Abstract
Background and Aims
Heteroblasty is an encompassing term referring to ontogenetic changes in the plant shoot. A shaded environment is known to affect the process of heteroblastic development; however, it is not known whether crowded or high density growing conditions can also alter heteroblasty. Compound leaves of the shade-intolerant Acacia implexa allocate less biomass per unit photosynthetic area than transitional leaves or phyllodes and it is hypothesized that this trait will convey an advantage in a crowded environment. Compound leaves also have larger photosynthetic capture area – a trait known to be advantageous in shade. This studied tested the hypothesis that more compound leaves will be developed under shade and crowded environments. Furthermore, this species should undergo optimal allocation of biomass to shoots and roots given shaded and crowded environments.
Methods
A full factorial design of irradiance (high and low) and density levels (high, medium and low) on three populations sourced from varying rainfall regions (high, medium and low) was established under controlled glasshouse conditions. Traits measured include the number of nodes expressing a compound leaf, biomass allocation to shoots and roots, and growth traits.
Key Results
A higher number of nodes expressed a compound leaf under low irradiance and in high density treatments; however, there were no significant interactions across treatments. Phenotypes strongly associated with the shade avoidance syndrome were developed under low irradiance; however, this was not observed under high density. There was no significant difference in relative growth rates across light treatments, but growth was significantly slower in a crowded environment.
Conclusions
Heteroblastic development in Acacia can be altered by shade and crowded environments. In this experiment, light was clearly the most limiting factor to growth in a shaded environment; however, in a crowded environment there were additional limiting resources to growth.
Keywords: Acacia implexa, discriminant function analysis, LMR, LAR, NAR, RMR, SLA, relative growth rate, heteroblastic development, optimal allocation theory, shade avoidance syndrome, density dependence
INTRODUCTION
Plants allocate biomass to organs where critical resources for growth are most limiting (Thornley, 1972; Bloom et al., 1985). Generally, across species, an increase in allocation to roots occurs in nutrient-poor or moisture deficit environments, whereas allocation to the shoot occurs in light-limited environments (Tilman, 1988). A great amount of interest has been directed towards understanding patterns of allocation under various light environments as plants not only respond to a direct decrease in light energy but can anticipate possible future decreases in light availability (Ballaré, 1999).
A change in allocation pattern to above-ground growth and the ability to compete for light when light energy becomes limiting is known as the shade avoidance syndrome. The syndrome is expressed as an increase in stem mass and stem elongation at the expense of root and leaf mass (e.g. Smith, 1982; Schmitt and Wulff, 1993; Dudley and Schmitt, 1996; Franklin and Whitelam, 2005). Total leaf area, however, tends to increase and the ratio of leaf area to whole-plant biomass also increases (Morgan and Smith, 1981; Mitchell and Woodward, 1988; Yu and Ong, 2003; Poorter et al., 2006).
These trait responses are typical of plants with low shade tolerance growing under low irradiance and low density or uncrowded environments. In crowded environments, above-ground space and below-ground resources are additional limitations, and these environments may favour different suites of plant traits. For example, competition for below-ground resources may force the allocation of biomass to roots at the expense of the shoot (Weiner, 1990; Weiner et al., 1997). Therefore, in spite of light being a limiting factor to growth, we may expect different phenotypes to be developed by plants competing near neighbours of similar size compared with those produced by more asymmetric interactions such as those between adult plants and seedlings.
Shading and competition from neighbours not only affect patterns of biomass allocation but may also affect sequential patterns of development. For example, many plant species express dramatically different leaf types along the shoot – a developmental pattern known as heteroblasty (Jones, 1999). Ontogenetic change in leaf type is highly variable between species and can be highly variable within species (Jones, 1999). The timing of ontogenetic changes can be plastic, and the light environment in particular has been shown to play an important role in this flexibility. Goebel (1898; cited in Wells and Pigliucci, 2000), for example, noted that low light can prolong the expression of juvenile leaves. Increasing experimental evidence supports the idea that ontogenetic change can be hastened, or delayed, depending on which leaf type confers the greatest benefit for a given light level (Ashton 1956; Cameron, 1970; Ashton and Turner, 1979; James and Bell, 2000; Burns, 2005; Forster and Bonser, 2009a, b). Heteroblastic leaf development, however, has only been examined under conditions of experimental shading, and to our knowledge it has yet to be examined in a competitive context.
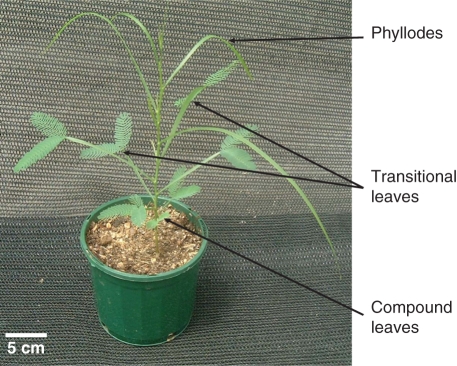
Heteroblastic development is a particularly prominent phenomenon in Acacia subgenus Phyllodineae (New, 1984). Heteroblasty in Acacia involves a shift from bipinnate compound, to transitional leaves (modified petiole/rachis with bipinnate pinnae distally attached) and then to phyllodes (vertically flattened petiole/rachis; New, 1984; Forster and Bonser, 2009a, b; Fig. 1). Generally, species from more arid environments express phyllodes at an earlier ontogenetic stage than species from more mesic environments (Atkin et al., 1998). Additionally, there is a strong genetic component within species as different populations are also known to vary in the timing of leaf change (Farrell and Ashton, 1978; Daehler et al., 1999; Forster and Bonser, 2009b). Species and population differences in the timing of leaf changes have been attributed to varying moisture regimes (Farrell and Ashton, 1978); however, the light environment has been experimentally demonstrated to be more important (Forster and Bonser, 2009a, b), i.e. under a low irradiance, compound leaves are retained for longer (Withers, 1979).
