Abstract
Multidrug resistance associated protein 2 (Mrp2) is a canalicular transporter responsible for organic anion secretion into bile. Mrp2 activity is regulated by insertion into the plasma membrane; however, the factors that control this are not understood. Calcium (Ca2+) signaling regulates exocytosis of vesicles in most cell types, and the type II inositol 1,4,5-triphosphate receptor (InsP3R2) regulates Ca2+ release in the canalicular region of hepatocytes. However, the role of InsP3R2 and of Ca2+ signals in canalicular insertion and function of Mrp2 is not known. The aim of this study was to determine the role of InsP3R2-mediated Ca2+ signals in targeting Mrp2 to the canalicular membrane. Livers, isolated hepatocytes, and hepatocytes in collagen sandwich culture from wild-type (WT) and InsP3R2 knockout (KO) mice were used for western blots, confocal immunofluorescence, and time-lapse imaging of Ca2+ signals and of secretion of a fluorescent organic anion. Plasma membrane insertion of green fluorescent protein (GFP)-Mrp2 expressed in HepG2 cells was monitored by total internal reflection microscopy. InsP3R2 was concentrated in the canalicular region of WT mice but absent in InsP3R2 KO livers, whereas expression and localization of InsP3R1 was preserved, and InsP3R3 was absent from both WT and KO livers. Ca2+ signals induced by either adenosine triphosphate (ATP) or vasopressin were impaired in hepatocytes lacking InsP3R2. Canalicular secretion of the organic anion 5-chloromethylfluorescein diacetate (CMFDA) was reduced in KO hepatocytes, as well as in WT hepatocytes treated with 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA). Moreover, the choleretic effect of tauroursodeoxycholic acid (TUDCA) was impaired in InsP3R2 KO mice. Finally, ATP increased GFP-Mrp2 fluorescence in the plasma membrane of HepG2 cells, and this also was reduced by BAPTA.
Conclusion
InsP3R2-mediated Ca2+ signals enhance organic anion secretion into bile by targeting Mrp2 to the canalicular membrane.
Cytosolic Ca2+ is a ubiquitous intracellular messenger.1 In the liver, Ca2+ signals have been linked to the regulation of such diverse functions as gene expression,2,3 cell proliferation,4 apoptosis,5-7 paracellular permeability,8 glucose release,9,10 and bile flow.11 Ca2+ signaling in hepatocytes is mainly controlled by the inositol 1,4,5-trisphosphate receptor (InsP3R), an InsP3-gated Ca2+ channel in the endoplasmic reticulum. InsP3Rs type I (InsP3R1) and type II (InsP3R2)12,13 are the two InsP3R isoforms expressed in hepatocytes. InsP3R2 is the most highly expressed of these isoforms and is concentrated in the region of the endoplasmic reticulum near the canalicular membrane. Thus it is in close proximity to canalicular transporters,14 which are either inserted into the canalicular plasma membrane or localized to subplasmalemmal vesicles.15 Activation of InsP3Rs in the apical region of other epithelia generates a spatially restricted domain with Ca2+ concentrations as high as approximately 10 μM,16 which is sufficient to induce vesicular exocytosis.17
Secretion of a wide variety of amphiphilic organic anions into bile, including bilirubin, glutathione S-conjugates, and oxidized glutathione, occurs through multidrug resistance-associated protein 2 (Mrp2), a member of the ATP binding cassette family of transporters.18 Mrp2 activity is partially regulated by its dynamic trafficking between the canalicular plasma membrane and a nearby endosomal compartment. Cholestatic agents such as estradiol,19 lipopolysaccha-ride,20 lithocholic acid,21 and phalloidin22 act in part by decreasing insertion of Mrp2 into the plasma membrane. Fusion of vesicles with the plasma membrane involves Ca2+-dependent proteins in nearly every cell type,23 and Ca2+ signals induced by tauroursodeoxycholic acid (TUDCA) have been linked to exocytosis in hepatocytes24,25; yet the role of Ca2+ in regulating organic anion secretion is not known. Therefore, we investigated the role of InsP3R2-mediated Ca2+ release in the localization and activity of Mrp2.
Materials and Methods
Animals and Materials
Male Swiss black wild-type (WT) mice obtained from Taconic Farms Inc (Hudson, NY) and InsP3R2 knockout (KO) mice described previously26 were used for experiments. Animals were maintained on a standard diet and housed under a 12-hour light/dark cycle. All animal procedures were approved by the Yale University Institutional Animal Care and Use Committee. Vasopressin, ATP, bovine serum albumin, penicillin-streptomycin, and protease inhibitor cocktails were purchased from Sigma Chemical Co (St. Louis, MO). Dulbecco's modified Eagle medium, Liebowitz 15, Fluo-4/acetoxymethyl ester (AM), Cell tracker green 5-chloromethylfluorescein diacetate (CMFDA), the nuclear stain TO-PRO-3, rhodamine-conjugated phalloidin, and Alexa-488 secondary antibodies were obtained from Invitrogen (Eugene, OR). InsP3R1 antibodies from affinity-purified specific rabbit polyclonal antiserum directed against the 19 C-terminal residues of the mouse InsP3R1 were from Affinity Bioreagents (Golden, CO). InsP3R2 antibodies from affinity-purified specific rabbit polyclonal antiserum directed against the 18 C-terminal residues of the rat InsP3R2 were provided by Richard Wojcikiewicz (SUNY Syracuse, NY). Monoclonal antibodies directed against the N-terminal of InsP3R3 were obtained from Becton Dickinson (Lexington, KY). Bilirubin total reagent was obtained from ClinicQA (San Marcos, CA). Mammalian protein extraction reagent cell lysis buffer was obtained from Pierce (Rockford, IL). Rat GFP-MRP2 expression vector was provided by Dietrich Keppler (German Cancer Research Center, Heidelberg, Germany).27 HepG2 cells were purchased from ATCC (Manassas, VA). All other chemicals were of the highest quality commercially available.
