standard stimulus and the modified stimulus was randomized. Subjects were required to respond by saying that either the first or the second stimulus was larger (two alternatives, with a required decision).
The force ramped up and down from the resting force to the stimulus force in 0.2 s. Stimuli lasted 1 s each, with a 1-s interval between stimuli and a 3-s interval between trials. A resting force of 0.1 N was applied between stimuli to retain contact of the stimulator with the skin. Baseline forces of 1 N, 2.5 N, and 4 N were used, unless otherwise noted. A limit of 4 N was chosen because in preliminary testing subjects experienced discomfort for repeated application of higher forces, given the size of the stimulator head.
A weighted up–down stochastic approximation staircase technique [16] was used to evaluate the force difference limen (threshold). This technique generates a force difference based on the following equation:
| (1) |
where xn+1 is the next force difference, xn is the force difference just evaluated, and c is the initial difference. To provide rapid convergence, c was set to 0.8σ [16], where σ is defined as the difference in force between the 51% and 99% detection level [17]. σ was set to 0.36 N per N of baseline force and x1 was set to 0.6 N per N of baseline force based on pilot data gathered previously. nreversal was the number of reversals, z was set to 0.75 to find the force difference at which the subject chose the correct stimuli 75% of the time, and Φ equaled 0 for an incorrect response and 1 for a correct response. The stochastic approximation effect of c/nreversals + 1 prevented step size selection from influencing the actual targeted probability [16], [17].
Two staircases were interwoven for testing. In the first staircase, the modified force was larger than the baseline force. In the second staircase, the modified force was lower than the baseline force. The experiment was terminated after both staircases contained at least 25 reversals [18], [19]. The force difference limens from the upper and lower staircases following 25 reversals were averaged to obtain the difference limen for that baseline force. WR was calculated for each baseline force as the ratio of the average force difference limens to the baseline force.
Using a staircase above the standard and a staircase below the standard prevented the average force difference limen from being biased due to an average presentation force higher or lower than the baseline force. In addition, simultaneously presenting alternating trials from each staircase preserved the SM/MS effect by preventing the subject from acquiring a memory that the baseline force was always the same magnitude: an effect which can change the optimum choice paradigm and affect the resulting force difference limen [15].
In an effort to model effects of experimental design choice on the results of the study, the staircase testing paradigm was simulated using a sigmoidal function to represent the subject’s ability to discriminate force. This model provided the estimated population standard deviation, which in turn allowed the authors to model the smallest detectable difference that would be expected for a given sample size. The simulation also allowed for inclusion of force attenuation between the two samples of a trial, which was not found to bias the convergence value.
Experimental sessions typically lasted 30 min. Only one session was performed per day, except for which subject SD2, where time constraints dictated two sessions per day. Completion of all testing trials typically took one to two weeks. Baseline force was randomized, and testing sites were randomized for each baseline force.
B. Able-Bodied Subjects
Ten able-bodied subjects (mean age 37 ± 13 years) including five males and five females with no known sensory deficits performed this test for a single 1 N baseline on the right index finger and chest. WR was calculated for this baseline force. The chest testing location was standardized by establishing the point midway between the acromion and sternoclavicular joint. The testing point was placed directly inferior to the midpoint at a distance that was equal to the midway distance. These data were analyzed independently for both locations using a linear regression model that included age, gender, compliance of the soft tissue, and all interaction terms.
C. TR Subjects
TR amputee subjects were independently evaluated over three baseline forces at each testing point. WR was calculated for each baseline force, and the average WR was calculated across the three baseline forces. For each TR subject, a two-way univariate ANOVA was performed with one factor (location) and one covariate (baseline force). A statistically significant interaction term between the location and baseline force signifies a different value of WR between locations. WR for chest values of the TR population (n = 3) were compared to the able-bodied population (n = 9) using an independent sample t-test, with no assumption of equal variance given the unequal population sizes.
1) Subject SD1
The TR surgery involves transferring the large severed peripheral nerves that once served the limb to denervated target muscles proximal to the amputation site. Subject SD1, a 55-year-old male bilateral shoulder disarticulation amputee, had four of these nerve-to-muscle transfers on his left side. The musculocutaneous, median, radial, and ulnar nerves were redirected to the clavicular head of the pectoralis (p.) major, the upper sternal head of the p. major, the lower sternal head of the p. major and the p. minor. The p. minor was moved lateral to the p. major [2].