Fig. 1.
An example of heteroblastic development in Acacia implexa. Compound leaves, transitional leaves and phyllodes indicated with an arrow. The plant was grown in full sunlight and the photograph was taken on the 86th day of growth.
Physiological and morphological traits of compound leaves are better adapted to lower irradiance, whereas the traits of phyllodes are better adapted to more intense light environments (Brodribb and Hill, 1993; Yu and Li, 2007). Moreover, compound leaves are less expensive to construct than phyllodes in terms of the amount of leaf biomass per unit of photosynthetic tissue, or specific leaf area (SLA: Yu and Li, 2007; Forster and Bonser, 2009a, b), consistent with other species grown at low irradiance (Poorter et al., 2006). Leaves with lower construction costs and higher returns under low quality light may also be beneficial in high density or competitive growing conditions, and therefore heteroblastic species may retain more of these leaves in crowded or low light conditions. The timing of the ontogenetic shift of compound leaves to phyllodes should be environmentally dependent and under strong selection across environments. Thus, we should see differences in the expression of heteroblasty across populations, and variations in the expression of heteroblasty within populations.
We ran a full factorial experiment on the species Acacia implexa, with population, shading and intraspecific seedling density level as factors, to examine patterns of heteroblastic development and the expression of shade avoidance traits. We hypothesized that a shift to transitional leaves will be delayed under a shaded treatment, a high density treatment and more so under the interaction of shade and density. We also hypothesized that shaded and high density treatments should induce a strong shade avoidance syndrome in the shade-intolerant, A. implexa. Lastly, we examined how heteroblasty and shade avoidance syndrome affected growth rates.
MATERIALS AND METHODS
Acacia implexa is a tree growing to approx. 12 m inhabiting woodland and forest habitats across south-east and eastern Australia (Kodela, 2002). Acacia implexa is a pioneer species with seeds typically needing intense heat from fire to germinate, and saplings are fast growing (Maslin and McDonald, 2004).
In mid-December 2006 seeds were pre-treated in boiling water (to simulate heat from fire) for approx. 2 min to promote germination. Seeds were sown directly into potted soil as previous trials had found this led to uniform germination. Pots were 115 mL in size and consisted of soil with 33 % Australian Native Landscape supply of ‘Organic Garden Mix’, 33 % washed river sand and 33 % cocopeat. A 4 month Osmocote low phosphorus slow release fertilizer was added with the N:P:K ratio of 17:1·5:8·5. No additional fertilizer was added throughout the experiment due to the sensitivity of this species to excess nutrients and the fact the experiment ran for <4 months. All soil, pots and any other equipment used in the experiment were completely sterilized to prevent infection of roots by symbiotic rhizobia (which fix atmospheric nitrogen and thus improve growth). At the time of final harvest, no plants were found to have rhizobia. Pots were spaced evenly across a glasshouse bench using a randomized complete block experimental design in the School of Biological, Earth and Environmental Sciences glasshouse at the University of New South Wales.
There were three experimental factors: three populations (from high, medium and low rainfall sites), two levels of light environment (full sunlight and shaded, from here on termed high and low light, respectively) and three levels of intraspecific competition (seeds sown at high, medium and low density) with 15 replicates per treatment combination, giving a total of 270 pots. Mortality over the experiment led to a total sample size of 182 (replicates per treatment are summarized in Supplementary Data, Table S1, available online).
Seeds from three populations were obtained from the CSIRO Australian Tree Seed Centre, Canberra, Australia. The three populations were chosen because they naturally inhabit different points along a rainfall gradient (Table 1). Farrell and Ashton (1978) found that heteroblastic development was delayed along a rainfall gradient in the closely related Acacia melanoxylon, and this motivated our choice of populations.
Table 1.
Brief description of populations of sourced seeds
| Population | Seedlot | Location | Rainfall (mm year−1) | Average temperature (°C) |
|---|---|---|---|---|
| Low rainfall | 19780 | 35°57′S, 145°45′E | 450 | 23 |
| Medium rainfall | 19770 | 32°37′S, 150°03′E | 650 | 23 |
| High rainfall | 18859 | 31°56′S, 151°11′E | 830 | 24 |
Rainfall and temperature data were collated from Bureau of Meteorology, Australia climate data of the nearest weather station.
Plants in the high light treatment received natural sunlight whereas the plants in the low light treatment were grown inside a 57 cm cylinder (open ended) of green filter plastic (Lee Filters, Andover, UK; number 121 Lee Green). The light filters simulate a natural canopy; the photosynthetic flux density was reduced by 35 % and the ratio of red to far red light was reduced from 1·0 to 0·2 (Bonser and Geber, 2005). This experimental approach induces a strong shade avoidance response in A. implexa (Forster and Bonser, 2009a, b).
The density treatment was established at the time of seed sowing. Fifty, 15 and six seeds were sown in the high, medium and low density treatments. Following the emergence of the first double compound leaf, seedlings in the low density treatment were thinned to one plant per pot. At the emergence of the first double compound leaf, five target plants were randomly selected in the medium and high density treatments for measurement. Five plants were chosen as a precaution against mortality during the course of the experiment. A marked toothpick was carefully placed next to each target plant as an identifier and a toothpick was also placed next to stems in the low density treatment as a control measure.
Stem height and diameter were made as initial measurements on each target plant. The number of germinated stems was also counted to give initial levels of density. Plants were allowed to grow until at least three mature transitional leaves had fully expanded. Thereafter, plants were checked weekly for at least three fully expanded transitional leaves. Harvesting began 80 d following initial measurements and, after 170 d of growth, any remaining plants were harvested.