Isolation and Culture of Hepatocytes and Bile Flow Studies
Isolated mouse hepatocyte couplets were used for single-cell imaging. Cells were isolated in the Cell Isolation Core of the Yale Liver Center, as described.28,29 Briefly, mouse livers were perfused with Hanks' A and then Hanks' B medium containing 0.05% collagenase (Roche Applied Science, Indianapolis, IN) and 0.8 units of trypsin inhibitor (Sigma) per unit of tryptic activity. Livers were minced and passed through serial nylon mesh filters, and the resultant cells were washed. Isolated hepatocytes were resuspended in Liebowitz 15 medium with 50 units penicillin and 50 mg streptomycin. Cells were then seeded onto collagen-I-coated coverslips and incubated at 37°C for 2 to 4 hours before use in isolated hepatocyte experiments. For hepatocytes in collagen sandwich culture, cells were incubated for 2 hours at 37°C before being coated with a second layer of collagen-I and used 3 to 5 days after plating.30 For bile flow studies, bile was collected using Intramedic Polyethylene Tubing (Becton Dickinson, Franklin Lakes, NJ) inserted into the gallbladder. Bile was collected in pre-tared tubes, and then flow measurements were normalized by the liver weight and expressed as microliters per gram of liver per minute (μL/g liver/minute). During the entire experiment (1 hour), animals were under anesthesia by inhalation of a mixture of oxygen (0.5 L/minute) and isoflurane (2.5%-3.0%) and their temperature monitored.
Immunoblotting
Immunoblots were performed as described previously.13 Briefly, cells were lysed at 4°C with mammalian protein extraction reagent lysis buffer; the lysate underwent centrifugation, and the protein concentration of the supernatant was determined spectrophotometrically. Sixty micrograms total cellular protein was separated by sodium dodecyl sul-fate polyacrylamide gel electrophoresis, using a 7.5% polyacrylamide gel. Membranes were blocked with nonfat milk and then incubated overnight at 4°C with InsP3R1 or InsP3R2, Mrp2, and alpha tubulin-specific antibodies. A rabbit anti-InsP3R1 was used at a dilution of 1:3000, an affinity-purified rabbit antibody against the C-terminus of InsP3R2 isoform was used at 1:100, and a mouse antibody against the N-terminus of the InsP3R3 was used at a dilution of 1:3000. For Mrp2 immunoblotting, an affinity-purified rabbit antibody31 was used at dilution of 1:2000; the positive control (alpha tubulin) was used at a dilution of 1:5000. Membranes were washed and incubated with peroxidase-conjugated secondary antibodies at a dilution of 1:5000. The protein-antibody conjugates were then detected by enhanced chemiluminescence (Millipore, Billerica, MA).
Immunofluorescence
Confocal immunofluorescence to detect the subcellular distribution of InsP3R iso-forms was performed as described previously.13,14,32 Briefly, frozen mouse liver sections were fixed in 4% formaldehyde and permeabilized with 0.1% Triton X-100. Nonspecific binding was blocked with 1% bovine serum albumin and 5% normal goat serum. Liver sections were incubated at room temperature with primary antibody directed against specific InsP3R iso-forms or against Mrp2 (Alexis Biochemicals, Farming-dale, NY) and then rinsed with phosphate-buffered saline. The specimens were then incubated with Alexa 488 secondary antibody and co-labeled with Rhodamine-conjugated Phalloidin and TO-PRO-3 to facilitate recognition of hepatocyte morphology and nucleus, respectively. Images were obtained using a LSM 510 confocal microscope (Zeiss, Thornwood, NY) by excitation at 488 nm and observation at 505 to 530nm to detect Alexa 488. Rhodamine phalloidin was excited at 543 nm and observed at 585 nm and TO-PRO3 was detect by using a 647 nm laser line and observed above 665 nm.
Time-Lapse Imaging
Cytosolic Ca2+ was monitored in isolated mouse hepatocytes and HepG2 cells. CMFDA secretion was monitored in the canalicular spaces between mouse hepatocytes in collagen sandwich culture by time-lapse confocal microscopy as described previously.13,33 Briefly, hepatocytes were incubated with either Fluo-4/AM (6 μM) for 30 minutes at 37°C for Ca2+ imaging or Cell tracker Green CMFDA (1 μm) to monitor organic anion secretion. Cover slips seeded with the cells were transferred to a custom-built perfusion chamber on the stage of an LSM 510 confocal microscope (Zeiss, Thornwood, NY), and the cells were then perfused with 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid-buffered solution. Cells were excited at 488 nm and observed at 505 to 550 nm. In most experiments, 40× or 63× objectives were used to observe the cells. Increases in Ca2+ or organic anion secretion were expressed as percent increase in Fluo-4 or CMFDA fluorescence intensity and normalized by baseline fluorescence, respectively.
Total Internal Reflection Microscopy
HepG2 cells were seeded on 35-mm glass-bottom culture dishes at 2.0 × 105 cells/dish 2 days before total internal reflection fluorescence (TIRF) imaging. Cells were transfected 24 hours later with rat GFP-Mrp2 and transferred to the stage of a custom-built TIRF microscope 1 day after transfection. Cells were kept at 37°C during the experiment. Images were acquired using an Olympus inverted microscope equipped with a 1.45 NA 60× TIRFM lens, back-illuminated electron multiplying charge-coupled device camera (16-bit; iXon887; Andor Technologies), and controlled by Andor iQ software. Cells were excited using the 488-nm line of an argon laser, with exposure times of 0.15 second and an acquisition rate of 0.5Hz. Cells were imaged for 5 minutes before the addition of ATP (100 μM) to determine the average baseline membrane fluorescence. Fluorescence changes were monitored for 20 minutes in the presence of ATP. In control experiments, cells were pretreated with the intracellular Ca2+ chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)/AM (50 μM). The resulting series of images were background subtracted using Image J software (National Institutes of Health). The calculated evanescent field depth was approximately 150 nm.
Statistics
All results are expressed as mean ± standard error of the mean of at least three individual experiments. Student t test or analysis of variance (ANOVA) was used for comparisons between groups. A P value less than 0.05 was used to indicate a statistically significant difference. GraphPad Prism software (San Diego, CA) was used for all statistical tests.