This experiment was conducted five and a half years after SD1’s TR surgery. He was tested on both his reinnervated and contralateral chest [Fig. 2(a)]. SD1’s reinnervated skin testing site was placed at a location that elicited sensations referred to the back of the hand when touched. This region did not contain any native chest sensation [1]. His contralateral normal testing point was situated lower on the right chest than on the reinnervated side, over an area devoid of scar tissue. This location also previously showed normal physiologic response to vibratory input [20].
Fig. 2.
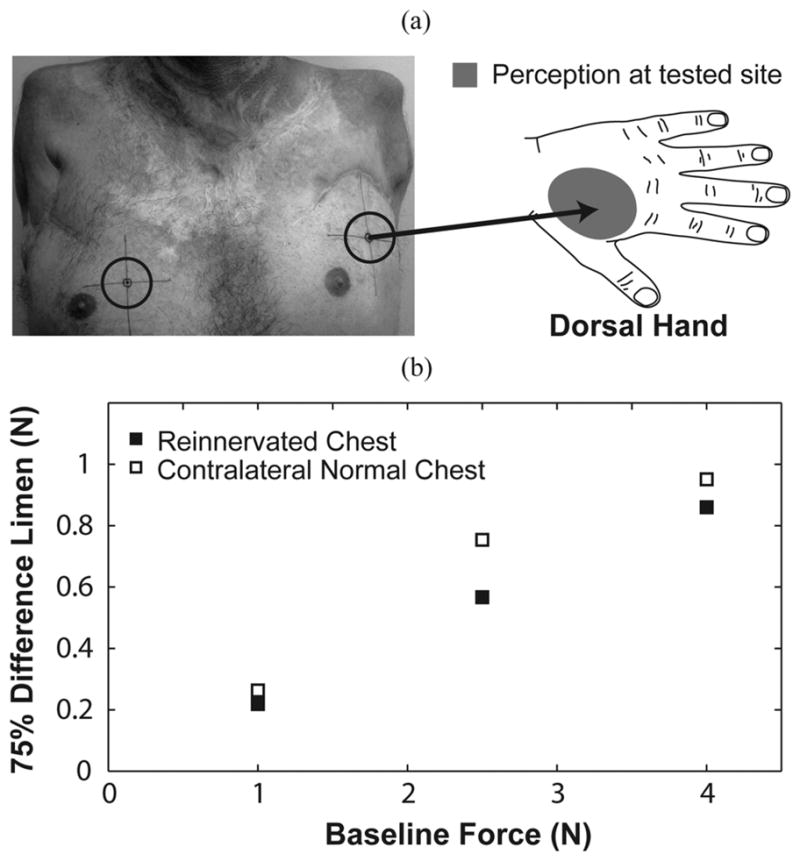
Subject SD1 was tested on two locations: his left reinnervated chest and right normal chest. (a) Force applied to the testing site elicited referred sensations that were projected to the thenar aspect on the dorsal aspect of his missing hand. The right normal chest location was chosen in a place devoid of scar tissue. Figure adapted from [1]. (b) TR subject plots are presented as a function of baseline force for the chest.
2) Subject SD2
Subject SD2, a 25-year-old female left unilateral short transhumeral (functional shoulder disarticulation) amputee, had four nerve-to-muscle transfers. The ulnar and musculocutaneous nerves were redirected to the medial and lateral halves of the clavicular head of the p. major. The median nerve was split and each end was redirected to the upper and lower portions of the sternal head of the p. major. The radial nerve was transferred to the long thoracic nerve to reinnervate the distal slips of the serratus anterior. Two sensory nerve transfers were also performed to provide additional conduits for reinnervation to the skin surface. The supraclavicular cutaneous nerve was cut—denervating the surface of the chest inferior to the clavicle—and sewn end-to-side to the ulnar nerve. The intercostobrachial nerve was sewn end-to-side to the median nerve [3].
Subject SD2 was tested two and a half years after her TR surgery on her reinnervated chest skin and on her normal contralateral chest, just inferior to the clavicle [Fig. 3(a)]. The reinnervated testing site was situated where she felt only sensations projected to the index finger, thumb, and outer edge of the palm of her missing hand [Fig. 3(a)]. No native chest sensation was present at this location [1]. Subject SD2 was also tested on her right index finger. Due to time constraints, subject SD2 was sometimes required to perform two trials on the same day: one 30-min trial in the morning and another trial following her 1-h-long lunch break.
Fig. 3.