At the time of harvest the following traits were measured: number of nodes with a compound leaf and total number of nodes, stem height and diameter. These data were used to calculate height to diameter ratio and average internode length. Leaves were separated from the stem, flattened and kept refrigerated at 4 °C until area measurements were taken. This prevented pinnules on the compound leaves from folding and modifying leaf area. Individual leaves were measured on a flatbed scanner, and LeafA software (G. Williamson, University of Adelaide, Australia, pers. comm.) was used to determine area. Individual leaves were oven dried at 60 °C for at least 72 h before determination of dry weight. SLA was calculated as fresh leaf area divided by dried mass. Soil was carefully removed from the roots over a tray to ensure all root material was collected. Stem and roots were oven dried at 60 °C for at least 72 h and the mass was determined. The addition of leaf, stem and root mass gave the total plant dry weight at time of harvest. The determination of stem mass ratio (StMR), root mass ratio (RMR), leaf mass ratio (LMR) and leaf area ratio (LAR) was derived from these mass and area measurements.
Relative growth rate (RGR) and relative height growth rate were determined by:
| (1) |
where x is harvest biomass or height and y is initial biomass or height. Net assimilation rate (NAR) was determined by (e.g. Portsmuth and Niinemets, 2006):
| (2) |
Data analyses
The number of nodes with compound leaves was tested with a three-way type III univariate analysis of variance (ANOVA) with population, light quality and density level as fixed factors and number of nodes that developed compound leaves as the dependent variable. Population was used as a fixed factor rather than a random factor because each population was sourced from a pre-defined rainfall regime and was not randomly selected. The same model was used to test for differences in relative growth rates, relative height growth and total plant dry weight at time of harvest.
A multivariate approach was then adopted to determine how experimental treatments shaped phenotypes and the relationships of traits relative to each other. Type III multivariate analysis of variance (MANOVA) considered population, light quality and density level as fixed factors, and variables included: height to diameter (HtoD), internode length (Internode), StMR, RMR, LMR, LAR, NAR and SLA (see Table 2 for full definitions of abbreviations). Homogeneity of variances was checked with Levene's test of equality (Supplementary Data Table S2, available online). The significance of model terms and interactions was assessed using Pillai's trace test statistics. Multivariate sets of covarying traits were further characterized using discriminant function analysis (DFA). DFA is a classification procedure that can be applied on factorial multivariate data sets to assess whether groups can be uniquely separated (Quinn and Keough, 2002). We performed four sets of DFAs: (1) population × light × density; (2) population × light; (3) population × density; and (4) light × density. Group centroids with 95 % confidence intervals (CIs) of experimental treatments were plotted against the first and second discriminant functions using procedures outlined in Johnson et al. (2007). A series of univariate ANOVAs were then performed on each dependent variable to assess for treatment differences. All P-values were corrected for Type I error using the sequential Bonferroni technique.
Table 2.
Summary of traits measured in this study with abbreviations and transformations made for statistical analysis
| Trait | Abbreviation | Definition | Transformation |
|---|---|---|---|
| Compound leaf nodes | The total number of nodes that developed a compound leaf before the onset of transitional leaves | log | |
| Height to diameter (mm cm−2) | HtoD | Ratio of stem length to basal stem diameter | Sqrt |
| Internode length (mm) | Internode | Stem length divided by the total number of nodes | Sqrt |
| Stem mass ratio (g g−1) | StMR | Ratio of total stem mass to sum of root and leaf mass | log |
| Root mass ratio (g g−1) | RMR | Ratio of total root mass to sum of stem and leaf mass | log |
| Leaf mass ratio (g g−1) | LMR | Ratio of total leaf mass to sum of stem and root mass | log |
| Leaf area ratio (cm2 g−1) | LAR | Ratio of total leaf area to total biomass | Sqrt |
| Net assimilation rate (g cm−2 d−1) | NAR | The amount of carbon fixed per day for a given amount of photosynthetic tissue | log |
| Specific leaf area (cm2 g−1) | SLA | Ratio of leaf area to leaf mass | log |
| Relative growth rate (g g−1 d−1) | RGR | Amount of biomass fixed per day and determined by eqn (1) | |
| Height growth (mm d−1) | Amount of height accrued each day and determined by eqn (1) | ||
| Total dry weight (g) | Amount of dry weight of all plant material at time of harvest | log |
All traits were assessed for normality using the Shapiro–Wilk test and were transformed where appropriate. Traits and their transformations have been summarized in Table 2. Following transformations, traits were then standardized with a mean of zero and s.d. of 1. All statistical analyses were performed using SPSS 15·0·1.
RESULTS
Heteroblastic development
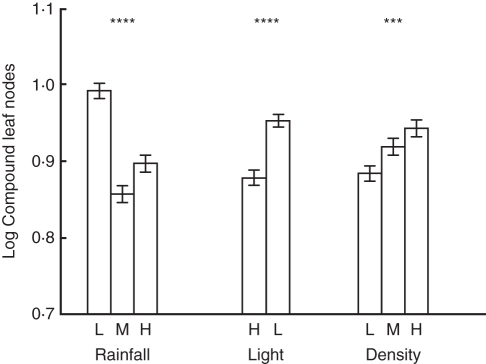
Compound leaves were retained for significantly longer in the low light over the high light treatment and in the high density over the medium and low density treatments (Table 3; Fig. 2). The low rainfall population developed more compound leaves than the medium and high rainfall populations (Table 3; Fig. 2).
Table 3.