Results
Expression and Distribution of InsP3R Isoforms in Mouse Liver
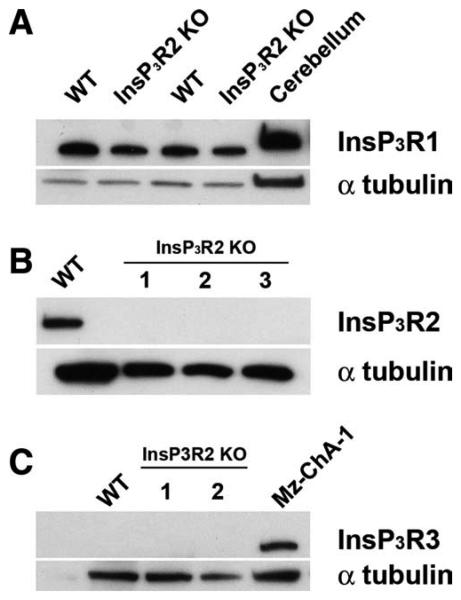
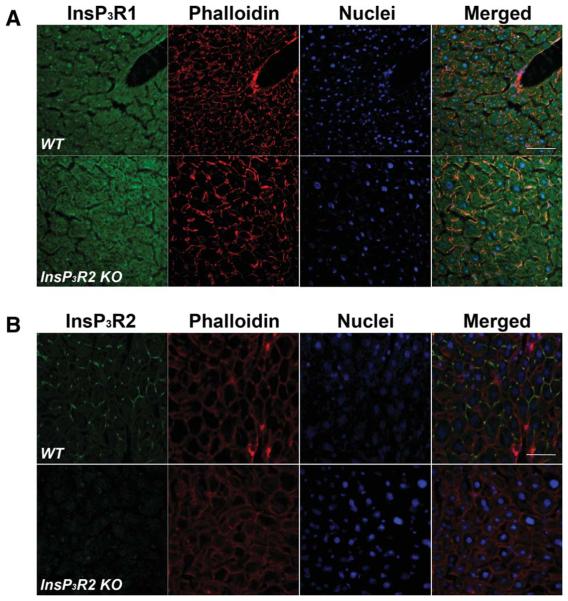
Immunoblotting demonstrated expression of InsP3R1 and InsP3R2 but not InsP3R3 (Fig. 1A-C) in wild-type (WT) mouse liver, similar to what has been observed in rat13 and human liver.34 In InsP3R2 KO liver, InsP3R1 was detected but both InsP3R2 and InsP3R3 were absent (Fig. 1A-C). Confocal immunofluorescence of WT mouse liver slices (Fig. 2) revealed that InsP3R2 is highly concentrated close to the canalicular membrane, whereas InsP3R1 is distributed throughout the hepatocyte, also similar to what is observed in rats.13 Immunofluorescence for InsP3R2 revealed no specific staining in InsP3R2 KO liver, plus no appreciable modification in the diffuse subcellular distribution of InsP3R1 (Fig. 2). Expression of InsP3R3 was absent from hepatocytes in both types of mice. Together these results confirm the absence of InsP3R2 in the livers of KO animals and show that there is no significant compensatory up-regulation of InsP3R1 in the InsP3R2 KO mice.
Fig. 1.
Expression of InsP3R isoforms in WT and InsP3R2 KO hepatocytes. (A) InsP3R1 is not up-regulated in the liver of InsP3R2 KO animals. Each lane is loaded with 60 μg total cell lysate prepared from freshly isolated hepatocytes. Blots were probed with an anti-InsP3R1 antibody and show that InsP3R1 expression levels are similar between WT and InsP3R2 KO livers. Cerebellum was used as positive control for InsP3R1, and alpha-tubulin was used as a loading control. (B) Blot for InsP3R2 confirms that this isoform is absent in InsP3R2 KO animals (lanes 1-3). (C) InsP3R3 is absent in both WT and InsP3R2 KO hepatocytes. Mz-ChA-1 cell extracts were used as positive controls for detection of InsP3R3.
Fig. 2.
Cellular localization of InsP3R isoforms in WT and InsP3R2 KO liver. Confocal immunofluorescence microscopy was used to determine the subcellular localization of each InsP3R isoform. (A) InsP3R1 is diffusely distributed in the cytosol of both types of hepatocytes. Top row shows the diffuse distribution of InsP3R1 (green) in WT liver. Subcortical f-actin is shown in red to reveal the outline of individual hepatocytes. Nuclei (blue) were stained with TO-PRO3. The rightmost panel shows the merged image. Note that the expression level and subcellular distribution of InsP3R1 is similar in InsP3R2 KO liver (bottom row). (B) InsP3R2 is concentrated near the canalicular membrane of WT (top left panel) hepatocytes but is absent in InsP3R2 KO liver (bottom left panel). Expression of InsP3R3 is absent from hepatocytes in livers from both types of mice (data not shown). Scale bar = 50 μm.
Effects of InsP3R2 on Ca2+ Signaling in Mouse Hepatocytes
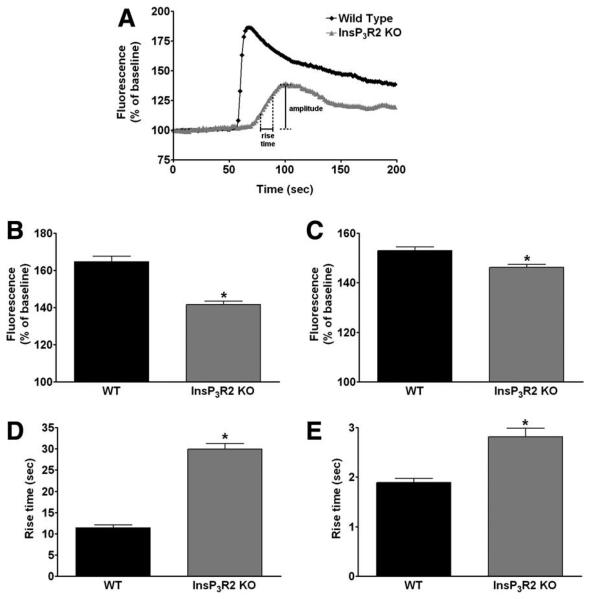
To determine the effects of loss of InsP3R2 on Ca2+ signaling, hepatocytes isolated from WT and InsP3R2 KO mice were stimulated with ATP (100 μM) or concentrations ranging from 0.1 to 100 nM of arg8-vasopressin (AVP) to induce InsP3-mediated cytosolic Ca2+ release.12-14,35 Either stimulus increased Ca2+ in both WT and InsP3R2 KO hepatocytes, although differences in the nature of the Ca2+ signal could be detected between the two types of cells (Fig. 3). The two kinetic parameters used to characterize these differences were the amplitude of the Ca2+ signal and the rise time, which is the time required for the signal to increase from 25% to 75% of the maximum response (Fig. 3A). The amplitude of the Ca2+ signal was significantly reduced in InsP3R2 KO cells stimulated with either AVP (Fig. 3B) or ATP (Fig. 3C). The rise time for both agonists was significantly prolonged in InsP3R2 KO hepatocytes compared with WT cells (Fig. 3D, E). These differences demonstrate impaired InsP3-mediated Ca2+ signaling in InsP3R2 KO hepatocytes, similar to what has been reported in rat hepatocytes in which InsP3R2 expression was reduced by antisense.12
Fig. 3.