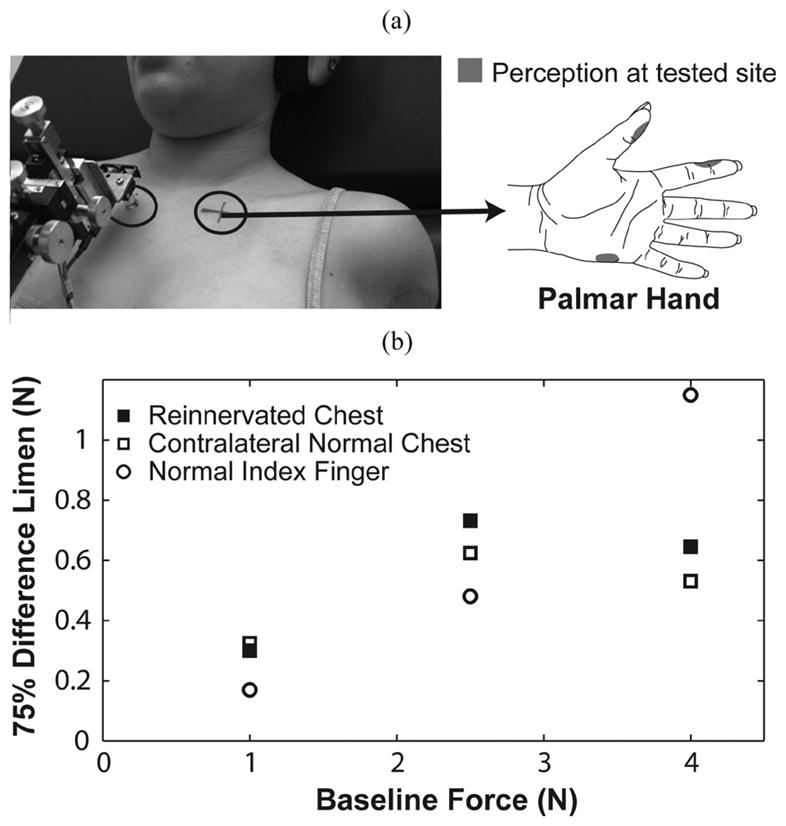
Subject SD2 was tested on three locations: her left reinnervated chest, right chest, and right index finger. (a) Force applied to the testing site elicited referred sensations that were projected to three distinct locations on the palmar aspect of her missing hand. The right chest location was chosen to provide symmetry with the reinnervated location. Figure adapted from [1]. (b) TR subject plots are presented as a function of baseline force for the chest and index finger.
3) Subject TH3
Subject TH3, a 39-year-old female left trans-humeral amputee, underwent two nerve–muscle transfers. The distal radial and median nerves were redirected to the lateral head of the triceps and medial head of the biceps. The distal end of the residual limb was also purposely denervated.
Subject TH3 was tested one year following her TR surgery. Testing points included the medial aspect of her reinnervated upper arm and a location mirroring the position on her contralateral normal upper arm [Fig. 4(a)]. Subject TH3 was also tested on her right index finger. TH3’s reinnervated testing site was situated where she only felt sensations projected to the palm and palmar aspect of her lateral three fingers. No native arm sensation was evident at this location.
Fig. 4.
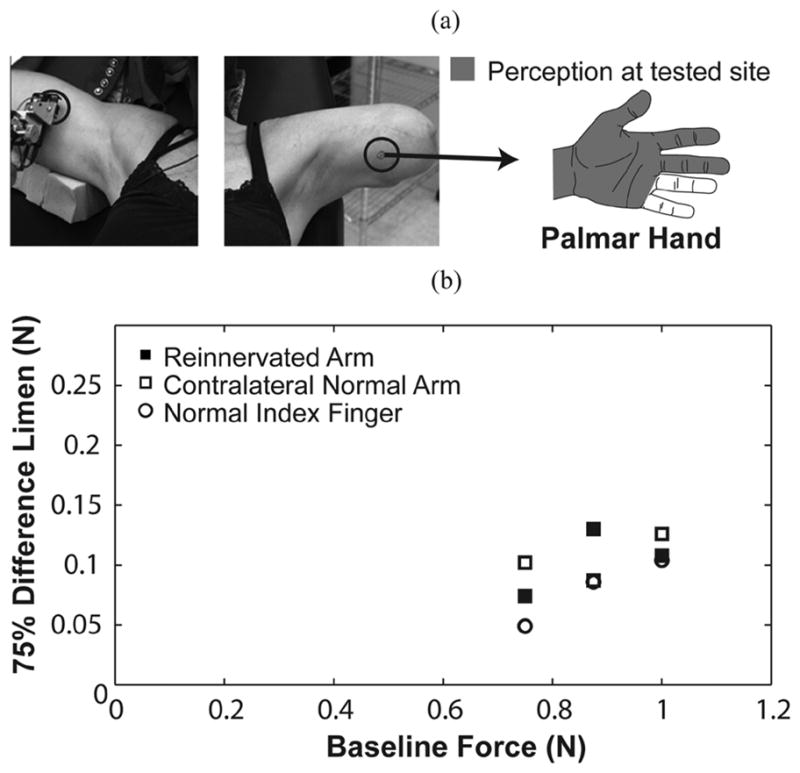
Subject TH3 was tested on three locations: her left reinnervated arm, right arm, and right index finger. (a) Force applied to the testing site elicited referred sensations that were projected to a broad region of the palmar aspect of her missing hand. The right arm location was chosen to provide symmetry with the reinnervated location. (b) TR subject plots are presented as a function of baseline force for the chest and index finger.