Treatment effects on the node at which plants switched from compound leaves to transitional leaves
| Treatment | F-value | Variance explained (%) |
|---|---|---|
| Population | 43·466**** | 25·61 |
| Light | 34·719**** | 10·17 |
| Density | 8·660*** | 4·62 |
| Population × light | 0·547 | |
| Population × density | 1·885 | 1·07 |
| Light × density | 1·865 | 0·52 |
| Population × light × density | 0·483 |
*** P < 0·001; **** P < 0·0001. Variance explained determined using the omega squared (ω2) method. The complete ANOVA table is supplied in Supplementary Data Table S3 (available online).
Fig. 2.
Compound leaf nodes represents the total number of nodes that developed a compound leaf before the onset of transitional leaves. Displayed are means (± s.e.) for low (L), medium (M) and high (H) levels for each variable. *** P < 0·001; **** P < 0·0001. Complete statistical results are presented in Supplementary Data Table S3 (available online).
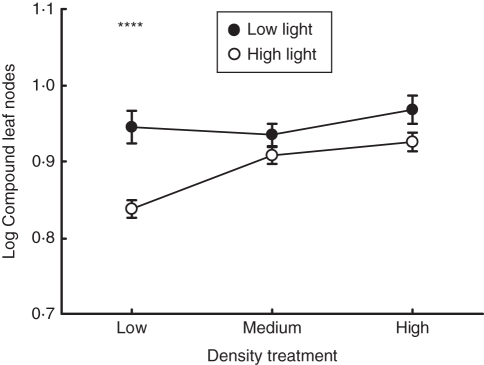
It was expected that there would be a strong interaction between light and density treatments; however, there was no significant effect (Fig. 3). Furthermore, there were no significant interactions between the experimental treatments (Table 3). A planned comparison within the light and density treatments revealed a significant difference between high and low light at low density levels (Tukey's HSD, P < 0·0001).
Fig. 3.
Compound leaf nodes and the interaction of shade and density treatments. Displayed are means (± s.e.) for low and high light, as indicated. Comparisons were made by Tukey's HSD; **** P < 0·0001.
Shade avoidance syndrome
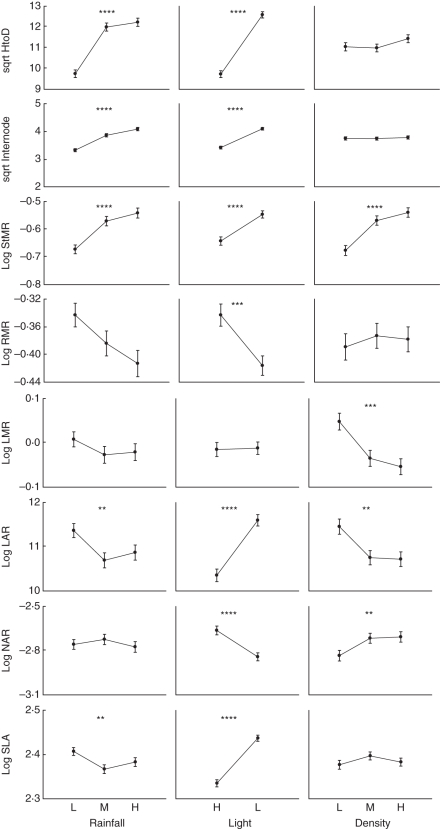
The shade avoidance response differed significantly among the population, light quality and density treatments, and there were no significant differences across treatment interactions (MANOVA, Table 4). A shade avoidance syndrome was expressed to a greater extent in the high rainfall population relative to the medium and low rainfall populations and in the low light relative to the high light treatment. For the high rainfall population HtoD was higher, internodes were longer, there was a greater allocation to stem mass, and less allocation to root mass in the low light treatments (Table 5, Fig. 4). Plants in the low light treatment had a higher LAR and SLA (Table 5, Fig. 4). The low rainfall population had significantly higher LAR and SLA than the medium and high rainfall populations (Fig. 4). NAR was also lower under low light; however, there were no significant differences across populations (Table 5, Fig. 4). A greater allocation towards stem mass in the high density treatment was the only indication of shade avoidance across density treatments (Fig. 4).
Table 4.
Results from multivariate analysis of variance on whole-plant traits
| Pillai's trace | F-value | d.f. | P-value | |
|---|---|---|---|---|
| Population | 0·497 | 6·249 | 16, 302 | <0·0001 |
| Light | 0·665 | 37·193 | 8, 150 | <0·0001 |
| Density | 0·308 | 3·437 | 16, 302 | <0·0001 |
| Population × light | 0·140 | 1·422 | 16, 302 | 0·1294 |
| Population × density | 0·238 | 1·210 | 32, 612 | 0·2003 |
| Population × light × density | 0·088 | 0·871 | 16, 302 | 0·6034 |
Table 5.
Results from analysis of variance on whole-plant traits
| Population (P) | Light (L) | Density (D) | P × L | P × D | L × D | P × L × D | |
|---|---|---|---|---|---|---|---|
| HtoD | 44·280**** | 166·570**** | 1·528 | 0·066 | 2·049 | 1·154 | 1·968 |
| Internode | 44·134**** | 100·767**** | 0·010 | 0·099 | 2·658* | 1·926 | 0·536 |
| StMR | 16·824**** | 25·482**** | 15·262**** | 2·022 | 0·494 | 1·461 | 0·223 |
| RMR | 2·808 | 12·512*** | 0·331 | 0·297 | 0·819 | 0·886 | 0·892 |
| LMR | 1·541 | 0·084 | 8·131*** | 1·295 | 1·110 | 1·114 | 1·262 |
| LAR | 6·072** | 45·830**** | 6·592** | 4·457* | 0·110 | 0·744 | 1·297 |
| NAR | 0·982 | 23·751**** | 5·234** | 2·364 | 0·822 | 1·219 | 1·393 |
| SLA | 5·194** | 83·117**** | 1·303 | 3·784* | 0·599 | 0·064 | 0·701 |
Significant differences are given by: * P < 0·05; ** P < 0·01; *** P < 0·001; **** P < 0·0001. Full results are found in Supplementary Data Table S5 (available online).