Ca2+ signaling is impaired in InsP3R2 KO hepatocytes. (A) Representative tracing of Fluo4 fluorescence changes over time in WT and InsP3R2 KO hepatocytes stimulated with ATP (100 μM). Hepatocytes were stimulated with either ATP or AVP and examined by time-lapse confocal microscopy. Using these individual tracings, the amplitude and the rise time of the Ca2+ signal from individual hepatocytes was determined. (B) The increase in Ca2+ induced by AVP (10 nM) was greater in WT as compared with InsP3R2 KO hepatocytes (165 ± 3%, n = 309 cells versus 148 ± 2%, n = 423 cells; P < 0.0001). (C) ATP (100 μM)-induced Ca2+ release also was greater in WT as compared with InsP3R2 KO cells (152.9 ± 1.16%, n = 149 versus 146.2 ± 1.18%, n = 176; P < 0.001). (D) Rise time of Ca2+ signal was faster in WT hepatocytes than in InsP3R2 KO cells perfused with AVP 10 nM (11.43 ± 0.71 seconds, n = 251 versus 29.96 ± 1.30 seconds, n = 256; P < 0.0001). (E) Rise time also was shorter in WT than in InsP3R2 KO cells when ATP 100 μM was used to induced Ca2+ release (1.89 ± 0.08 seconds, n = 149 cells versus 2.81 ± 0.17 seconds, n = 176 cells; P < 0.0001), reflecting slower Ca2+ release in cells lacking InsP3R2.
Bile Flow and Serum Bilirubin Are Not Altered in InsP3R2 KO Mice
To further characterize the effects of InsP3R2 expression on liver function, bile secretion and serum bilirubin concentration were examined in WT and InsP3R2 KO mice. Age-matched and weight-matched animals were used to measured basal bile flow in both groups of mice. The average liver weight in WT mice was greater than in InsP3R2 KO mice (1.483 g ± 0.0502, n = 9, and 1.234 g ± 0.0437, n = 6, respectively; P < 0.005). However, there was no significant difference in bile flow between the two groups (0.9486 μL/g liver/minute ± 0.0618, n = 9; and 1.116 μL/g liver/minute ± 0.030, n = 6, respectively). There also was no significant difference in the total bilirubin concentration in the serum of WT and InsP3R2 KO animals (0.2170 mg/dL ± 0.01813, n = 10; and 0.2096 mg/dL ± 0.02917, n = 9, respectively).
Role of Type II InsP3R in Organic Anion Secretion
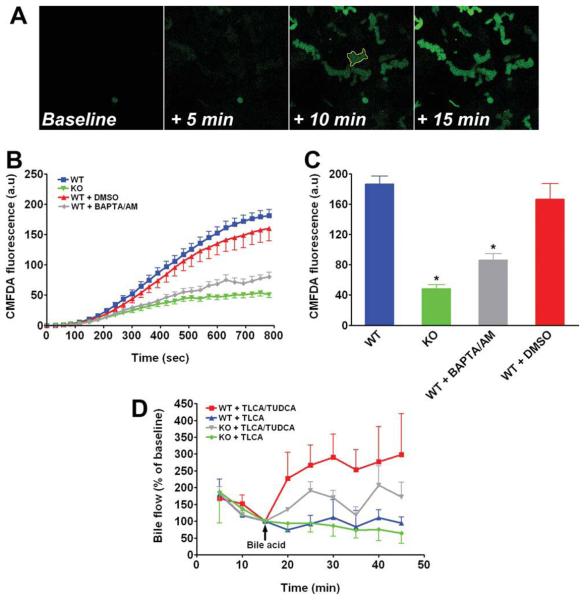
Because translocation of Mrp2 to the plasma membrane is stimulated by protein kinase C alpha (PKCα) and this kinase is Ca2+-activated, we hypothesized that organic anion secretion might be impaired in InsP3R2 KO animals. To test this, we first measured the secretion of cell tracker green CMFDA, a fluores-cent Mrp2 substrate,36 by hepatocytes in collagen sandwich cultures using time-lapse confocal microscopy. This cell system was used because it preserves structural and functional polarity of hepatocytes.37,38 CMFDA accumulated in the canalicular lumen of hepatocytes (Fig. 4A), and the kinetics could be quantified as a measure of Mrp2 function (Fig. 4B). Canalicular accumulation of CMFDA fluorescence was observed in both WT and InsP3R2 KO hepatocytes, but luminal fluorescence in InsP3R2 KO hepatocytes reached only 25.8% of what was observed in WT cells (Fig. 4C). Luminal fluorescence was reduced by a similar extent in WT cells treated with the cytosolic Ca2+ chelator BAPTA/AM (50 μM; Fig. 4C). To understand the significance of this finding in vivo, bile flow was measured in WT and InsP3R2 KO livers infused with the cholestatic bile acid TLCA (5 uM) with or without co-infusion of taurolithocholic acid (TUDCA) (25 μM). This model was chosen because TUDCA stimulates biliary exocytosis in a Ca2+-dependent fashion24,25 and reverses the cholestatic effect of TLCA.39 As shown in Fig. 4D, infusion of TLCA alone induced a similar decrease in bile flow in both groups of animals, but this effect was reversed more effectively in WT than in InsP3R2 KO animals by co-infusion of TUDCA (P < 0.02 by repeated-measures ANOVA). Confocal immunofluorescence studies also were performed in hepatocytes in collagen sandwich culture to determine whether the subcellular distribution of Mrp2 and InsP3R2 is preserved in this model system. Both InsP3R2 (Fig. 5A) and Mrp2 (Fig. 5b) were localized to the region of the canalicular membrane of these hepatocytes, similar to what was observed in liver sections. These results demonstrate that Mrp2 function in hepatocytes depends on expression of InsP3R2, and suggest that this is attributable to the function of the receptor as an intracellular Ca2+ release channel.
Fig. 4.