The choice of baseline forces for subject TH3 (0.75 N–1 N) differed from the substantially larger range of forces for the other subjects (1 N–4 N) because the testing location on her arm was situated in an area where there was a large amount of compliant tissue. The 12 mm range of the tactor could not displace enough tissue to obtain higher baseline forces.
II. RESULTS
A. Control Subjects
WRs for the fingertip ranged from 0.09–0.19, with a mean value of 0.14 ± 0.04 (Table I). WRs for the chest ranged from 0.11–0.20, with one value of 0.37 identified as a statistical outlier using Grubbs’ test [21] that was not included in analyses, and a mean value of 0.15 ± 0.03. No variables were significant for the index finger (p > 0.2) or the chest (p > 0.1). Importantly, we found no significant difference (p > 0.6) or trend between the chest and index finger using a repeated measures t-test. Neither WRs for the reinnervated skin nor the contralateral chest skin for the TR population was statistically significant from the WRs of the control population (p > 0.4).
TABLE I.
Weber’s Ratio (Mean ± SD)
| SD1 | SD2 | TH3 | Targeted Reinnervation (n=3) | Able bodied (n=10) | |
|---|---|---|---|---|---|
| Reinnervated Skin | 0.22 ± 0.01 | 0.25 ± 0.08 | 0.12 ± 0.03 | 0.19 ± 0.06 | N.A. |
| Contralateral Normal Skin | 0.25 ± 0.03 | 0.23 ± 0.10 | 0.12 ± 0.02 | 0.21 ± 0.08 | 0.15 ± 0.03 |
| Normal Fingertip | N.A. | 0.21 ± 0.06 | 0.09 ± 0.02 | 0.15 (n=2) | 0.14 ± 0.04 |
B. SD1
Subject SD1’s staircases for each trial were stable as they converged, and there was consistency between the upper and lower staircases, save for the 2.5 N baseline testing session on the normal chest. Subject SD1 demonstrated linearity in baseline force versus average 75% force difference limen on both sides of his chest [Fig. 2(b)]. The WR at the 2.5 N baseline for the normal chest was slightly higher than at the other baselines. This small deviation was likely because of the inconsistent upper staircase for this session. The reinnervated chest had a consistently lower WR (0.22 ± 0.01) than the contralateral normal chest (0.25 ± 0.03). WRs for the reinnervated site were within the 95% confidence interval for control subjects, but WRs for the contralateral normal side were outside the 95% confidence interval.
C. SD2
Subject SD2 exhibited less stability and consistency in her staircases than other subjects did. WRs for the reinnervated chest (0.25 ± 0.08), contralateral normal chest (0.23 ± 0.10), and normal index finger (0.21 ± 0.06) were similar. There was a trend of better sensitivity for her index finger than for either side of her chest for the two lower force levels [Fig. 3(b)]. In contrast, her chest appeared to be more sensitive than her index finger did at the higher force level. In comparison to the able-bodied population, the WRs for both sides of subject SD2’s chest fell within the control subject 95% confidence range for the chest; however, her WR for the index finger was outside the control subject 95% confidence range for the index finger.