Fig. 4.
Means (± s.e.) of whole-plant traits across experimental treatments: height-to-diameter ratio (HtoD, square-root transformed); average internode length (sqare-root transformed); stem mass ratio (StMR); root mass ratio (RMR); leaf mass ratio (LMR); leaf area ratio (LAR); net assimilation rate (NAR); and specific leaf area (SLA). * P < 0·05; ** P < 0·01; *** P < 0·001; **** P < 0·0001. Units of measurement are given in Table 2. Complete ANOVA results can be found in Supplementary Data Table S4 (available online).
Discriminant function analysis
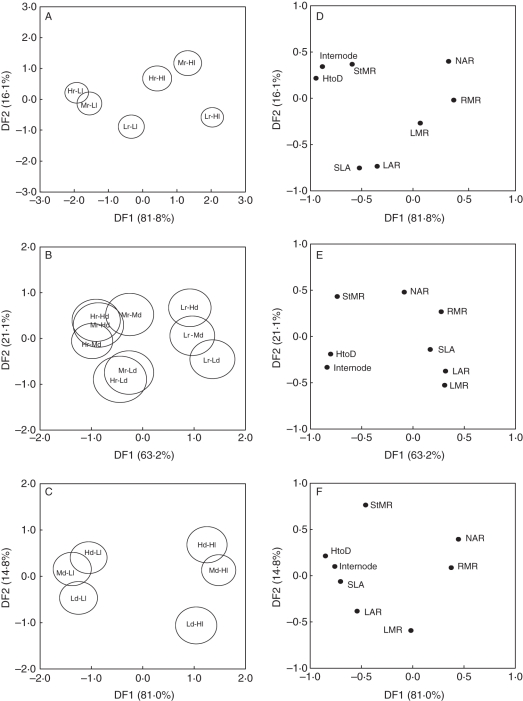
DFA of the population by light treatments separated the light treatment across the first discriminant function for whole-plant traits (Fig. 5A). Traits positively associated with the first discriminant function were RMR and NAR, whereas stem elongation traits were negatively associated, which corresponded to the high light and low light treatments, respectively (Fig. 5D). This same pattern was observed following DFA of the light by density treatments (Fig. 5C, F). DFA of the population by density treatments produced considerable overlap of CIs; however, populations were discriminated along the first axis and density treatments across the second axis (Fig. 5B).
Fig. 5.
Grouping of population, light and density treatments following discriminant function analysis for whole-plant traits. Displayed are group centroids with circles indicating 95 % CIs for whole-plant and leaf-level traits (overlapping circles indicate no difference between groups). Variance explained by first and second discriminant functions are displayed in brackets. (A) Hr-Ll, Mr-Ll, Lr-Ll, high rainfall, medium rainfall, low rainfall population by low quality light treatments; and Hr-Hl, Mr-Hl, Lr-Hl, high rainfall, medium rainfall, low rainfall population by high quality light treatments. (B) Hr-Hd, Mr-Hd, Lr-Hd, high rainfall, medium rainfall, low rainfall population by high density treatments; Hr-Md, Mr-Md, Lr-Md, high rainfall, medium rainfall, low rainfall population by medium density treatments; and Hr-Ld, Mr-Ld, Lr-Ld, high rainfall, medium rainfall, low rainfall population by low density treatments. (C) Hd-Ll, Md-Ll, Ld-Ll, low quality light by high, medium and low density treatments; and Hd-Hl, Md-Hl, Ld-Hl, high quality light by high, medium and low density treatments. (D–F) Factor loadings of whole-plant traits corresponding to DFA of treatments across first and second discriminant functions. See Table 2 for abbreviations.
Growth traits
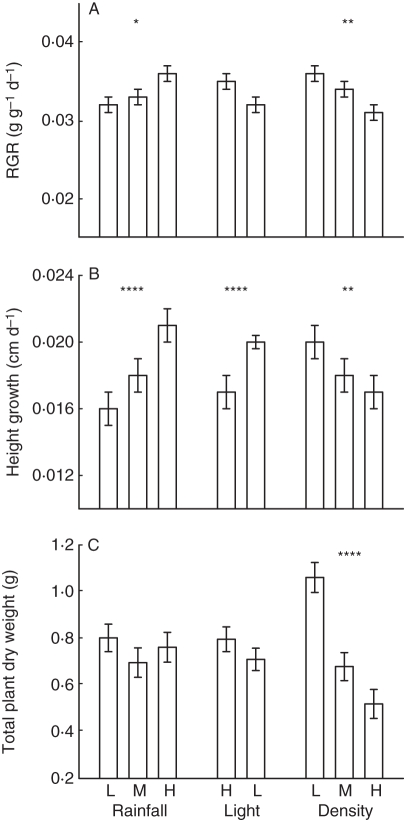
Within the population treatment there was a significant difference in RGR (F2,163 = 3·654, P = 0·0280; Fig. 6A) and height growth (F2,163 = 17·114, P < 0·0001; Fig. 6B), but no significant difference in total dry weight at time of harvest (F2,163 = 2·738, P = 0·0677; Fig. 6C). RGR and total dry weight did not differ between high and low quality light treatments (F1,163 = 3·793, P = 0·0532; F1,163 = 2·100, P = 0·1492; Fig. 6A, C); however, height growth was significantly faster in low quality light (F1,163 = 25·875, P < 0·0001; Fig. 6B). RGR and height growth were significantly faster in the low density treatment (F2,163 = 7·177, P = 0·0010; F2,163 = 4·865, P = 0·0089; Fig. 6A, B), and total dry weight at time of harvest was also significantly greater (F2,163 = 15·574, P < 0·0001; Fig. 6C).