Lack of InsP3R2 impairs bile flow and organic ion secretion. (A) Representative time-lapse series of images of CMFDA secretion in WT mouse hepatocytes in collagen sandwich culture. The cell-permeant fluorescent Mrp2 substrate was added to cells, and its secretion into the canalicular spaces was monitored every 2.5 seconds for 15 minutes by confocal microscopy. Regions of interest such as the one outlined in yellow (third panel) were used to measure the canalicular accumulation of fluorescence over time. Scale bar = 20 μm. (B) Canalicular accumulation of fluorescence is decreased in cells lacking InsP3R2, as well as in cells in which cytosolic Ca2+ is chelated. Results reflect mean ± standard error of the mean increases over time in WT cells (77 canaliculi on seven coverslips; blue), InsP3R2 KO cells (74 canaliculi on five coverslips; green), WT pretreated with 50 μM BAPTA/AM (82 canaliculi on six coverslips; gray) and WT incubated with dimethylsulfoxide (32 canaliculi on three coverslips; red), which was the vehicle for BAPTA/AM. Data are pooled from three separate WT and InsP3R2 KO cell preparations. (C) Increases in CMDFA fluorescence were significantly greater in canaliculi from WT cells (186.7 ± 10.2 au) than from InsP3R2 KO cells (48.2 ± 5.6 au; P < 0.0001), as well as in WT pretreated with the intracellular Ca2+ chelator BAPTA/AM (86.0 ± 8.4 au) as compared with WT pretreated with dimethylsulfoxide vehicle alone (166.5 ± 20.8 au; P < 0.0001 by one-way ANOVA with Bonferroni post-test). (D) Portal infusion of TLCA (5 μM) caused a similar reduction in bile flow in WT (blue tracing, n = 3 animals) and InsP3R2 KO (green tracing, n = 4) mice. TLCA-induced cholestasis was reversed by co-infusion of TUDCA (25 μM) in WT (red tracing, n = 3) and to a lesser extent in InsP3R2 KO animals (gray tracing, n = 4; P < 0.02 by repeated-measures ANOVA). Arrow indicates time of addition of bile acids.
Fig. 5.
InsP3R2 localization is maintained in mouse hepatocytes in collagen sandwich culture. Cells were maintained in culture for 4 days and then examined by confocal immunofluorescence. (A) Immunofluorescence for InsP3R2 (green) reveals that this intracellular Ca2+ release channel is distributed in the vicinity of the canalicular network that develops between hepatocytes in collagen sandwich culture, similar to the localization pattern in the liver. Labeling of f-actin by Rhodamine phalloidin (red) identifies the canalicular membrane, and TO-PRO3 is used to label cell nuclei (blue). Scale bar = 20 μm. (B) Labeling of Mrp2 shows that the canalicular distribution of this transporter is preserved in this cell system as well.
Role of InsP3R2 in Mrp2 Expression and Localization
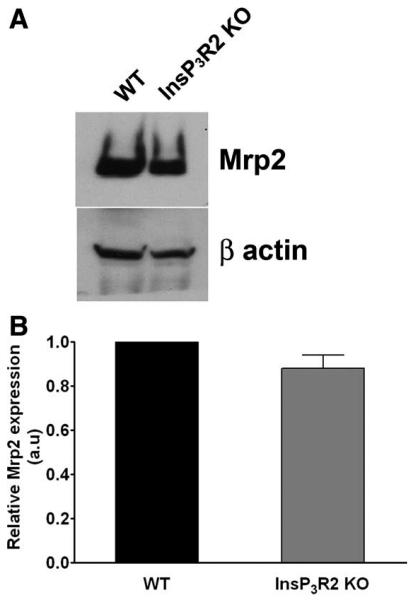
To determine whether reduced secretion of CMFDA in InsP3R2 KO hepatocytes might reflect effects of InsP3R2 on Mrp2 expression, the expression of Mrp2 in total cell lysates of WT and InsP3R2 KO hepatocytes was analyzed. Both western blot (Fig. 6A) and densitometric analysis (Fig. 6B) demonstrated that there is no significant difference in Mrp2 protein expression in the InsP3R2 KO animals when compared with the WT. The subcellular distribution of Mrp2 also was examined, in liver slices from both WT and InsP3R2 KO animals. Confocal immunofluorescence demonstrated that Mrp2 is localized to the canalicular domain of hepatocytes in both InsP3R2 KO and WT mice (Fig. 7A). Higher magnification images revealed that Mrp2 does not colocalize with submembranous f-actin in either WT or InsP3R2 KO mice (Fig. 7B). Together, these results demonstrate that Mrp2 expression and localization is not altered in InsP3R2 KO liver.
Fig. 6.
Mrp2 protein expression is not altered in InsP3R2 KO cells. (A) Representative western blot comparing total Mrp2 protein expression in WT and InsP3R2 KO hepatocytes shows equivalent expression levels in both cell types. Beta-actin was used as a control for protein loading. (B) Densitometric analysis of three separate blots shows that Mrp2 expression is unchanged in InsP3R2 KO hepatocytes (87.9% ± 10.5% relative to WT; P > 0.3).
Fig. 7.
Mrp2 localization is not altered in InsP3R2 KO cells. (A) Confocal immunofluorescence localization of Mrp2 (green), f-actin (red), and nuclei (blue) in WT (top row) and InsP3R2 KO (bottom row) liver. Note that the canalicular localization of Mrp2 is preserved in InsP3R2 KO liver. Scale bar = 50 μm. (B) Higher-magnification view of a canalicular region reveals that Mrp2 (green) is adjacent to but does not co-localize with subcortical f-actin (red) in both WT and InsP3R2 hepatocytes. Scale bar = 5 μm.