D. TH3
Most of the TH3’s staircases from testing trials were stable and consistent. There was a general trend of improved sensitivity in WR on the index finger (0.09 ± 0.02) compared to the arm sites [0.12 ± 0.03 on reinnervated skin, 0.12 ± 0.02 on contralateral skin, Fig. 4(b)]. WRs for all three of subject TH3’s sites fell within the control group’s 95% confidence intervals.
III. Discussion
This study demonstrated that persons with targeted reinnervation could discriminate between gradations in force. When the chest or arm skin that had been reinnervated with hand afferents was touched, they could accurately perceive changes in force. Despite small variations across baseline force, all of the TR subjects demonstrated near-normal WR. The WR values for reinnervated skin were always within the control group 95% confidence interval and closely matched to contralateral skin, although WR values for SD1’s contralateral chest and SD2’s index finger fell outside the control group 95% confidence interval. There was no trend towards impaired WR on their reinnervated skin, suggesting that their reinnervated skin had retained high fidelity force discrimination.
The calculated WR typically provided a good estimate of the relative force difference limen within the range of tested forces. The WR values for SD1 appear to provide a good description of his perception due to their strong linearity and zero offset, and values for TH3 were likewise stable. SD2 did not have as stable and consistent staircases or linear WRs. Several issues may have contributed to this result. SD2 was often tested twice in one day and she appeared to be less alert during the experimental sessions than other subjects, which may have contributed to the higher variance of her results. The decreased force difference limen found at high baseline forces for both sides of her chest locations may possibly be explained by the proximity of the stimulus location to the clavicle. The harder the tactor pushed, the less compliant the tissue became from being sandwiched between the tactor and the tissue supporting the clavicle.
A necessary limitation of this study was the use of controlled force instead of controlled indentation. Although studies focusing on manipulation typically use controlled force [7], [8], [22], studies on cutaneous mechanoreceptive afferents typically use controlled indentation rates [23]–[25], due to the nonlinear relationship between force and depth [26], [27]. Perceived intensity may correlate better with controlled indentation rates than with controlled force, but only when indentation is the controlled variable [23]. Although using controlled indentation would have provided a more robust experimental protocol for this study than controlled force did, it would have been difficult to implement in the laboratory, and almost impossible to implement in a clinical feedback system, due to the necessity of tracking chest motion as subjects breathed. In the presence of breathing, determining indentation rate becomes a significantly more difficult challenge, whereas force tracking is still feasible as long as a high fidelity, low impedance stimulator is used.
This observation still does not explain why SD2 had lower WR for higher stiffness (4 N baseline) than she did for lower stiffness (1 N baseline), but it may provide evidence for why both force perception thresholds and force discrimination limen of the chest were similar to the index finger. Mechanoreceptors are stimulated by strain [28], which is caused either by indentation or force. In order to examine the interaction between indentation and force as they pertain to perceived intensity, we turn to an elasticity solution derived by Hayes [26], [29] that models indentation on a thin elastic layer bonded to a rigid foundation by a rigid frictionless cylindrical plane-ended indenter. This solution suggests that strain is proportional to depth of indentation, regardless of force, and that for a given force, tissue with higher stiffness will produce less strain, and by implication, a less intense stimulation. Thus, from a mechanical viewpoint we would expect a constant force to evoke a more intense stimulation in the lower-stiffness chest than in the higher-stiffness index finger. It is conceivable that this mechanical advantage in the chest, coupled with the disadvantage of a fewer number of mechanoreceptors, results in similar intensity of perception for both monofilament pressure thresholds and graded force discrimination.
This protocol maintained a constant ramp time to apply each force, which in a compliant environment resulted in different ramp velocities. It is possible that subjects used the difference in indentation velocity, rather than the difference in the constant force, to determine the intensity of the stimulus. For example, SA-I Merkel Cell receptors, which are routinely found in reinnervated skin [30], [31] code both static pressure intensity following reinnervation [32] and velocity of indentation.
The results obtained by this study for the normal index finger were consistent with a recent study [7], which found an average WR for graded force discrimination of 0.12 on the index finger. There appears to be no literature reporting force difference limens for graded force discrimination on normal chest or other non-glabrous skin. Data on our able-bodied subjects indicates that glabrous index finger skin and hairy chest skin have similar WRs and thus are able to detect similar relative changes in graded force. Although sensory stimuli such as two-point discrimination and point localization are more sensitive on the fingertip than on the chest [13], monofilament pressure thresholds for the fingertip, thenar eminence, forearm, upper arm, and chest, however, are not appreciably different from each other [13], [14]. This paper suggests that there is no appreciable difference in force discrimination either. This trend is important because it suggests that when TR is used to provide sensory feedback for prosthetic hands, the target tissue environment will likely not adversely affect the ability of the individual to sense gradations in force.