Fig. 6.
Mean (± s.e.) of growth traits across experimental treatments. * P < 0·05; ** P < 0·01; *** P < 0·001; **** P < 0·0001. Complete ANOVA results can be found in Supplementary Data Table S5 (available online).
DISCUSSION
Light is fundamental for growth in plants, and a lack of it induces shifts in the expression of a range of traits, particularly in shade-avoiding species. The shift from one leaf type to another has previously been related to the light environment (Ashton, 1956; Cameron, 1970; Ashton and Turner, 1979; James and Bell, 2000; Burns, 2005; Forster and Bonser, 2009a, b). The current experiment demonstrated that the onset of transitional leaves in A. implexa is not only delayed in a shaded environment but it can also be delayed in a crowded environment (Fig. 2). There are advantages of retaining compound leaves in both situations: under shade compound leaves have greater photosynthetic capture area (per unit of biomass) and perform better under low irradiance than phyllodes (Brodribb and Hill, 1993; Yu and Li, 2007); and compound leaves are cheaper to construct than transitional leaves or phyllodes (in terms of amount of biomass allocated per photosynthetic area, or SLA: Yu and Li, 2007; Forster and Bonser, 2009a, b) which is beneficial in a highly competitive environment. Delaying the onset of transitional leaves may be a strategy across Acacia species more generally that assists in the establishment of seedlings in relatively adverse environments (Hansen, 1996).
In spite of the strong evidence of a change in development of transitional leaves within light and density treatments, there was no significant interaction between treatments. However, when the data from the light and density treatments were examined more closely, they revealed a significant difference in the number of nodes that expressed a compound leaf at low density but not medium and high density (Fig. 3). For the species used in this experiment, A. implexa, there is a capacity to develop transitional leaves at a developmentally earlier stage in a high light environment; however, in a lower resource environment (i.e. shade or crowded conditions) there is not a capacity to retain compound leaves indefinitely. There is a developmental constraint on the expression of plasticity (Valladares et al., 2007) in the species A. implexa, a result supported by Forster and Bonser (2009b). For the species A. implexa the switch from compound to transitional leaves appears to be genetically fixed to occur at least after the fourth node and no later than the 14th node (pers. obs.). In the shaded treatment, plants were switching to transitional leaves as close to the 14th node as genetically possible. When an additional experimental treatment was included, in this case crowding, the study species did not have the developmental capacity to express more compound leaves – there was a developmental constraint on the expression of this plastic trait.
In a more general context, the plastic expression of heteroblasty in A. implexa is one response along a continuum that plants express. Heteroblastic development has recently been defined as changes that occur along the stem as a normal expression of whole-plant growth (Jones, 1999). In some plants this expression is fixed (canalized; Wells and Pigliucci, 2000), such as many divaricate shrubs in the New Zealand flora that switch from small, carbon-rich leaves to large, nitrogen-rich leaves at defined heights thought to be out of reach of browsing herbivorous birds (Greenwood and Atkinson, 1977; Atkinson and Greenwood, 1989; Bond et al., 2004). Other plants exhibit different levels of plasticity depending on environmental context (e.g. Eucalyptus globulus; James and Bell, 2000). By the definition of heteroblasty, inevitably plastic expression must be developmentally constrained as plants go through the process of whole-plant growth.
The reduction of light by competing neighbours or an overhanging canopy is perceived by photoreceptors in different parts of the plant, yet both crowding and shading should induce a shade avoidance syndrome (Schmitt and Wulff, 1993). The expression of traits in the shaded treatment is clearly consistent with shade avoidance strategies (Smith, 1982; Schmitt and Wulff, 1993; Dudley and Schmitt, 1996; Yu and Ong, 2003; Franklin and Whitelam, 2005): elongated stem, increased SLA, increased LAR and StMR, and less allocation of biomass to roots (Fig. 4). Plants in the high density treatment not only competed for light but faced competition for other resources, such as moisture and nutrients (Casper and Jackson, 1997). RGRs and total plant dry weight at time of harvest were significantly lower in the higher density treatments, suggesting that these plants were limited across a range of critical resources. Plants grown at high and low light levels, on the other hand, had similar growth rates and total plant biomass. Relative height growth was greatly enhanced in the shaded environment and, coupled with the shade avoidance response, suggests that light was the most limiting resource to these plants.
In the genus Acacia the expression of different phenotypes across populations has previously been related to different rainfall regimes (e.g. Farrell and Ashton, 1978; Cody, 1989, 1991). However, this hypothesis has not been widely tested. Although the current experiment only examined the expression of traits from three populations within a single species, there is a suggestion that the development of different phenotypes may not be directly related to rainfall (and moisture availability) per se, and that other environmental variables may be important. Acacia species have excellent water use efficiency when compared with other species (Hansen, 1986, 1996) and Acacia frequently dominates vegetation communities in Australian arid and semi-arid regions (Specht and Specht, 1999). These facts suggest that Acacia species have physiological mechanisms that allow individuals to tolerate arid environments or environments experiencing periods of soil moisture deficits. Populations within species may therefore be differentiated along alternative environmental axes, and recent evidence suggests light availability could be an important variable (Forster and Bonser, 2009a, b). In this experiment, the high and medium rainfall populations were discriminated along axes related to traits associated with the low light treatment, whereas the low rainfall population was grouped with traits associated with the high light treatment (Fig. 5). Plants from the high and medium rainfall populations expressed traits in a manner comparable with the shade avoidance syndrome evident from the low light treatment (Fig. 5). Data of the exact light regime of the three populations used in this experiment were not available and therefore a direct comparison is not possible. However, it is reasonable to expect that as rainfall increases canopy cover may also increase and light availability in the understorey will decrease (Forster and Bonser, 2009b; Ladd et al., 2009). Dominant vegetation communities from the high and medium rainfall populations were forests and woodlands (McRae and Cooper, 1985; Benson, 1986), whereas open woodlands dominated the region from where the low rainfall population was sourced (Scott, 1992). Nevertheless, this is anecdotal evidence, and a more extensive study is needed to support the hypothesis that populations are differentiated along a light gradient and/or along another alternative gradient, as has been suggested for A. koa (Daehler et al., 1999).