Ca2+ Regulates Insertion of Mrp2 into the Plasma Membrane
Because Mrp2 activity is mediated by its dynamic trafficking, we investigated whether InsP3R2-depent Ca2+ release was involved in insertion of Mrp2 into the plasma membrane. Rat GFP-Mrp2 was transiently expressed in the HepG2 liver cell line, and then GFP was imaged by total internal reflection fluorescence (TIRF) microscopy (Fig. 8). This microscopy technique allows for the visualization of fluorescence within approximately 150 nm of the plasma membrane,40,41 without affecting resolution in the focal plane, and so is ideal to monitor events such as exocytic insertion into the plasma membrane of vesicles containing GFP-tagged proteins.42 Stimulation of HepG2 cells with ATP (100 μM) to increase cytosolic Ca2+ led to an increase in plasma membrane GFP fluorescence as compared with the increased fluorescence in unstimulated cells observed over the same time interval (125% ± 2% and 107% ± 1% for cells perifused with ATP versus N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer; P < 0.0001). This increase was reduced significantly by pretreating the cells with the intracellular Ca2+ chelator BAPTA/AM (P < 0.0006). In control experiments, treatment of cells expressing GFP-Mrp2 with BAPTA for 30 minutes had no effect on GFP fluorescence (not shown). To verify that ATP induces InsP3-mediated Ca2+ signals in these cells, confocal microscopy was used to measure Ca2+ signals in HepG2 cells stimulated with ATP (100 μM) in the presence or absence of BAPTA/AM, the phospholipase C inhibitor U-73122, or the InsP3R blocker Xestospongin C. Buffering intracellular Ca2+ nearly completely abolished ATP-induced Ca2+ signals (Fig. 9A, B). Moreover, when formation of InsP3 or its target channels were blocked, there was also a significant reduction in ATP-induced Ca2+ release (Fig. 9C, D). These results provide evidence that intracellular Ca2+ signals are required for insertion of Mrp2 into the plasma membrane, and in particular suggest that Ca2+ released by an InsP3R-dependent mechanism is responsible for targeting of Mrp2.
Fig. 8.
Intracellular Ca2+ promotes insertion of Mrp2 into the plasma membrane. (A) Representative HepG2 cell expressing rat GFPMrp2 and imaged by TIRF microscopy. The same cell is shown before and 15 minutes after addition of ATP (100 μM) to the bath. Yellow arrows indicated regions of increased fluorescence. Cells are pseudo-colored according to the scale at right. (B) Serial magnified images of the region indicated by the white square in (A) reveals progressive accumulation of GFP-Mrp2 in that region over time. Images are separated by 2-minute intervals. (C) Mean fluorescence increases in control cells, cells stimulated with ATP alone, or pretreated with Bapta-AM (50 μM). Note that there is a significant increase in GFP-Mrp2 membrane fluorescence in cells treated with ATP (124.9% ± 2.2%, n = 81 regions in 19 cells) as compared with control cells (106.7% ± 0.8%, n = 83; P < 0.0001) and that this increase is attenuated by pretreatment with BAPTA (112.9% ± 2.2%, n = 42; P < 0.001 by oneway ANOVA with Bonferroni post-test). Data represent the average of four independent experiments.
Fig. 9.
ATP-induced Ca2+ signals are mediated by InsP3R in HepG2 cells. (A) Representative tracings of ATP (100 μM)-induced Ca2+ signals in control and BAPTA/AM-treated cells. (B) Pretreatment with BAPTA/AM significantly reduces the amplitude of Ca2+ signals (12.57% ± 0.36% increase above baseline, n = 550 cells) as compared with control (109.5% ± 1.95% increase, n = 550). (C) Representative tracings of Ca2+ signals in control cells or cells pretreated with either U73122 (10 μM) or Xestospongin C (1 μM). (D) Ca2+ release was partially reduced by U73122 pretreatment (86.19% ± 5.87% increase above baseline fluorescence, n = 233 versus 178.3% ± 4.50% increase, n = 504 in control cells, P < 0.0001). Pre-incubation with the InsP3R blocker Xestospongin C greatly reduced ATP-induced Ca2+ release (27.69% ± 2.31%, n= 184 versus 178.3% ± 4.50%, n = 504, P < 0.0001) by one-way ANOVA with Bonferroni post-test.
Discussion
The current work shows that InsP3R2 KO hepatocytes have defective Ca2+ signaling and organic anion secretion, and that insertion of Mrp2 into the plasma membrane is Ca2+ dependent. InsP3R2 KO mice develop normally, and no gross phenotypical alteration has been described. However, double-knockout mice lacking both InsP3R2 and InsP3R3 have deficient secretion of saliva and pancreatic zymogens, which results in an inability to fully digest and absorb nutrients. Pancreatic acinar cells isolated from these double-knockout animals have decreased Ca2+ release and accumulate zymogen granules, presumably reflecting impaired exocytosis.43 In the current work, Ca2+ signals in hepatocytes isolated from InsP3R2 KO mice showed reduced amplitude, and there was an increase in rise time of the Ca2+ signals. Together these kinetic parameters of Ca2+ signaling indicate a significant impairment in Ca2+ release in the InsP3R2 KO cells. This effect is consistent with the observation that pericanalicular InsP3R2 are essential to create a trigger zone to initiate and propagate Ca2+ waves in rat hepatocytes,12,14 much like what is observed in pancreatic acinar cells16,44 and in cholangiocytes.32,45
The results presented here also demonstrate decreased Mrp2 activity in sandwich cultures of InsP3R2 KO hepatocytes. This effect is likely attributable to the action of InsP3R2 as an intracellular Ca2+ release channel, because the effect was duplicated by chelation of cytosolic Ca2+. This apparent effect of Ca2+ could be attributable to regulation of transporter activity, trafficking, or expression. The absence of InsP3R2 did not affect either expression levels or localization of Mrp2, however. Instead, TIRF measurements suggested that release of intracellular Ca2+ promotes insertion of rat Mrp2 into the plasma membrane. This is consistent with previous observations that Ca2+ and PKC act as regulators of exocytosis in the liver46 and also with reports showing that tauroursodeoxycholic acid induces Ca2+ release24 and Ca2+-dependent exocytosis25 and activates conventional PKCs, leading to Mrp2 insertion into the canalicular membrane.39 However, the current work cannot exclude the possibility that Ca2+ also may directly regulate transporter activity, perhaps through PKC-dependent phosphorylation.39
Mutations that affect Mrp2 expression and trafficking are found in patients with Dubin-Johnson syndrome,47 an inherited form of hyperbiluribinemia, as well as in the GY/TR− and Eisai rat strains,48,49 both of which exhibit a specific defect in organic anion transport. One might therefore predict that InsP3R2 KO animals would also have increased serum bilirubin levels. However, our results show serum bilirubin levels in InsP3R2 KO mice that are similar to what is found in WT mice. This may reflect appropriate localization of Mrp2 in basal conditions in the KO animals (Fig. 7). Together, these findings suggest that InsP3R2-dependent Ca2+ release may be important for recruitment and insertion of additional Mrp2 transporters into the canalicular membrane but would not be essential for the behavior of this transporter under basal conditions. An alternative explanation is that any reduction in bile acid-independent bile flow, the fraction of bile flow that is directly regulated by Mrp2 activity,50 is compensated for by transport events taking place downstream, at the level of the biliary tree.