The goal of this study was to investigate the ability of targeted reinnervation subjects to perceive differences in graded force applied to reinnervated skin. Although the sample size was small—by virtue of there being only a few individuals who have undergone the TR surgery—there appeared to be no outward trend towards functional deficit of the reinnervated skin versus the normal contralateral skin of the TR subjects. These results suggest that reinnervated sites were as sensitive to changes in force as contralateral normal sites and the intact index finger. Although TR subjects were tested on the skin of the chest or upper arm, they felt as if they were being touched on their missing hand. This perception of touch is different from the phantom sensations reported by one of the subjects and described in detail by other investigators (for review see [33]). The new reinnervated hand sensation may provide a direct portal from the skin to the sensory pathways that once served the missing limb. The reinnervated skin responds to a variety of stimuli, including temperature and pain. Moreover, sharp/dull discrimination is also intact [3].
Several commercial stimulators are being developed to provide normal and tangential force feedback, as well as vibration feedback, for subjects with TR. This feedback may improve the ability of amputees to control advanced prostheses in the future, as they may be able to use physiologically appropriate feedback to aid in task completion, rather than relying solely on visual feedback to complete all tasks or using autonomous grip adjustment by the prosthesis [34]–[36]. Although the ability to sense graded pressure may play an important role in such feedback systems [10], [11], perception of tangential force discrimination [8] and scaling between normal and tangential forces [8], [37]–[39] are required for the manipulation of small objects. Future studies must investigate these areas as they pertain to targeted reinnervation before any concrete statements may be made regarding the ability of these subjects to intuitively manipulate objects in space.
Acknowledgments
This work was supported in part by the NIH National Institute of Child and Human Development under Grant R01 HD044798 and Grant NO1-HD-5-3402 and in part by the Defense Advanced Research Projects Agency.
The authors would like to thank B. Lock and R. Weir for their substantial role in preliminary research, P. Marasco for his assistance in experimental design, data interpretation, and manuscript development, and K. Koerding for his advice regarding statistical methods.
Biographies

Jonathon W. Sensinger (M’09) received the B.S. degree in bioengineering from the University of Illinois, Chicago, in 2002, and the Ph.D. degree in biomedical engineering from Northwestern University, Chicago, IL, in 2007.
He is a Research Assistant Professor in the Department of Physical Medicine and Rehabilitation of Northwestern University. He is a Research Scientist within the Neural Engineering Center for Artificial Limbs at the Rehabilitation Institute of Chicago. His research interests include myoelectric and body-powered prosthesis theory and design.

Aimee E. Schultz received the B.S. degree in engineering from Swarthmore College in 2003, and the M.S. degree in mechanical engineering from Northwestern University in 2007.
She is currently a Research Engineer and Technical Writer in the Neural Engineering Center for Artificial Limbs at the Rehabilitation Institute of Chicago.

Todd A. Kuiken (M’99–SM’07) received the B.S. degree in biomedical engineering from Duke University, Durham, NC, in 1983, and the Ph.D. degree in biomedical engineering and the M.D. degree from Northwestern University, Chicago, IL, in 1989 and 1990, respectively. He completed his residency in Physical Medicine and Rehabilitation at the Rehabilitation Institute of Chicago and Northwestern University Medical School, Chicago, IL, in 1995.
He is currently the Director of the Neural Engineering Center for Artificial Limbs and Director of Amputee Services at the Rehabilitation Institute of Chicago. He is an Associate Professor in the Departments of Physical Medicine and Rehabilitation and Biomedical Engineering of Northwestern University. He is also the Associate Dean, Feinberg School of Medicine, for Academic Affairs at the Rehabilitation Institute of Chicago.
Contributor Information
Jonathon W. Sensinger, Email: sensinger@ieee.org, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, IL 60611 USA and the Department of Physical Medicine and Rehabilitation at Feinberg School of Medicine, Northwestern University, Chicago, IL 60611 USA.
Aimee E. Schultz, Email: aes@northwestern.edu, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, IL 60611 USA
Todd A. Kuiken, Email: tkuiken@northwestern.edu, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, IL 60611 USA, and the Department of Physical Medicine and Rehabilitation at Feinberg School of Medicine, Northwestern University, Chicago, IL 60611 USA, and the Biomedical Engineering Department, Northwestern University, Evanston, IL 60208 USA.