Overall, this study found general similarities between shaded and crowded treatments, yet there were many differences. These differences may have arisen because plants in a crowded environment not only have to adjust phenotype to altered light conditions, but may also have limited access to other critical resources. The most significant finding of this study was that heteroblastic development can be affected by the light environment as well as the presence of neighbours (i.e. crowding). The fact that heteroblastic development is regulated by density dependence has not previously been demonstrated. Lastly, low light levels led to the expression of phenotypic traits that were characteristic of the high rainfall population, which suggests that populations within Acacia species may be differentiated along a light gradient.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following tables. Table S1: Summary of sample size per treatment. Table S2: Levene's test of equality of error variances. Table S3: Full ANOVA table for the number of nodes with a compound leaf (i.e. heteroblastic development). Table S4: Full ANOVA table for shade-avoidance traits. Table S5: Full ANOVA tables for relative growth rate, relative height growth and total plant dry weight at time of harvest.
ACKNOWLEDGEMENTS
We thank Geoff McDonnell, Kylie Mallit and Natalie Wynyard for glasshouse assistance, and Grant Williamson for leaf area software. Funded by an UNSW Faculty Research Grant and Early Career Research grant to S.P.B.
LITERATURE CITED
- Atkin OK, Schortemeyer M, McFarlane N, Evans JR. Variation in the components of relative growth rate in ten Acacia species from contrasting environments. Plant, Cell and Environment. 1998;21:1007–1017. [Google Scholar]
- Atkinson IAE, Greenwood RM. Relationships between moas and plants. New Zealand Journal of Ecology. 1989;12S:67–98. [Google Scholar]
- Ashton DH. Studies on the autecology of Eucalyptus regnans, F.v.M. 1956 PhD Thesis, University of Melbourne. [Google Scholar]
- Ashton DH, Turner JS. Studies on the light compensation point of Eucalyptus regnans F. Muell. Australian Journal of Botany. 1979;27:589–607. [Google Scholar]
- Ballaré CL. Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends in Plant Sciences. 1999;4:97–102. doi: 10.1016/s1360-1385(99)01383-7. [DOI] [PubMed] [Google Scholar]
- Benson DH. The vegetation of the Gosford and Lake Macquarie 1:100 000 vegetation map sheet. Cunninghamia. 1986;1:467–490. [Google Scholar]
- Bloom AJ, Chapin FS, Mooney HA. Resource limitations in plants – an economic analogy. Annual Review of Ecology and Systematics. 1985;16:363–392. [Google Scholar]
- Bond WJ, Lee WG, Craine JM. Plant structural defenses against browsing birds: a legacy of New Zealand's extinct moas. Oikos. 2004;104:500–508. [Google Scholar]
- Bonser SP, Geber MA. Growth form evolution and shifting habitat specialization in annual plants. Journal of Evolutionary Biology. 2005;18:1009–1018. doi: 10.1111/j.1420-9101.2005.00904.x. [DOI] [PubMed] [Google Scholar]
- Brodribb T, Hill RS. A physiological comparison of leaves and phyllodes in Acacia melanoxylon. Australian Journal of Botany. 1993;41:293–305. [Google Scholar]
- Burns KC. Plastic heteroblasty in beach groundsel (Senecio lautus) New Zealand Journal of Botany. 2005;43:665–672. [Google Scholar]
- Cameron RJ. Light intensity and the growth of Eucalyptus seedlings. I. Ontogenetic variation in E. fastigata. Australian Journal of Botany. 1970;18:29–43. [Google Scholar]
- Casper BB, Jackson BB. Plant competition underground. Annual Review of Ecology and Systematics. 1997;28:545–570. [Google Scholar]
- Cody ML. Morphological variation in mulga. I. Variation and covariation within and among Acacia aneura populations. Israeli Journal of Botany. 1989;38:241–257. [Google Scholar]
- Cody ML. Morphological variation in mulga. II. Covariation among morphology, phenology, and spatial patterns in Acacia-dominated vegetation. Israeli Journal of Botany. 1991;40:41–59. [Google Scholar]
- Daehler CC, Yorkston M, Sun WG, Dudley N. Genetic variation in morphology and growth characters of Acacia koa in the Hawaiian Islands. International Journal of Plant Sciences. 1999;160:567–573. [Google Scholar]
- Dudley SA, Schmitt J. Testing the adaptive plasticity hypothesis: density-dependent selection on manipulated stem length in Impatiens capensis. American Naturalist. 1996;147:445–465. [Google Scholar]
- Farrell TP, Ashton DH. Population studies of Acacia melanoxylon. I. Variation in seed and vegetation characteristics. Australian Journal of Botany. 1978;26:365–379. [Google Scholar]
- Forster MA, Bonser SP. Heteroblastic development and the optimal partitioning of traits among contrasting environments in Acacia implexa. Annals of Botany. 2009a;103:95–105. doi: 10.1093/aob/mcn210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MA, Bonser SP. Heteroblastic development and shade-avoidance in response to blue and red light signals in Acacia implexa. Photochemistry and Photobiology. 2009b;85:1375–1383. doi: 10.1111/j.1751-1097.2009.00605.x. [DOI] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC. Phytochromes and shade-avoidance responses in plants. Annals of Botany. 2005;96:169–175. doi: 10.1093/aob/mci165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel K. Organographie der Pflanzen. Jena: Fischer; 1898. [Google Scholar]