Other second messengers and signaling pathways have been implicated in transporter trafficking in hepatocytes. Most notably, cyclic adenosine monophosphate (cAMP) stimulates insertion of Mrp2 into the plasma membrane in rat hepatocytes in short-term culture,36 and this is partially mediated by activation of PI3K and PKCδ.51 Cyclic AMP also potentiates Ca2+ oscillations in isolated rat hepatocytes.52,53 Moreover, cAMP specifically enhances InsP3R2-dependent Ca2+ release independent of the activation of protein kinase A.54 In light of our findings that InsP3R-mediated (most likely InsP3R2) Ca2+ release enhances canalicular insertion of Mrp2, these observations raise the interesting possibility that cAMP-mediated canalicular targeting of Mrp2 occurs via the effects of cAMP on InsP3R2 and Ca2+ release. However, the importance of this particular cross-talk pathway between Ca2+ and cAMP signaling remains to be demonstrated in hepatocytes. Moreover, it remains to be determined whether InsP3R2-dependent Ca2+ signals also control the trafficking and canalicular targeting of other transporters that are important for bile formation.
Acknowledgment
The authors thank Kathy Harry for help with hepatocyte isolations and Agnes Ferguson for assistance with TIRF microscopy.
Supported by National Institutes of Health grants DK54710, DK34989, DK57751, and DK61747 and by Fundaçaão de Amparo à Pesquisa do Estado de Saão Paulo (FAPESP, Brazil).
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, et al. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem. 2002;277:27517–27527. doi: 10.1074/jbc.M203002200. [DOI] [PubMed] [Google Scholar]
- 3.Thompson M, Andrade VA, Andrade SJ, Pusl T, Ortega JM, Goes AM, et al. Inhibition of the TEF/TEAD transcription factor activity by nuclear calcium and distinct kinase pathways. Biochem Biophys Res Commun. 2003;301:267–274. doi: 10.1016/s0006-291x(02)03024-3. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, et al. Nucleoplasmic calcium is required for cell proliferation. J Biol Chem. 2007;282:17061–17068. doi: 10.1074/jbc.M700490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- 6.Joseph SK, Hajnoczky G. IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis. 2007;12:951–968. doi: 10.1007/s10495-007-0719-7. [DOI] [PubMed] [Google Scholar]
- 7.Minagawa N, Kruglov EA, Dranoff JA, Robert ME, Gores GJ, Nathanson MH. The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals. J Biol Chem. 2005;280:33637–33644. doi: 10.1074/jbc.M503210200. [DOI] [PubMed] [Google Scholar]
- 8.Nathanson MH, Gautam A, Ng OC, Bruck R, Boyer JL. Hormonal regulation of paracellular permeability in isolated rat hepatocyte couplets. Am J Physiol Gastrointest Liver Physiol. 1992;262:G1079–G1086. doi: 10.1152/ajpgi.1992.262.6.G1079. [DOI] [PubMed] [Google Scholar]
- 9.Blackmore PF, Strickland WG, Bocckino SB, Exton JH. Mechanism of hepatic glycogen synthase in activation induced by Ca2+-mobilizing hormones. Biochem J. 1986;237:235–242. doi: 10.1042/bj2370235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathanson MH, Rios-Velez L, Burgstahler AD, Mennone A. Communication via gap junctions modulates bile secretion in the isolated perfused rat liver. Gastroenterology. 1999;116:1176–1183. doi: 10.1016/s0016-5085(99)70021-1. [DOI] [PubMed] [Google Scholar]
- 11.Nathanson MH, Gautam A, Bruck R, Isales CM, Boyer JL. Effects of Ca2+ agonists on cytosolic Ca2+ in isolated hepatocytes and on bile secretion in the isolated perfused rat liver. Hepatology. 1992;15:107–116. doi: 10.1002/hep.1840150119. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez E, Leite MF, Guerra MT, Kruglov EA, Bruna-Romero O, Rodrigues MA, et al. The spatial distribution of inositol 1,4,5-trisphosphate receptor isoforms shapes Ca2+ waves. J Biol Chem. 2007;282:10057–10067. doi: 10.1074/jbc.M700746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirata K, Pusl T, O'Neill AF, Dranoff JA, Nathanson MH. The type II inositol 1,4,5-trisphosphate receptor can trigger Ca2+ waves in rat hepatocytes. Gastroenterology. 2002;122:1088–1100. doi: 10.1053/gast.2002.32363. [DOI] [PubMed] [Google Scholar]
- 14.Nagata J, Guerra MT, Shugrue CA, Gomes DA, Nagata N, Nathanson MH. Lipid rafts establish calcium waves in hepatocytes. Gastroenterology. 2007;133:256–267. doi: 10.1053/j.gastro.2007.03.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oude Elferink RPJ, Bakker CTM, Roelofsen H, Middelkoop E, Ottenhoff R, Heijn M, et al. Accumulation of organic anion in intracellular vesicles of cultured rat hepatocytes is mediated by the canalicular multispecific organic anion transporter. Hepatology. 1993;17:434–444. [PubMed] [Google Scholar]
- 16.Ito K, Miyashita Y, Kasai H. Micromolar and submicromolar Ca2+ spikes regulating distinct cellular functions in pancreatic acinar cells. EMBO J. 1997;16:242–251. doi: 10.1093/emboj/16.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 18.Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflugers Arch. 2007;453:643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 19.Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. Altered localization and activity of canalicular Mrp2 in estradiol-17beta-D-glucuronide-induced cholestasis. Hepatology. 2002;35:1409–1419. doi: 10.1053/jhep.2002.33327. [DOI] [PubMed] [Google Scholar]
- 20.Kubitz R, Wettstein M, Warskulat U, Haussinger D. Regulation of the multidrug resistance protein 2 in the rat liver by lipopolysaccharide and dexamethasone. Gastroenterology. 1999;116:401–410. doi: 10.1016/s0016-5085(99)70138-1. [DOI] [PubMed] [Google Scholar]
- 21.Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, et al. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33:1206–1216. doi: 10.1053/jhep.2001.24034. [DOI] [PubMed] [Google Scholar]
- 22.Rost D, Kartenbeck J, Keppler D. Changes in the localization of the rat canalicular conjugate export pump Mrp2 in phalloidin-induced cholestasis. Hepatology. 1999;29:814–821. doi: 10.1002/hep.510290319. [DOI] [PubMed] [Google Scholar]