References
- 1.Kuiken TA, Maraco PD, Lock BA, Harden RN, Dewald JPA. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc Nat Acad Sci USA. 2007 Dec;104:20061–20066. doi: 10.1073/pnas.0706525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthetics Orthotics Int. 2004 Dec;28:245–253. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 3.Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: A case study. Lancet. 2007 Feb;369:371–380. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- 4.Marasco PD, Schultz AE, Kuiken TA. Sensory capacity of reinnervated skin after redirection of amputated upper limb nerves to the chest. Brain. 2009 Jun;132:1441–1448. doi: 10.1093/brain/awp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P, Lowery M, Englehart K, Hargrove L, Dewald JPA, Kuiken TA. Decoding a new neural-machine interface for superior artificial limb control. J Neurophysiol. 2007;98:2974–2982. doi: 10.1152/jn.00178.2007. [DOI] [PubMed] [Google Scholar]
- 6.Gardner EP, Martin JH. Coding of sensory information. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4. New York: McGraw-Hill; 2000. pp. 411–429. [Google Scholar]
- 7.Kotani K, Ito S, Miura T, Horii K. Evaluating tactile sensitivity adaptation by measuring the differential threshold of archers. J Physiological Anthropol. 2007;26:143–148. doi: 10.2114/jpa2.26.143. [DOI] [PubMed] [Google Scholar]
- 8.Wheat HE, Salo LM, Goodwin AW. Human ability to scale and discriminate forces typical of those occurring during grasp and manipulation. J Neurosci. 2004 Mar;24:3394–3401. doi: 10.1523/JNEUROSCI.4822-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Childress DS. Closed-loop control in prosthetic systems—Historical perspective. Ann Biomed Eng. 1980;8:293–303. doi: 10.1007/BF02363433. [DOI] [PubMed] [Google Scholar]
- 10.Meek SG, Jacobsen SC, Goulding P. Extended physiologic taction: Design and evaluation of a proportional force feedback system. J Rehabil Res Develop. 1989;26:53–62. [PubMed] [Google Scholar]
- 11.Patterson PE, Katz JA. Design and evaluation of a sensory feedback-system that provides grasping pressure in a myoelectric hand. Bull Prosthetics Res. 1992;29:1–8. doi: 10.1682/jrrd.1992.01.0001. [DOI] [PubMed] [Google Scholar]
- 12.Phillips CA. Sensory feedback-control of upper-extremity and lower-extremity motor prostheses. Crit Rev Biomed Eng. 1988;16:105–140. [PubMed] [Google Scholar]
- 13.Weinstein S, Kenshalo DR, editors. Intensive and extensive aspects of tactile sensitivity as a function of body part, sex, and laterality; The skin senses: Proceedings of the first International Symposium on the Skin Senses, held at the Florida State University in Tallahassee, Florida; Springfield, IL: Charles C Thomas; 1968. pp. 195–222. [Google Scholar]
- 14.Voerman VF, van Egmond J, Crul B. Normal values forsensory thresholds in the cervical dermatomes: A critical note on the use of semmes-weinstein monofilaments. Amer J Phys Med Rehabil. 1999;78:24–29. doi: 10.1097/00002060-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KO. Sensory discrimination—Decision-process. J Neurophysiol. 1980;43:1771–1792. doi: 10.1152/jn.1980.43.6.1771. [DOI] [PubMed] [Google Scholar]
- 16.Faes L, Nollo G, Ravelli F, Ricci L, Vescovi M, Turatto M, Pavani F, Antolini R. Small-sample characterization of stochastic approximation staircases in forced-choice adaptive threshold estimation. Perception Psychophys. 2007 Feb;69:254–262. doi: 10.3758/bf03193747. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Perez MA. Forced-choice staircases with fixed step sizes asymptotic and small-sample properties. Vis Res. 1998 Jun;38:1861–1881. doi: 10.1016/s0042-6989(97)00340-4. [DOI] [PubMed] [Google Scholar]
- 18.Kaernbach C. Simple adaptive testing with the weighted up-down method. Perception Psychophys. 1991 Mar;49:227–229. doi: 10.3758/bf03214307. [DOI] [PubMed] [Google Scholar]
- 19.Alcala-Quintana R, Garcia-Perez MA. A comparison of fixed-step-size and bayesian staircases for sensory threshold estimation. Spatial Vis. 2007;20:197–218. doi: 10.1163/156856807780421174. [DOI] [PubMed] [Google Scholar]