- Greenwood RM, Atkinson IAE. Evolution of divaricating plants in relation to moa browsing. Proceedings of the New Zealand Ecological Society. 1977;24:21–29. [Google Scholar]
- Hansen DH. Water relations of compound leaves and phyllodes in Acacia koa var latifolia. Plant, Cell and Environment. 1986;9:439–445. [Google Scholar]
- Hansen DH. Establishment and persistence characteristics in juvenile leaves and phyllodes of Acacia koa (Leguminosae) in Hawaii. International Journal of Plant Sciences. 1996;157:123–128. [Google Scholar]
- James SA, Bell DT. Influence of light availability on leaf structure and growth of two Eucalyptus globulus ssp. globulus provenances. Tree Physiology. 2000;20:1007–1018. doi: 10.1093/treephys/20.15.1007. [DOI] [PubMed] [Google Scholar]
- Johnson HE, Llyod AJ, Mur LAJ, Smith AR, Causton DR. The application of MANOVA to analyse Arabidopsis thaliana metabolomic data from factorially designed experiments. Metabolomics. 2007;3:517–530. [Google Scholar]
- Jones CS. An essay on juvenility, phase change, and heteroblasty in seed plants. International Journal of Plant Sciences. 1999;160:S105–S111. doi: 10.1086/314215. [DOI] [PubMed] [Google Scholar]
- Kodela PG. In: Acacia. Harden GJ, editor. Sydney: University of New South Wales Press; 2002. pp. 381–476. Flora of New South Wales. [Google Scholar]
- Ladd B, Bonser SP, Peri PL, et al. Towards a physical description of habitat: quantifying environmental adversity (abiotic stress) in temperate forest and woodland ecosystems. Journal of Ecology. 2009;97:964–971. [Google Scholar]
- Maslin BR, McDonald MW. AcaciaSearch: evaluation of Acacia as a woody crop option for southern Australia. Canberra: Rural Industries Research and Development Corportation; 2004. [Google Scholar]
- McRae RHD, Cooper MG. Vegetation of the Merriwa area, New South Wales. Cunninghamia. 1985;1:351–370. [Google Scholar]
- Mitchell PL, Woodward FI. Responses of three woodland herbs to reduced photosynthetically active radiation and low Red to Far-Red ratio in shade. Journal of Ecology. 1988;76:807–825. [Google Scholar]
- Morgan DC, Smith H. Control of development in Chenopodium album L. by shadelight: the effect of light quantity (total fluence rate) and light quality (Red:Far-Red ratio) New Phytologist. 1981;88:239–248. [Google Scholar]
- New TR. A biology of Acacias. Melbourne: Oxford University Press; 1984. [Google Scholar]
- Poorter H, Pepin S, Rijkers T, Jong Yd, Evans JR, Korner C. Construction costs, chemical composition and payback time of high- and low-irradiance leaves. Journal of Experimental Botany. 2006;57:355–371. doi: 10.1093/jxb/erj002. [DOI] [PubMed] [Google Scholar]
- Portsmuth A, Niinemets Ü. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Functional Ecology. 2006;21:61–77. [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologist. Port Melbourne: Cambridge University Press; 2002. [Google Scholar]
- Schmitt J, Wulff RD. Light spectral quality, phytochrome and plant competition. Trends in Ecology and Evolution. 1993;8:47–51. doi: 10.1016/0169-5347(93)90157-K. [DOI] [PubMed] [Google Scholar]
- Scott JA. The natural vegetation of Balranald – Swan Hill area. Cunninghamia. 1992;2:597–652. [Google Scholar]
- Smith H. Light quality, photoreception, and plant strategy. Annual Review of Plant Physiology. 1982;33:481–518. [Google Scholar]
- Specht RL, Specht A. Australian plant communitites: dynamics of structure, growth and biodiversity. South Melbourne: Oxford University Press; 1999. [Google Scholar]
- Thornley JHM. A balanced quantitative model for root:shoot ratios in vegetative plants. Annals of Botany. 1972;36:431–441. [Google Scholar]
- Tilman D. Plant strategies and the dynamics and structure of plant communities. Princeton: Princeton University Press; 1988. [Google Scholar]
- Valladares F, Gianoli E, Gómez JM. Ecological limits to plant phenotypic plasticity. New Phytologist. 2007;176:749–763. doi: 10.1111/j.1469-8137.2007.02275.x. [DOI] [PubMed] [Google Scholar]
- Weiner J. Asymmetric competition in plant populations. Trends in Ecology and Evolution. 1990;5:360–364. doi: 10.1016/0169-5347(90)90095-U. [DOI] [PubMed] [Google Scholar]
- Weiner J, Wright DB, Castro S. Symmetry of belowground competition between Holcus lanatua and Dactylis glomera. Oikos. 1997;79:85–91. [Google Scholar]
- Wells CL, Pigliucci M. Adaptive phenotypic plasticity: the case of heterophylly in aquatic plants. Perspectives in Plant Ecology, Evolution and Systematics. 2000;3:1–18. [Google Scholar]
- Withers JR. Studies on the status of unburnt Eucalyptus woodland at Ocean Grove, Victoria. IV. The effect of shading on seedling establishment. Australian Journal of Botany. 1979;27:47–66. [Google Scholar]
- Yu H, Li JT. Physiological comparisons of true leaves and phyllodes in Acacia mangium seedlings. Photosynthetica. 2007;45:312–316. [Google Scholar]
- Yu H, Ong BL. Effect of radiation quality on growth and photosynthesis of Acacia mangium seedlings. Photosynthetica. 2003;41:349–355. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.