- 23.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beuers U, Nathanson MH, Boyer JL. Effects of tauroursodeoxycholic acid on cytosolic Ca2+ signals in isolated rat hepatocytes. Gastroenterology. 1993;104:604–612. doi: 10.1016/0016-5085(93)90433-d. [DOI] [PubMed] [Google Scholar]
- 25.Beuers U, Nathanson MH, Isales CM, Boyer JL. Tauroursodeoxycholic acid stimulates hepatocellular exocytosis by mobilization of extracellular Ca2+, a mechanism defective in cholestasis. J Clin Invest. 1993;92:2984–2993. doi: 10.1172/JCI116921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- 27.Nies AT, Konig J, Cui Y, Brom M, Spring H, Keppler D. Structural requirements for the apical sorting of human multidrug resistance protein 2 (ABCC2) Eur J Biochem. 2002;269:1866–1876. doi: 10.1046/j.1432-1033.2002.02832.x. [DOI] [PubMed] [Google Scholar]
- 28.Graf J, Gautam A, Boyer JL. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc Natl Acad Sci USA. 1984;81:6516–6520. doi: 10.1073/pnas.81.20.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathanson MH, Burgstahler AD, Fallon MB. Multi-step mechanism of polarized Ca2+ wave patterns in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 1994;267:G338–G349. doi: 10.1152/ajpgi.1994.267.3.G338. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, et al. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131:878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nies AT, Cantz T, Brom M, Leier I, Keppler D. Expression of the apical conjugate export pump, Mrp2, in the polarized hepatoma cell line, WIF-B. Hepatology. 1998;28:1332–1340. doi: 10.1002/hep.510280523. [DOI] [PubMed] [Google Scholar]
- 32.Hirata K, Dufour JF, Shibao K, Knickelbein R, O'Neill AF, Bode HP, et al. Regulation of Ca(2+) signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology. 2002;36:284–296. doi: 10.1053/jhep.2002.34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Correa PR, Guerra MT, Leite MF, Spray DC, Nathanson MH. Endotoxin unmasks the role of gap junctions in the liver. Biochem Biophys Res Commun. 2004;322:718–726. doi: 10.1016/j.bbrc.2004.07.192. [DOI] [PubMed] [Google Scholar]
- 36.Roelofsen H, Soroka CJ, Keppler D, Boyer JL. Cyclic AMP stimulates sorting of the canalicular organic anion transporter (Mrp2/cMoat) to the apical domain in hepatocyte couplets. J Cell Sci. 1998;111:1137–1145. doi: 10.1242/jcs.111.8.1137. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, et al. Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol. 1999;277:G12–G21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- 38.Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10:1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 39.Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. doi: 10.1136/gut.2007.140871. [DOI] [PubMed] [Google Scholar]
- 40.Tsien RY. Imagining imaging's future. Nat Rev Mol Cell Biol. 2003;(Suppl):SS16–SS21. [PubMed] [Google Scholar]
- 41.Toomre D, Manstein DJ. Lighting up the cell surface with evanescent wave microscopy. Trends Cell Biol. 2001;11:298–303. doi: 10.1016/s0962-8924(01)02027-x. [DOI] [PubMed] [Google Scholar]
- 42.Huang S, Lifshitz LM, Jones C, Bellve KD, Standley C, Fonseca S, et al. Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes. Mol Cell Biol. 2007;27:3456–3469. doi: 10.1128/MCB.01719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, et al. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 44.Leite MF, Burgstahler AD, Nathanson MH. Ca2+ waves require sequential activation of inositol 1,4,5-trisphosphate receptors and ryanodine receptors in pancreatic acinar cells. Gastroenterology. 2002;122:415–427. doi: 10.1053/gast.2002.30982. [DOI] [PubMed] [Google Scholar]
- 45.Nathanson MH, Burgstahler AD, Mennone A, Boyer JL. Characterization of cytosolic Ca2+ signaling in rat bile duct epithelia. Am J Physiol Gastrointest Liver Physiol. 1996;271:G86–G96. doi: 10.1152/ajpgi.1996.271.1.G86. [DOI] [PubMed] [Google Scholar]
- 46.Bruck R, Nathanson MH, Roelofsen H, Boyer JL. Effects of protein kinase C and cytosolic Ca2+ on exocytosis in the isolated perfused rat liver. Hepatology. 1994;20:1032–1040. doi: 10.1002/hep.1840200436. [DOI] [PubMed] [Google Scholar]
- 47.Kartenbeck J, Leuschner U, Mayer R, Keppler D. Absence of the canalicular isoform of the MRP gene-encoded conjugate export pump from the hepatocytes in Dubin-Johnson syndrome. Hepatology. 1996;23:1061–1066. doi: 10.1053/jhep.1996.v23.pm0008621134. [DOI] [PubMed] [Google Scholar]
- 48.Kitamura T, Jansen P, Hardenbrook C, Kamimoto Y, Gatmaitan Z, Arias IM. Defective ATP-dependent bile canalicular transport of organic anions in mutant (TR-) rats with conjugated hyperbilirubinemia. Proc Natl Acad Sci USA. 1990;87:3557–3561. doi: 10.1073/pnas.87.9.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takikawa H, Sano N, Narita T, Uchida Y, Yamanaka M, Horie T, et al. Biliary excretion of bile acid conjugates in a hyperbilirubinemic mutant Sprague-Dawley rat. Hepatology. 1991;14:352–360. [PubMed] [Google Scholar]
- 50.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 51.Schonhoff CM, Gillin H, Webster CR, Anwer MS. Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology. 2008;47:1309–1316. doi: 10.1002/hep.22162. [DOI] [PubMed] [Google Scholar]
- 52.Chatton JY, Cao Y, Liu H, Stucki JW. Permissive role of cAMP in the oscillatory Ca2+ response to inositol 1,4,5-trisphosphate in rat hepatocytes. Biochem J. 1998;330:1411–1416. doi: 10.1042/bj3301411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez-Bueno A, Marrero I, Cobbold PH. Different modulatory effects of elevated cyclic AMP on cytosolic Ca2+ spikes induced by phenylephrine or vasopressin in single rat hepatocytes. Biochem J. 1993;291:163–168. doi: 10.1042/bj2910163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tovey SC, Dedos SG, Taylor EJ, Church JE, Taylor CW. Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates direct sensitization of IP3 receptors by cAMP. J Cell Biol. 2008;183:297–311. doi: 10.1083/jcb.200803172. [DOI] [PMC free article] [PubMed] [Google Scholar]