- 20.Schultz AE, Marasco PD, Kuiken TA. Vibrotactile detection thresholds for chest skin of amputees following targeted reinnervation surgery. Brain Res. 2009 Jan;1251:121–129. doi: 10.1016/j.brainres.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 21.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969 Feb;11:1–21. [Google Scholar]
- 22.Goodwin AW, Wheat HE. Sensory signals in neural populations underlying tactile perception and manipulation. Ann Rev Neurosci. 2004;27:53–77. doi: 10.1146/annurev.neuro.26.041002.131032. [DOI] [PubMed] [Google Scholar]
- 23.Greenspan JD. A comparison of force and depth of skin indentation upon psychophysical functions of tactile intensity. Somatosensory Motor Res. 1984;2:33–48. [PubMed] [Google Scholar]
- 24.Blake D, Johnson KO, Hsiao S. Monkey cutaneous SA1 and RA responses to raised and depressed scanned patterns: Effects of width, height, orientation, and a raised surround. J Neurophysiol. 1997;78:2503–2517. doi: 10.1152/jn.1997.78.5.2503. [DOI] [PubMed] [Google Scholar]
- 25.Jones LA, Lederman SJ. Human Hand Function. New York: Oxford Univ. Press; 2006. [Google Scholar]
- 26.Zheng Y, Mak AFT, Leung AK. State-of-the-art methods for geometric and biomechanical assessments of residual limbs: A review. J Rehabil Res Development. 2001;38:487–504. [PubMed] [Google Scholar]
- 27.Pawluck D, Howe R. Dynamic contact of the human fingerpad against aflat surface. J Biomechan Eng—Trans ASME. 1999;121:605–611. doi: 10.1115/1.2800860. [DOI] [PubMed] [Google Scholar]
- 28.Gardner EP, Martin JH, Jessell TM. The bodily senses. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4. New York: McGraw-Hill; 2000. pp. 430–450. [Google Scholar]
- 29.Hayes W, Keer L, Herrmann G, Mockros L. A mathematical analysis for indentation tests of articular cartilage. J Biomechan. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 30.Dubovy P, Aldskogius H. Degeneration and regeneration of cutaneous sensory nerve formations. Microscopy Res Techn. 1996 Jul;34:362–375. doi: 10.1002/(SICI)1097-0029(19960701)34:4<362::AID-JEMT7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Kinnman E, Wiesenfeld-Hallin Z. Time-course and characteristics of the capacity of sensory nerves to reinnervate skin territories outside their normal innervation zones. Somatosensory Motor Res. 1993;10:445–454. doi: 10.3109/08990229309028849. [DOI] [PubMed] [Google Scholar]
- 32.Dykes RW, Terzis JK, Turnbull BG. Properties of mechanoreceptive fibers serving skin-grafts transferred to the hands of adult baboons (Papio-Anubis) J Physiol-London. 1984;357:1–1. doi: 10.1113/jphysiol.1984.sp015485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. hebb lecture. Brain. 1998;121:1603–30. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 34.Kyberd PJ, Chappell PH. The southampton hand—An intelligent myoelectric prosthesis. J Rehabil Res Development. 1994;31:326–334. [PubMed] [Google Scholar]
- 35.Kyberd PJ, Evans M, Winkel ST. An intelligent anthropomorphic hand, with automatic grasp. Robotica. 1998 Sep.–Oct;16:531–536. [Google Scholar]
- 36.Kyberd PJ, Light C, Chappell PH, Nightingale JM, Whatley D, Evans M. The design of anthropomorphic prosthetic hands: A study of the southampton hand. Robotica. 2001 Nov./Dec;19:593–600. [Google Scholar]
- 37.Jenmalm P, Johansson RS. Visual and somatosensory information about object shape control manipulative fingertip forces. J Neurosci. 1997;17:4486–4499. doi: 10.1523/JNEUROSCI.17-11-04486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cadoret G, Smith AM. Friction, not texture, dictates grip forces used during object manipulation. J Neurophysiol. 1996;75:1963–1969. doi: 10.1152/jn.1996.75.5.1963. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin AW, Jenmalm P, Johansson RS. Control of grip force when tilting objects: Effect of curvature of grasped surfaces and applied tangential torque. J Neurosci. 1998;18:10724–10734. doi: 10.1523/JNEUROSCI.18-24-10724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


